Multi-Objective Optimization of Cost Saving and Emission Reduction in Blast Furnace Ironmaking Process
Abstract
1. Introduction
2. Modeling
2.1. Blast Furnace Carbon Loss Calculation Model
2.1.1. Calculation of Direct Reduction Degree
2.1.2. Calculation of Carbon Loss
- (1)
- Indirect reduction zone. In this region, the direct reduction reaction has not occurred. The indirect reduction reaction equations as follows:
- (2)
- Coupled direct reduction zone. In this region, solution loss reaction and indirect reduction reaction can be coupled into direct reduction reaction. The solution loss reaction equations are shown as:Equation (4) + Equation (6) and Equation (5) + Equation (7) can obtain direct reduction reaction equation as follows:
- (3)
- Molten direct reduction zone. In this region, the indirect reduction reaction has not occurred. And coke is the only material that remains a solid phase, the direct reduction reaction takes place between coke and molten slag. The molten direct reduction reaction equation is shown as:
2.2. Blast Furnace Emission Reduction Optimization Mathematical Model
2.2.1. Optimization Variables
2.2.2. Objective Functions
2.2.3. Constraint Conditions
3. Results and Discussions
3.1. Different Objective Optimization Results
3.2. Analysis of Main Influence Factors
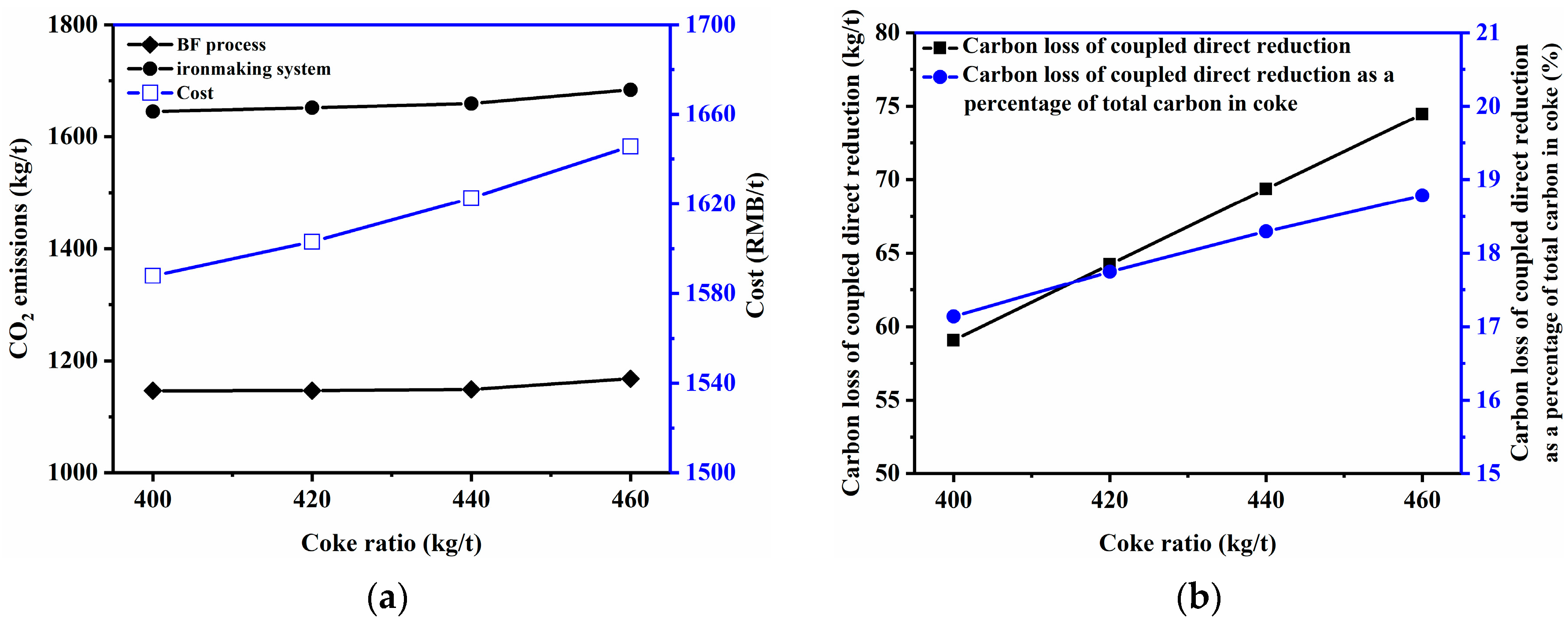
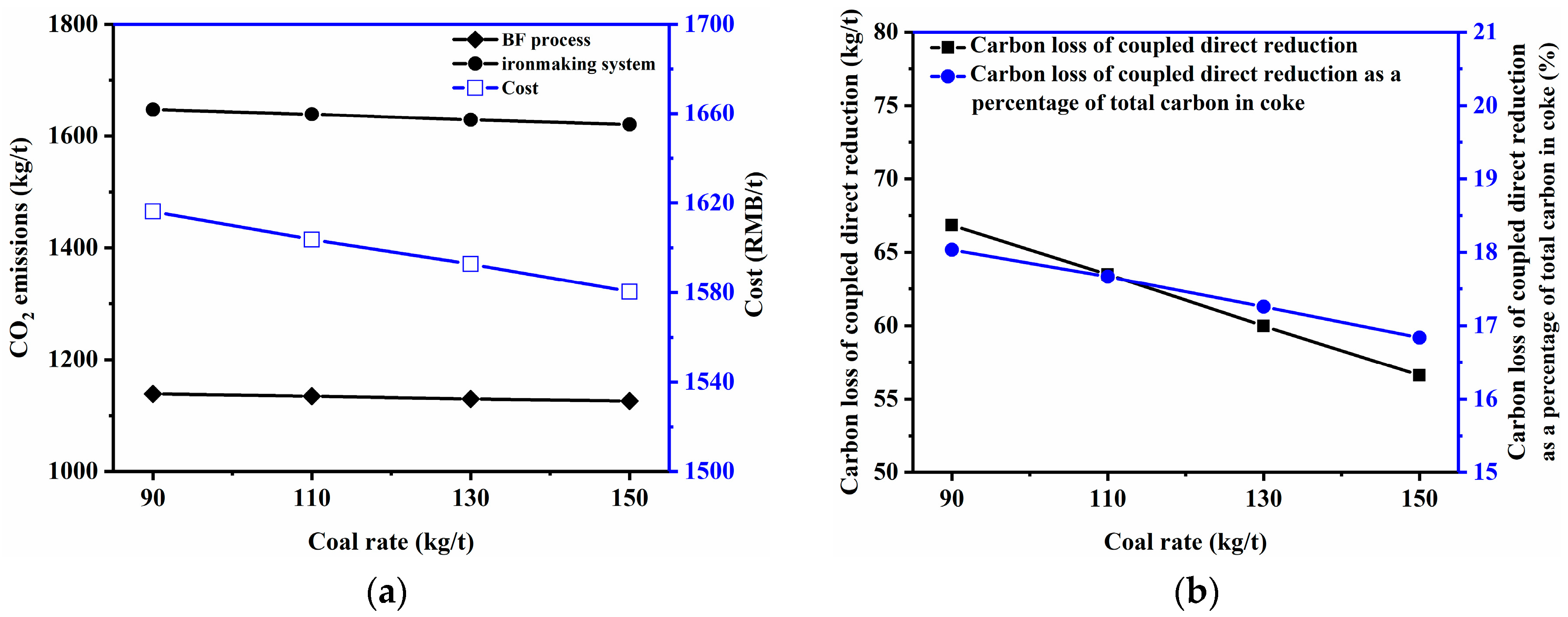
3.2.1. Coke Ratio and Coal Rate
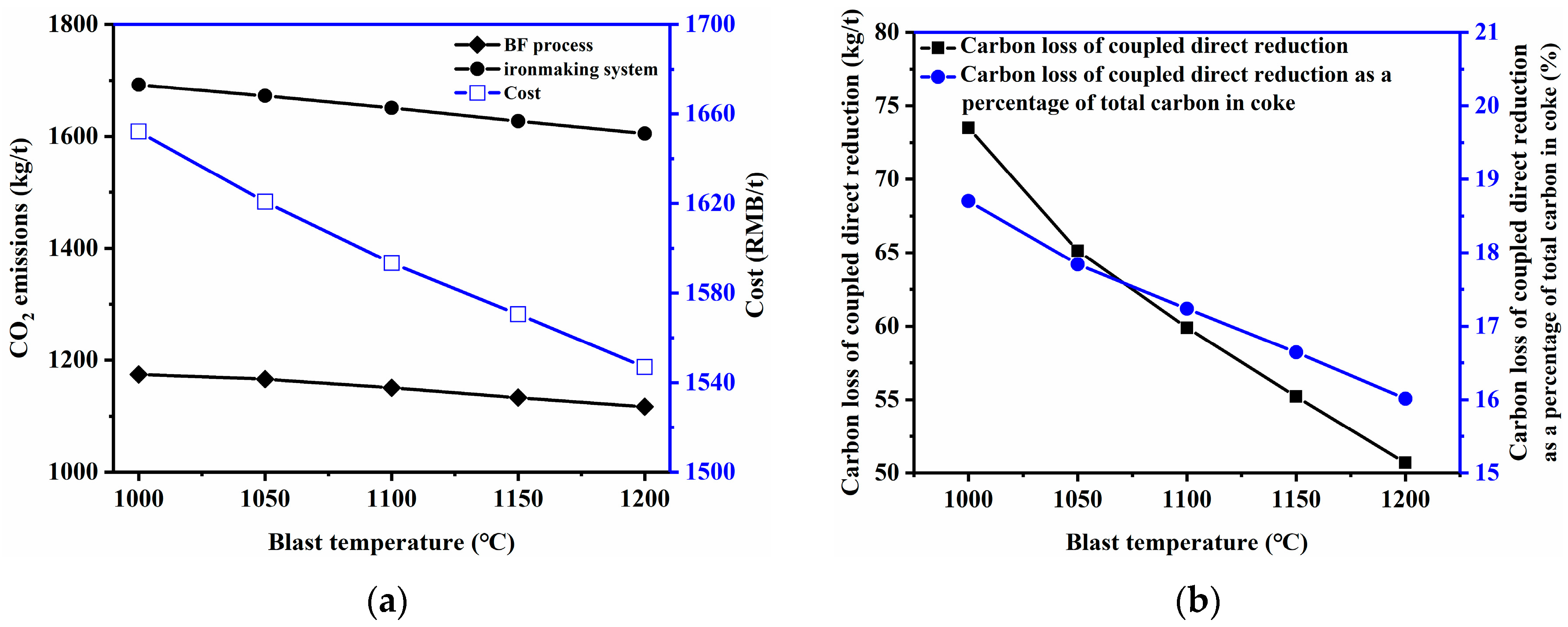
3.2.2. Blast Temperature
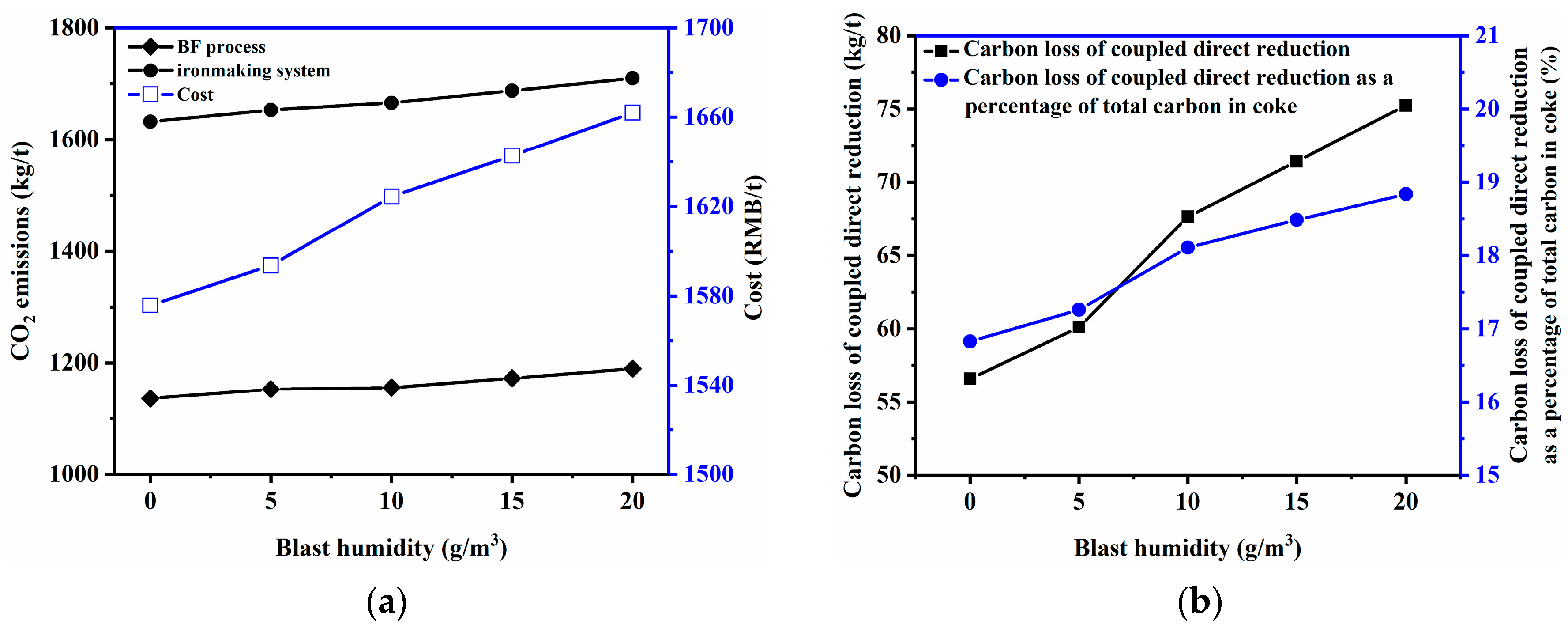
3.2.3. Blast Humidity
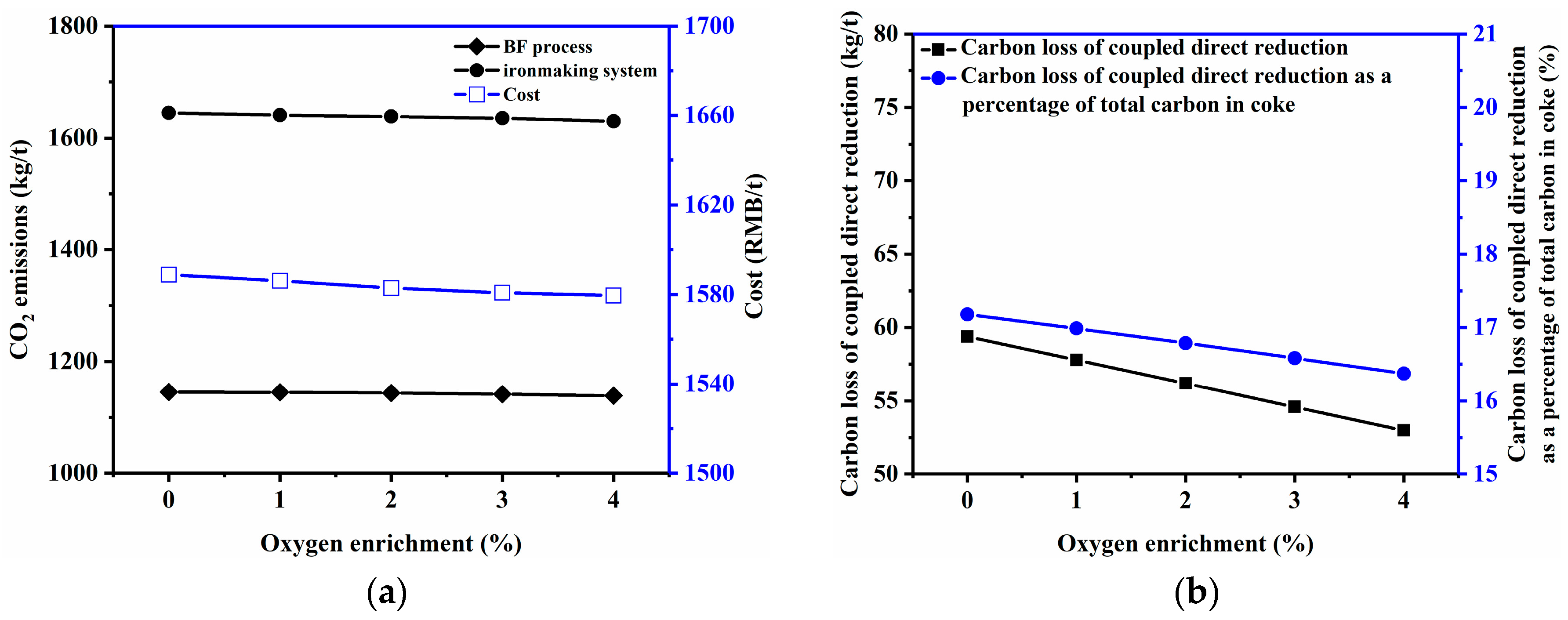
3.2.4. Oxygen Enrichment Rate
3.2.5. Burden Metallization Ratio
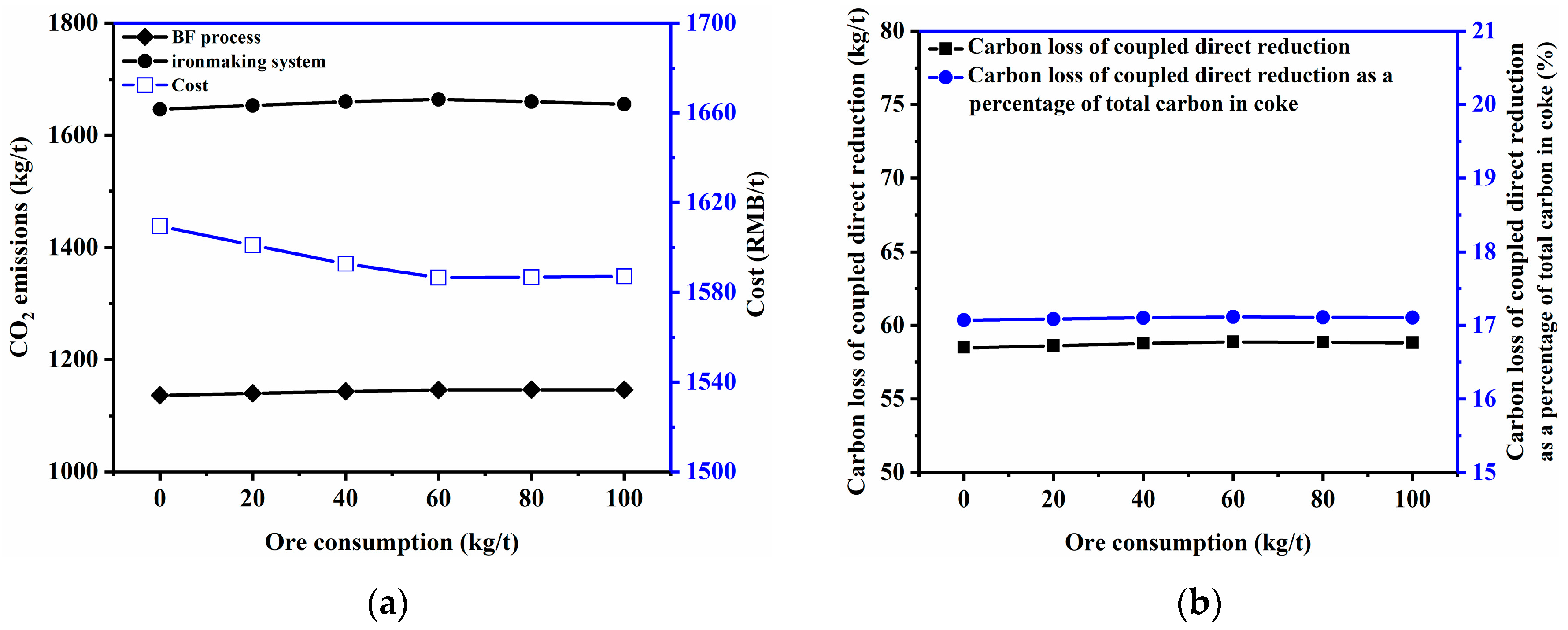
3.2.6. Ore Consumption
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| rd | direct reduction degree |
| ωFe(DR) | the amount of Fe that is generated by direct reduction, kg/t |
| ωFe(HM) | the amount of Fe in hot metal, kg/t |
| ωC(DR) | the carbon loss of direct reduction, kg/t |
| ωC(total) | the total amount of carbon in coke, kg/t |
| ωC(Vad) | the amount of carbon in volatiles of coke, kg/t |
| ωC([C]) | the amount of carbon consumed by hot metal carburization, kg/t |
| ωC(XO) | the amount of carbon consumed by non-ferrous oxide direct reduction, kg/t |
| ωC(H2O) | the amount of carbon consumed by oxygen of blast moisture, kg/t |
| ωC(combustion) | the amount of carbon consumed by combustion of coke in raceway, kg/t |
| ωC(dust) | the amount of carbon in dust, kg/t |
| ωC(CDR) | the amount of carbon loss of coupled direct reduction, kg/t |
| ϕLC | the coke carbon loss rate before coke falling into the raceway |
| ϕCC | the completed ratio of hot metal carburization |
| ωC(MR) | the amount of carbon loss of molten direct reduction, kg/t |
References
- International Energy Agency. Global Energy & CO2 Status Report. Available online: https://www.iea.org/geco/ (accessed on 23 March 2018).
- Xu, C.; Cang, D.Q. A Brief Overview of Low CO2 Emission Technologies for Iron and Steel Making. J. Iron Steel Res. 2010, 17, 1–7. [Google Scholar] [CrossRef]
- Liu, Z.J.; Zhang, J.L.; Yang, T.J. Low Carbon Operation of Super-Large Blast Furnaces in China. ISIJ Int. 2015, 55, 1146–1156. [Google Scholar] [CrossRef]
- Zhang, Q.; Jia, G.Y.; Cai, J.J.; Shen, F.M. Carbon Flow Analysis and CO2 Emission Reduction Strategies of Iron-Making System in Steel Enterprise. J. Northeast. Univ. 2013, 34, 392–394. (In Chinese) [Google Scholar] [CrossRef]
- Yang, T.J.; Zhang, J.L.; Liu, Z.J.; Li, K.J. The Transformation, Upgrade and Innovation of China’s Blast Furnace Ironmaking Technologies in the “New Normal” Economy. In Proceedings of the 10th CSM Steel Congress & the 6th Baosteel Biennial Academic Conference, Beijing, China, 21–23 October 2015; pp. 1–17. [Google Scholar]
- Yang, T.J.; Zhang, J.L.; Liu, Z.J.; Li, K.J. Transformation and Upgrade of Ironmaking Industry via Addressing Over Capacity, Dufusing Difficulty and by Technical Innovation. Ironmaking 2016, 35, 1–10. (In Chinese) [Google Scholar]
- Zou, Z.P.; Xiang, Z.Y.; Zhao, R.H.; Luo, Y.W. Development Status and Trend of Blast Furnace Ironmaking Technology at Home and Abroad. Iron Steel Technol. 2011, 2, 4–10. (In Chinese) [Google Scholar]
- Zhang, Q.; Yao, T.H.; Cai, J.J.; Shen, F.M. On the Multi-objective Optimal Model of Blast Furnace Iron-Making Process and Its Application. J. Northeast. Univ. 2011, 32, 270–273. (In Chinese) [Google Scholar] [CrossRef]
- Zou, Z.P.; Guo, X.Z.; Wang, G.; Xiang, Z.Y.; Wang, R.L. Discussion of calculation method of BF’s CO2 emission. J. Cent. South Univ. 2012, 43, 257–261. (In Chinese) [Google Scholar]
- Wang, C.; Ryman, C.; Dahl, J. Potential CO2 Emission Reduction for BF-BOF Steelmaking Based on Optimized Use of Ferrous Burden Materials. Int. J. Greenh. Gas Control 2009, 3, 29–38. [Google Scholar] [CrossRef]
- Shangguan, F.Q.; Zhang, C.X.; Li, X.P.; Fan, B.; Huang, D. Discussion on the CO2 Emission Calculation Methods in Iron and Steel Industry. J. Iron Steel Res. 2010, 11, 1–5. (In Chinese) [Google Scholar] [CrossRef]
- Xu, W.R.; Wu, X.C.; Chen, J.M.; Qian, H.; Lin, J.J. Influence of High PCR Operation on Coke Degradation in Blast Furnace. Iron Steel 2003, 38, 4–7, 24. (In Chinese) [Google Scholar] [CrossRef]
- Cao, J.; Zhang, Q.; Wu, X.C. Coke Size Degradation of Baosteel in Blast Furnace. Bao Steel Technol. 2003, 1, 39–43. (In Chinese) [Google Scholar] [CrossRef]
- Ren, R.X.; Fang, J.; Zhang, J.; Zhang, H. Strength Distribution of Coke in Blast Furnace. J. Hebei Inst. Technol. 2007, 29, 21–26. (In Chinese) [Google Scholar] [CrossRef]
- Liu, P.X.; Fang, J.; Guo, L.; Gong, R.J. Research on the Distribution of Coke Decarbonization. Henan Metall. 2006, 14, 9–16. (In Chinese) [Google Scholar] [CrossRef]
- Wang, P.; Li, J.X.; Zhou, L.Y. Fundamental Study on Coke Degradation in BF with Oxygen Enriched Blast and High Hydrogen Atmosphere. J. Iron Steel Res. 2005, 12, 1–4, 10. [Google Scholar] [CrossRef]
- Haapakangas, J.; Suopajärvi, H.; Iljana, M.; Kemppainen, A.; Mattila, O.; Heikkinen, E.P.; Samuelsson, C.; Fabritius, T. Coke Reactivity in Simulated Blast Furnace Shaft Conditions. Metall. Mater. Trans. B 2016, 47, 2357–2370. [Google Scholar] [CrossRef]
- Xing, X.; Rogers, H.; Zhang, G.Q.; Hockings, K.; Zulli, P.; Ostrovski, O. Coke Degradation under Simulated Blast Furnace Conditions. ISIJ Int. 2016, 56, 786–793. [Google Scholar] [CrossRef]
- Fang, J.; Wang, X.Y.; Guo, L.; Shao, J.H. Research on High Temperature Compressive Strength of Coke. Iron Steel 2006, 41, 20–23. (In Chinese) [Google Scholar] [CrossRef]
- Cui, P.; Wang, W.W.; Wang, Z.L.; Huang, L.; Qu, K.L. Study on High Temperature Compressive Strength of Coke in the Presence of CO2. Fuel Chem. Process. 2014, 45, 1–7. [Google Scholar] [CrossRef]
- Guo, R.; Wang, Q.; Zhang, S. Influence of Solution Loss Reaction on Post Reaction Strength of Coke. Coal Convers. 2012, 35, 12–16. [Google Scholar] [CrossRef]
- Liu, Z.S.; Wang, Q.; Zou, Z.S.; Tan, G.L. Non-isothermal thermogravimetric investigation on kinetics of coke gasification with CO2. Res. Iron Steel 2010, 38, 1–3, 43. [Google Scholar]
- Wu, S.L.; Han, H.L.; Xu, S.B.; Niu, B. Study on Mathematical Model for Burden Optimization of Blast Furnace. Iron Steel 2007, 42, 19–23. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Che, X.R.; Zhang, H.B. Establishment and Validation of Multi-objective Optimization Model of Blast Furnace. Chin. J. Process Eng. 2017, 17, 178–182. (In Chinese) [Google Scholar] [CrossRef]
- Zheng, Y.T.; Fang, J.; Cai, G.B. Comparison of multi-objective optimization methods on engineering design. J. Beijing Univ. Aeronaut. Astronaut. 2006, 32, 860–864. [Google Scholar] [CrossRef]
- Suopajärvi, H.; Pongrácz, E.; Fabritius, T. Bioreducer use in Finnish blast furnace ironmaking – Analysis of CO2 emission reduction potential and mitigation cost. Appl. Energy 2014, 124, 82–93. [Google Scholar] [CrossRef]
- Wang, L.; Buvarp, F.; Skreiberg, Ø.; Bartocci, P.; Fantozzi, F. A Study on Densification and CO2 Gasification of Biocarbon. Chem. Eng. Trans. 2018, 65, 145–150. [Google Scholar] [CrossRef]
- Goleczka, J.; Tucker, J. Coke quality and its assessment in the CRE laboratory. J. Proc. Ironmak. Conf. 1985, 44, 217–232. [Google Scholar]
- Deng, S.Q. Process of iron and slag formation in the B. F. Gangtie 1982, 11, 69–71. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, Z.J.; Zhang, J.L.; Jiao, K.X.; Liu, Z.L. Research Progress of Iron Carburization in Blast Furnace. In Proceedings of the 6th International Symposium on High-Temperature Metallurgical & TMS (The Minerals, Metals & Materials Society), Orlando, FL, USA, 15–19 March 2015; pp. 627–633. [Google Scholar]

| Parameter | Variable | Single Objective Optimization | Multi-Objective Optimization | Actual Value | ||
|---|---|---|---|---|---|---|
| Cost | CO2 Emissions of Blast Furnaces Process | CO2 Emissions of Ironmaking System | ||||
| Sinter consumption (kg/t) | x1 | 1518 | 1005 | 1005 | 1337 | 1309 |
| Pellet 1 consumption (kg/t) | x2 | 115 | 605 | 605 | 202 | 211 |
| Pellet 2 consumption (kg/t) | x3 | 0 | 0 | 0 | 0 | 65 |
| Pellet 3 consumption (kg/t) | x4 | 0 | 0 | 0 | 0 | 50 |
| Ore consumption (kg/t) | x5 | 56 | 0 | 0 | 150 | 28 |
| Flux consumption (kg/t) | x6 | 0 | 0 | 0 | 0 | 4 |
| Coke consumption (kg/t) | x7 | 400 | 410 | 410 | 401 | 425 |
| Pulverized coal consumption (kg/t) | x8 | 152 | 97 | 97 | 149 | 110 |
| Blast volume (m3/t) | x9 | 1394 | 1268 | 1268 | 1390 | 1371 |
| Oxygen enrichment rate (%) | x10 | 0 | 0 | 0 | 0 | 0 |
| Blast humidity (g/m3) | x11 | 3 | 3 | 3 | 3 | 3 |
| Blast temperature (°C) | x12 | 1112 | 1112 | 1112 | 1112 | 1112 |
| Gas volume (m3/t) | x13 | 1863 | 1684 | 1684 | 1856 | 1676 |
| Pig iron Fe content (%) | x14 | 93.871 | 93.911 | 93.911 | 93.871 | 94.23 |
| Pig iron C content (%) | x15 | 5.2 | 5.2 | 5.2 | 5.2 | 4.80 |
| Pig iron Si content (%) | x16 | 0.4 | 0.4 | 0.4 | 0.4 | 0.43 |
| Pig iron P content (%) | x17 | 0.077 | 0.077 | 0.077 | 0.077 | 0.097 |
| Pig iron Mn content (%) | x18 | 0.32 | 0.28 | 0.28 | 0.32 | 0.308 |
| Pig iron S content (%) | x19 | 0.047 | 0.047 | 0.047 | 0.047 | 0.02 |
| Pig iron Ti content (%) | x20 | 0.085 | 0.085 | 0.085 | 0.085 | 0.12 |
| Theoretical combustion temperature (°C) | — | 2073 | 2141 | 2141 | 2077 | 2133 |
| Cost (RMB/t) | — | 1586 | 1657 | 1657 | 1589 | 1650 |
| CO2 emissions of blast furnaces process (kg/t) | — | 1146 | 1112 | 1112 | 1145 | 1158 |
| CO2 emissions of ironmaking system (kg/t) | — | 1665 | 1600 | 1600 | 1645 | 1672 |
| Carbon loss of coupled direct reduction (kg/t) | — | 59.00 | 61.80 | 61.80 | 59.37 | 68.68 |
| Carbon loss of coupled direct reduction as a percentage of total carbon in coke (%) | — | 17.13 | 17.51 | 17.51 | 17.17 | 18.77 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, S.; Wu, S.; Song, B.; Kou, M.; Zhou, H.; Gu, K. Multi-Objective Optimization of Cost Saving and Emission Reduction in Blast Furnace Ironmaking Process. Metals 2018, 8, 979. https://doi.org/10.3390/met8120979
Yao S, Wu S, Song B, Kou M, Zhou H, Gu K. Multi-Objective Optimization of Cost Saving and Emission Reduction in Blast Furnace Ironmaking Process. Metals. 2018; 8(12):979. https://doi.org/10.3390/met8120979
Chicago/Turabian StyleYao, Shun, Shengli Wu, Bo Song, Mingyin Kou, Heng Zhou, and Kai Gu. 2018. "Multi-Objective Optimization of Cost Saving and Emission Reduction in Blast Furnace Ironmaking Process" Metals 8, no. 12: 979. https://doi.org/10.3390/met8120979
APA StyleYao, S., Wu, S., Song, B., Kou, M., Zhou, H., & Gu, K. (2018). Multi-Objective Optimization of Cost Saving and Emission Reduction in Blast Furnace Ironmaking Process. Metals, 8(12), 979. https://doi.org/10.3390/met8120979






