Effects of CaO Addition on the Iron Recycling from Nickel Slags by Oxidation-Magnetic Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
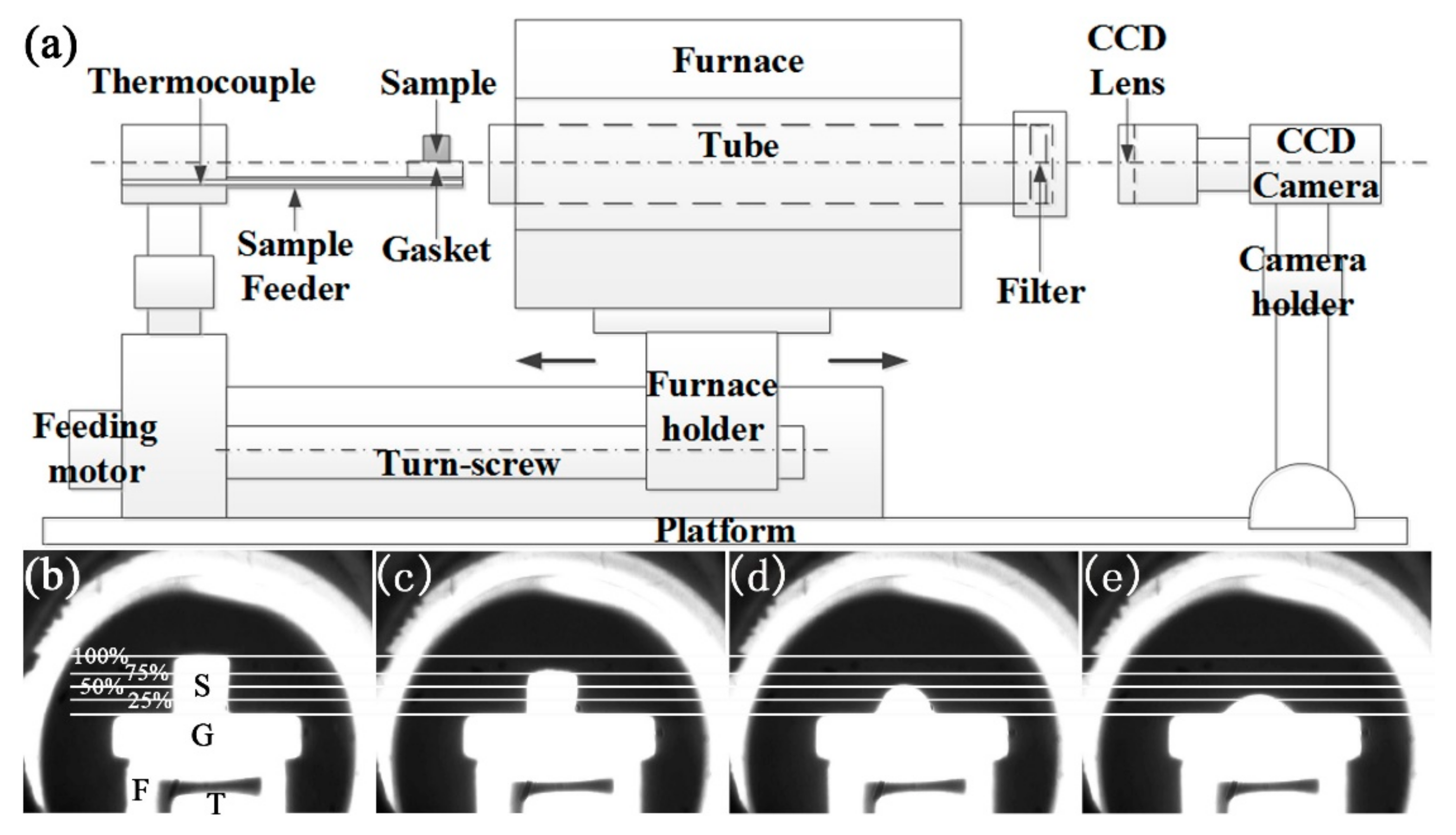
2.2. Equipment
2.3. Methods
3. Results and Discussion
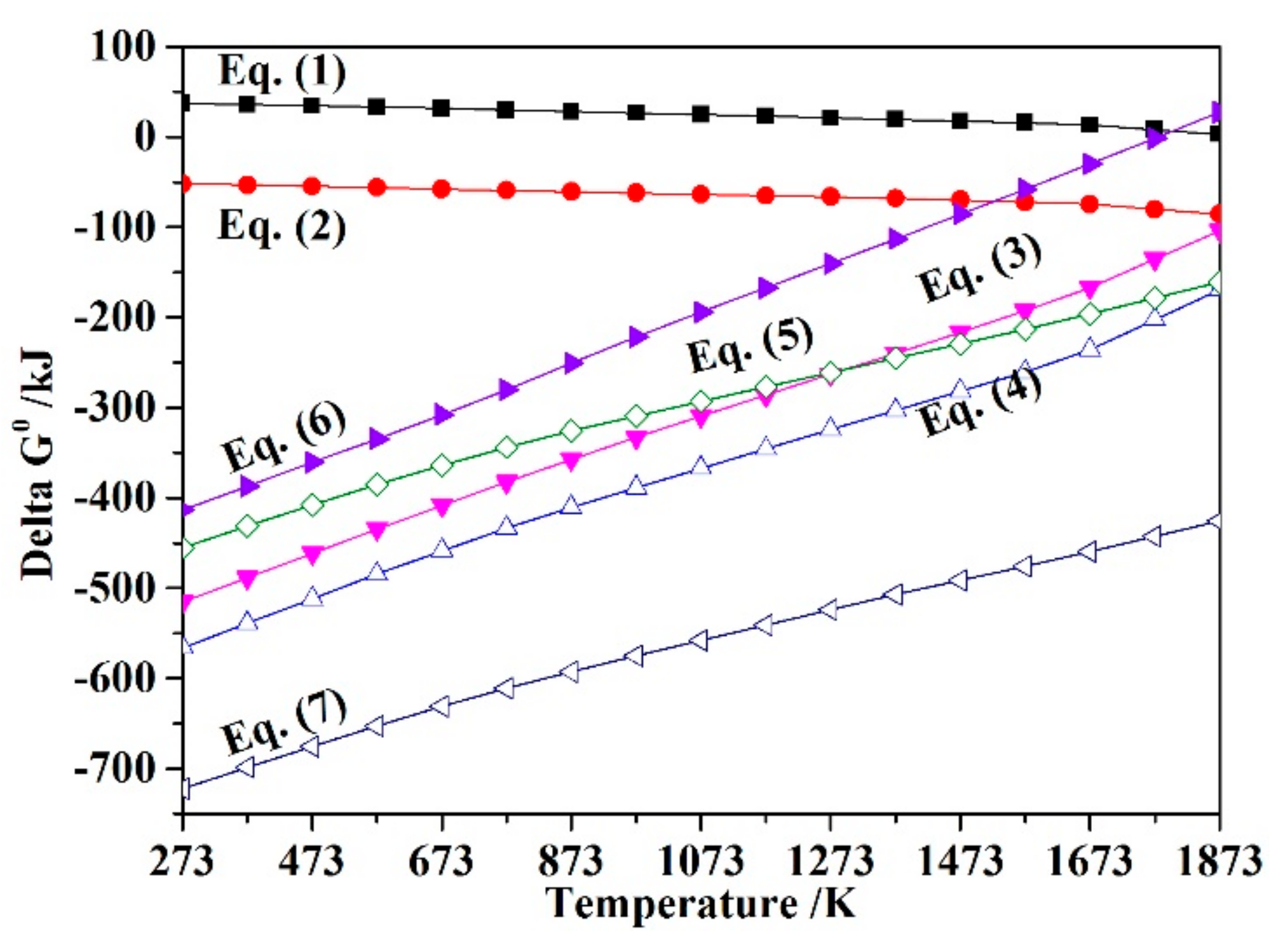
3.1. Thermodynamic Analysis
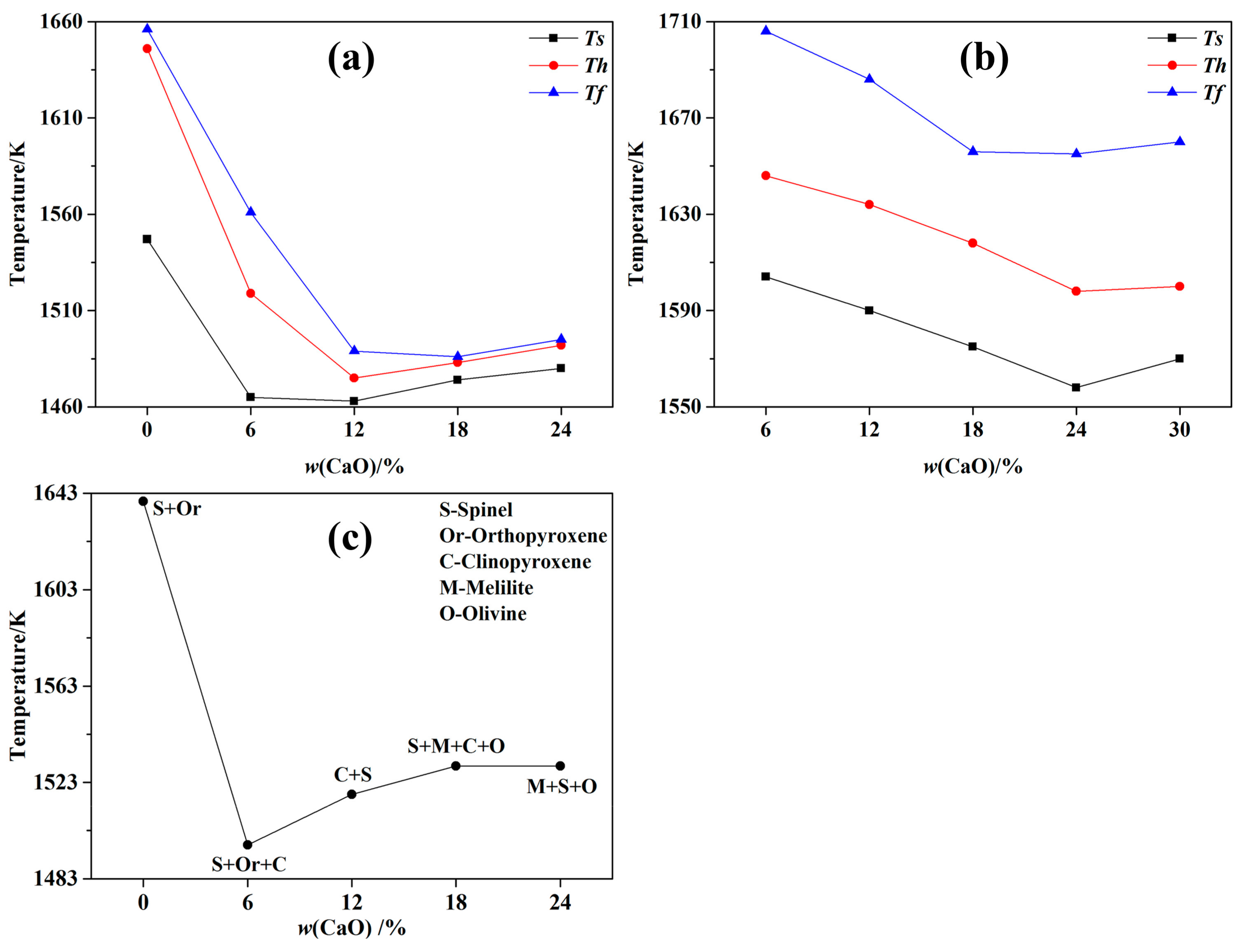
3.2. Fusion Characteristic Temperatures
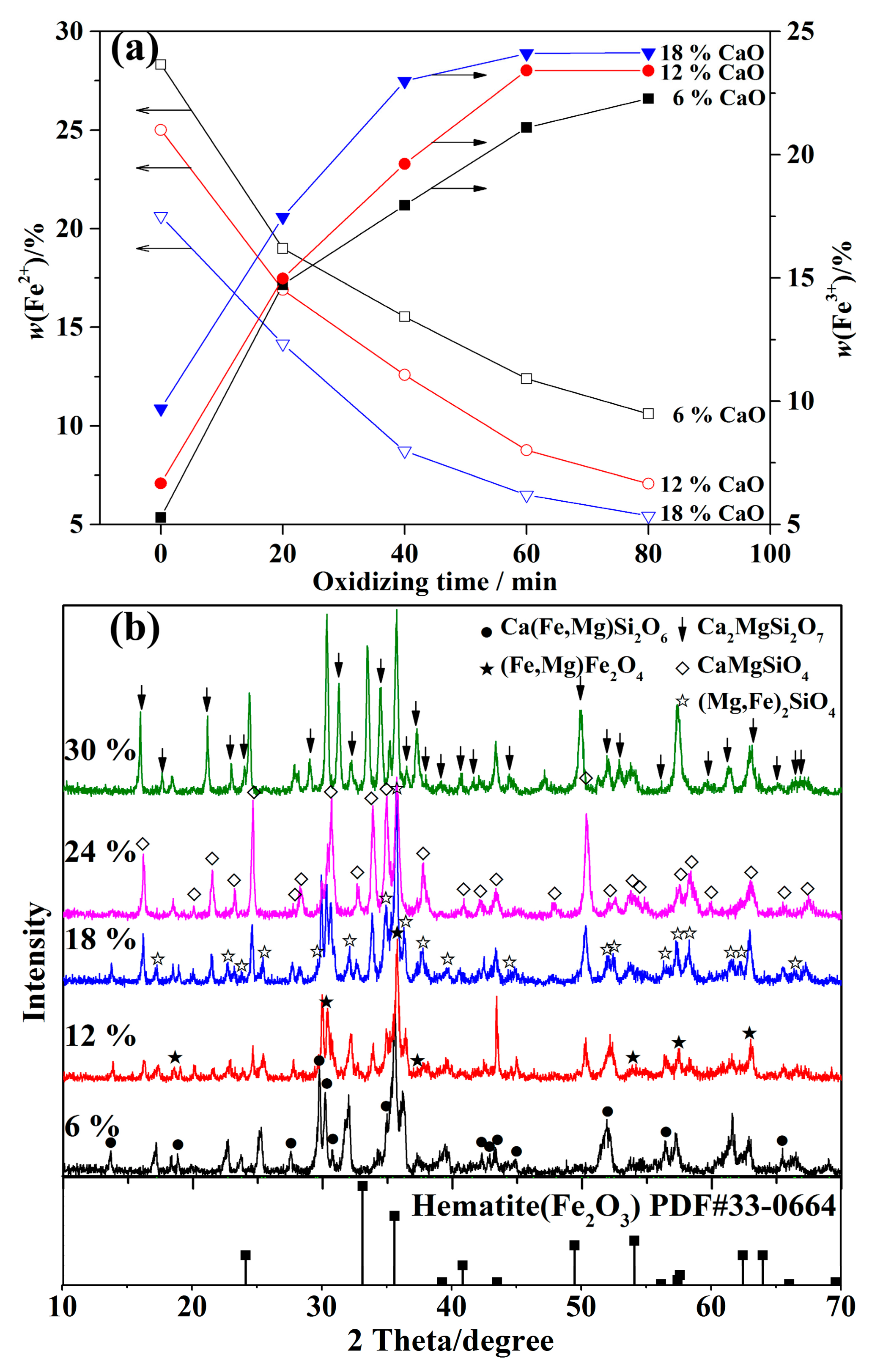
3.3. Oxidation of Fayalite
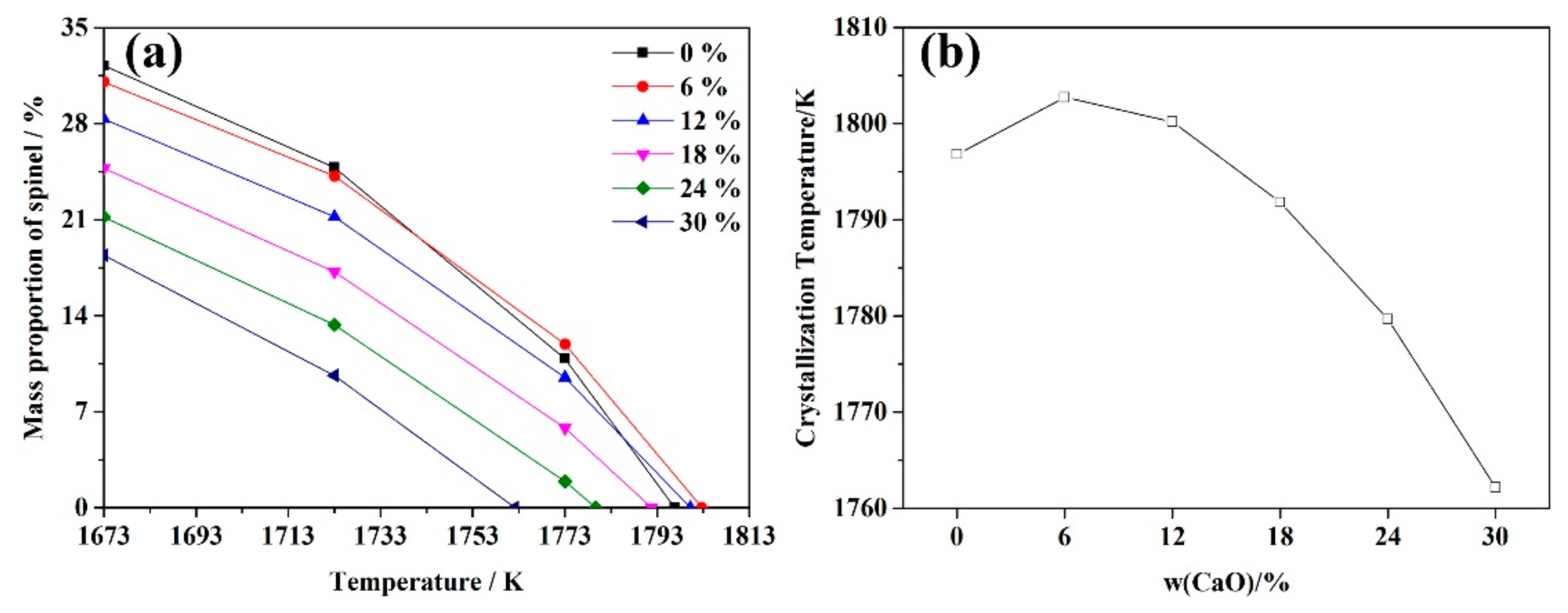
3.4. Crystallization Processing
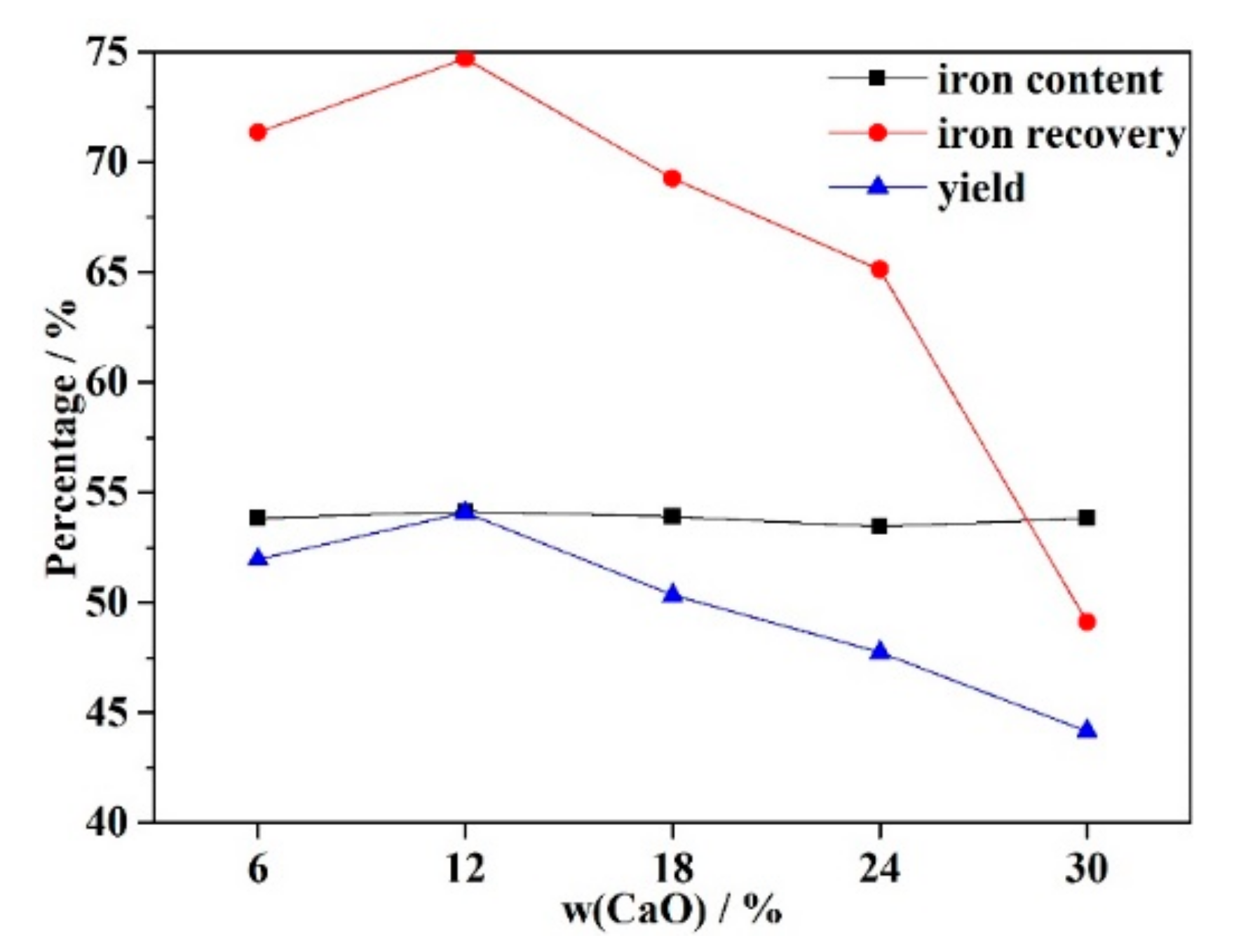
3.5. Magnetic Separation
4. Conclusions
- Thermodynamic analysis proved that Fe2SiO4 cannot decompose spontaneously, but the addition of CaO plays a positive role in the oxidization of fayalite in nickel slag to produce magnetite. Diagrams of FeO-SiO2-MgO-CaO slag in an air atmosphere drawn by FactSage 7.1 showed that the phase components, as well as the area of spinel phase, can be affected by the addition of CaO.
- With the increase of CaO content, the fusion characteristic temperatures decreased rapidly until a CaO content of 12 wt.% and then slightly increased. This was mainly caused by the variation of phase components at various CaO contents.
- Experiments proved that the oxidization of Fe2SiO4 in nickel slags can be accelerated significantly by the addition of CaO. Due to the addition of CaO, a(FeO) increased and thus led to a decrease of a(Fe2O3), which promotes the reaction between MgO and Fe2O3 to form MgFe2O4. However, excess addition of CaO only formed more silicates.
- The crystallization temperature can be reduced by the increasing CaO content. As the CaO content increased, the crystallization temperature decreased, and fewer spinel crystals formed.
- The recovery rate and yield of iron were first increased and later decreased as the CaO increased, while the iron content remained nearly constant. XRD patterns showed that magnetite and magnesium ferrite were the dominant phases in the iron concentrate, while the tailing slag mainly consisted of several silicates. Ultimately, the maximum iron recovery and yield of concentrate achieved were 74.71% and 54.09%, while the iron content was 54.13%.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, J.X.; Zhao, Z.Y.; Cui, Y.R.; Shi, R.M.; Tang, W.D.; Li, X.M.; Shang, N. New slag for nickel matte smelting process and subsequent Fe extraction. Metall. Mater. Trans. B 2018, 49, 304–310. [Google Scholar] [CrossRef]
- Wolf, A.; Mitrašinović, A.M. Nickel, copper and cobalt coalescence in copper cliff converter slag. J. Min. Metall. Sect. B 2016, 52, 143–150. [Google Scholar] [CrossRef]
- Li, Y.J.; Papangelakis, V.G.; Perederiy, I. High pressure oxidative acid leaching of nickel smelter slag: Characterization of feed and residue. Hydrometallurgy 2009, 97, 185–193. [Google Scholar] [CrossRef]
- Krogerus, E.V.; Talonen, T.T. Method for Continuous Reduction of Molten Metallurgical Slag in an Electric Furnace. U.S. Patent 4,737,186, 12 April 1988. [Google Scholar]
- Ma, Y.B.; Du, X.Y.; Shen, Y.Y.; Li, G.Z.; Li, M. Crystallization and beneficiation of magnetite for iron recycling from nickel slags by oxidation-magnetic separation. Metals 2017, 7, 321–332. [Google Scholar] [CrossRef]
- Zhao, J.X.; Cui, Y.R.; Gao, X.T.; Lu, X.T.; Tang, W.R.; Li, X.M.; Shi, R.M. Method for Smelting Nickel-Copper from Sulfide Ores by Virtue of Pyrogenic Process and Extracting Iron. CN. Patent 201410376672, 30 March 2016. [Google Scholar]
- Hao, W.Y. Comprehensive utilization of the smelting slags in Jinchuan. Gansu Metall. 1995, 2, 23–27. [Google Scholar]
- Ducret, A.C.; Rankin, W.J. Liquidus temperatures and viscosities of FeO-Fe2O3-SiO2-CaO-MgO slags at compositions relevant to nickel matte smelting. Scand. J. Metall. 2002, 31, 59–67. [Google Scholar] [CrossRef]
- Fujino, K.; Murakami, T.; Kasai, E. Effect of addition of CaO component on the oxidation reaction of wustite particles in sintering bed. ISIJ Int. 2015, 55, 940–946. [Google Scholar] [CrossRef]
- Mackwell, S.J. Oxidation kinetics of fayalite (Fe2SiO4). Phys. Chem. Miner. 1992, 19, 220–228. [Google Scholar] [CrossRef]
- Semykina, A.; Shatokha, V.; Iwase, M.; Seetharaman, S. Kinetics of oxidation of divalent iron to trivalent state in liquid FeO-CaO-SiO2 slags. Metall. Mater. Trans. B 2010, 41, 1230–1239. [Google Scholar] [CrossRef]
- Semykina, A. The kinetics of oxidation of liquid FeO-MnO-CaO-SiO2 slags in air. Metall. Mater. Trans. B 2012, 43, 56–63. [Google Scholar] [CrossRef]
- Heo, J.H.; Park, S.S.; Park, J.H. Effect of slag composition on the distribution behavior of Pb between FetO-SiO2(-CaO, Al2O3) slag and molten copper. Metall. Mater. Trans. B 2012, 43, 1098–1105. [Google Scholar] [CrossRef]
- Heo, J.H.; Kim, B.S.; Park, J.H. Effect of CaO addition on iron recovery from copper smelting slags by solid carbon. Metall. Mater. Trans. B 2013, 44, 1352–1363. [Google Scholar] [CrossRef]
- Zhao, Z.L.; Tang, H.Q.; Guo, Z.C. Effects of CaO on precipitation morphology of metallic iron in reduction of iron oxides under CO atmosphere. J. Iron Steel Res. Int. 2013, 20, 16–24. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, W.D.; Zhao, Z.X.; Wang, D.Q.; Evans, T.; Zhao, B.J. High temperature softening behaviours of iron blast furnace feeds and their correlations to the microstructures. In The 6th International Symposium on High-Temperature Metallurgical Processing; Jiang, T., Hwang, J.-Y., Eds.; Springer: Cham, Switzerland, 2016; pp. 67–74. [Google Scholar]
- Yu, Y.; Wang, D.; Li, J.L.; Zhu, H.Y.; Xue, Z.L. Thermodynamic calculation of FeO effect on precipitation of spinel containing chromium in CaO-SiO2-MgO-Al2O3-Cr2O3 system. J. Wuhan Uni. Sci. Technol. 2018, 41, 15–19. [Google Scholar] [CrossRef]
- Sagadin, C.; Luidold, S.; Wagner, C.; Wenzl, C. Melting behaviour of ferronickel slags. JOM 2016, 68, 3022–3028. [Google Scholar] [CrossRef]
- Talapaneni, T.; Yedla, N.; Sarkar, S.; Pal, S. Effect of Basicity, Al2O3 and MgO content on the softening and melting properties of the CaO-MgO-SiO2-Al2O3 high alumina quaternary slag system. Metall. Res. Technol. 2016, 113, 501–511. [Google Scholar] [CrossRef]
- Seetharaman, S. Fundamentals of Metallurgy; Woodhead Publishing Limited: Abington, UK, 2005; pp. 115–116. [Google Scholar]
- Wang, L.; Zhang, C.; Cai, D.X.; Zhang, J.Q.; Sasaki, Y.; Ostrovski, O. Effects of CaO/SiO2 ratio and Na2O content on melting properties and viscosity of SiO2-CaO-Al2O3-B2O3-Na2O mold fluxes. Metall. Mater. Trans. B 2017, 48, 516–526. [Google Scholar] [CrossRef]


| TFe | Si | Mg | Ca | S | Ni | Co | Cu | Pb | Zn | As |
|---|---|---|---|---|---|---|---|---|---|---|
| 36.22 | 14.28 | 8.94 | 3.75 | 0.63 | 0.65 | 0.12 | 0.27 | 0.001 | 0.043 | 0.001 |
| Products | TFe | Si | Mg | Ca | Ni | Co | Cu | Pb | Zn | As |
|---|---|---|---|---|---|---|---|---|---|---|
| Iron concentrate | 54.13 | 4.96 | 3.28 | 0.79 | 0.22 | 0.13 | 0.26 | 0.006 | 0.047 | 0.001 |
| Tailing slag | 19.60 | 21.36 | 5.48 | 7.16 | 0.08 | 0.06 | 0.12 | 0.015 | 0.050 | 0.001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Du, X. Effects of CaO Addition on the Iron Recycling from Nickel Slags by Oxidation-Magnetic Separation. Metals 2018, 8, 956. https://doi.org/10.3390/met8110956
Ma Y, Du X. Effects of CaO Addition on the Iron Recycling from Nickel Slags by Oxidation-Magnetic Separation. Metals. 2018; 8(11):956. https://doi.org/10.3390/met8110956
Chicago/Turabian StyleMa, Yongbo, and Xueyan Du. 2018. "Effects of CaO Addition on the Iron Recycling from Nickel Slags by Oxidation-Magnetic Separation" Metals 8, no. 11: 956. https://doi.org/10.3390/met8110956
APA StyleMa, Y., & Du, X. (2018). Effects of CaO Addition on the Iron Recycling from Nickel Slags by Oxidation-Magnetic Separation. Metals, 8(11), 956. https://doi.org/10.3390/met8110956




