Effective Gold Recovery from Near-Surface Oxide Zone Using Reductive Microwave Roasting and Magnetic Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ore in the Near-Surface Oxide Zone
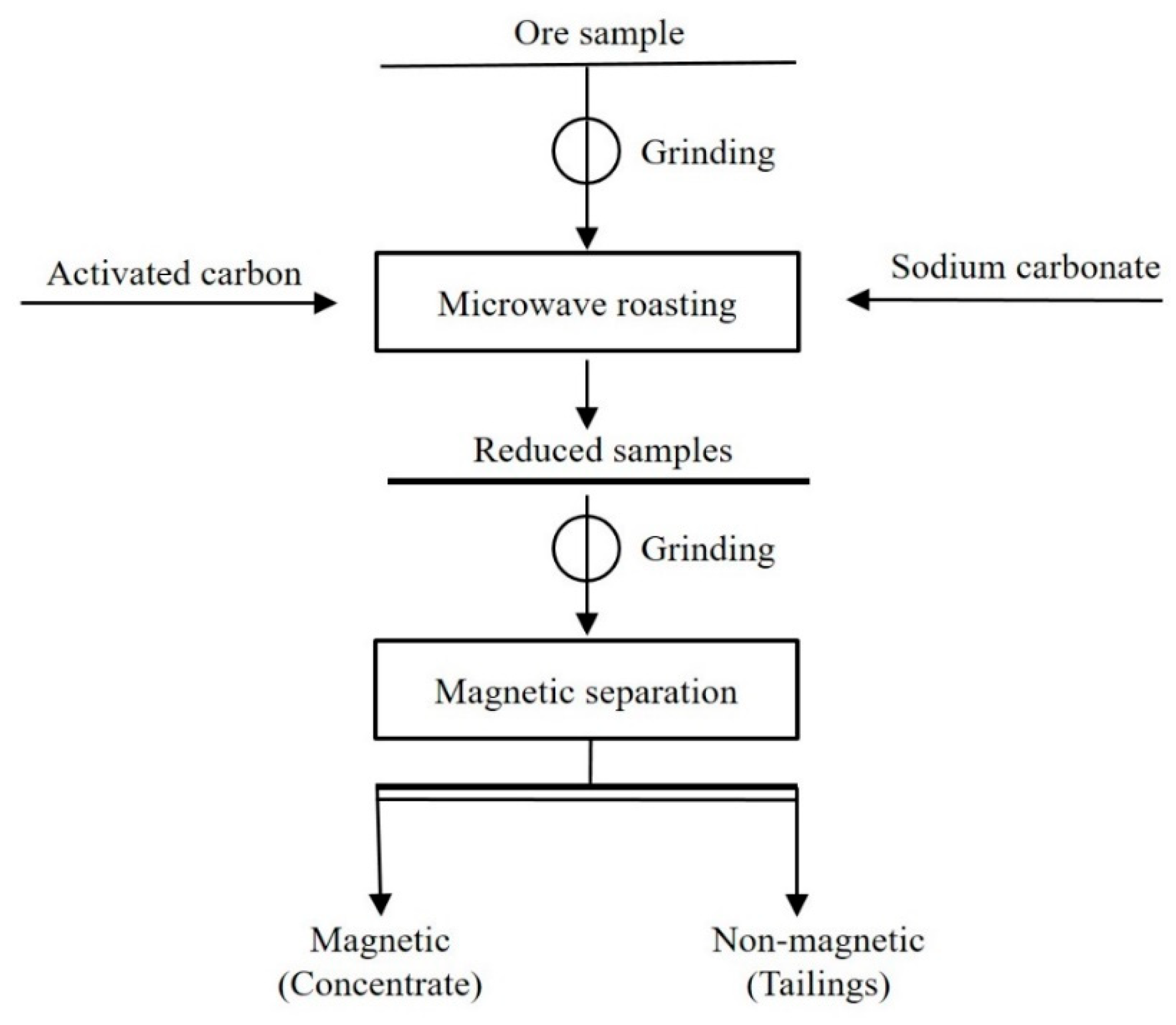
2.2. Reductive Microwave Roasting (RMR)-Magnetic Separation (MS) Process
2.2.1. RMR Process
2.2.2. MS Process
3. Results and Discussion
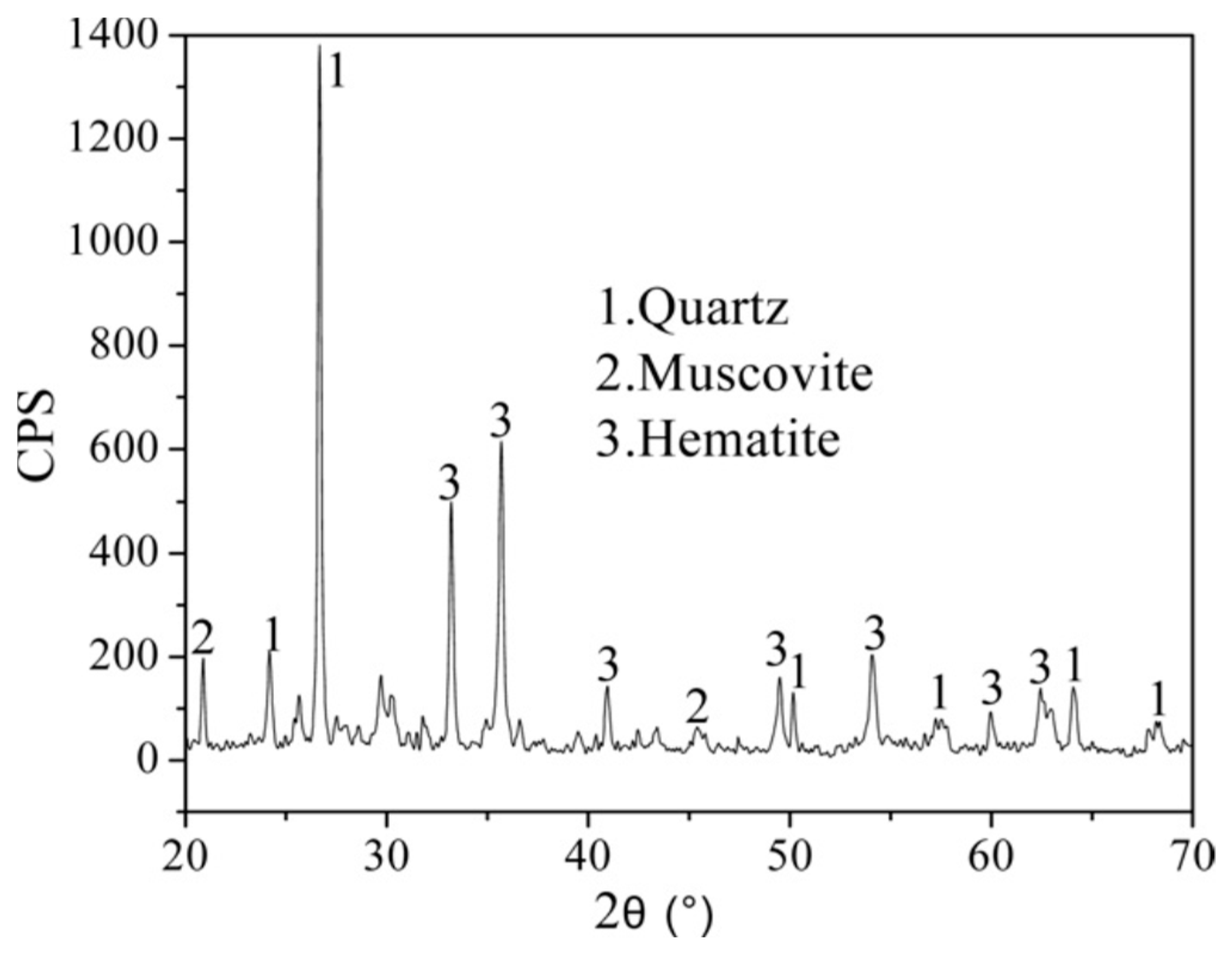
3.1. Characteristics of Ore in the Near-Surface Oxide Zone
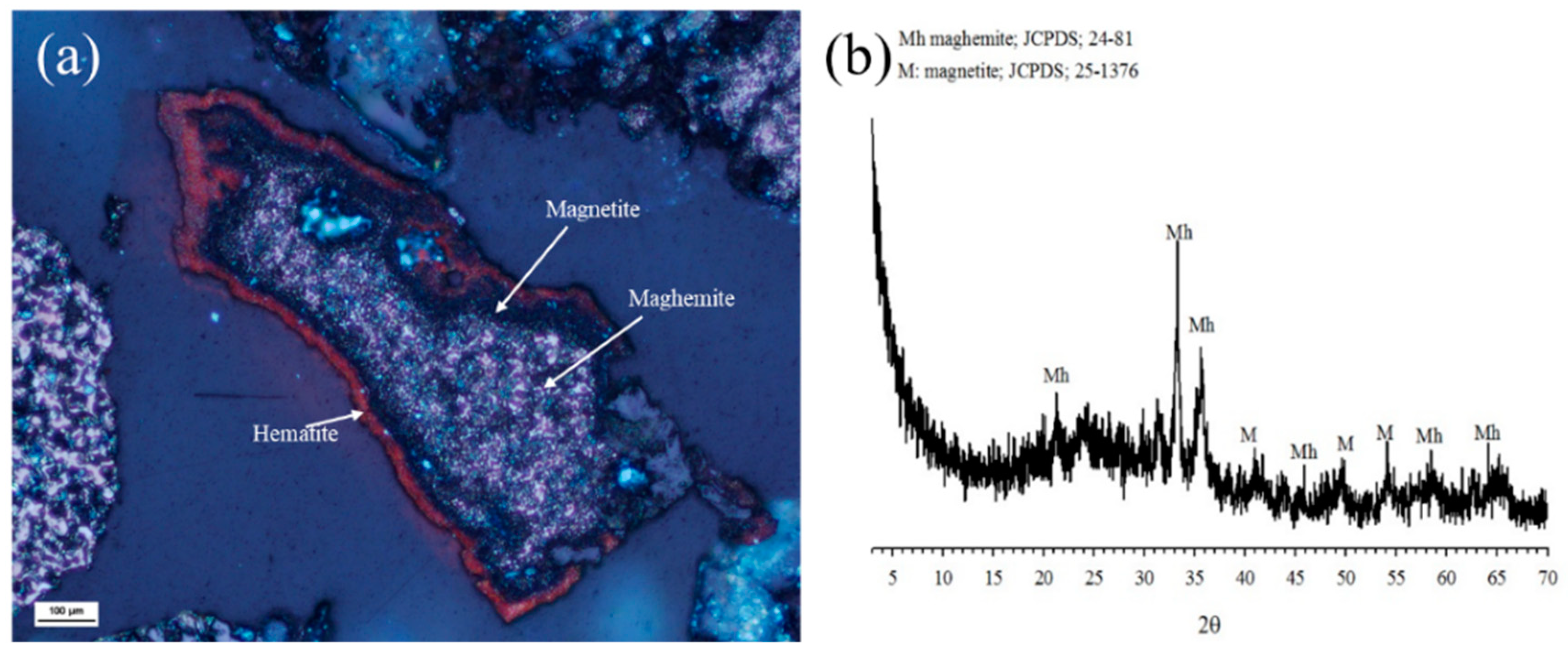
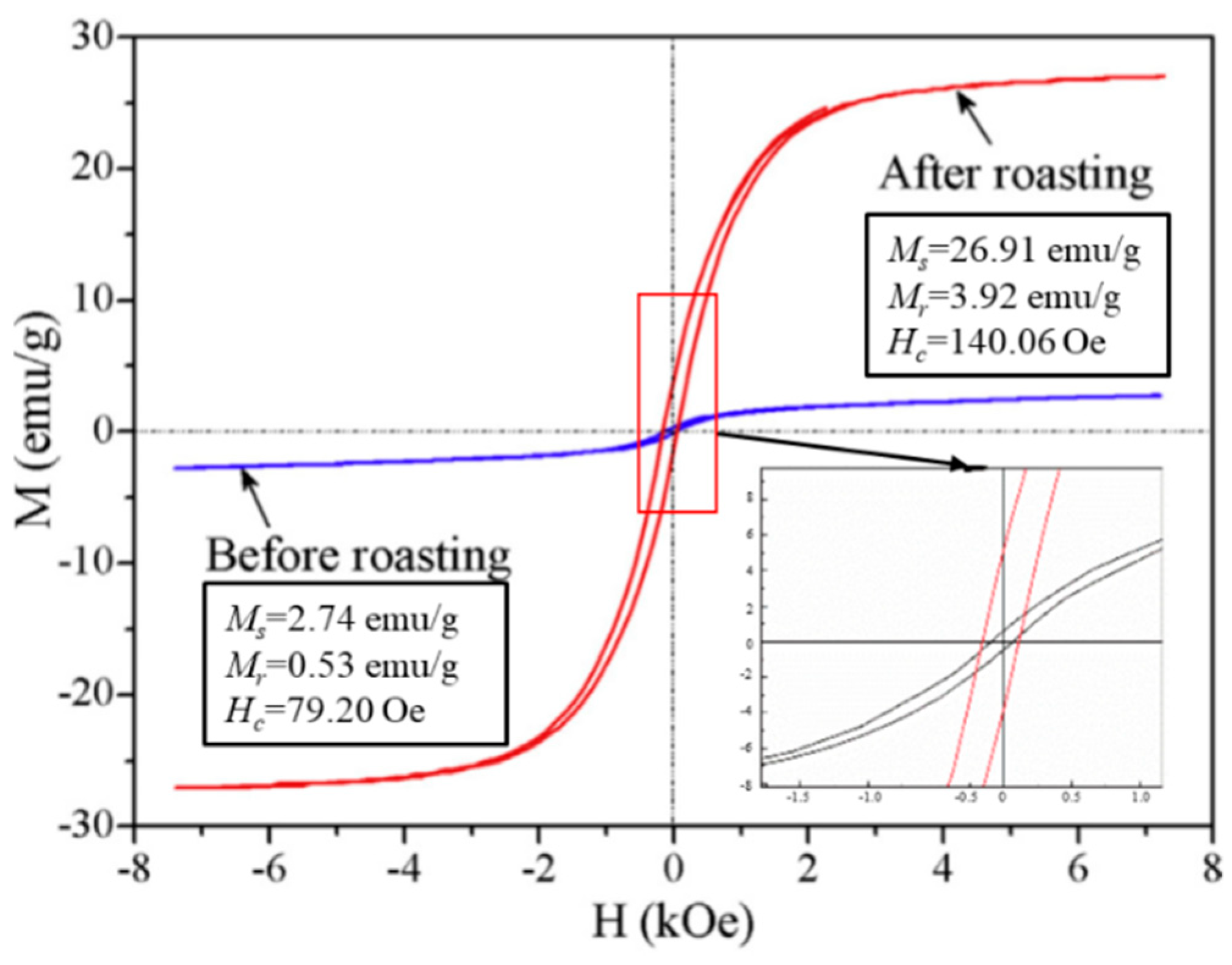
3.2. RMR-MS for Recovery of Au Concentrate from Oxide Ore
3.3. Effects of RMR Conditions on Iron Recovery
3.3.1. Effect of Activated Carbon and Sodium Carbonate
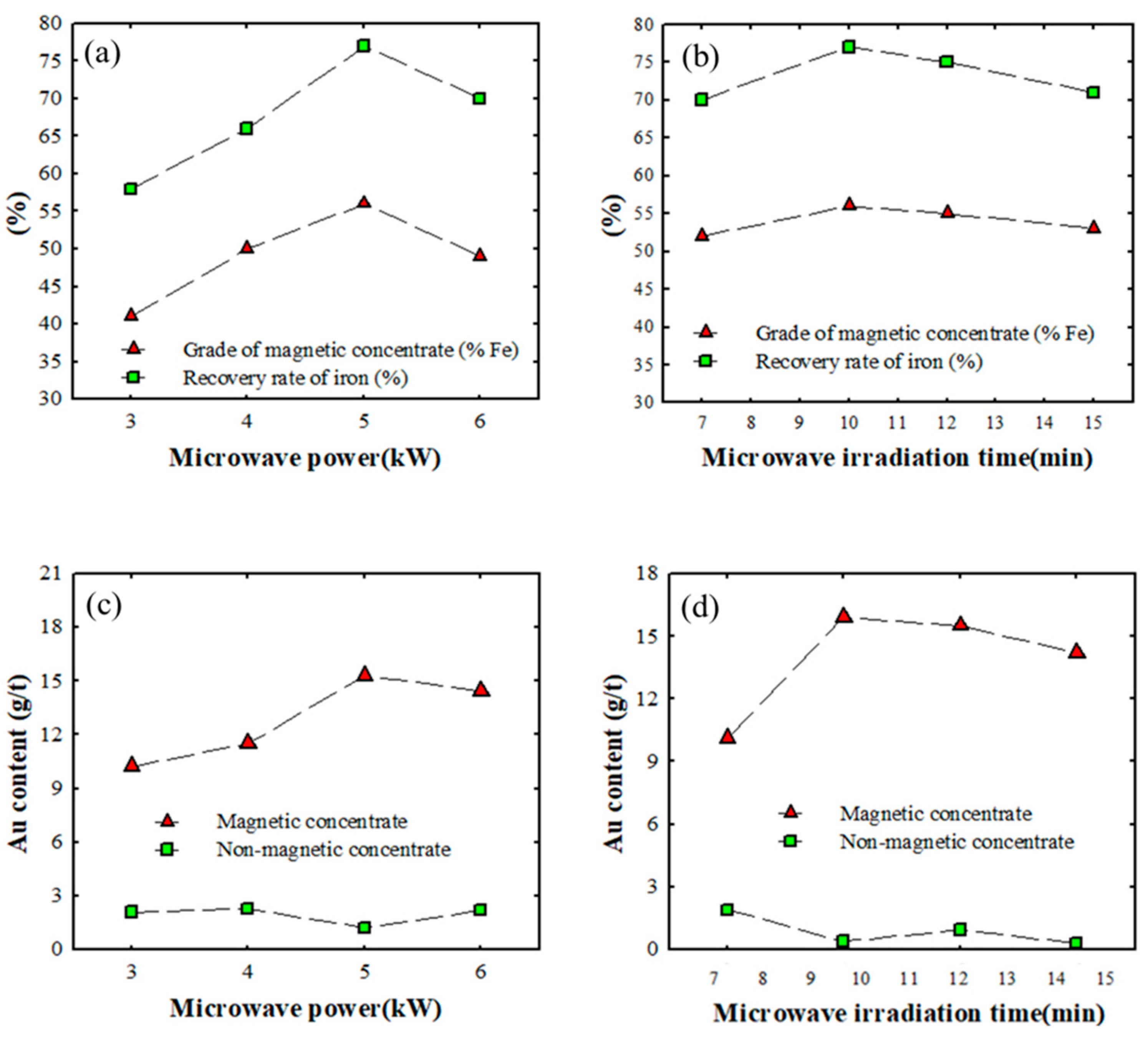
3.3.2. Effect of Microwave Power and Irradiation Time
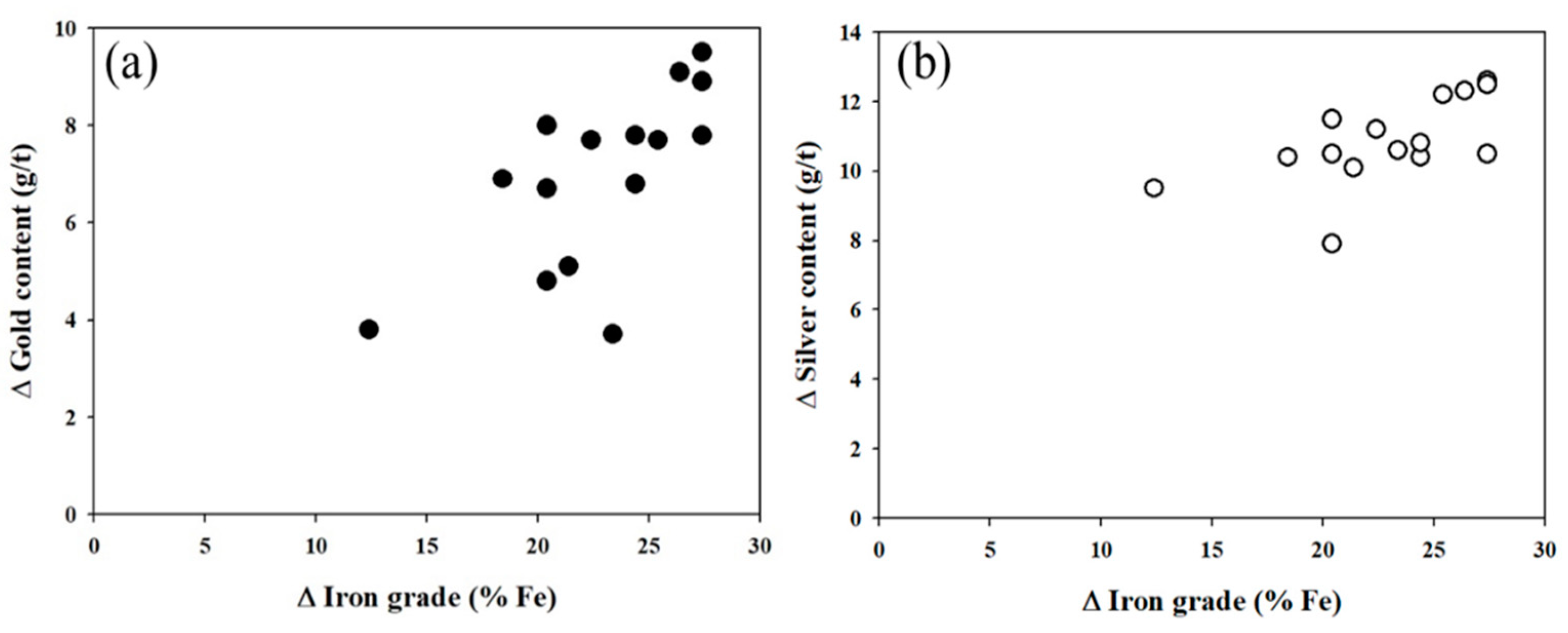
3.4. Au Concentration during RMR-MS
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, Y.; Fang, M.; Lan, L.D.; Zhang, P.; Rao, K.V.; Bao, Z.Y. Rapid and direct magnetization of goethite ore roasted by biomass fuel. Sep. Purif. Technol. 2012, 94, 34–38. [Google Scholar] [CrossRef]
- Gao, G.; Li, D.; Zhou, Y.; Sun, X.; Sun, W. Kinetics of high-sulphur and high-arsenic refractory gold concentrate oxidation by dilute nitric acid under mild conditions. Miner. Eng. 2009, 22, 111–115. [Google Scholar] [CrossRef]
- Mari, D.; Bolognini, S.; Feusier, G.; Cutard, T.; Verdon, C.; Viatte, T.; Benoit, W. Timocn based cermets: Part I. Morphology and phase composition. Int. J. Refract. Met. Hard Mater. 2003, 21, 37–46. [Google Scholar] [CrossRef]
- Hayashi, M.; Takeda, K.; Kashimura, K.; Watanabe, T.; Nagata, K. Carbothermic reduction of hematite powders by microwave heating. Isij Int. 2013, 53, 1125–1130. [Google Scholar] [CrossRef]
- Li, G.H.; Zhang, S.H.; Rao, M.J.; Zhang, Y.B.; Jiang, T. Effects of sodium salts on reduction roasting and Fe–P separation of high-phosphorus oolitic hematite ore. Int. J. Miner. Process. 2013, 124, 26–34. [Google Scholar] [CrossRef]
- Zhang, M.; Peng, Y.J. Effect of clay minerals on pulp rheology and the flotation of copper and gold minerals. Miner. Eng. 2015, 70, 8–13. [Google Scholar] [CrossRef]
- Celep, O.; Alp, İ.; Paktunç, D.; Thibault, Y. Implementation of sodium hydroxide pretreatment for refractory antimonial gold and silver ores. Hydrometallurgy 2011, 108, 109–114. [Google Scholar] [CrossRef]
- Dunn, J.G.; Chamberlain, A.C. The recovery of gold from refractory arsenopyrite concentrates by pyrolysis-oxidation. Miner. Eng. 1997, 10, 919–928. [Google Scholar] [CrossRef]
- Gudyanga, F.P.; Mahlangu, T.; Roman, R.J.; Mungoshi, J.; Mbeve, K. An acidic pressure oxidation pre-treatment of refractory gold concentrates from the kwekwe roasting plant, zimbabwe. Miner. Eng. 1999, 12, 863–875. [Google Scholar] [CrossRef]
- Iglesias, N.; Carranza, F. Refractory gold-bearing ores: A review of treatment methods and recent advances in biotechnological techniques. Hydrometallurgy 1994, 34, 383–395. [Google Scholar] [CrossRef]
- Celep, O.; Alp, İ.; Deveci, H. Improved gold and silver extraction from a refractory antimony ore by pretreatment with alkaline sulphide leach. Hydrometallurgy 2011, 105, 234–239. [Google Scholar] [CrossRef]
- Celep, O.; Serbest, V. Characterization of an iron oxy/hydroxide (gossan type) bearing refractory gold and silver ore by diagnostic leaching. Trans. Nonferrous Met. Soc. China 2015, 25, 1286–1297. [Google Scholar] [CrossRef]
- Lunt, D.; Weeks, T. Chapter 7—Process flowsheet selection. In Gold ore Processing, 2nd ed.; Adams, M.D., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 113–129. [Google Scholar]
- Faraz, S.; Hossna, D.; Rezgar, B.; Piroz, Z. Improved recovery of a low-grade refractory gold ore using flotation-preoxidation-cyanidation methods. Int. J. Min. Sci. Technol. 2014, 24, 537–542. [Google Scholar] [CrossRef]
- La Brooy, S.R.; Linge, H.G.; Walker, G.S. Review of gold extraction from ores. Miner. Eng. 1994, 7, 1213–1241. [Google Scholar] [CrossRef]
- Learmont, M.; Iwasaki, I. Effect of grinding media on galena flotation. Min. Metall. Explor. 1984, 1, 136–143. [Google Scholar] [CrossRef]
- Bandini, P.; Prestidge, C.A.; Ralston, J. Colloidal iron oxide slime coatings and galena particle flotation. Miner. Eng. 2001, 14, 487–497. [Google Scholar] [CrossRef]
- Parsonage, P. Effects of slime and colloidal particles on the flotation of galena. Flotat. Sulphide Miner. 1984, 111–139. [Google Scholar]
- Peng, Y.J.; Zhao, S.L. The effect of surface oxidation of copper sulfide minerals on clay slime coating in flotation. Miner. Eng. 2011, 24, 1687–1693. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Yu, X. Recovery of iron from cyanide tailings with reduction roasting-water leaching followed by magnetic separation. J. Hazard. Mater. 2012, 213–214, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Viñals, J.; Roca, A.; Cruells, M.; Núñez, C. Characterization and cyanidation of rio tinto gossan ores. Can. Metall. Q. 1995, 34, 115–122. [Google Scholar] [CrossRef]
- Znamenáčková, I.; Lovas, M.; Mockovčiaková, A.; Jakabský, Š.; Briančin, J. Modification of magnetic properties of siderite ore by microwave energy. Sep. Purif. Technol. 2005, 43, 169–174. [Google Scholar] [CrossRef]
- Omran, M.; Fabritius, T.; Elmandy, A.M.; Abdel-Khalek, N.A.; El-Aref, M.; Elmanawi, A.E.H. Effect of microwave pre-treatment on the magnetic properties of iron ore and its implications on magnetic separation. Sep. Purif. Technol. 2014, 136, 223–232. [Google Scholar] [CrossRef]
- Barani, K.; Koleini, S.M.J.; Rezaei, B. Magnetic properties of an iron ore sample after microwave heating. Sep. Purif. Technol. 2011, 76, 331–336. [Google Scholar] [CrossRef]
- Nanthakumar, B.; Pickles, C.A.; Kelebek, S. Microwave pretreatment of a double refractory gold ore. Miner. Eng. 2007, 20, 1109–1119. [Google Scholar] [CrossRef]
- Roca, A. Characterization and alkaline decomposition/cyanidation of beudantite–jarosite materials from rio tinto gossan ores. Can. Metall. Q. 1999, 38, 93–103. [Google Scholar] [CrossRef]
- Choi, S.-G.; Ryu, I.-C.; Pak, S.J.; Wee, S.-M.; Kim, C.S.; Park, M.-E. Cretaceous epithermal gold–silver mineralization and geodynamic environment, korea. Ore Geol. Rev. 2005, 26, 115–135. [Google Scholar] [CrossRef]
- Kim, C.S.; Choi, S.G. Potassium–argon ages of the epithermal gold–silver mineralization in the haenam–jindo area, southwestern korea. Resour. Geol. 2009, 59, 415–421. [Google Scholar] [CrossRef]
- Choi, N.-C.; Kim, B.-J.; Cho, K.; Lee, S.; Park, C.-Y. Microwave pretreatment for thiourea leaching for gold concentrate. Metals 2017, 7, 404. [Google Scholar] [CrossRef]
- Li, J.; Li, B.W.; Wang, L.; Wang, S.B. Study of reduction characteristic on microwave magnetizing roast of hematite. Adv. Mater. Res. 2011, 284–286, 1237–1243. [Google Scholar] [CrossRef]
- Foo, K.; Hameed, B. Potential of jackfruit peel as precursor for activated carbon prepared by microwave induced naoh activation. Bioresour. Technol. 2012, 112, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Amankwah, R.; Pickles, C. Microwave roasting of a carbonaceous sulphidic gold concentrate. Miner. Eng. 2009, 22, 1095–1101. [Google Scholar] [CrossRef]
- Paktunc, D.; Kingston, D.; Pratt, A.; McMullen, J. Distribution of gold in pyrite and in products of its transformation resulting from roasting of refractory gold ore. Can. Mineral. 2006, 44, 213–227. [Google Scholar] [CrossRef]
- Xu, C.; Sun, T.; Jue, K.; Li, Y.; Mo, X.; Tang, L. Mechanism of phosphorus removal in beneficiation of high phosphorous oolitic hematite by direct reduction roasting with dephosphorization agent. Trans. Nonferrous Met. Soc. China 2012, 22, 2806–2812. [Google Scholar] [CrossRef]
- Pracejus, B. The Ore Minerals under the Microscope: An Optical Guide; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Samouhos, M.; Hutcheon, R.; Paspaharis, L. Microwave reduction of copper(ii) oxide and malachite concentrate. Miner. Eng. 2011, 24, 903–913. [Google Scholar] [CrossRef]
- Aguilar, J.A.; Gomez, I. Microwaves applied to carbothermic reduction of iron ore pellets. J. Microw. Power Electromagn. Energy 1997, 32, 67–73. [Google Scholar] [CrossRef]
- Seyrankaya, A.; Ozalp, B. Dehydration of sodium carbonate monohydrate with indirect microwave heating. Thermochim. Acta 2006, 448, 31–36. [Google Scholar] [CrossRef]
- Bai, S.J.; Wen, S.M.; Liu, D.W.; Zhang, W.B.; Cao, Q.B. Beneficiation of high phosphorus limonite ore by sodium-carbonate-added carbothermic reduction. ISIJ Int. 2012, 52, 1757–1763. [Google Scholar] [CrossRef]
- Zhu, D.Q.; Chun, T.J.; Pan, J.; He, Z. Recovery of iron from high-iron red mud by reduction roasting with adding sodium salt. J. Iron Steel Res. Int. 2012, 19, 1–5. [Google Scholar] [CrossRef]
- Bai, S.J.; Wen, S.M.; Liu, D.W.; Zhang, W.B.; Xian, Y.J. Catalyzing carbothermic reduction of siderite ore with high content of phosphorus by adding sodium carbonate. ISIJ Int. 2011, 51, 1601–1607. [Google Scholar] [CrossRef]
- Basumallick, A. Influence of CaO and Na2CO3 as additive on the reduction of hematite-lignite mixed pellets. ISIJ Int. 1995, 35, 1050–1053. [Google Scholar] [CrossRef]
- Huang, Z.; Guo, Z.; Calka, A.; Wexler, D.; Lukey, C.; Liu, H.-K. Effects of iron oxide (Fe2O3, Fe3O4) on hydrogen storage properties of Mg-based composites. J. Alloys Compd. 2006, 422, 299–304. [Google Scholar] [CrossRef]
- Lavaud, T.; Beziat, D.; Blot, A.; Debat, P.; Lompo, M.; Martin, F.; Ouangrawa, M.; Tollon, F. Paleo-gossans within the lateritic iron crust: Example of the nickeliferous prospect of bonga, burkina faso. J. Afr. Earth. Sci. 2004, 39, 465–471. [Google Scholar] [CrossRef]


| Experiments | A | B | C | D |
|---|---|---|---|---|
| Ore Sample (g) | 50 | 50 | 50 | 50 |
| Activated Carbon Mass (g) | 25–100 | 75 | 75 | 75 |
| Sodium Carbonate (g) | 6 | 2–14 | 10 | 10 |
| Microwave Intensity (kW) | 5 | 5 | 3–6 | 5 |
| Irradiation time (min) | 10 | 10 | 10 | 7–15 |
| Element/Mineral | Au | Ag | As | T Fe | Fe2O3 | SiO2 | Al2O3 | Na2O | SO3 | K2O | CaO | TiO2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Contents (wt. %) | 6.4 a | 35.6 a | 911.6 a | 28.6 | 26.2 | 24.2 | 6.8 | 2.1 | 6.8 | 2.4 | 3.1 | 0.9 |
| Sample | Au | Ag | As | T Fe (wt. %) | Fe2O3 (wt. %) |
|---|---|---|---|---|---|
| Raw | 6.4 | 35.6 | 911.6 | 28.6 | 26.2 |
| RMR-MS processed a | 10.8 | 43.2 | 10.5 | 41.9 | 1.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.-J.; Cho, K.H.; Lee, S.-G.; Park, C.-Y.; Choi, N.-C.; Lee, S. Effective Gold Recovery from Near-Surface Oxide Zone Using Reductive Microwave Roasting and Magnetic Separation. Metals 2018, 8, 957. https://doi.org/10.3390/met8110957
Kim B-J, Cho KH, Lee S-G, Park C-Y, Choi N-C, Lee S. Effective Gold Recovery from Near-Surface Oxide Zone Using Reductive Microwave Roasting and Magnetic Separation. Metals. 2018; 8(11):957. https://doi.org/10.3390/met8110957
Chicago/Turabian StyleKim, Bong-Ju, Kang Hee Cho, Sang-Gil Lee, Cheon-Young Park, Nag-Choul Choi, and Soonjae Lee. 2018. "Effective Gold Recovery from Near-Surface Oxide Zone Using Reductive Microwave Roasting and Magnetic Separation" Metals 8, no. 11: 957. https://doi.org/10.3390/met8110957
APA StyleKim, B.-J., Cho, K. H., Lee, S.-G., Park, C.-Y., Choi, N.-C., & Lee, S. (2018). Effective Gold Recovery from Near-Surface Oxide Zone Using Reductive Microwave Roasting and Magnetic Separation. Metals, 8(11), 957. https://doi.org/10.3390/met8110957





