Characteristics and Formation Mechanism of Inclusions in 304L Stainless Steel during the VOD Refining Process
Abstract
1. Introduction
2. Experiments
2.1. Experimental Procedure and Sampling
2.2. Composition Analysis and Inclusion Characterization
3. Results
3.1. Composition of the Molten Steel and Slag
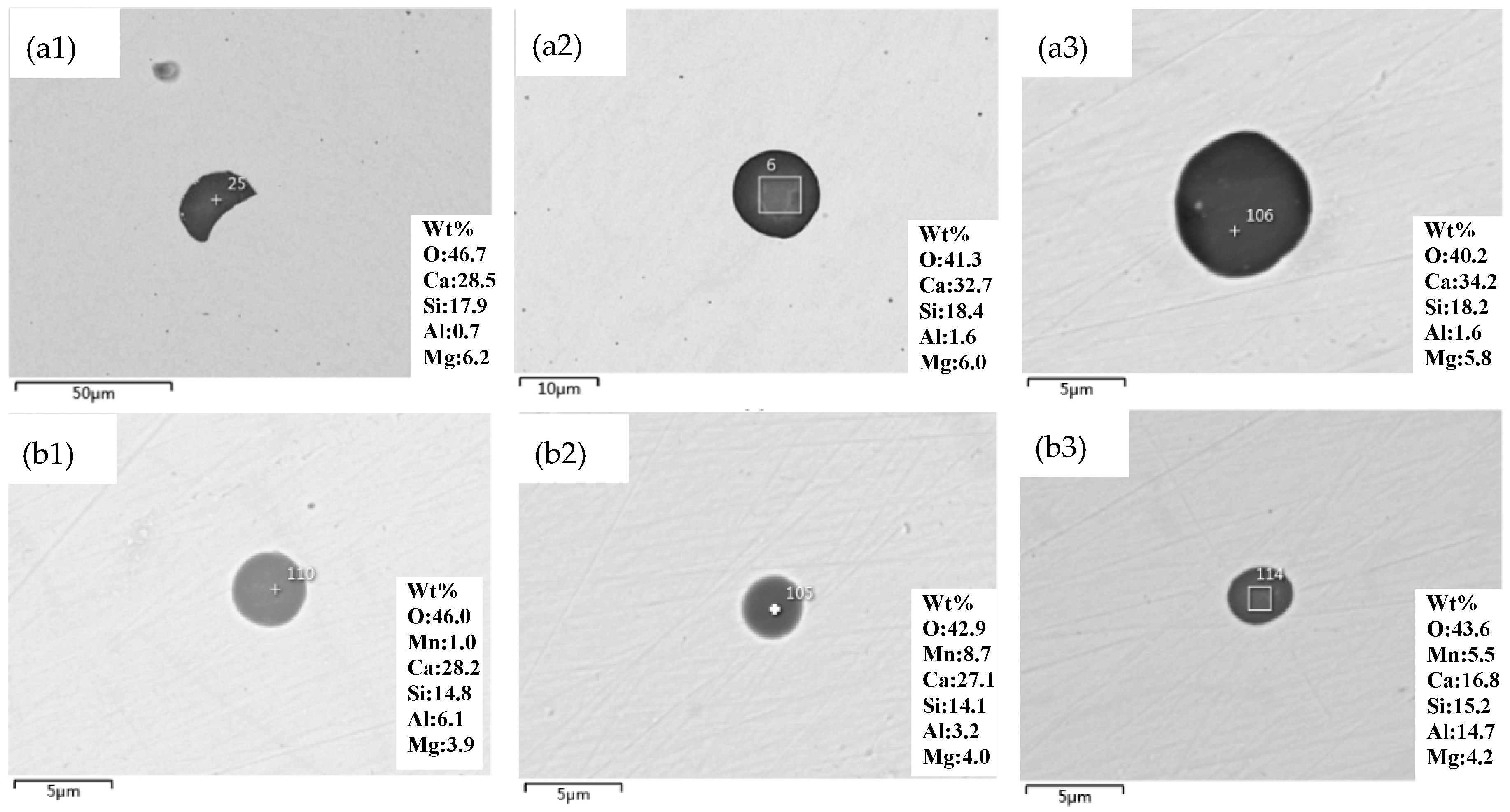
3.2. Characterization of the Inclusions
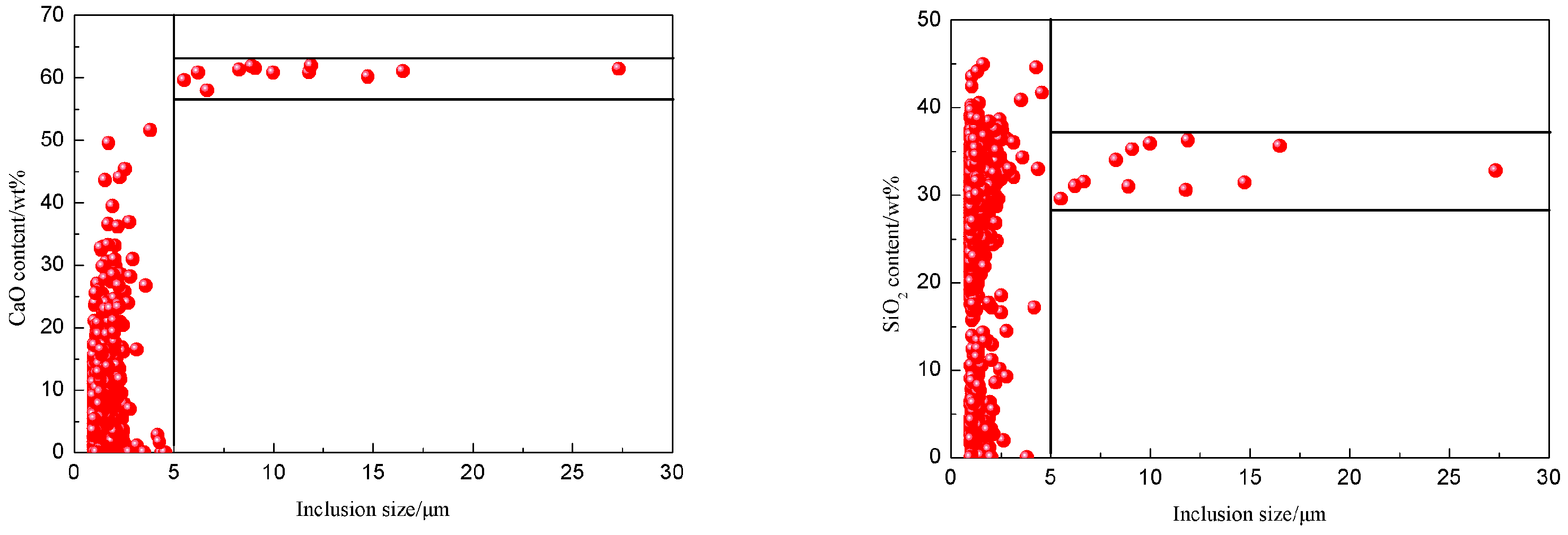
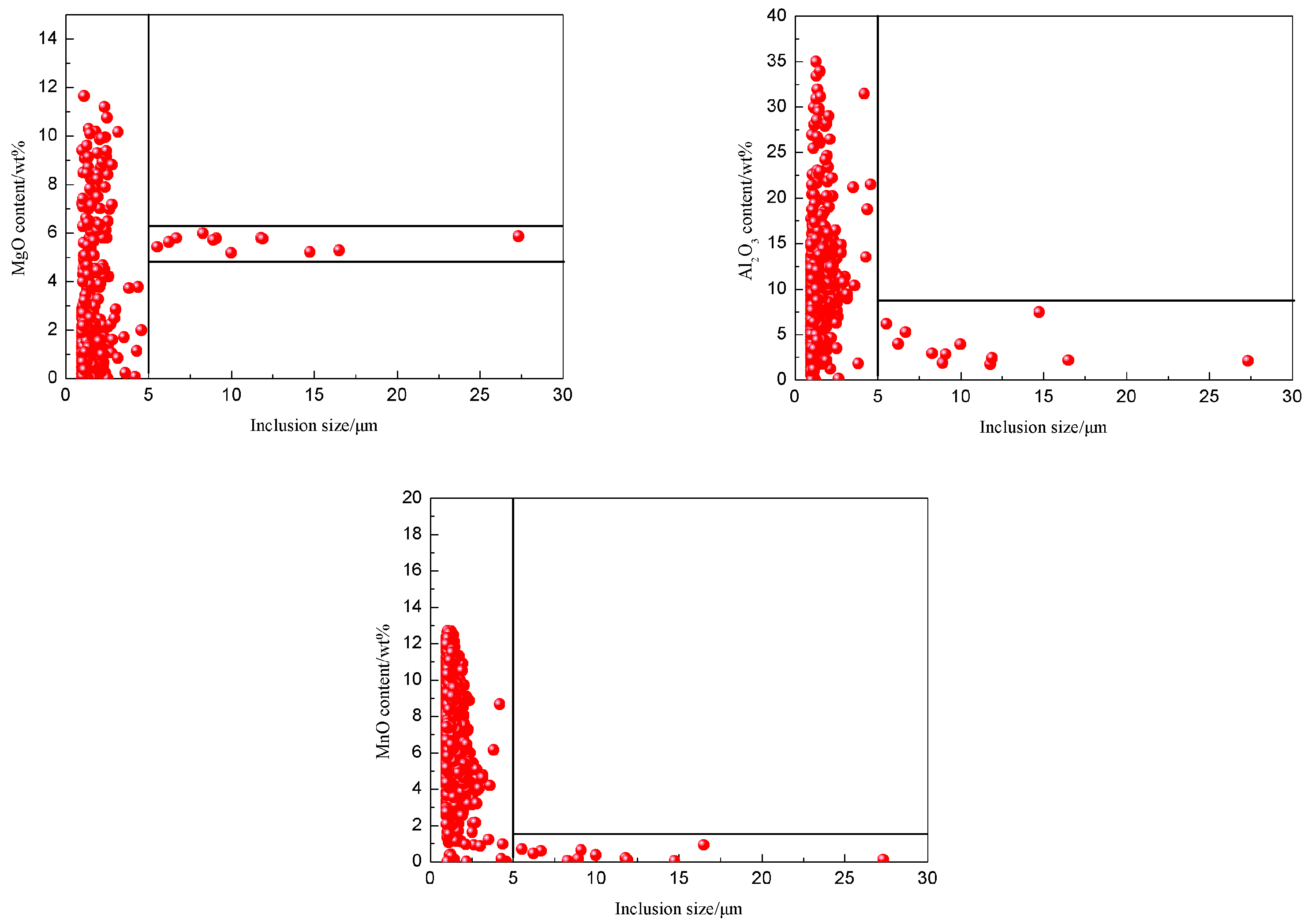
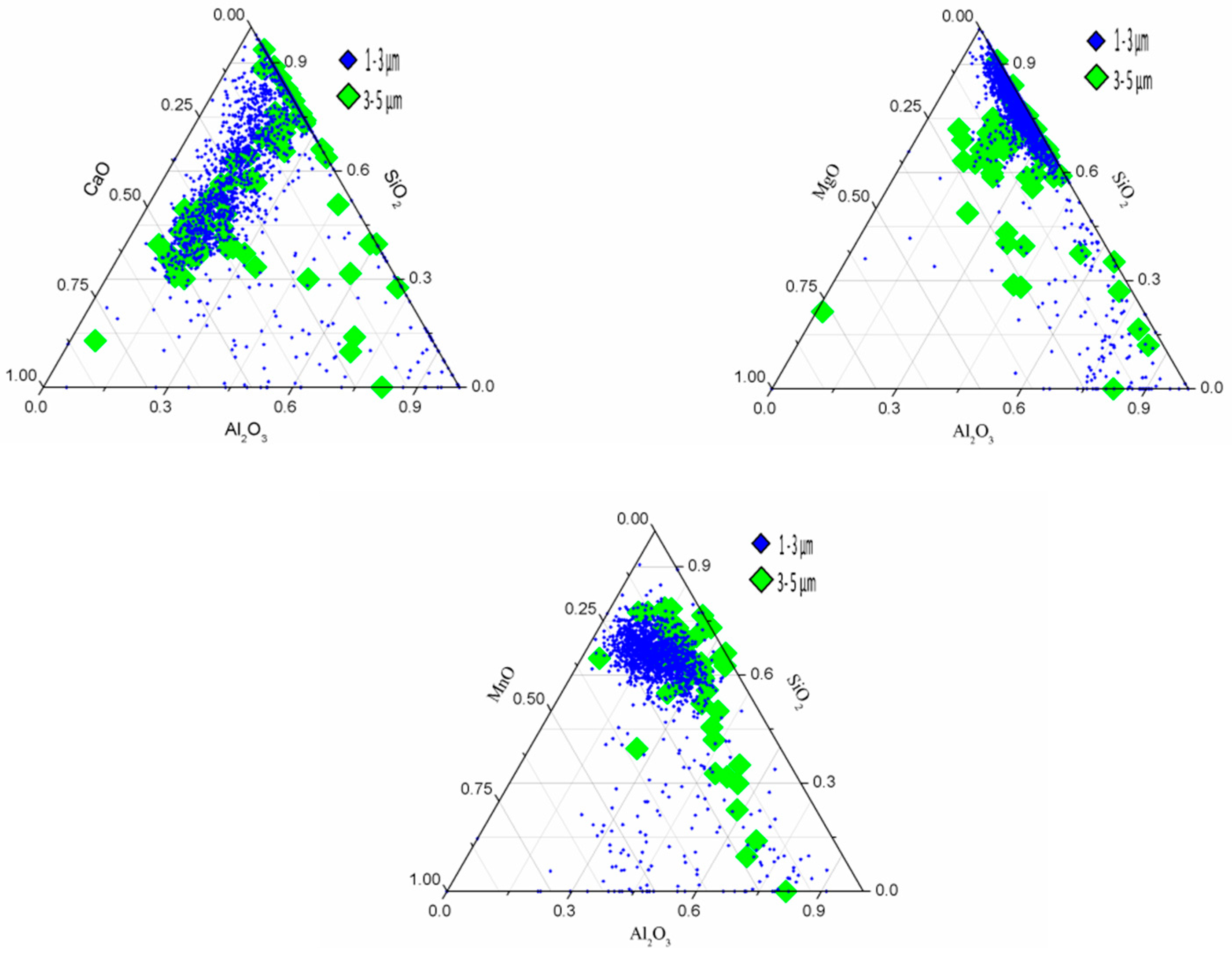
3.3. Corresponding Relation between the Size and Composition of the Inclusions
4. Discussion
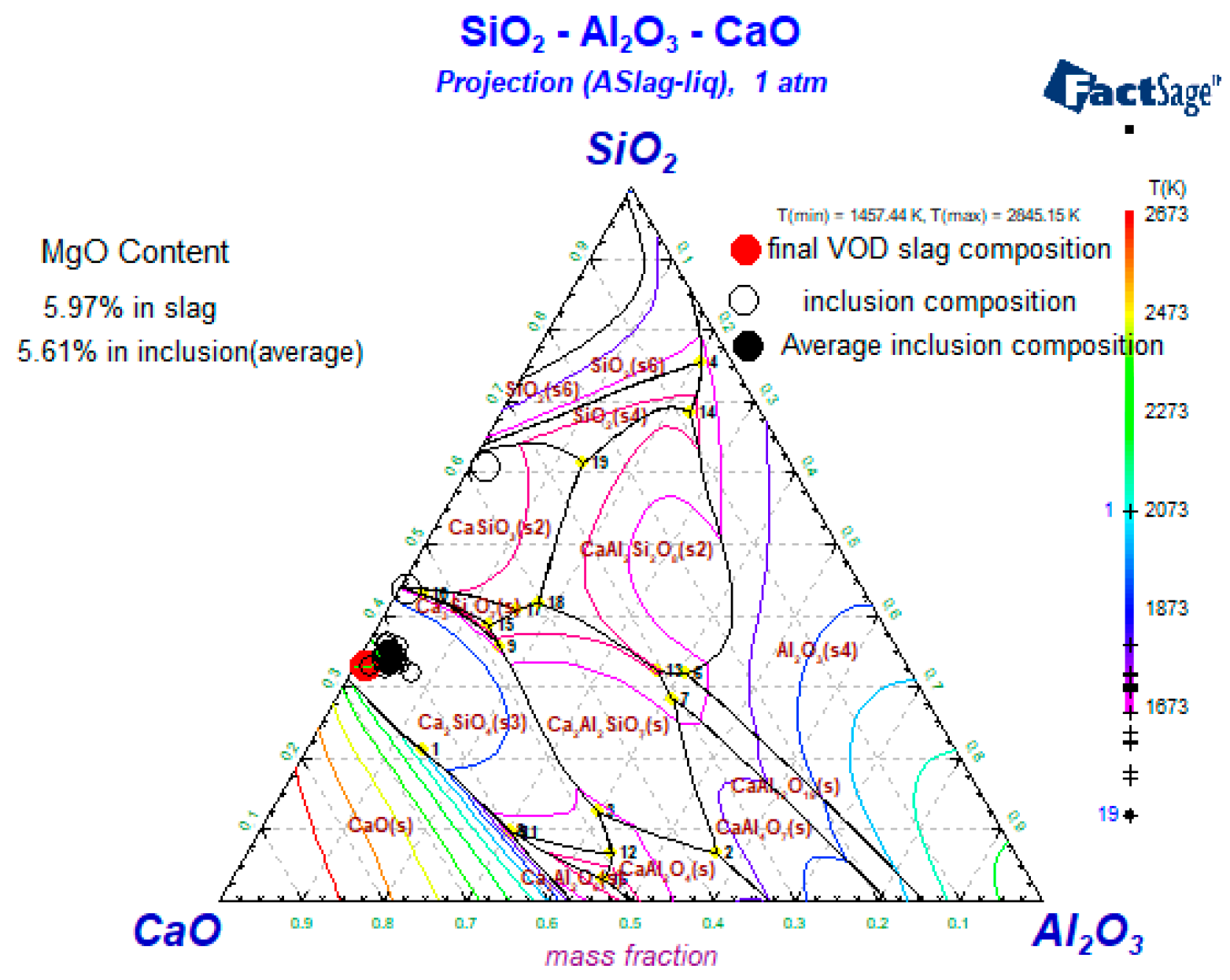
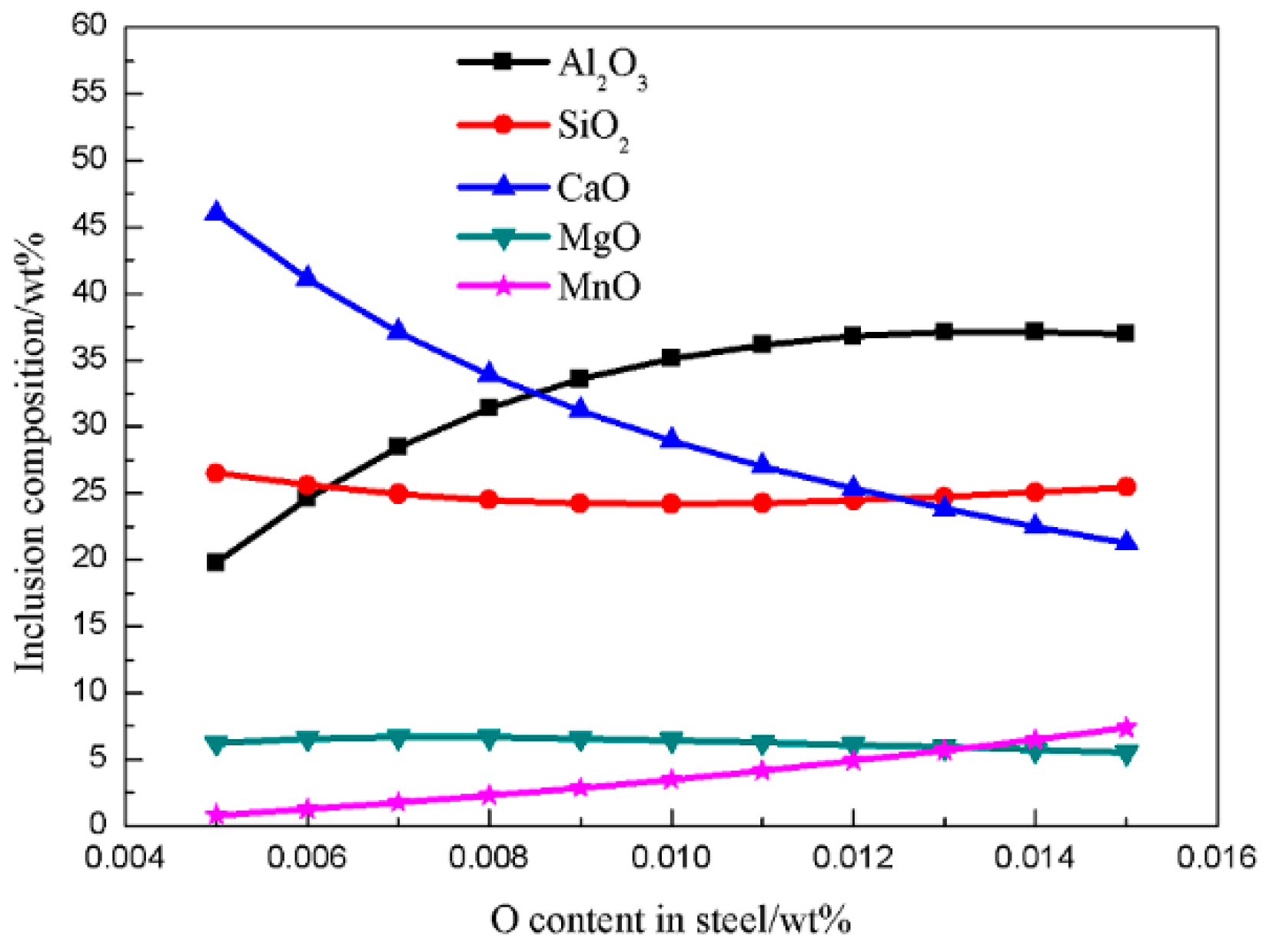
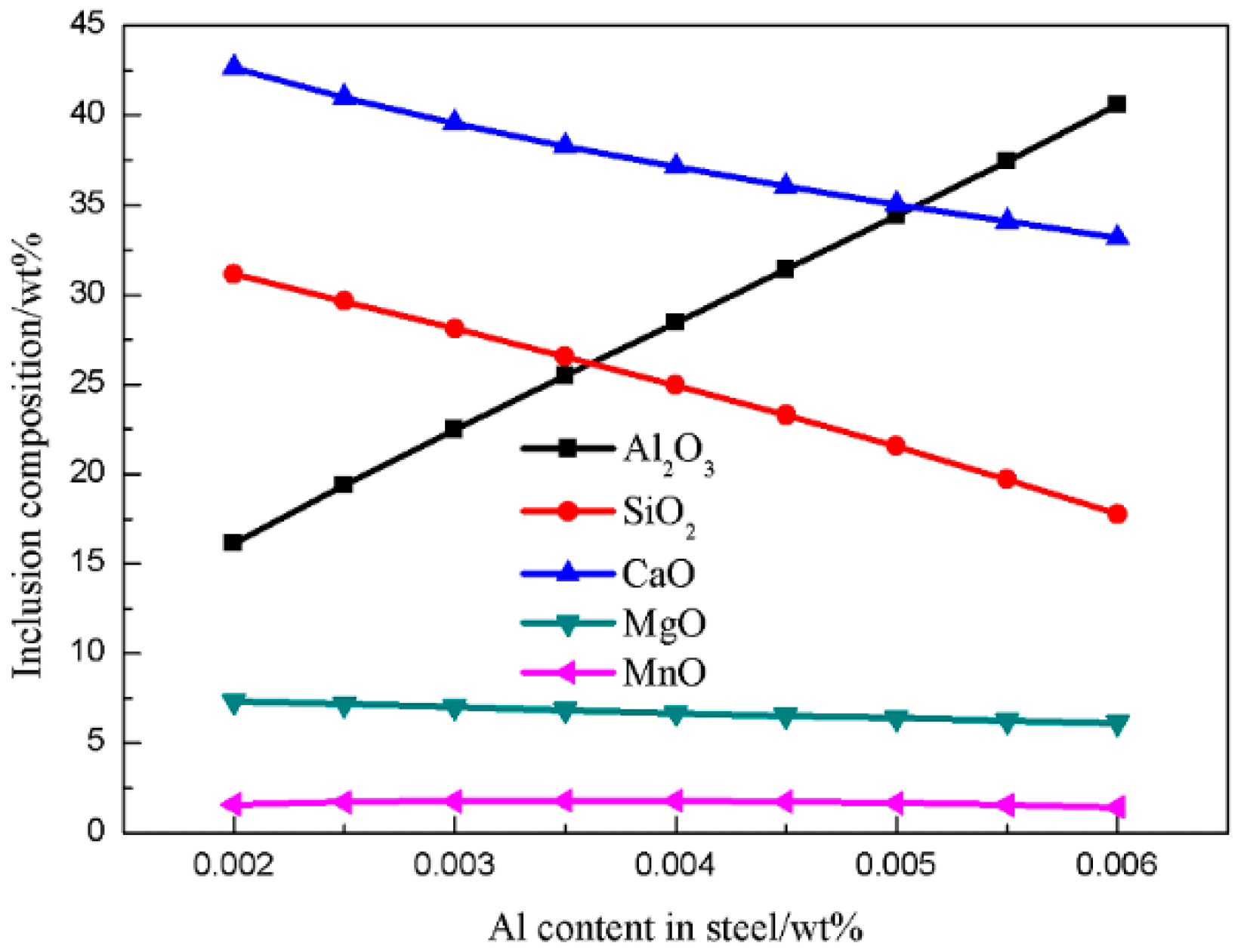
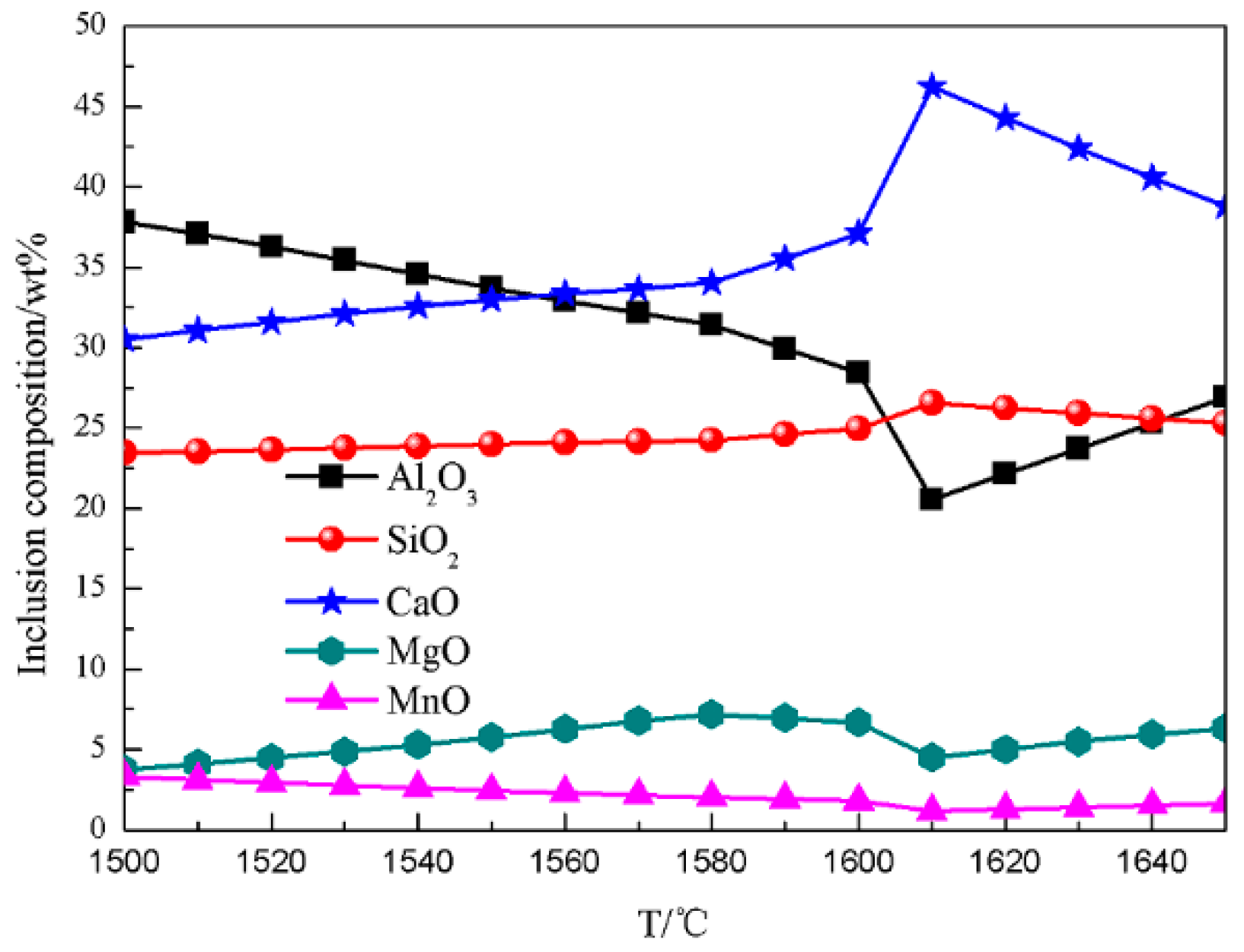
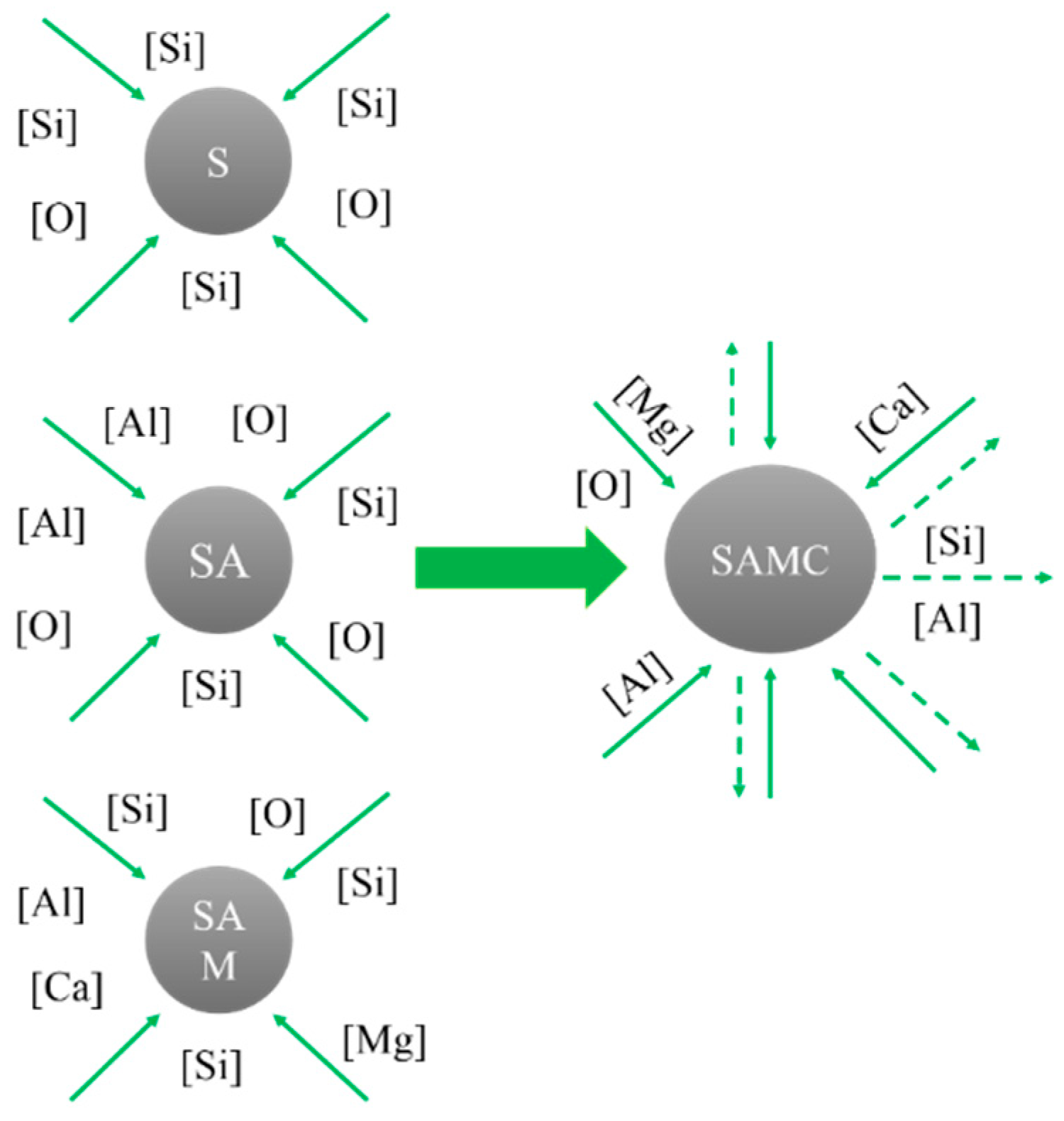
4.1. Generating the Mechanism of CaO-SiO2-MgO-Al2O3 Inclusions
4.2. Generating the Mechanism of CaO-SiO2-Al2O3-MgO-MnO Inclusions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Pan, J. Analysis on microstructure and inclusions of slab surface layer in 304L stainless steel. Contin. Casting 2018, 43, 44–48. [Google Scholar]
- Momeni, A.; Abbasi, S.M. Repetitive Thermomechanical processing towards ultra fine grain structure in 301, 304 and 304L stainless steels. J. Mater. Sci. Technol. 2011, 27, 338–343. [Google Scholar] [CrossRef]
- Amine, T.; Kriewall, C.S.; Newkirk, J.W. Long-term effects of temperature exposure on SLM 304L stainless steel. JOM 2018, 70, 384–389. [Google Scholar] [CrossRef]
- Padhy, N.; Ningshen, S.; Panigrahi, B.K.; Mudali, U.K. Corrosion behaviour of nitrogen ion implanted AISI type 304L stainless steel in nitric acid medium. Corros. Sci. 2010, 52, 104–112. [Google Scholar] [CrossRef]
- Mizuno, K.; Todoroki, H.; Noda, M.; Tohge, T. Effects of Al and Ca in ferrosilicon alloys for deoxidation on inclusion composition in type 304 stainless steel. Iron Steelmak. 2001, 28, 93–101. [Google Scholar]
- Park, J.H.; Kang, Y.B. Effect of ferrosilicon addition on the composition of inclusions in 16Cr-14Ni-Si stainless steel melts. Metall. Mater. Trans. B 2006, 37, 791–797. [Google Scholar] [CrossRef]
- Li, L.; Cheng, G.; Hu, B.; Wang, C.; Qian, G. Formation of Non-metallic Inclusions of Si-killed Stainless Steel during GOR Refining Process. High Temp. Mater. Proc. 2018, 37, 521–529. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, S.K.; Kim, D.S.; Lee, Y.D.; Yang, P.K. Formation mechanism of Ca-Si-Al-Mg-Ti-O inclusions in type 304 stainless steel. ISIJ Int. 1996, 36, S140–S143. [Google Scholar] [CrossRef]
- Ehara, Y.; Yokoyama, S.; Kawakami, M. Control of formation of spinel inclusion in type 304 stainless steel by slag composition. Tetsu-to-Hagané 2007, 93, 475–482. [Google Scholar] [CrossRef]
- Yin, X.; Sun, Y.H.; Yang, Y.D.; Bai, X.F.; Deng, X.X.; Barati, M.; McLean, A. Inclusion evolution during refining and continuous casting of 316L stainless steel. Ironmak. Steelmak. 2016, 43, 533–540. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, S.B.; Kim, D.S. Inclusion control of ferritic stainless steel by aluminum deoxidation and calcium treatment. Metall. Mater. Trans. B 2005, 36, 67–73. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, L.; Fang, W.; Shao, S.; Yang, J.; Mao, W. Effect of Slag Composition on Inclusions in Si-Deoxidized 18Cr-8Ni Stainless Steels. Metall. Mater. Trans. B 2016, 47, 1024–1034. [Google Scholar] [CrossRef]
- Yan, P.; Huang, S.; Pandelaers, L.; Van Dyck, J.; Guo, M.; Blanpain, B. Effect of the CaO-Al2O3 based top slag on the cleanliness of stainless steel during secondary metallrugy. Metall. Mater. Trans. B 2013, 44, 1105–1119. [Google Scholar] [CrossRef]
- Yan, P.; Huang, S.; Guo, M.; Blanpain, B. Desulphurisation and inclusion behaviour of stainless steel refining by using CaO-Al2O3 based slag at low sulphur levels. ISIJ Int. 2014, 54, 72–81. [Google Scholar] [CrossRef]
- Sakata, K. Technology for production of austenite type clean stainless steel. ISIJ Int. 2006, 46, 1795–1799. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, S.B.; Gaye, H.R. Thermodynamics of the formation of MgO-Al2O3-TiOx inclusions in Ti-stabilized 11Cr ferritic stainless steel. Metall. Mater. Trans. B 2008, 39, 853–861. [Google Scholar] [CrossRef]
- Kang, Y.B.; Lee, H.G. Inclusions chemistry for Mn/Si deoxidized steels: Thermodynamic predictions and experimental confirmations. ISIJ Int. 2004, 44, 1006–1015. [Google Scholar] [CrossRef]
- Qian, G.; Qu, Z.; Cheng, G. Effect of Al in FeSi alloy on continuous casting and surface defect of stainless steel hot-rolled sheet. Iron Steel 2016, 51, 76–81. [Google Scholar]
- Qian, G. The Technologies and Theory of Desulfurization and Inclusion Control for 304 Stainless Steel on GOR Process. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2015. [Google Scholar]
- Swinbourne, D.R.; Kho, T.S.; Langberg, D.; Blanpain, B.; Arnout, S. Understanding stainless steelmaking through computational thermodynamics Part 2–VOD converting. Miner. Process. Extr. Metall. 2010, 119, 107–115. [Google Scholar] [CrossRef]
- Wei, J.H.; Li, Y. Study on Mathematical Modeling of Combined Top and Bottom Blowing VOD Refining Process of Stainless Steel. Steel Res. Int. 2015, 86, 189–211. [Google Scholar] [CrossRef]

| Stage | C | Si | Mn | P | S | Ni | Cr | Ca | Al | N | Mg | O |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VOD | 0.008 | 0.22 | 1.14 | 0.015 | 0.003 | 8.04 | 18.00 | 0.002 | 0.004 | 0.015 | 0.0005 | 0.007 |
| Stage | CaO | SiO2 | MgO | Al2O3 | Cr2O3 | FeO |
|---|---|---|---|---|---|---|
| VOD | 59.16 | 29.12 | 5.97 | 1.87 | 0.43 | 0.15 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Cheng, G.; Li, J.; Hou, Y.; Pan, J.; Ruan, Q. Characteristics and Formation Mechanism of Inclusions in 304L Stainless Steel during the VOD Refining Process. Metals 2018, 8, 1024. https://doi.org/10.3390/met8121024
Chen X, Cheng G, Li J, Hou Y, Pan J, Ruan Q. Characteristics and Formation Mechanism of Inclusions in 304L Stainless Steel during the VOD Refining Process. Metals. 2018; 8(12):1024. https://doi.org/10.3390/met8121024
Chicago/Turabian StyleChen, Xingrun, Guoguang Cheng, Jingyu Li, Yuyang Hou, Jixiang Pan, and Qiang Ruan. 2018. "Characteristics and Formation Mechanism of Inclusions in 304L Stainless Steel during the VOD Refining Process" Metals 8, no. 12: 1024. https://doi.org/10.3390/met8121024
APA StyleChen, X., Cheng, G., Li, J., Hou, Y., Pan, J., & Ruan, Q. (2018). Characteristics and Formation Mechanism of Inclusions in 304L Stainless Steel during the VOD Refining Process. Metals, 8(12), 1024. https://doi.org/10.3390/met8121024





