New Insight on the Hydrogen Absorption Evolution of the Mg–Fe–H System under Equilibrium Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation
2.2. Characterization
2.3. Handling
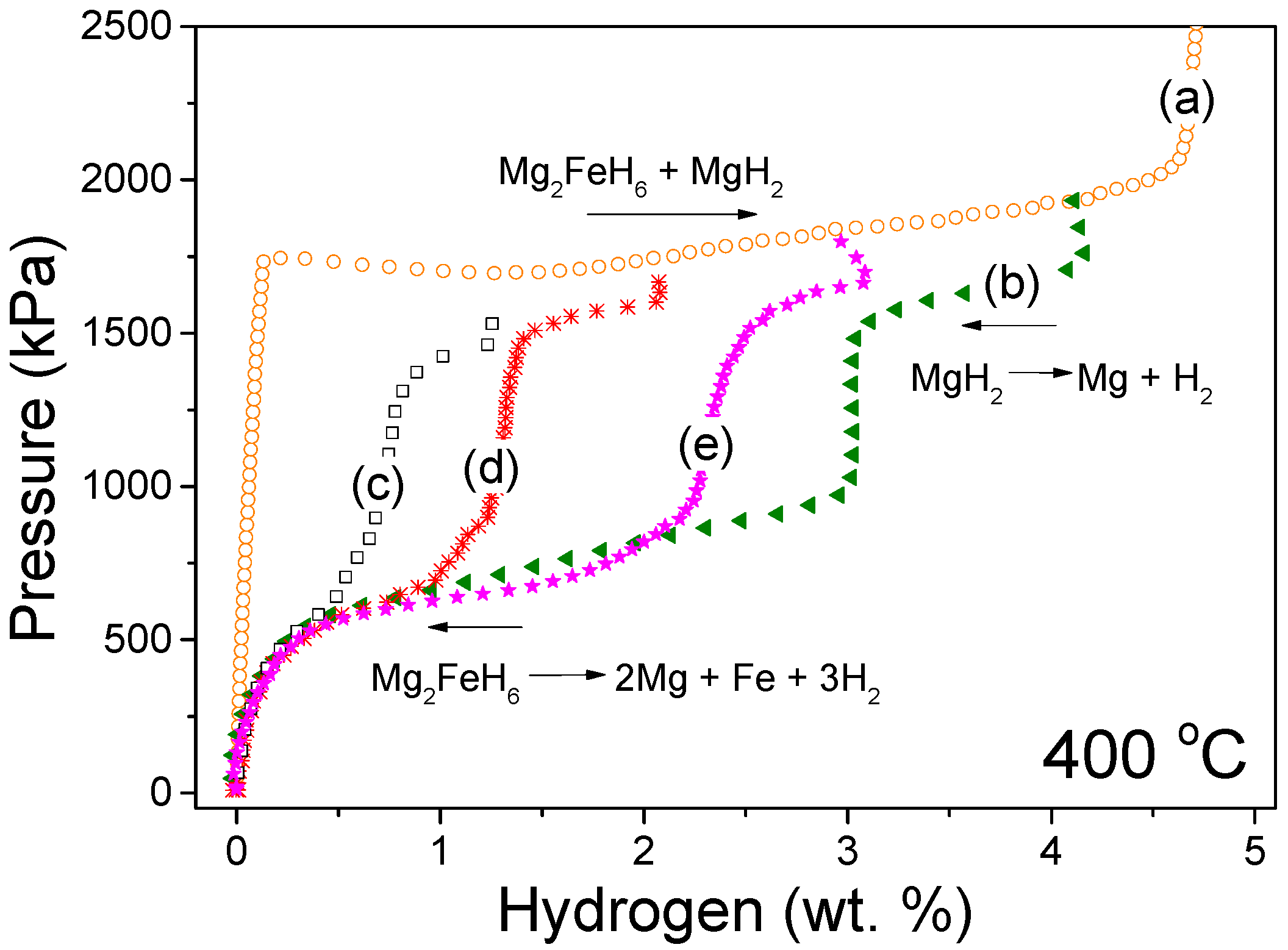
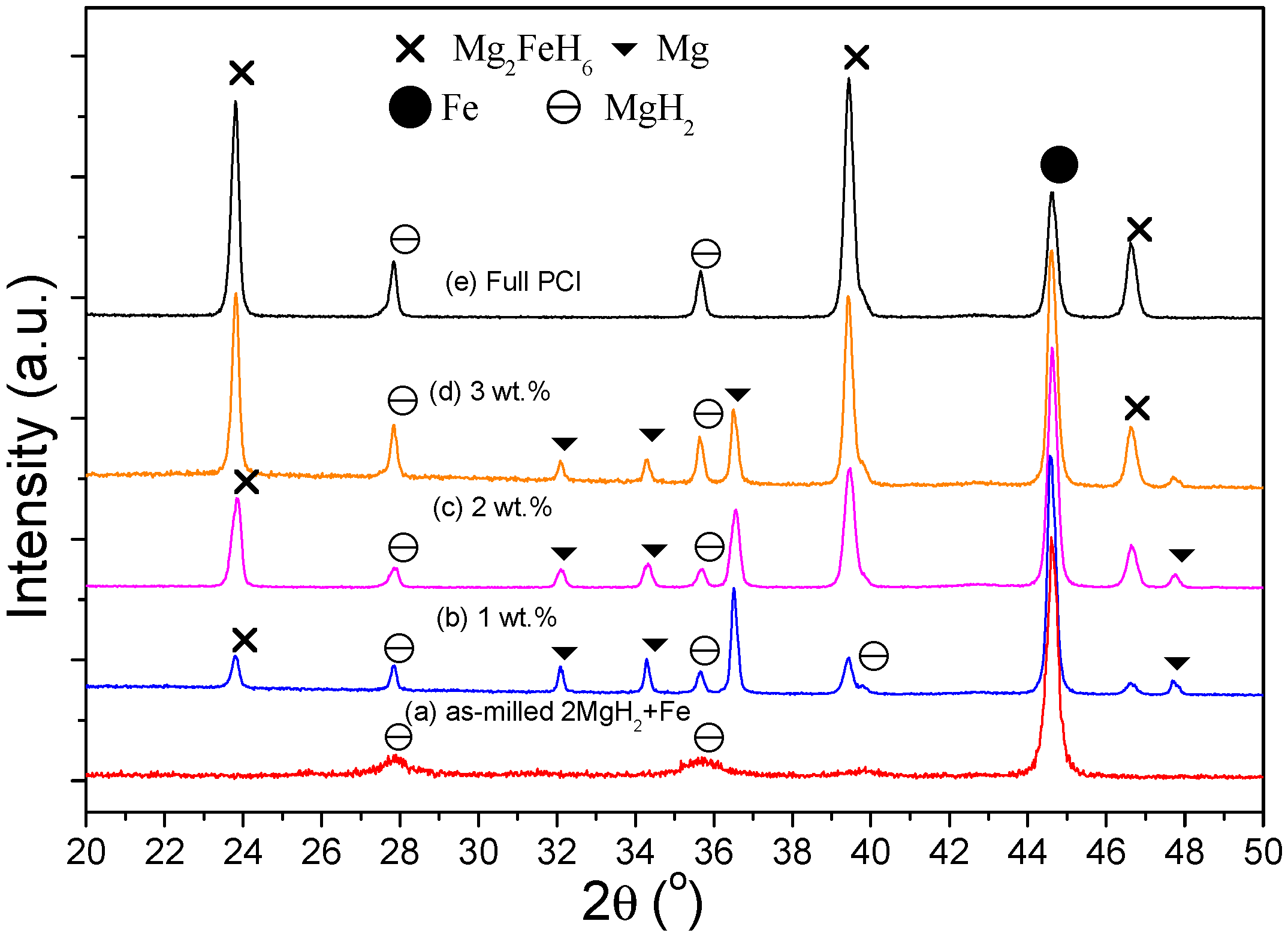
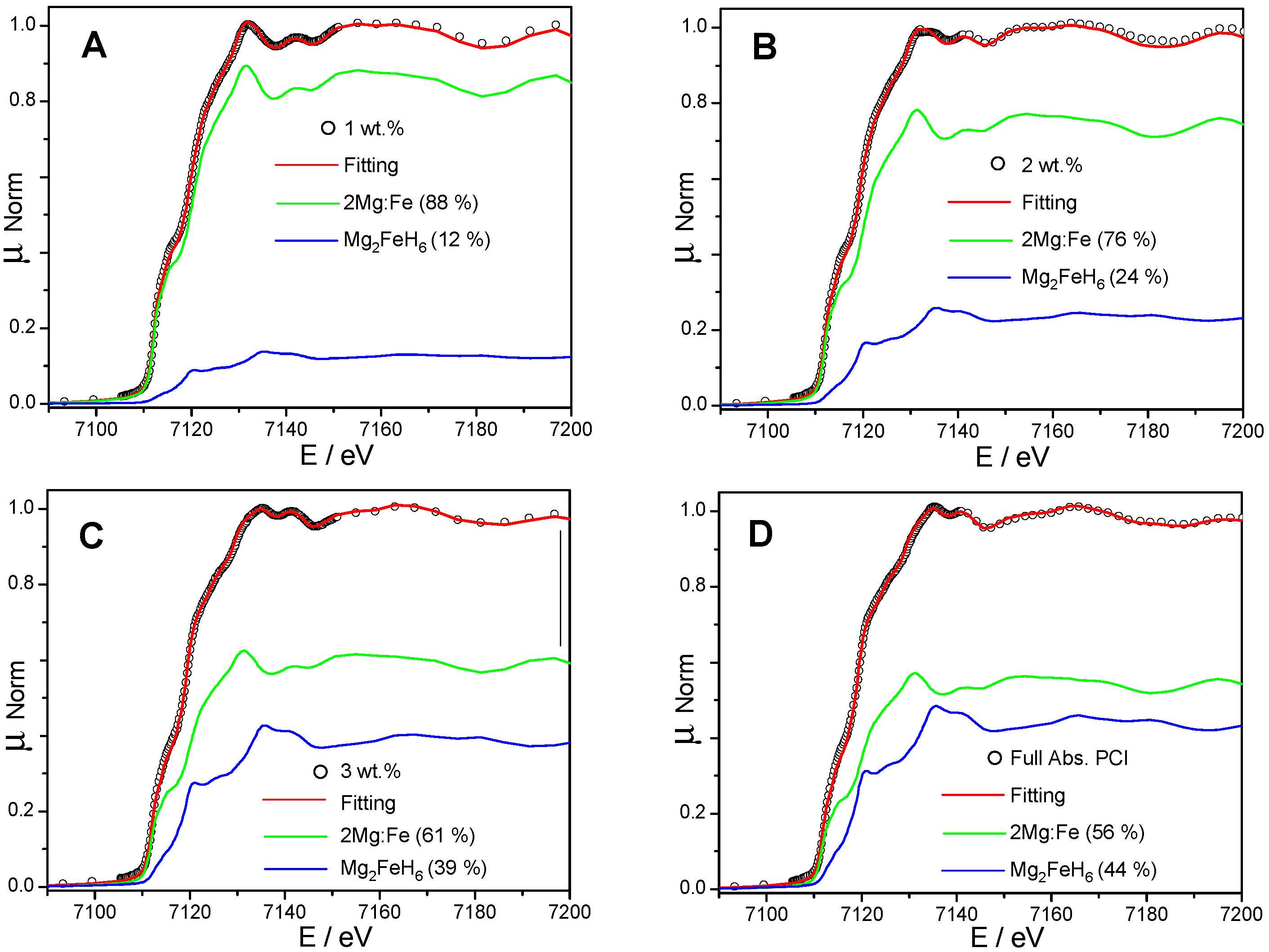
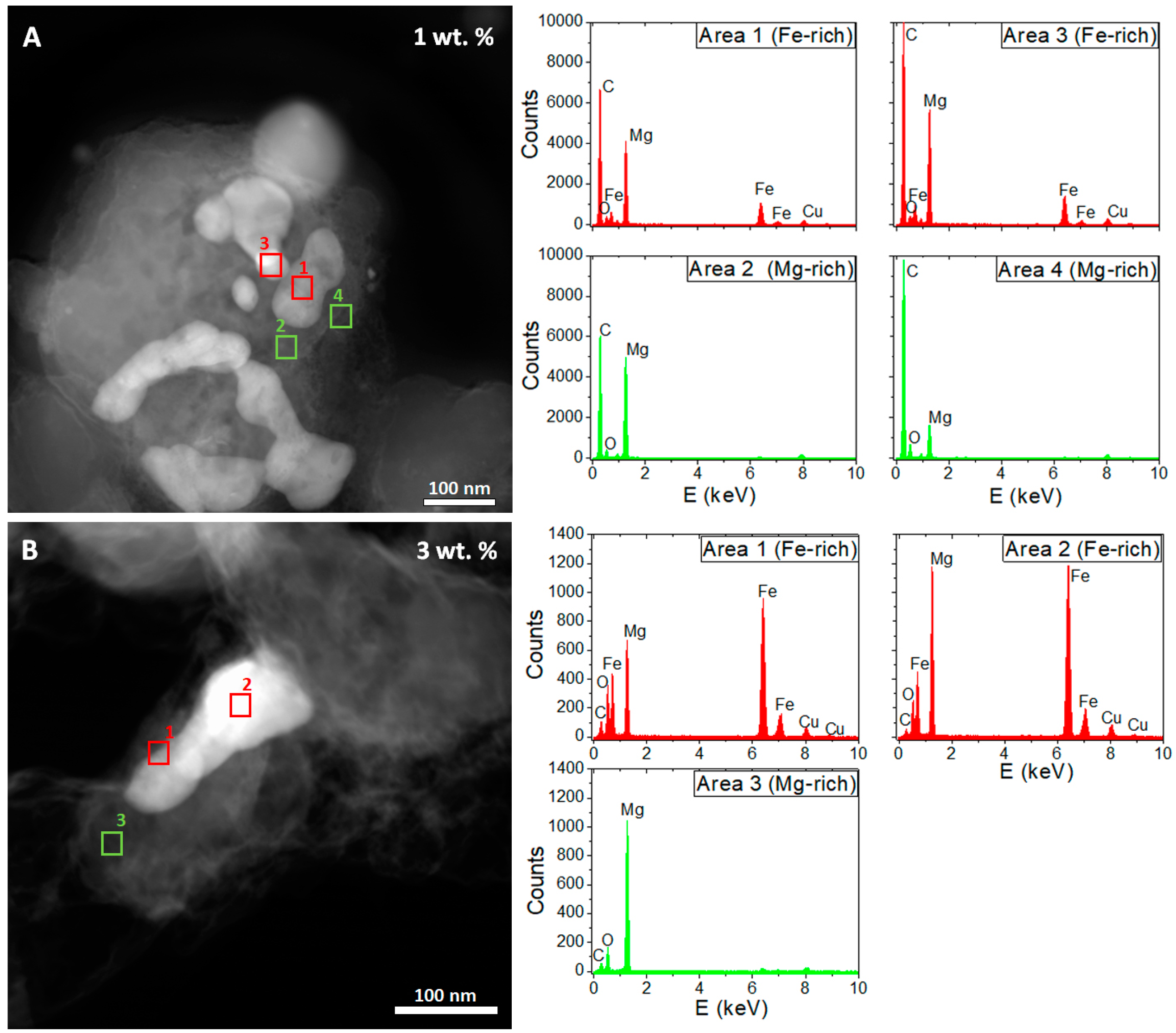
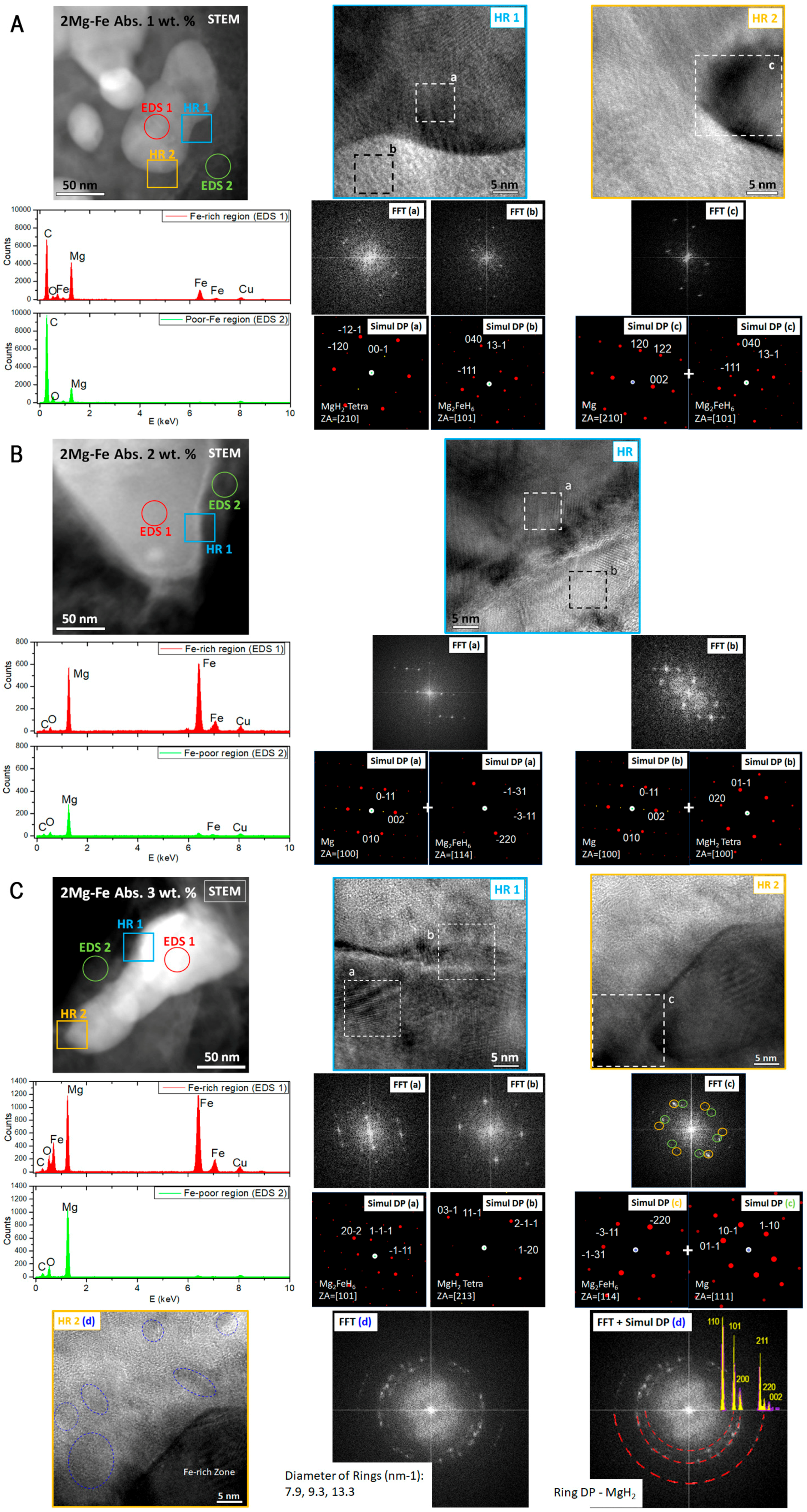
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bogdanović, B.; Reiser, A.; Schlichte, K.; Spliethoff, B.; Tesche, B. Thermodynamics and dynamics of the Mg–Fe–H system and its potential for thermochemical thermal energy storage. J. Alloy. Compd. 2002, 345, 77–89. [Google Scholar] [CrossRef]
- Puszkiel, J.; Andrade-Gamboa, J.; Gennari, F.C. Recent Progress in Mg–Co–H and Mg–Fe–H Systems for Hydrogen Energy Storage Applications. In Emerging Materials for Energy Conversion and Storage, 1st ed.; Cheong, K.Y., Impellizzeri, G., Fraga, M., Eds.; Elsevier: Cambridge, UK, 2018; pp. 393–428. [Google Scholar]
- Reiser, A.; Bogdanović, B. The application of Mg-based metal-hydrides as heat energy storage systems. Int. J. Hydrogen Energy 2000, 25, 425–430. [Google Scholar] [CrossRef]
- Crivello, J.-C.; Denys, R.V.; Dornheim, M.; Felderhoff, M.; Grant, D.M.; Huot, J.; Jensen, T.R.; de Jongh, P.; Latroche, M.; Walker, G.S.; et al. Mg-based compounds for hydrogen and energy storage. Appl. Phys. A 2016, 122, 1–17. [Google Scholar] [CrossRef]
- Felderhoff, M.; Bogdanović, B. High temperature metal hydrides as heat storage materials for solar and related applications. Int. J. Mol. Sci. 2009, 10, 325–344. [Google Scholar] [CrossRef] [PubMed]
- Urbanczyk, R.; Meggouh, M.; Moury, R.; Peinecke, K.; Peil, S.; Felderhoff, M. Demonstration of Mg2FeH6 as heat storage material at temperatures up to 550 °C. Appl. Phys. A 2016, 122, 315–320. [Google Scholar] [CrossRef]
- Urbanczyk, R.; Peinecke, K.; Peil, S.; Felderhoff, M. Development of a heat storage demonstration unit on the basis of Mg2FeH6 as heat storage material and molten salt as heat transfer media. Int. J. Hydrogen Energy 2017, 42, 13818–13826. [Google Scholar] [CrossRef]
- Massalski, T. Binary Alloy Phase Diagram, 2nd ed.; ASM International: Metals Park, OH, USA, 1990; pp. 1722–1723. [Google Scholar]
- Gennari, F.C.; Castro, F.J.; Andrade Gamboa, J.J. Synthesis of Mg2FeH6 by reactive mechanical alloying: Formation and decomposition properties. J. Alloy. Compd. 2002, 339, 261–267. [Google Scholar] [CrossRef]
- Polanski, M.; Nielsen, T.K.; Cerenius, Y.; Bystrzycki, J.; Jensen, T.R. Synthesis and decomposition mechanisms of Mg2FeH6 studied by in-situ synchrotron X-ray diffraction and high-pressure DSC. Int. J. Hydrogen Energy 2010, 35, 3578–3582. [Google Scholar] [CrossRef]
- Puszkiel, J.A.; Gennari, F.; Arneodo Larochette, P.; Karimi, F.; Pistidda, C.; Gosalawit-Utke, R.; Jepsen, J.; Jensen, T.R.; Gundlach, C.; von Colbe, J.B.; et al. Sorption behavior of the MgH2-Mg2FeH6 hydride storage system synthesized by mechanical milling followed by sintering. Int. J. Hydrogen Energy 2013, 38, 14618–14630. [Google Scholar] [CrossRef]
- Danaie, M.; Asselli, A.A.C.; Huot, J.; Botton, G.A. Formation of the ternary complex hydride Mg2FeH6 from magnesium hydride (β-MgH2) and iron: An electron microscopy and energy-loss spectroscopy study. J. Phys. Chem. C 2012, 116, 25701–25714. [Google Scholar] [CrossRef]
- Didisheim, J.J.; Zolliker, P.; Yvon, K.; Fischer, P.; Schefer, J.; Gubelmann, M. Dimagnesium iron (II) hydride, Mg2FeH6, containing octahedral [FeH6]4− anions. Inorg. Chem. 1984, 23, 1953–1957. [Google Scholar] [CrossRef]
- Selvam, P.; Yvon, K. Synthesis of Mg2FeH6, Mg2CoH5 and Mg2NiH4 by high-pressure sintering of the elements. Int. J. Hydrogen Energy 1991, 16, 615–617. [Google Scholar] [CrossRef]
- Huot, J.; Hayakawa, H.; Akiba, E. Preparation of the hydrides Mg2FeH6 and Mg2CoH5 by mechanical alloying followed by sintering. J. Alloy. Compd. 1997, 248, 164–167. [Google Scholar] [CrossRef]
- Konstanchuk, I.G.; Ivanov, E.Y.; Pezat, M.; Darriet, B.; Boldyrev, V.V.; Hagenmuller, P. The hydriding properties of a mechanical alloy with composition Mg-25%Fe. J. Less Common Met. 1987, 131, 181–189. [Google Scholar] [CrossRef]
- Retuerto, M.; Sánchez-Benítez, J.; Rodríguez-Cañas, E.; Serafini, D.; Alonso, J.A. High-pressure synthesis of Mg2FeH6 complex hydride. Int. J. Hydrogen Energy 2010, 35, 7835–7841. [Google Scholar] [CrossRef]
- Niaz, N.A.; Ahmad, I.; Khalid, N.R.; Ahmed, E.; Abbas, S.M.; Jabeen, N. Preparation of Mg2FeH6: Nanoparticles for hydrogen storage properties. J. Nanomater. 2013, 1–7. [Google Scholar] [CrossRef]
- Ivanov, E.; Konstanchuk, I.; Stepanov, A.; Boldyrev, V. Magnesium mechanical alloys for hydrogen storage. J. Less Common Met. 1987, 131, 25–29. [Google Scholar] [CrossRef]
- Puszkiel, J.A.; Arneodo Larochette, P.; Gennari, F.C. Thermodynamic and kinetic studies of Mg-Fe-H after mechanical milling followed by sinterin. J. Alloy. Compd. 2008, 463, 134–142. [Google Scholar] [CrossRef]
- Puszkiel, J.A.; Arneodo Larochette, P.; Gennari, F.C. Thermodynamic and kinetic characterization of the synthesized Mg2FeH6–MgH2 hydrides mixture. Int. J. Hydrogen Energy 2008, 33, 3555–3560. [Google Scholar] [CrossRef]
- Riktor, M.D.; Deledda, S.; Herrich, M.; Gutfleisch, O.; Fjellvåg, H.; Hauback, B.C. Hydride formation in ball-milled and cryomilled Mg-Fe powder mixtures. Mater. Sci. Eng. B 2009, 158, 19–25. [Google Scholar] [CrossRef]
- Polanski, M.; Płociński, T.; Kunce, I.; Bystrzycki, J. Dynamic synthesis of ternary Mg2FeH6. Int. J. Hydrogen Energy 2010, 35, 1257–1266. [Google Scholar] [CrossRef]
- Polanski, M.; Witek, K.; Nielsen, T.K.; Jaroszewicz, L.; Bystrzycki, J. The influence of the milling time on the yield of Mg2FeH6 from a two-step synthesis conducted in a custom-made reactor. Int. J. Hydrogen Energy 2013, 38, 2785–2789. [Google Scholar] [CrossRef]
- Zhang, J.; Cuevas, F.; Zaïdi, W.; Bonnet, J.P.; Aymard, L.; Bobet, J.L.; Latroche, M. Highlighting of a single reaction path during reactive ball milling of Mg and TM by quantitative H2 gas sorption analysis to form ternary complex hydrides (TM = Fe, Co, Ni). J. Phys. Chem. C 2011, 115, 4971–4979. [Google Scholar] [CrossRef]
- Huot, J.; Boily, S.; Akiba, E.; Schulz, R. Direct synthesis of Mg2FeH6 by mechanical alloying. J. Alloy. Compd. 1998, 280, 306–309. [Google Scholar] [CrossRef]
- Castro, F.J.; Gennari, F.C. Effect of the nature of the starting materials on the formation of Mg2FeH6. J. Alloy. Compd. 2004, 375, 292–296. [Google Scholar] [CrossRef]
- Herrich, M.; Ismail, N.; Lyubina, J.; Handstein, A.; Pratt, A.; Gutfleisch, O. Synthesis and decomposition of Mg2FeH6 prepared by reactive milling. Mater. Sci. Eng. B 2004, 108, 28–32. [Google Scholar] [CrossRef]
- Varin, R.A.; Li, S.; Calka, A.; Wexler, D. Formation and environmental stability of nanocrystalline and amorphous hydrides in the 2Mg-Fe mixture processed by controlled reactive mechanical alloying (CRMA). J. Alloy. Compd. 2004, 373, 270–286. [Google Scholar] [CrossRef]
- Li, S.; Varin, R.A.; Morozova, O.; Khomenko, T. Controlled mechano-chemical synthesis of nanostructured ternary complex hydride Mg2FeH6 under low-energy impact mode with and without pre-milling. J. Alloy. Compd. 2004, 384, 231–248. [Google Scholar] [CrossRef]
- Varin, R.A.; Li, S.; Wronski, Z.; Morozova, O.; Khomenko, T. The effect of sequential and continuous high-energy impact mode on the mechano-chemical synthesis of nanostructured complex hydride Mg2FeH6. J. Alloy. Compd. 2005, 390, 282–296. [Google Scholar] [CrossRef]
- Zhou, D.W.; Li, S.L.; Varin, R.A.; Peng, P.; Liu, J.S.; Yang, F. Mechanical alloying and electronic simulations of 2Mg-Fe mixture powders for hydrogen storage. Mater. Sci. Eng. A 2006, 427, 306–315. [Google Scholar] [CrossRef]
- Wronski, Z.; Varin, R.A.; Chiu, C.; Czujko, T.; Calka, A. Mechanochemical synthesis of nanostructured chemical hydrides in hydrogen alloying mills. J. Alloy. Compd. 2007, 434, 743–746. [Google Scholar] [CrossRef]
- Varin, R.A.; Czujko, T.; Wronski, Z.S. Nanomaterials for Solid State Hydrogen Storage, 1st ed.; Springer: New York, NY, USA, 2008; pp. 1–325. [Google Scholar]
- Sai Raman, S.S.; Davidson, D.J.; Bobet, J.-L.; Srivastava, O.N. Investigations on the synthesis, structural and microstructural characterization of Mg based K2PtCl6-type (Mg2FeH6) hydrogen storage material prepared by mechanical alloying. J. Alloy. Compd. 2002, 333, 282–290. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, F.; Li, C.; Tao, Z.; Chen, J. Preparation and characterization of nanocrystalline Mg2FeH6. J. Alloy. Compd. 2010, 508, 554–558. [Google Scholar] [CrossRef]
- Asselli, A.C.C.; Leiva, D.R.; Jorge, A.M.; Ishikawa, T.T.; Botta, W.J. Synthesis and hydrogen sorption properties of MgH2-Mg2FeH6 nanocomposites prepared by reactive milling. J. Alloy. Compd. 2012, 536, S250–S254. [Google Scholar] [CrossRef]
- Nyamsi, S.N.; Yartys, V.; Lototskyy, M. Synthesis of Mg2FeH6 Assisted by Heat Treatment of Starting Materials. In Proceedings of the 1st Africa Energy Materials Conference, Pretoria, South Africa, 28–31 March 2017. [Google Scholar]
- Jung, J.Y.; Fadonougbo, J.O.; Suh, J.-Y.; Lee, Y.-S.; Huh, Y.-J.; Cho, Y.W. Synthesis of Mg2FeH6 by hydrogenation of Mg/Fe powder mixture prepared by cold roll milling in air: Effects of microstructure and oxygen distribution. Int. J. Hydrogen Energy 2018, 43, 16758–16765. [Google Scholar] [CrossRef]
- Alexander, L.; Klug, P.H. Determination of crystallite size with the x-ray spectrometer. J. Appl. Phys. 2004, 21, 137. [Google Scholar] [CrossRef]
- Laboratorio Nacional de Luz Síncrotron (LNLS). Available online: http://www.lnls.br/28 (accessed on 11 October 2018).
- XAFSmass, Freeware. Available online: www.cells.es/Beamlines/CLAESS/software/xafsmass.html (accessed on 11 October 2018).
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Zabinsky, S.I.; Rehr, J.J.; Ankudinov, A.; Albers, R.C.; Eller, M.J. Multiple-scattering calculations of x-ray-absorption spectra. J. Phys. Rev. B 1995, 52, 2995–3009. [Google Scholar] [CrossRef]
- Roine, A. Outokumpu HSC Chemistry for Windows; Outokumpu Research Oy: Pori, Finland, 2009. [Google Scholar]
- Zhou, H.L.; Yu, Y.; Zhang, H.F.; Gao, T. Structural, vibrational and thermodynamic properties of Mg2FeH6 complex hydride. Eur. Phys. J. B 2011, 79, 283–288. [Google Scholar] [CrossRef]
- Ravel, B. ATOMS: Crystallography for the X-ray absorption spectroscopist. J. Synchrotron Radiat. 2001, 8, 314–316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zaïdi, W.; Paul-Boncour, V.; Provost, K.; Michalowicz, A.; Cuevas, F.; Latroche, M.; Belin, S.; Bonnet, J.-P.; Aymard, L. XAS investigations on nanocrystalline Mg2FeH6 used as a negative electrode of Li-ion batteries. J. Mater. Chem. A 2013, 1, 4706–4717. [Google Scholar] [CrossRef]
- Zareii, S.M.; Sarhaddi, R. Structural, electronic properties and heat of formation of Mg2FeH6 complex hydride: An ab initio study. Phys. Scr. 2012, 86, 015701. [Google Scholar] [CrossRef]
| Hydrogenation State | Amounts of Phases (wt %) | |||
|---|---|---|---|---|
| Mg | Fe | MgH2 | Mg2FeH6 | |
| 2Mg:Fe | 46 | 54 | - | - |
| 1 wt % | 34 | 46 | 6 | 14 (12) |
| 2 wt % | 26 | 40 | 10 | 24 (24) |
| 3 wt % | 18 | 31 | 10 | 41 (39) |
| Complete PCI | 4 | 28 | 22 | 46 (44) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puszkiel, J.; Castro Riglos, M.V.; Ramallo-López, J.M.; Mizrahi, M.; Gemming, T.; Pistidda, C.; Arneodo Larochette, P.; Bellosta von Colbe, J.; Klassen, T.; Dornheim, M.; et al. New Insight on the Hydrogen Absorption Evolution of the Mg–Fe–H System under Equilibrium Conditions. Metals 2018, 8, 967. https://doi.org/10.3390/met8110967
Puszkiel J, Castro Riglos MV, Ramallo-López JM, Mizrahi M, Gemming T, Pistidda C, Arneodo Larochette P, Bellosta von Colbe J, Klassen T, Dornheim M, et al. New Insight on the Hydrogen Absorption Evolution of the Mg–Fe–H System under Equilibrium Conditions. Metals. 2018; 8(11):967. https://doi.org/10.3390/met8110967
Chicago/Turabian StylePuszkiel, Julián, M. Victoria Castro Riglos, José M. Ramallo-López, Martin Mizrahi, Thomas Gemming, Claudio Pistidda, Pierre Arneodo Larochette, José Bellosta von Colbe, Thomas Klassen, Martin Dornheim, and et al. 2018. "New Insight on the Hydrogen Absorption Evolution of the Mg–Fe–H System under Equilibrium Conditions" Metals 8, no. 11: 967. https://doi.org/10.3390/met8110967
APA StylePuszkiel, J., Castro Riglos, M. V., Ramallo-López, J. M., Mizrahi, M., Gemming, T., Pistidda, C., Arneodo Larochette, P., Bellosta von Colbe, J., Klassen, T., Dornheim, M., & Gennari, F. (2018). New Insight on the Hydrogen Absorption Evolution of the Mg–Fe–H System under Equilibrium Conditions. Metals, 8(11), 967. https://doi.org/10.3390/met8110967







