Abstract
At present, gold mines are increasingly scarce in the world. The yield of cyanidation tailing (CT) of refractory gold ores with a grade of 10 g/T is huge; however, the effective capitalization of the cyanidation tailing is a significant problem in the gold industry. In this work, a new treatment method, a microwave-roasting process developed. The effect of roasting temperature, calcium chloride concentration, holding time and mineral size on the recovery of Au researched under conventional and microwave conditions. It found that, under the same processing conditions, the Au recovery in the microwave field is much higher than that of conventional conditions. The preliminary reinforcing role of the microwave discussed in the recovery of gold. This might be because of the microwave absorption ability of CaCl2 is much better, heated quickly and the reactions intensified among CaCl2, O2, and H2O. As a result, the extraction of gold in CT drastically increased in the microwave field, and the microwave roasting technology shows the characteristics of environmental protection in terms of low energy consumption and high efficiency.
1. Introduction
Gold is a kind of precious metal, and its ductility is the highest among the known metals. In modern life, gold is mainly used for international reserves, jewelry decoration, industrial, science, and technology application [1,2,3]. In some countries, gold is the standard for currency trading and used for making coins and jewelry. However, gold is a rare metal, it is not easy to exploit, and the rich gold mines decreasing day-by-day. Today, the cyanide residue of refractory gold ore with a grade of 10 g/T is huge, and it is a valuable secondary resource [4]. The conventional cyanidation process [5,6], thiourea method [7], mercury mixing method [8], and acid-base method [9] to treat the cyanide residue of refractory gold ore cannot meet the requirements of gold extraction so that the refractory gold deposits have not been effectively extracted and separated, thus causing serious waste of resources. According to the previous reports, the gold particles are very small, and hard to extract from cyanide residue of refractory gold ore [10,11,12]. In our previous work, the roasting method turned out to be an effective and easy way to treat with the cyanide residue [13,14]. However, the technological conditions, roasting temperature has to optimize, as the roasting temperature is high. At the same time, the microwave roasting technology has the characteristics of environmental protection with high efficiency.
The microwave is a kind of electromagnetic wave with a shorter wavelength. It has the advantages of low energy consumption, environmental protection, and cleanliness [15,16,17]. By transferring the energy required by the chemical reaction to the molecules and atoms through the dielectric loss in the mineral [15,17], forming temperature gradient, and then the chemical reaction of the interface promoted. Thus, the efficient separation and extraction of valuable elements achieved from the ores. In general, the chloride has an excellent absorbing property with preferred heating, which reflects the selective heating and creates good conditions for the conversion and decomposition of chlorine/HCl to strengthen the chlorination roasting process.
In this study, a new treatment method, microwave-roasting process, was developed. During the roasting process, the chlorination reagent, CaCl2 reacts with mineral components as well as the O2 and H2O in the atmosphere [13]. As a result, Cl2 and HCl gas will generate/release, and chlorinate the gold. Due to the feature of low boiling point and high volatile of metal chlorides, the gold chloride volatilizes from the cyanide residue.
Based on the unique advantages of internal heating and selective heating of microwave chlorination [15,16], the microwave roasting of cyanidation tailings was carried out. Under the condition of the microwave, the gold inclusions destroyed, and the gold can be transforming into chloride. The effect of roasting temperature, calcium chloride adding amount, holding time, mineral size on the recovery of Au studied with conventional muffle furnace and microwave furnace, and the reinforcing role of the microwave on the recovery of the microwave discussed preliminarily.
2. Materials and Methods
2.1. Materials
The cyanidation tailing obtained from Yantai city, China. Its main elements analyzed by X Ray Fluorescence (XRF) as shown in Table 1. The content of valuable element Au in CT detected by fire assaying is 10 g/T, and the fire assaying considered as the most reliable analytical method in metallurgy at home and abroad [18]. In addition, the cyanide content in CT is 6 g/T. The phase composition of CT were detected by X Ray Diffraction (XRD) and displayed in Figure 1, and it can be found that the main components in CT are Fe2O3, SiO2, and Fe1.833(OH)0.5O2.5. The microstructure of CT observed with Scanning Electron Microscope (SEM) and the element distribution analyzed with Energy Dispersive Spectrometer (EDS), as shown in Figure 2.

Table 1.
Elemental composition of cyanidation tailings (mass fraction, %).
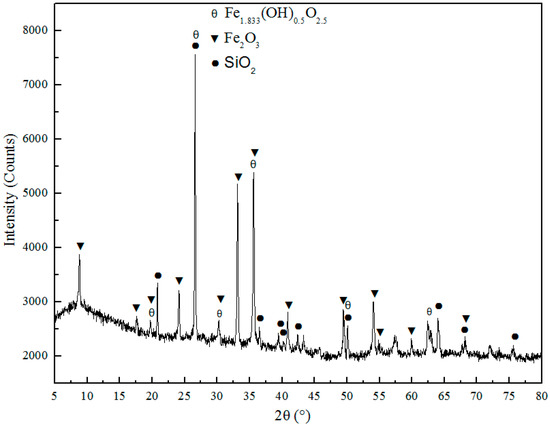
Figure 1.
XRD pattern of cyanide tailings.
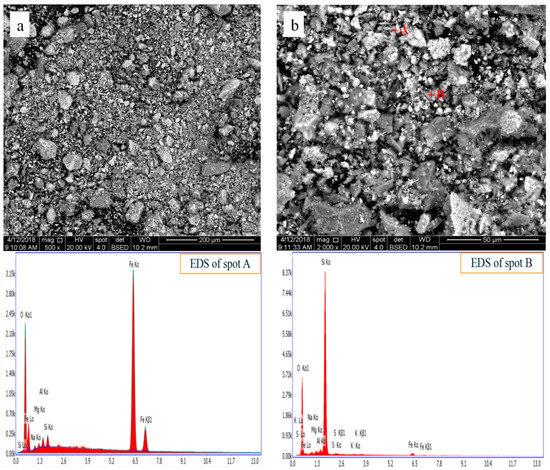
Figure 2.
SEM images and corresponding EDS pattern of cyanidation tailing. (a): low magnification image; (b): magnified image of local area in (a).
It is found that the CT consists of regular morphological particles with the size of 50 μm and amorphous powders with the size less than 30 μm. From the EDS spectra, the gold detected due to its too low content. Besides, the type of gold in CT is the main inclusive gold; maybe, it is another reason for the absence of Au from the EDS spectra.
2.2. Experimental Process
Before roasting experiments, the mixture of powder CT and the chlorinating agent was ball milled using a pelletizer with 1 to 2 cm diameter and dried in an oven at 120 °C for 3 h.
In order to explore the best experimental conditions, the CT roasted in the microwave reactor and the conventional muffle furnace, respectively. The process of roasting experiments is shown in Figure 3. The effect of reaction temperature (700 °C, 750 °C, 800 °C, 850 °C, 900 °C, 950 °C), CaCl2 concentration (1%, 3%, 5%, 7%, 9%, 11%), holding time (15 min, 20 min, 25 min, 30 min, 35 min, 40 min), and particle size (200 mesh, 250 mesh, 300 mesh, 350 mesh, 400 mesh, 450 mesh) on the recovery of the Au was investigated. The recovery of Au was calculated using below formula (1):
where, m1 is the weight of unroasted CT (g); m2 is the weight of roasted CT (g); w1 is the content of Au in unroasted CT (g/T); w2 is the content of Au in roasted CT (g/T).
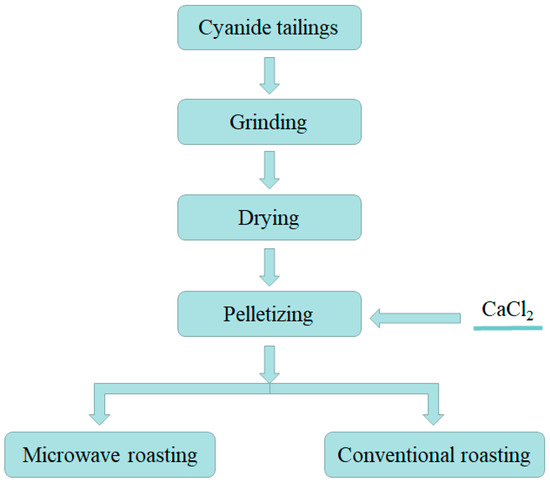
Figure 3.
Cyanide tailings gold extraction process.
After roasting, the sample roasted in the conventional muffle furnace was ground, and that roasted in the microwave field was polished with a metallographic polishing machine (P-1, Laizhou Weiyi Experiment Instrument Ltd., Laizhou, China). The microstructure and element distribution of the samples ground and polished contrasted with SEM and EDS, and their phase composition studied with XRD.
2.3. Technical Testing and Experimental Equipment
The equipment used in this experiment is a microwave box furnace (HAMiLab-M1500, Key Laboratory of Unconventional Metallurgy of the Ministry of Education, Kunming, China) and a conventional muffle furnace (YFX9/13Q-YC, Beijing Zhongyi Youxin Technology Ltd., Beijing, China). Under different equipment, the power controlled at 1400 W, and the cyanide tailings are treated. Under the control of different process parameters, the effect of gold extraction compared. At the same time, dynamic experiments of cyanide tailings carried out by microwave tube furnace to analyze its process mechanism.
The SEM with energy-dispersive X-ray spectroscopy (EDS-GENESIS, Mahwah, NJ, USA) measurements of Cyanide tailings performed to understand the structure, morphology and chemical composition. SEM-EDS at the voltage of 20 kV, multiples 2000, take out angle 35.9, activation time 29.3 s. The XRD patterns have recorded a pattern in the 2θ range of 5° to 80° at a scan rate of 4°·min−1.
3. Results and Discussion
3.1. Effect of Roasting Temperature on Au Recovery
Under the optimization conditions during conventional roasting as follows [13]: calcium chloride adding amount 5%, holding time of 30 min, and CT granularity of 300 mesh, the effects of roasting temperature on the recovery of Au in the microwave field were studied, and the results are displayed in Figure 4. Apparently, the Au recovery of CT during microwave roasting rises sharply from 69.1% to 95.7% with the increase of roasting temperature from 973 K to 1123 K. The recovery of Au during conventional roasting is only 59.9%, which is much lower than that (95.7%) of microwave roasting at the same conditions. Notably, the Au recovery of CT roasted using conventional roasting at 1223 K is much lower than that (95.7%) of microwave roasting at 1123 K, and the microwave roasting is more efficient. Hence, the roasting temperature of 1123 K is chosen as a constant parameter in the subsequent experiments.
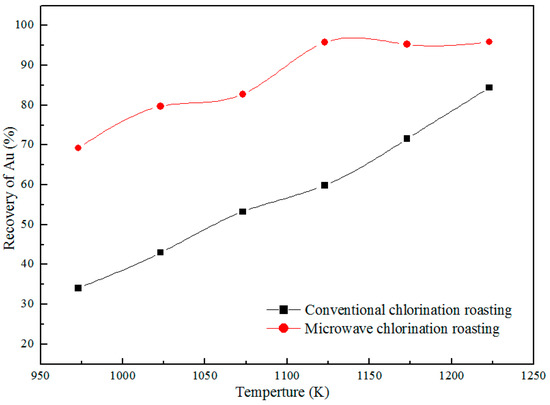
Figure 4.
Effect of different temperatures on the recovery of Au.
3.2. Effect of Calcium Chloride Concentration on Au Recovery
The effects of calcium chloride concentration on Au recovery studied in the experimental conditions as follows: roasting temperature of 1123 K, the reaction time of 30 min and CT granularity of 300 mesh, as shown in the Figure 5. As seen from Figure 5, the recovery of Au increases with increase in calcium chloride concentration from 1% to 5%. When the concentration of calcium chloride is 5%, the recovery of gold is 95.7%. The content of Au in CT is only 10 g/T, so the demand for HCl and Cl2 decomposed shown as in equations (2) and (3) is limited. Thereby, the recovery of Au increases will not continue to increase with the increase of calcium chloride concentration from 5% to 11%. The calcium chloride concentration of 5% is chosen as a constant parameter in the subsequent experiments.
CaCl2 + SiO2 + H2O (g) = CaSiO3 + 2HCl (g)
CaCl2 + 1/2O2 (g) = CaO + Cl2 (g)
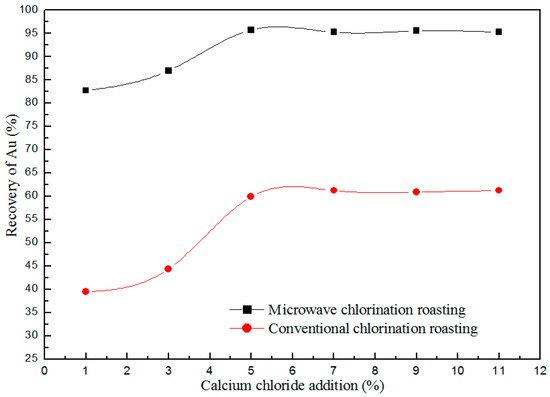
Figure 5.
Effect of calcium chloride with different additions on the recovery of Au.
Based on the selective heating of microwave [15,16], the dielectric constant of calcium chloride is large, which will become a hot spot (the point of high temperature), promote the (2) and (3) of the equation, so the chlorination process of gold increases.
3.3. Effect of Holding Time on Au Recovery
Under the conditions as follows: calcium chloride concentration amount 5%, roasting temperature of 1123 K and CT granularity of 300 mesh, the effects of holding time on the recovery of Au were investigated, and the results are shown in the Figure 6. It found that the recovery of Au reaches a maximum of 96.3% within 35 min during microwave roasting. It is worth to mention that the recovery of Au increases with the increasing of holding time in the conventional roasting. To reach higher level of Au recovery in the conventional roasting long roasting time needed. Therefore, the microwave roasting has the characteristics of high speed and high efficiency, and it shows the advantage of energy conservation. The holding time of 35 min is chosen as a constant parameter in the subsequent experiments.
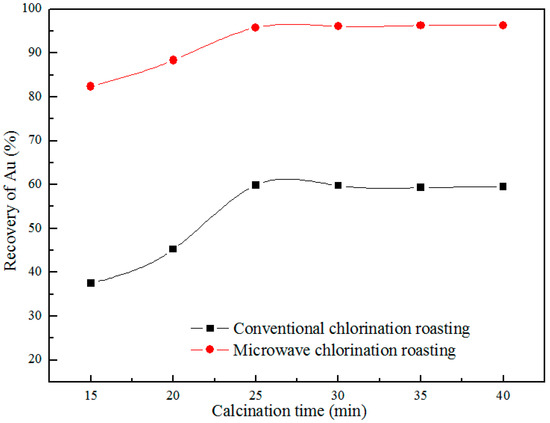
Figure 6.
Effect of holding time on recovery of Au.
3.4. Effect of Mineral Size on Au Recovery
The mineral size depends on the grinding process. If the mineral size is smaller, the cost of grinding process will be higher. Moreover, the recovery of Au related to the mineral (CT) size. Therefore, it is necessary to find the relationship between the recovery of Au and mineral size. The roasting experiments about the mineral size carried out as the following conditions: calcium chloride concentration 5%, roasting temperature of 1123 K, the holding time of 35 min, and the results shown in Figure 7. The recovery of Au reduces slightly with decrease in mineral size, and the recovery of Au is 96.6% when the size of CT is 200 mesh. The permeability of CT with a smaller mineral size is worse, so the conversion process as shown in equations (2) and (3) suppressed, as well as the chlorination roasting process of Au. Therefore, the mineral size of 200 mesh is better for the chlorination roasting process.
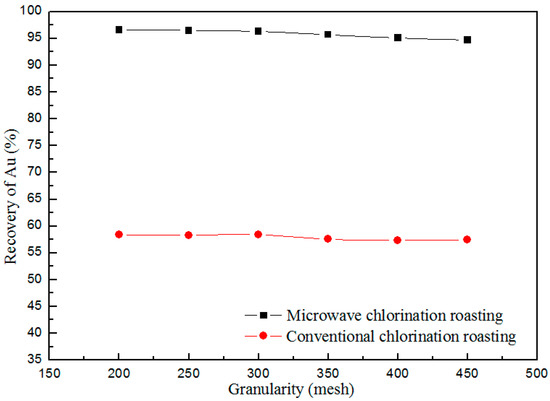
Figure 7.
Effect of mineral (cyanidation tailing (CT)) particle size on the recovery of Au.
3.5. Analysis of Techno-Mineralogical Properties
After conventional roasting, the sample was ground, and the microstructure and element distribution was analyzed with SEM and EDS, as displayed in Figure 8. It seen that the microstructures of unroasted CT (see Figure 2.) and CT roasted (as shown in the Figure 8.) with conventional roasting method do not show a significant difference. The elements of Ca and Cl in the white particles is rich (Figure 8b), which is due to a part of CaCl2 did not decompose and transform as shown in the equations (2) and (3). The phase composition of CT roasted with conventional roasting method was detected by XRD, as displayed in the Figure 9, and it can be seen that the main components roasted in CT are Fe2O3, SiO2, and Fe1.833(OH)0.5O2.5. The main phases of CT before and after roasting are same, and CaCl2 not able to detect due to its low content in the roasted CT.
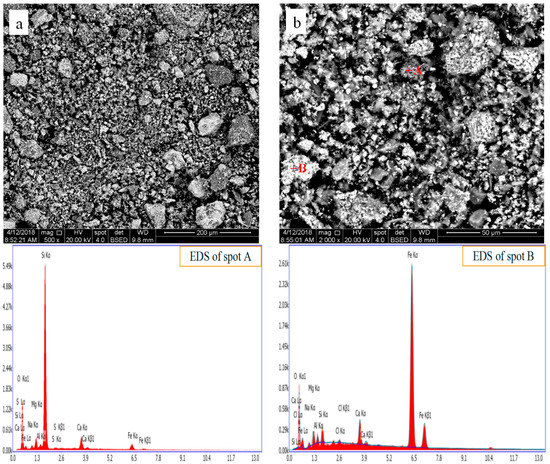
Figure 8.
SEM-EDS images of CT after chlorination roasting in the conventional field. (a): low magnification image; (b): magnified image of local area in (a).
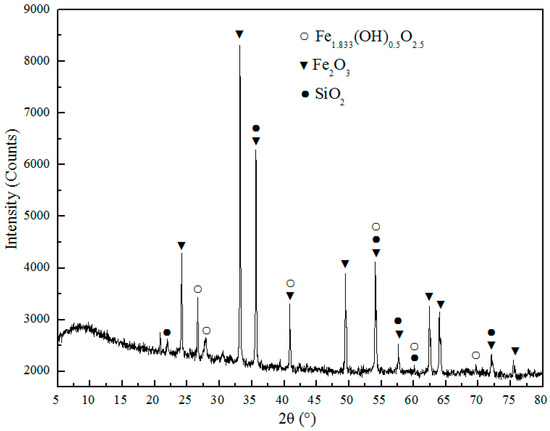
Figure 9.
X-ray diffraction pattern of conventional chlorination roasting.
Figure 10 shows the microstructure and element distribution of CT roasted in the microwave field, and it is seen that the mineral particle size is much smaller than that of conventional roasting method (as shown in Figure 8).
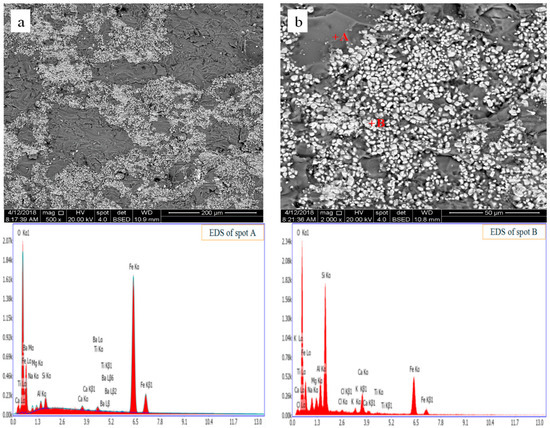
Figure 10.
SEM-EDS images of CT after chlorination roasting in the microwave field. (a): low magnification image; (b): magnified image of local area in (a).
The components of CT are very complex, and their dielectric properties are quite different. The temperature of the component with a high dielectric constant [15,19], such as CaCl2 will rise fast in the microwave field, while that of the component with a low dielectric constant will rise tardy. As a result, a huge temperature gradient generated, and the CT will disintegrate. The dissociation on a micro level will reduce the reaction resistance between Au and Cl2/HCl, which might be a reason for the superior recovery of Au roasted in the microwave field.
The phase composition of CT roasted in the microwave field detected by XRD, as displayed in Figure 11. It is seen that the main components of CT roasted with the conventional roasting method and microwave method are the same, thereby, the microwave roasting did not alert the main components of roasted CT compare to that of CT roasted with the conventional method.
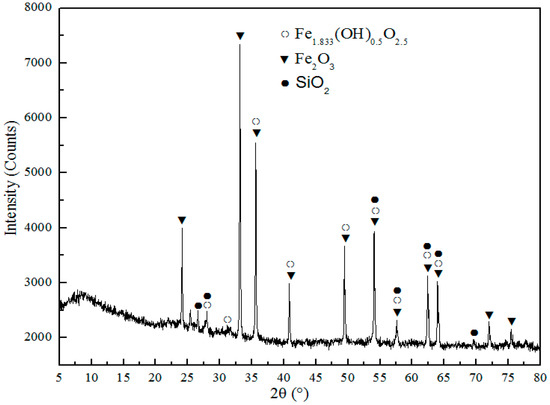
Figure 11.
XRD pattern of CT after chlorination roasting in the microwave field.
4. Conclusions
(1) The recovery of Au is as high as 96.6% in the microwave field under optimal process parameters as follows: roasting temperature 1123 K, holding time 35 min, calcium chloride adding amount 5%, mineral size 200 mesh. The microwave roasting technology shows improved environmental protection characteristics in terms of energy consumption and efficiency.
(2) Under the same conditions, the recovery rate of the microwave chlorination of gold is higher than that of the conventional chlorination. Especially, the microwave roasting temperature is much lower than that of the conventional method, and microwave calcination roasting shows the advantage of energy savings.
(3) The Microwave Chlorination process, harness the feature of low boiling point and high volatile of metal chlorides. It demonstrates that CaCl2, as a chlorination reagent, effectively break small particles and release gold.
(4) The diameter of the ore particles changed little after the conventional calcination. The different components show large electromagnetic properties difference in the microwave field, so the thermal stress between the different mineral phases generates easily. The dissociation reduces the activation energy between Au and Cl2/HCl in micro level, as a result the recovery of Au roasted in the microwave field was improved.
Author Contributions
Conceptualization, S.L.; Methodology, F.Z.; Software, H.L.; Validation, S.Y. and K.Y.; Formal Analysis, F.Z.; Investigation, S.L.; Resources, S.L.; Data Curation, F.Z.; Writing—Original Draft Preparation, F.Z.; Writing—Review & Editing, S.K.; Visualization, S.Y.; Supervision, S.L.; Project Administration, L.Z.; Funding Acquisition, L.Z.
Funding
This work was supported by National Natural Science Foundation of China (U1702252).
Acknowledgments
This work supported by the National Natural Science Foundation of China (51604135). Sivasankar Koppala acknowledges the Kunming University of Science and Technology Post-Doctoral fund (No. 10988804) and Yunnan Province Post-Doctoral training fund (No. 10988833).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Frimmel, H.E. Episodic concentration of gold to grade through Earth’s history. Earth Sci. Rev. 2018, 180, 148–158. [Google Scholar] [CrossRef]
- Smith, L.K.; Bruckard, W.J. The separation of arsenic from copper in a Northpark copper-gold ore using controlled-potential flotation. Int. J. Miner. Process. 2007, 84, 15–24. [Google Scholar] [CrossRef]
- Zhu, Y.; Fan, J.; Tucker, J. The impact of monetary policy on gold price dynamics. Res. Int. Bus. Finance. 2018, 44, 319–331. [Google Scholar] [CrossRef]
- Choudhary, B.C.; Paul, D.; Borse, A.U.; Garole, D.J. Surface functionalized biomass for adsorption and recovery of gold from electronic scrap and refinery wastewater. Sep. Purif. Technol. 2018, 195, 260–270. [Google Scholar] [CrossRef]
- Martens, M.; Prommer, H.; Dai, X.; Sun, J.; Breuer, P.; Fourie, A. Electrokinetic in situ leaching of gold from intact ore. Hydrometallurgy 2018, 178, 124–136. [Google Scholar] [CrossRef]
- Akcil, A. Destruction of cyanide in gol000000000d mill effluents: Biological versus chemical treatments. Biotechnol. Adv. 2003, 21, 501–511. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, X.; Wu, H.; Li, S.; Wang, G.; Liu, X.; Qiu, G.; Wang, D. A novel bio-oxidation and two-step thiourea leaching method applied to a refractory gold concentrate. Hydrometallurgy 2017, 171, 213–221. [Google Scholar] [CrossRef]
- Rex, M.; Campiglia, A. Pushing the Limits of Mercury Sensors with Gold Nanorods. Anal. Chem. 2006, 78, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Bain, C.; Whitesides, G. A study by contact angle of the acid-base behavior of monolayers containing .omega.-mercaptocarboxylic acids adsorbed on gold: An example of reactive sprea. Langmuir 1989, 5, 1370–1378. [Google Scholar] [CrossRef]
- Salinas-Rodríguez, E.; Hernández-Ávila, J.; Rivera-Landero, L.; Cerecedo-Sáenz, E.; Reyes-Valderrama, M.L. Leaching of silver contained in mining tailings, using sodium thiosulfate: A kinetic study. Hydrometallurgy 2016, 160, 6–11. [Google Scholar]
- Xu, B.; Yang, Y.; Li, Q.; Li, G.; Jiang, T. Fluidized roasting-stage leaching of a silver and gold bearing polymetallic sulfide concentrate. Hydrometallurgy 2014, 147–148, 79–82. [Google Scholar] [CrossRef]
- Mohammadnejad, S.; Provis, J.L.; van Deventer, J.S. Effects of grinding on the preg-robbing potential of quartz in an acidic chloride medium. Miner. Eng. 2013, 52, 31–37. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L.; Koppala, S.; Ma, A.; Peng, J.; Li, S.; Yin, S. Extraction of gold and silver in the selective chlorination roasting process of cyanidation tailing. Sep. Sci. Technol. 2018, 53, 458–466. [Google Scholar] [CrossRef]
- Li, H.; Ma, A.; Srinivasakannan, C.; Zhang, L.; Li, S.; Yin, S. Investigation on the recovery of gold and silver from cyanide tailings using chlorination roasting process. J. Alloys Compd. 2018, 763, 241–249. [Google Scholar] [CrossRef]
- Yang, K.; Li, S.; Zhang, L.; Peng, J.; Chen, W.; Xie, F.; Ma, A. Microwave roasting and leaching of an oxide-sulphide zinc ore. Hydrometallurgy 2016, 166, 243–251. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.; Ma, A.; Peng, J.; Li, J.; Liu, C. Dechlorination of Zinc Oxide Dust from Waelz Kiln by Microwave Roasting. High Temp. Mater. Processes. 2015, 34, 291–297. [Google Scholar] [CrossRef]
- Wei, Y.; Peng, J.; Zhang, L.; Ju, S.; Xia, Y.; Zheng, Q.; Wang, Y. Dechlorination of zinc dross by microwave roasting. J. Cent. South Uni. 2014, 21, 2627–2632. [Google Scholar] [CrossRef]
- Compernolle, S.; Pisonero, J.; Bordel, N. Evaluation of pulsed radiofrequency glow discharge time-of-flight mass spectrometry for precious metal determination in lead fire assay buttons. Anal. Chim. Acta. 2011, 701, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Chang, X.; Chen, J.; Zhao, W.; Peng, J. Investigation of BaCO3 Powders Synthesized by Microwave Homogeneous Precipitation. High Temp. Mater. Processes 2015, 34, 757–764. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).