Formation and Evolution of Inclusions with Different Adding Order of Magnesium and Sulfur in Al-Killed Free-Cutting Steel
Abstract
:1. Introduction
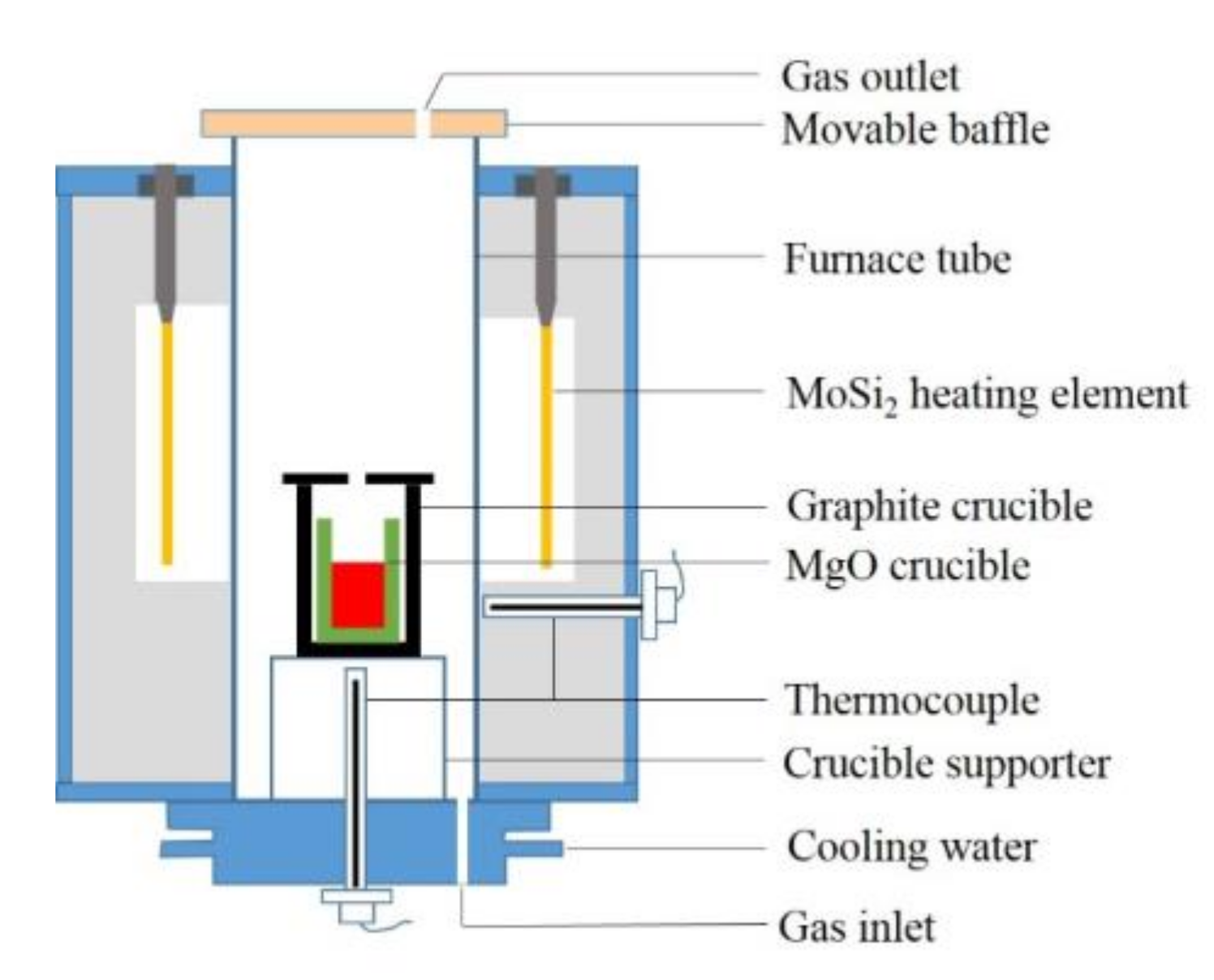
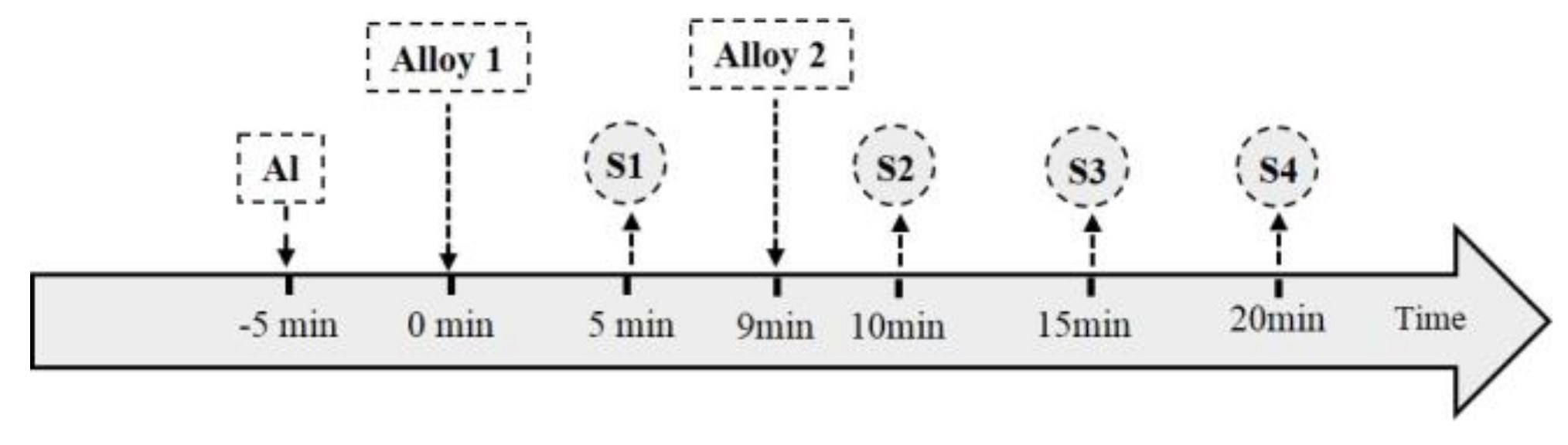
2. Experimental Methods
2.1. Experimental Procedure
2.2. Analysis Methods for Steels and Inclusions
3. Results and Discussions
3.1. Chemical Compositions of Steels
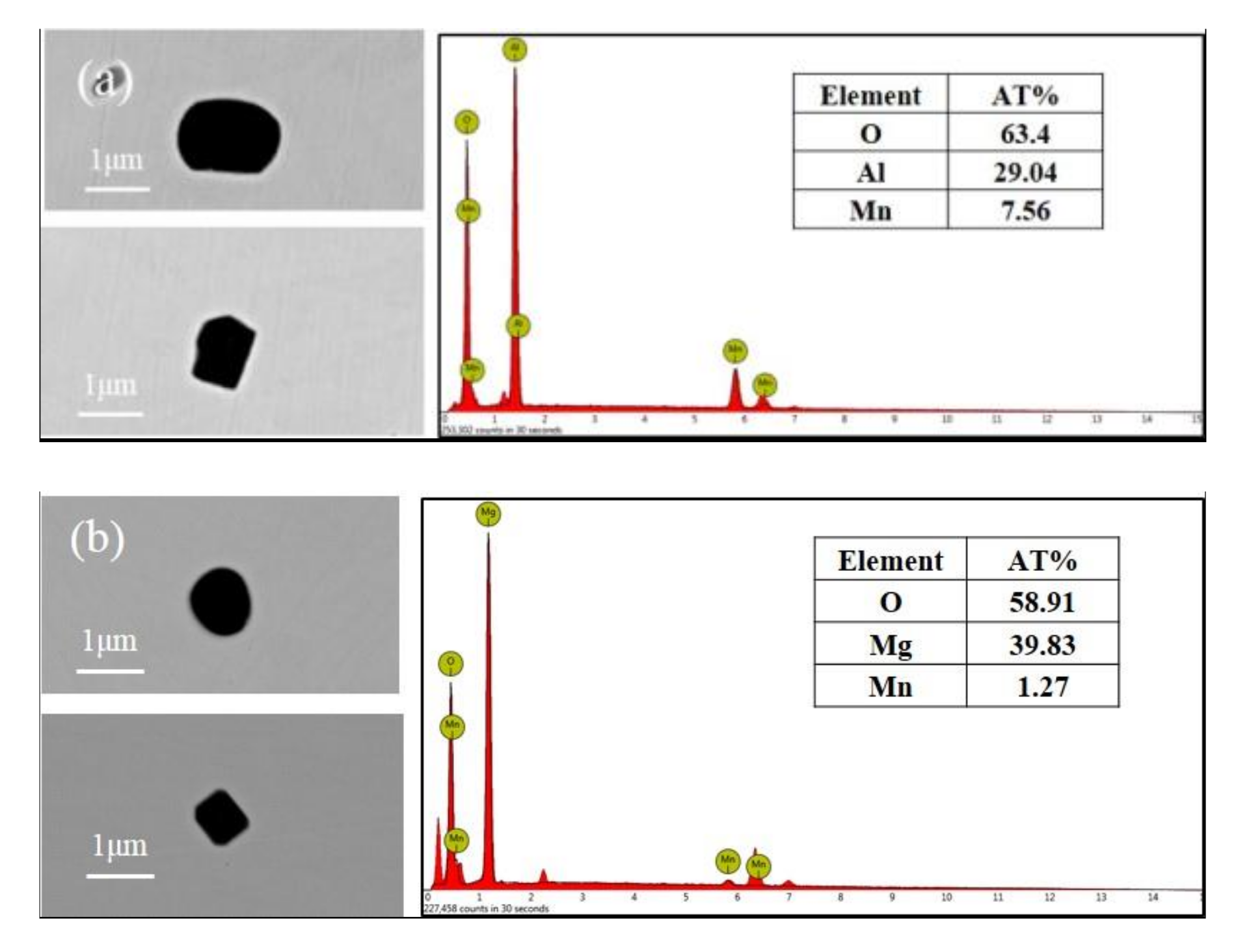
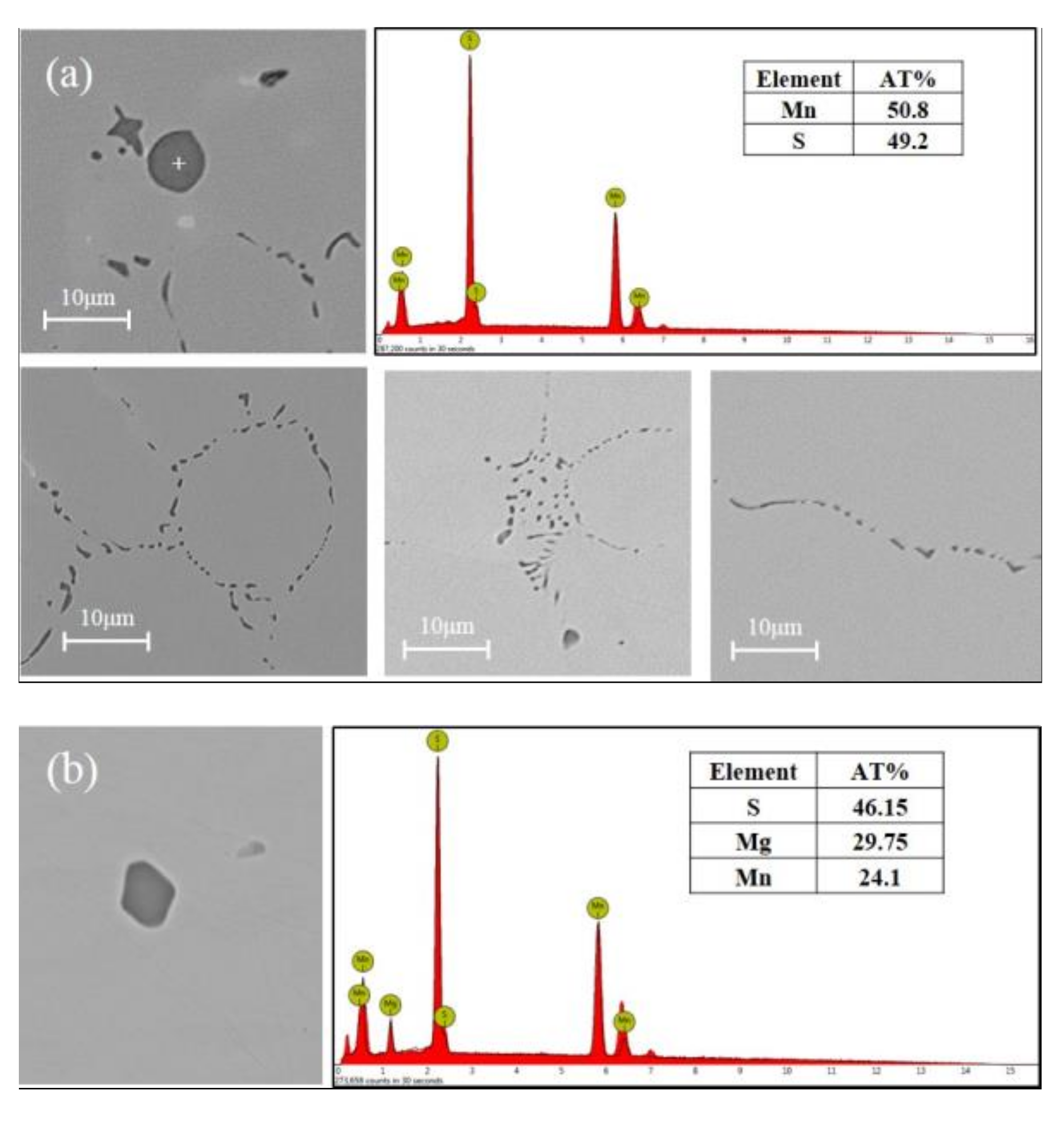
3.2. Inclusions in Steels
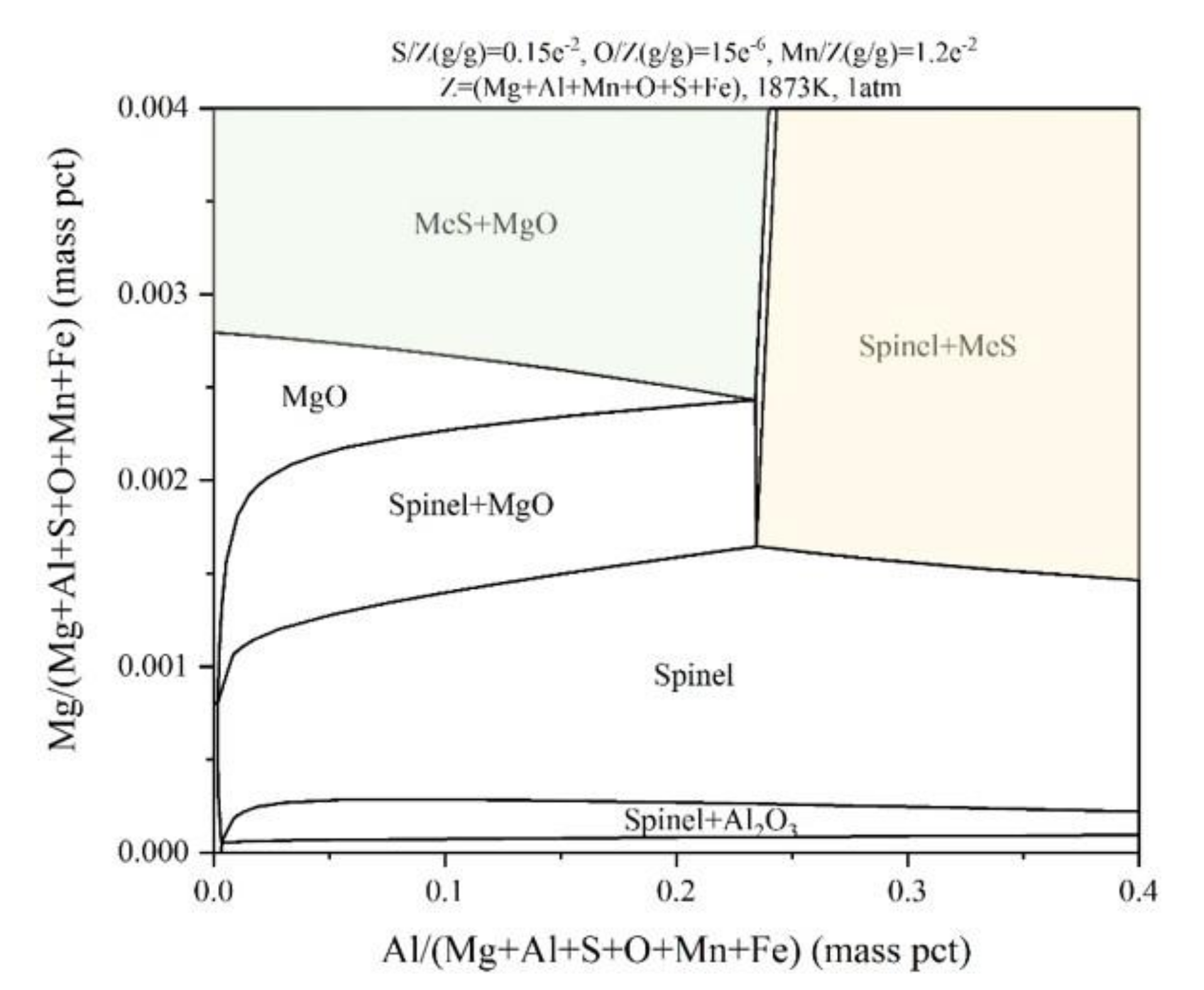
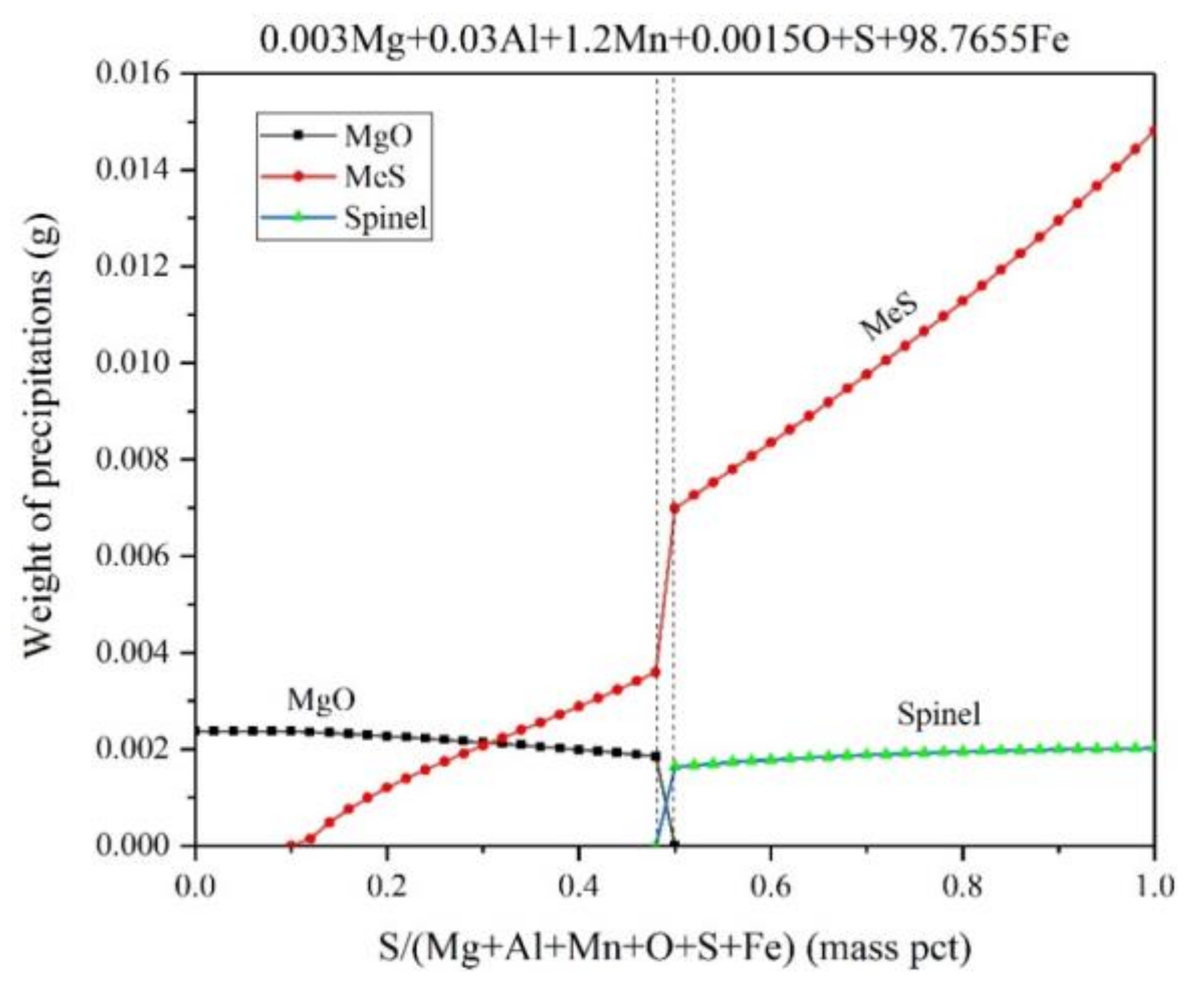
3.3. Thermodynamic Calculations for Fe-Mg-Al-Mn-O-S System
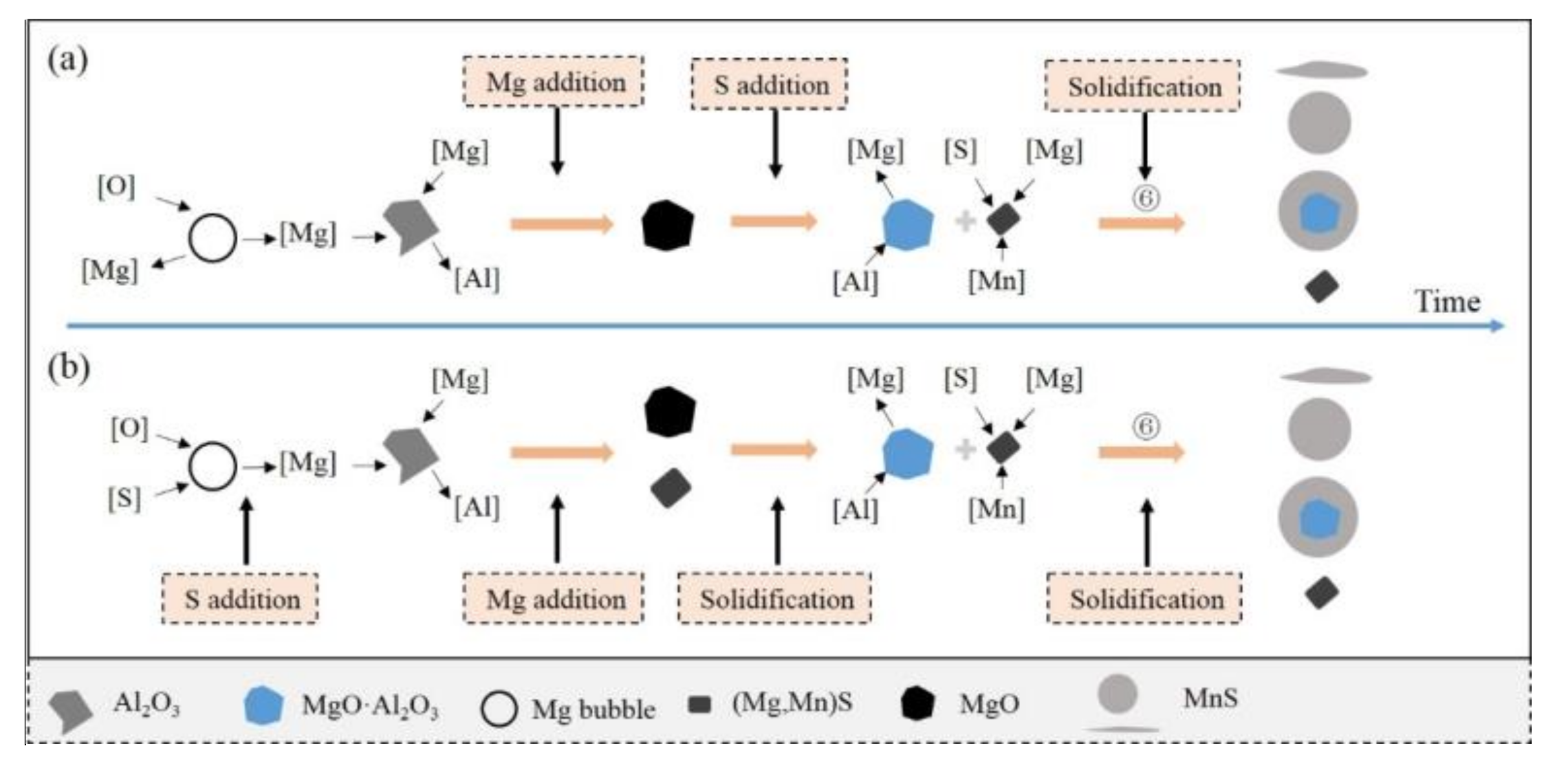
3.4. Formation and Transient Evolution Mechanism of Inclusions
4. Conclusions
- (1)
- In the case of magnesium added before sulfur, MgO is transformed into spinel immediately after sulfur addition, while this transformation is calculated to take place during the equilibrium solidification with the enrichment of [S] in the case of sulfur added before magnesium.
- (2)
- Under the experimental condition, MgS precipitates with a quantity of MnS and the stoichiometry is calculated to be (Mg0.9Mn0.1)S.
- (3)
- The stability diagram for Fe-Mg-Al-1.2Mn-0.0015O-0.15S system is obtained and the thermodynamic calculation results show that the equilibrium products are Al2O3/spinel/MgO/MeS at 1600 °C.
Author Contributions
Funding
Conflicts of Interest
References
- Laizhu, J.; Kun, C.; Hänninen, H. Effects of the composition, shape factor and area fraction of sulfide inclusions on the machinability of re-sulfurized free-machining steel. J. Mater. Process. Technol. 1996, 58, 160–165. [Google Scholar] [CrossRef]
- Ånmark, N.; Karasev, A.; Jönsson, P.G. The effect of different non-metallic inclusions on the machinability of steels. Materials 2015, 8, 751–783. [Google Scholar] [CrossRef] [PubMed]
- Yaguchi, H. Effect of MnS inclusion size on machinability of low-carbon, leaded, resulfurized free-machining steel. J. Appl. Metalwork. 1986, 4, 214–225. [Google Scholar] [CrossRef]
- Fujiwara, J. Cutting mechanism of sulfurized free-machining steel. In Scanning Electron Microscopy; IntechOpen: London, UK, 2012. [Google Scholar]
- Pollard, B. Effect of manganese content on sulphide shape control by zirconium in steel. Met. Technol. 1974, 1, 343–347. [Google Scholar] [CrossRef]
- Kim, H.; Kang, M.; Shin, S.Y.; Lee, S. Alligatoring phenomenon occurring during hot rolling of free-machining steel wire rods. Mater. Sci. Eng. A 2013, 568, 8–19. [Google Scholar] [CrossRef]
- Luyckx, L.; Bell, J.R.; McLean, A.; Korchynsky, M. Sulfide shape control in high strength low alloy steels. Metall. Trans. 1970, 1, 3341–3350. [Google Scholar]
- Jiang, L.; Cui, K. Quantitative study of modification of sulphide inclusions by calcium and its effect on the impact toughness of a resulfurized alloy steel. Steel Res. 1997, 68, 163–168. [Google Scholar] [CrossRef]
- Feild, A. Effect of zirconium on hot-rolling properties of high-sulfur steels and the occurrence of zirconium sulfide. Trans. Am. Inst. Min. Metall. Eng. 1924, 70, 201–223. [Google Scholar]
- Koch, W.; Artner, E. Beitrag zur Isolierung von Sulfiden in Stählen. Arch. Eisenhüttenwes. 1958, 29, 737–744. [Google Scholar] [CrossRef]
- Yang, J.; Okumura, K.; Kuwabara, M.; Sano, M. Behavior of magnesium in the desulfurization process of molten iron with magnesium vapor produced in-situ by aluminothermic reduction of magnesium oxide. ISIJ Int. 2002, 42, 685–693. [Google Scholar] [CrossRef]
- Hosohara, S.; Kato, Y.; Nakato, H.; Sorimachi, K. The effect of pressure in the atmosphere on desulfurization of hot metal and reaction mechanism with magnesium. Tetsu-to-hagane 2002, 88, 129–135. [Google Scholar] [CrossRef]
- Chen, B.; Jiang, M.; Wang, C.-g.; Wang, X.-h. Theoretical research of applying Mg to ultra clean steel production. Iron Steel 2007, 7, 007. (In Chinese) [Google Scholar]
- Zhang, T.S.; Min, Y.; Jiang, M.F. Effect of magnesium addition on evolution of inclusions in Mn-Si-Al deoxidized molten steels. Chem. Extr. Metall. Pyrometall. 2014, 54, 161–169. [Google Scholar]
- Park, J.H.; Todoroki, H. Control of MgO·Al2O3 spinel inclusions in stainless steels. ISIJ Int. 2010, 50, 1333–1346. [Google Scholar] [CrossRef]
- Zhang, L.; Ren, Y.; Duan, H.; Yang, W.; Sun, L. Stability Diagram of Mg-Al-O System Inclusions in Molten Steel. Metall. Mater. Trans. B 2015, 46, 1809–1825. [Google Scholar] [CrossRef]
- Sims, C.; Dahle, F. Effect of aluminum on the properties of medium carbon cast steel. Trans. Am. Foundry Soc. 1938, 46, 65–132. [Google Scholar]
- Ito, Y.; Yonezawa, N.; Matsubara, K. Effect of Carbon on the Composition of Eutectic Conjugation in the Fe--Mn-S System and Equilibrium Composition of Sulphide in Solid Steel. Trans. Iron Steel Inst. Jpn. 1980, 20, 301–308. [Google Scholar]
- Oikawa, K.; Ohtani, H.; Ishida, K.; Nishizawa, T. The control of the morphology of MnS inclusions in steel during solidification. ISIJ Int. 1995, 35, 402–408. [Google Scholar] [CrossRef]
- Ohta, H.; Suito, H. Precipitation and dispersion control of MnS by deoxidation products of ZrO2, Al2O3, MgO and MnO-SiO2 particles in Fe-10mass% Ni alloy. ISIJ Int. 2006, 46, 480–489. [Google Scholar] [CrossRef]

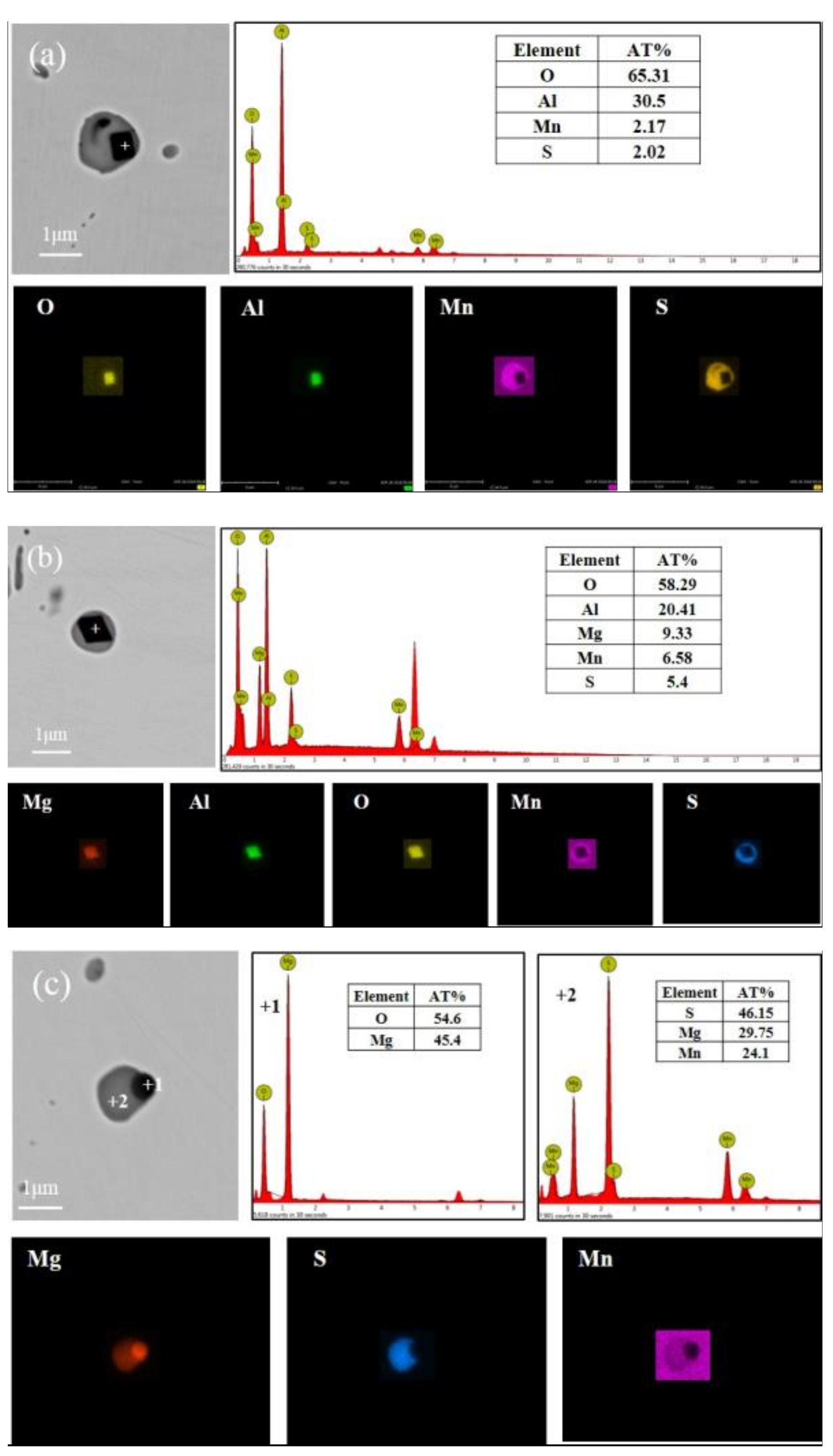


| Raw Material | Fe | Ni | Al | Mn | C | Si | Mg | S | Balance |
|---|---|---|---|---|---|---|---|---|---|
| Industrial pure iron | 99.90 | 0.01 | 0.018 | 0.03 | 0.0021 | 0.01 | - | 0.003 | 0.035 |
| Mn-Fe alloy | 12.57 | 84.20 | 1.50 | 1.50 | - | 0.03 | |||
| Pure aluminum | 99.90 | 0.10 | |||||||
| Ni-Mg alloy | 0.93 | 80.12 | 17.98 | 0.97 | |||||
| FeS | 63.32 | 36.18 | 0.50 |
| Exp. | Mn | Al | Mg | S |
|---|---|---|---|---|
| A | 1.41 | 0.035 | 0.0007 | 0.17 |
| B | 1.25 | 0.031 | 0.0021 | 0.15 |
| Types of Inclusions | Phase in Inclusions | Al2O3 | MgO | MnS | MgS |
|---|---|---|---|---|---|
| Al2O3 | Oxide | 73-100 | -- | -- | -- |
| MgO | Oxide | -- | 94-100 | -- | -- |
| Al2O3-MgO | Oxide | 28-76 | 24-72 | -- | -- |
| MnS | Sulfide | -- | -- | 100 | 0 |
| (Mg, Mn)S | Sulfide | -- | -- | 40-90 | 10-60 |
| Al2O3+MnS | Oxide | 100 | -- | -- | -- |
| sulfide | -- | -- | 100 | -- | |
| Al2O3-MgO+MnS | Oxide | 21-81 | 19-79 | -- | -- |
| Sulfide | -- | -- | 100 | -- | |
| MgO+(Mg, Mn)S | Oxide | -- | 100 | -- | -- |
| Sulfide | -- | -- | 42-56 | 44-58 |
| Types of Inclusions | Size (μm) | Sample Number in Exp. A | Sample Number in Exp. B | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | ||
| Al2O3 | 1~2 | × | × | × | × | √ | × | × | × |
| MgO | 1~2 | √√ | × | × | × | × | √ | √ | √ |
| Al2O3-MgO | 1~2 | × | √ | √ | √ | × | × | × | |
| MnS | 1~10 | × | √√ | √√ | √√ | √√ | √√ | √√ | √√ |
| (Mg, Mn)S | 1~2 | × | × | × | × | × | √√ | √ | × |
| Al2O3+MnS | 1~10 | × | × | × | × | √√ | × | × | × |
| Al2O3-MgO+MnS | 1~10 | × | √√ | √√ | √√ | × | × | × | × |
| MgO+(Mg, Mn)S | 1~10 | × | × | × | × | × | √√ | √ | √ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, Y.; Zhang, Q.; Xu, H.; Xu, J.; Liu, C. Formation and Evolution of Inclusions with Different Adding Order of Magnesium and Sulfur in Al-Killed Free-Cutting Steel. Metals 2018, 8, 1064. https://doi.org/10.3390/met8121064
Min Y, Zhang Q, Xu H, Xu J, Liu C. Formation and Evolution of Inclusions with Different Adding Order of Magnesium and Sulfur in Al-Killed Free-Cutting Steel. Metals. 2018; 8(12):1064. https://doi.org/10.3390/met8121064
Chicago/Turabian StyleMin, Yi, Qingsong Zhang, Haisheng Xu, Jiujian Xu, and Chengjun Liu. 2018. "Formation and Evolution of Inclusions with Different Adding Order of Magnesium and Sulfur in Al-Killed Free-Cutting Steel" Metals 8, no. 12: 1064. https://doi.org/10.3390/met8121064
APA StyleMin, Y., Zhang, Q., Xu, H., Xu, J., & Liu, C. (2018). Formation and Evolution of Inclusions with Different Adding Order of Magnesium and Sulfur in Al-Killed Free-Cutting Steel. Metals, 8(12), 1064. https://doi.org/10.3390/met8121064






