Phase Equilibria of the Co-Ti-Ta Ternary System
Abstract
1. Introduction
2. Experimental Procedures
3. Results and Discussion
3.1. Microstructure Morphology
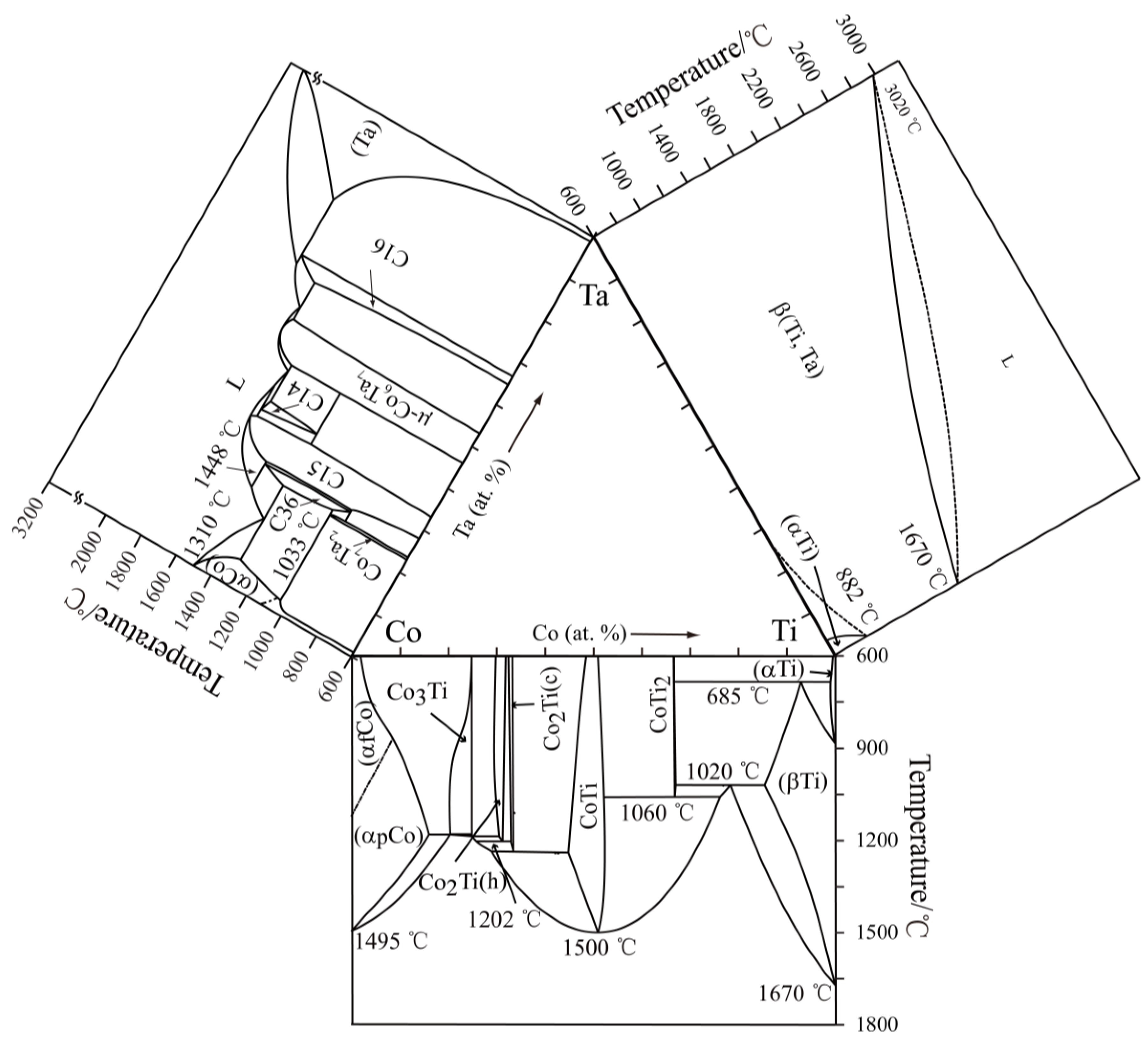
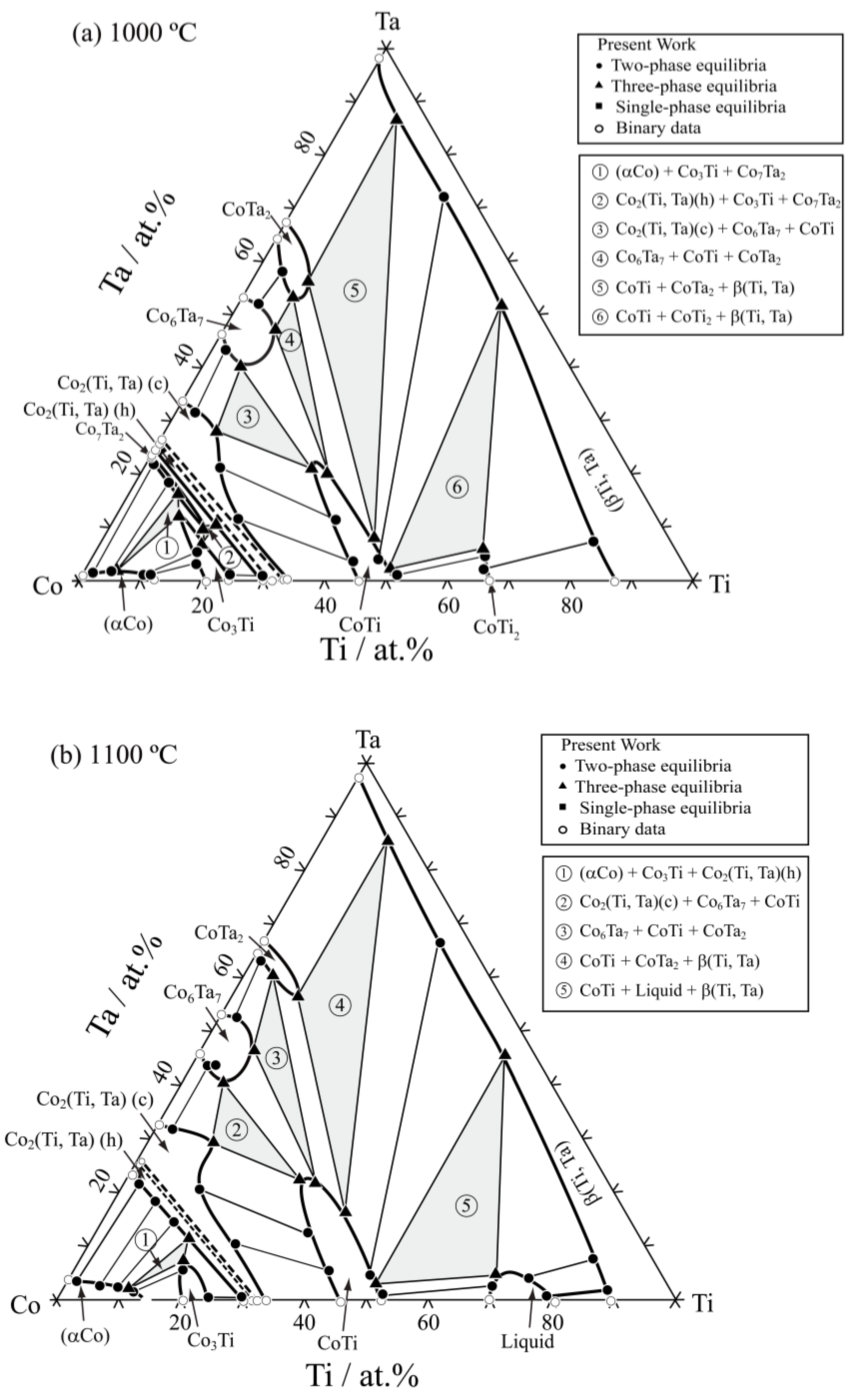
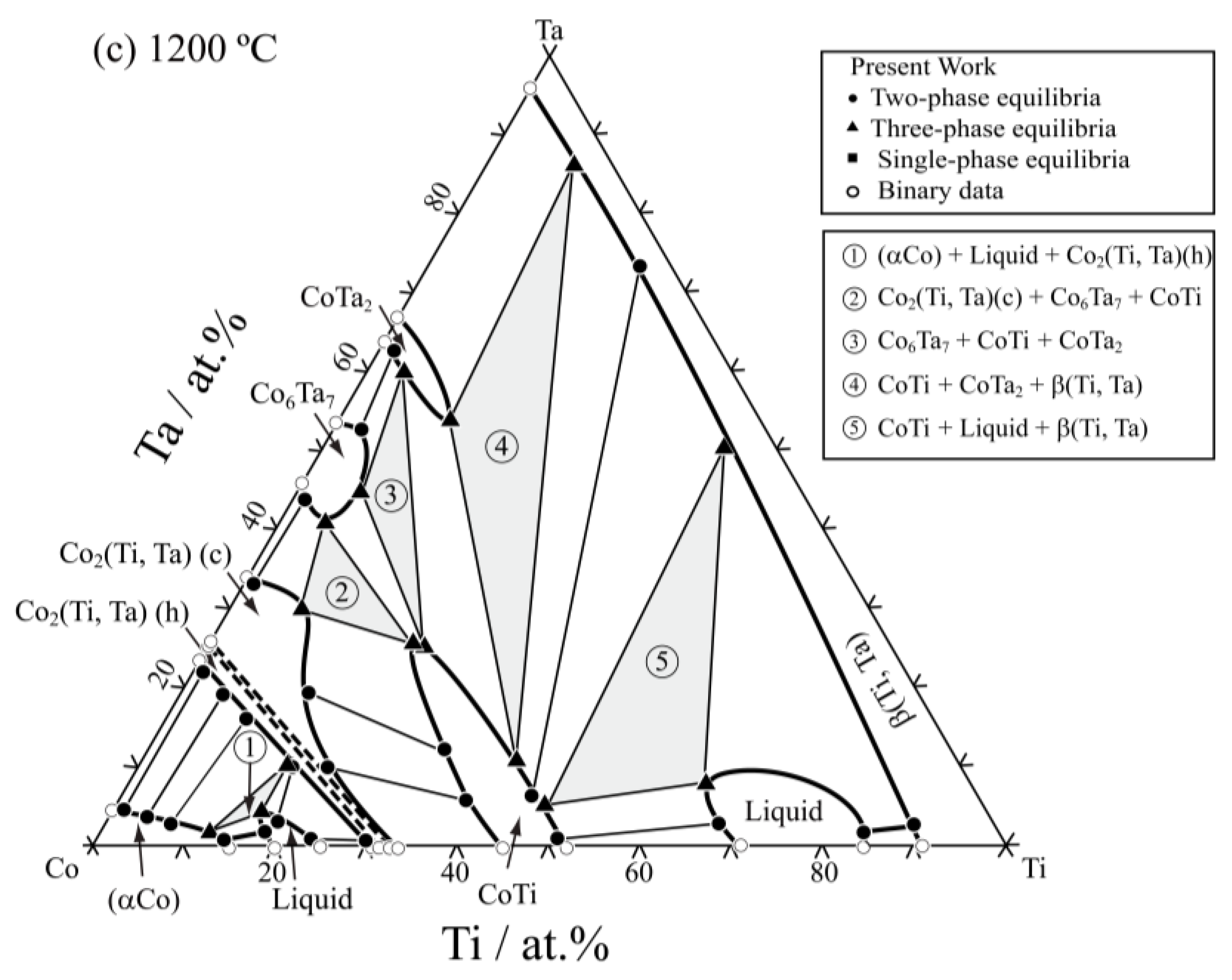
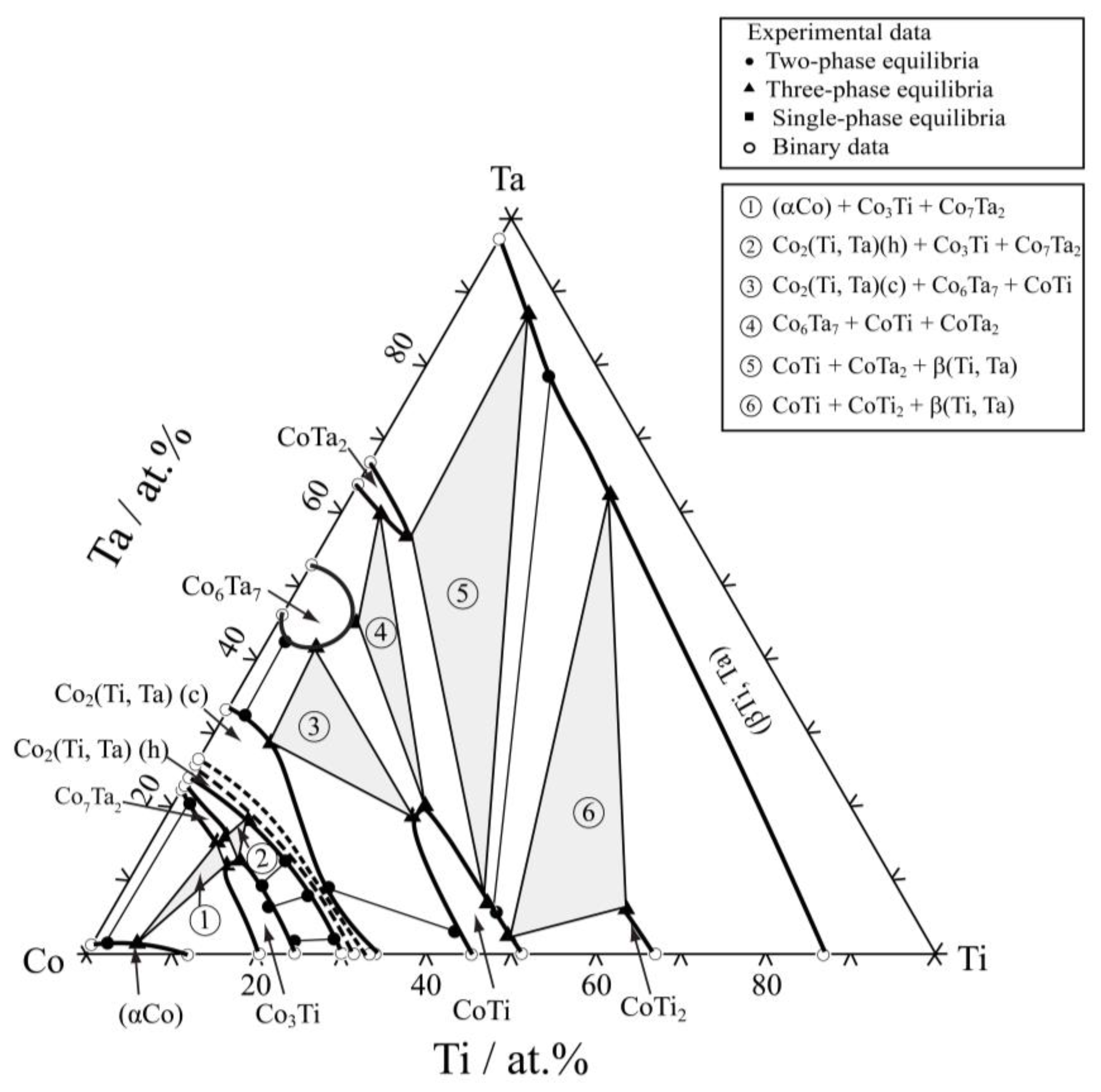
3.2. Isothermal Sections
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sato, J.; Omori, T.; Oikawa, K.; Ohnuma, I.; Kainuma, R.; Ishida, K. Cobalt-base high-temperature alloys. Science 2006, 312, 90–91. [Google Scholar] [CrossRef] [PubMed]
- Blaise, J.M.; Viatour, P.; Drapier, J.M. On the stability and precipitation of the Co3Ti phase in Co-Ti alloys. Cobalt 1970, 49, 192–195. [Google Scholar]
- Zenk, C.H.; Povstugar, I.; Li, R.F.; Neumeier, S.; Raabe, D.; Gökena, M. A novel type of Co–Ti–Cr-base γ/γ′ superalloys with low mass density. Acta Mater. 2017, 135, 244–251. [Google Scholar] [CrossRef]
- Ruan, J.J.; Wang, C.P.; Zhao, C.C.; Yang, S.Y.; Yang, T.; Liu, X.J. Experimental investigation of phase equilibria and microstructure in the Co-Ti-V ternary system. Intermetallics 2014, 49, 121–131. [Google Scholar] [CrossRef]
- Jia, C.C.; Ishida, K.; Nishizawa, T. Partition of alloying elements between γ(A1), γ′(L12), and β(B2) phases in Ni-Al base systems. Metall. Mater. Trans. A 1994, 25, 473–485. [Google Scholar] [CrossRef]
- Ooshima, M.; Tanaka, K.; Okamoto, N.L.; Kishida, K.; Inui, H. Effects of quaternary alloying elements on the γ′ solvus temperature of Co-Al-W based alloys with fcc/L12 two-phase microstructures. J. Alloys Compd. 2010, 508, 71–78. [Google Scholar] [CrossRef]
- Xu, H.; Xiong, X.; Du, Y.; Wang, P.; Hu, B.; He, Y. Phase equilibria of the Co-Ta-Ti system at 950 °C. J. Alloys Compd. 2009, 485, 249–254. [Google Scholar] [CrossRef]
- Jiang, M.; Saren, G.; Yang, S.; Li, H.; Hao, S. Phase equilibria in Co-rich region of Co-Ti-Ta system. Trans. Nonferr. Met. Soc. China 2011, 21, 2391–2395. [Google Scholar] [CrossRef]
- Pet’kov, V.V.; Kocherzhinskii, Y.A.; Markiv, V.Y. Investigations of the phase diagram in the system ta-co. Metall. Akad. Nauk Ukr. Inst. Metall. 1972, 41, 93–97. [Google Scholar]
- Kaufman, L. Coupled thermochemical and phase diagram data for tantalum based binary alloys. Calphad 1991, 15, 243–259. [Google Scholar] [CrossRef]
- Liu, Z.K.; Chang, Y.A. Thermodynamic assessment of the Co-Ta system. Calphad 1999, 23, 339–356. [Google Scholar] [CrossRef]
- Kumar, K.C.H.; Rompaey, T.V.; Wollants, P. Thermodynamic calculation of the phase diagram of the Co-Nb-Ta system. Z. Metall. 2002, 93, 1146–1153. [Google Scholar] [CrossRef]
- Okamoto, H. Co-Ta (Cobalt-Tantalum). J. Phase Equilib. Diffus. 2004, 25, 571. [Google Scholar] [CrossRef]
- Shinagawa, K.; Chinen, H.; Omori, T.; Oikawa, K.; Ohnuma, I.; Ishida, K.; Kniauma, R. Phase equilibria and thermodynamic calculation of the Co-Ta binary system. Intermetallics 2014, 49, 87–97. [Google Scholar] [CrossRef]
- Huthmann, H.; Inden, G. High-temperature neutron diffraction on FeTi and CoTi. Phys. Status Solidi A 1975, 28, K129–K130. [Google Scholar] [CrossRef]
- Kornilov, I.I.; Kachur, E.V.; Belousov, O.K. Study of the TiNi-TiCo System. Izv. Akad Nauk Metall. 1975, 2, 209–210. [Google Scholar]
- Murray, J.L. The Co-Ti (Cobalt-Titanium) system. Bull. Alloy Phase Diagrams 1982, 3, 74–85. [Google Scholar] [CrossRef]
- Nash, P.; Choo, H.; Schwarz, R.B. Thermodynamic calculation of phase equilibria in the Ti-Co and Ni-Sn systems. J. Mater. Sci. 1998, 33, 4929–4936. [Google Scholar] [CrossRef]
- Cacciamani, G.; Ferro, R.; Ansara, I.; Dupin, N. Thermodynamic modelling of the Co-Ti system. Intermetallics 2000, 8, 213–222. [Google Scholar] [CrossRef]
- Davydov, A.V.; Kattner, U.R.; Josell, D.; Waterstrat, R.M.; Boettinger, W.J.; Blendell, J.E.; Shapiro, A.J. Determination of the CoTi congruent melting point and thermodynamic reassessment of the Co-Ti system. Metall. Mater. Trans. A 2001, 32, 2175–2186. [Google Scholar] [CrossRef]
- Murray, J.L. Bulletin of Alloy Phase Diagram, 2nd ed.; Springer: Berlin, Germany, 1981; pp. 62–66. [Google Scholar]


| System | Phase | Pearson’s Symbol | Space Group | Prototype | Strukturbericht | References |
|---|---|---|---|---|---|---|
| Co-Ti | (αTi) | hP2 | P63/mmc | Mg | A3 | [20] |
| (βTi) | cI2 | Im-3m | W | A2 | [20] | |
| CoTi2 | cF96 | Fd-3m | Fe3W3C | E93 | [20] | |
| CoTi | cP2 | Pm-3m | CsCl | B2 | [20] | |
| Co2Ti(c) | cF24 | Fd-3m | Cu2Mg | C15 | [20] | |
| Co2Ti(h) | hP24 | P63/mmc | MgNi2 | C36 | [20] | |
| Co3Ti | cP4 | Pm-3m | Au3Cu | L12 | [20] | |
| (εCo) | hP2 | P63/mmc | Mg | A3 | [20] | |
| (αCo) | cF4 | Fm-3m | Cu | A1 | [20] | |
| Co-Ta | Ta | cI2 | Im-3m | W | A2 | [14] |
| Co6Ta7 | hR13 | R-3m | Fe7W6 | D8b | [14] | |
| CoTa2 | tI12 | I4/mcm | Al2Cu | C16 | [14] | |
| α-Co2Ta | hP12 | P63/mmc | Zn2Mg | C14 | [14] | |
| β-Co2Ta | cF24 | Fd-3m | Cu2Mg | C15 | [14] | |
| γ-Co2Ta | hP24 | P63/mmc | MgNi2 | C36 | [14] | |
| Co7Ta2 | hR36 | R-3m | BaPb3 | -- | [14] | |
| (εCo) | hP2 | P63/mmc | Mg | A3 | [14] | |
| (αCo) | cF4 | Fm-3m | Cu | A1 | [14] | |
| Ti-Ta | β(Ti,Ta) | cI2 | Im-3m | W | A2 | [21] |
| Alloys (at.%) | Annealed Time | Equilibria | Composition (at.%) | |||||
|---|---|---|---|---|---|---|---|---|
| Phase 1/Phase 2/Phase 3 | Phase 1 | Phase 2 | Phase 3 | |||||
| Ta | Ti | Ta | Ti | Ta | Ti | |||
| Co85Ti13Ta2 | 45 days | (αCo)/Co3Ti | 1.0 | 10.8 | 3.0 | 17.1 | — | — |
| Co85Ti2Ta13 | 45 days | (αCo)/Co7Ta2 | 0.9 | 2.3 | 1.0 | 22.5 | — | — |
| Co80Ti9Ta11 | 45 days | (αCo)/Co3Ti/Co7Ta2 | 2.1 | 5.3 | 11.5 | 10.3 | 16.4 | 7.7 |
| Co71Ti28Ta1 | 45 days | Co3Ti/Co2(Ti,Ta)(h) | 0.8 | 24.1 | 1.3 | 28.7 | — | — |
| Co74Ti16Ta10 | 45 days | Co3Ti/Co7Ta2/Co2(Ti,Ta)(h) | 7.0 | 15.8 | 8.4 | 15.3 | 10.5 | 15.4 |
| Co74Ti10Ta16 | 45 days | (αCo)/Co7Ta2 | 2.1 | 4.2 | 19.2 | 5.1 | — | — |
| Co75Ti1Ta14 | 45 days | Co2(Ti,Ta)(h) | 23.1 | 0.6 | — | — | — | — |
| Co60Ti2Ta38 | 45 days | Co2(Ti,Ta)(c)/Co6Ta7 | 31.5 | 2.1 | 43.2 | 1.8 | — | — |
| Co55Ti15Ta30 | 45 days | Co2(Ti,Ta)(c)/Co6Ta7/CoTi | 28.4 | 8.1 | 39.9 | 6.0 | 20.8 | 27.6 |
| Co60Ti30Ta10 | 45 days | Co2(Ti,Ta)(c)/CoTi | 10.8 | 19.4 | 4.5 | 41.6 | — | — |
| Co45Ti15Ta40 | 45 days | CoTi/Co6Ta7/CoTa2 | 19.8 | 30.3 | 47.1 | 7.9 | 53.2 | 7.9 |
| Co30Ti20Ta50 | 45 days | CoTi/CoTa2/β(Ti,Ta) | 8.1 | 43.7 | 55.6 | 9.8 | 86.0 | 8.3 |
| Co30Ti50Ta20 | 45 days | CoTi/CoTi2/β(Ti,Ta) | 2.1 | 43.3 | 6.3 | 61.4 | 51.3 | 42.5 |
| Co40Ti58Ta2 | 45 days | CoTi/CoTi2 | 1.1 | 51.1 | 3.5 | 64.1 | — | — |
| Co80Ti15Ta5 | 45 days | (αCo)/Co3Ti | 1.0 | 9.5 | 5.7 | 16.1 | — | — |
| Co62Ti20Ta18 | 45 days | Co2(Ti,Ta)(c)/CoTi | 22.3 | 11.9 | 11.3 | 35.7 | — | — |
| Co20Ti75Ta5 | 45 days | CoTi2/β(Ti,Ta) | 2.0 | 65.0 | 6.2 | 78.8 | — | — |
| Co42Ti3Ta55 | 45 days | Co6Ta7/CoTa2 | 52.0 | 3.0 | 57.5 | 3.5 | — | — |
| Co30Ti40Ta30 | 45 days | CoTi/β(Ti,Ta) | 3.1 | 46.2 | 71.2 | 22.9 | — | — |
| Alloys (at.%) | Annealed Time | Equilibria | Composition (at.%) | |||||
|---|---|---|---|---|---|---|---|---|
| Phase 1/Phase 2/Phase 3 | Phase 1 | Phase 2 | Phase 3 | |||||
| Ta | Ti | Ta | Ti | Ta | Ti | |||
| Co85Ti13Ta2 | 35 days | (αCo)/Co3Ti | 1.6 | 11.5 | 5.9 | 11.6 | — | — |
| Co80Ti5Ta15 | 35 days | (αCo)/Co2(Ti,Ta)(h) | 2.7 | 5.6 | 18.7 | 6.7 | — | — |
| Co85Ti2Ta13 | 35 days | (αCo)/Co2(Ti,Ta)(h) | 3.6 | 1.3 | 22.4 | 1.2 | — | — |
| Co71Ti28Ta1 | 35 days | Co3Ti/Co2(Ti,Ta)(h) | 0.6 | 23.8 | 1.0 | 28.7 | — | — |
| Co74Ti16Ta10 | 35 days | (αCo)/Co3Ti/Co2(Ti,Ta)(h) | 1.7 | 10.5 | 6.9 | 16.2 | 10.8 | 15.9 |
| Co74Ti10Ta16 | 35 days | (αCo)/Co2(Ti,Ta)(h) | 2.4 | 8.3 | 15.2 | 10.9 | — | — |
| Co75Ti1Ta24 | 35 days | Co2(Ti,Ta)(h) | 23.2 | 0.9 | — | — | — | — |
| Co60Ti2Ta38 | 35 days | Co2(Ti,Ta)(c)/Co6Ta7 | 31.4 | 2.2 | 44.7 | 1.9 | — | — |
| Co55Ti15Ta30 | 35 days | Co2(Ti,Ta)(c)/Co6Ta7/CoTi | 29.6 | 8.0 | 41.1 | 6.3 | 23.3 | 26.3 |
| Co60Ti30Ta10 | 35 days | Co2(Ti,Ta)(c)/CoTi | 11.6 | 22.7 | 5.5 | 41.1 | — | — |
| Co45Ti15Ta40 | 35 days | CoTi/Co6Ta7/CoTa2 | 21.4 | 29.6 | 46.4 | 8.3 | 61.3 | 3.7 |
| Co30Ti20Ta50 | 35 days | CoTi/CoTa2/β(Ti,Ta) | 16.8 | 38.5 | 56.6 | 9.9 | 86.1 | 9.9 |
| Co30Ti50Ta20 | 8 h | CoTi/Liquid/β(Ti,Ta) | 2.9 | 50.0 | 4.5 | 68.3 | 45.9 | 48.8 |
| Co40Ti58Ta2 | 8 h | CoTi/Liquid | 1.0 | 51.7 | 2.7 | 69.2 | — | — |
| Co15Ti84Ta1 | 8 h | Liquid/β(Ti,Ta) | 0.7 | 78.8 | 2.0 | 88.0 | — | — |
| Co62Ti20Ta18 | 35 days | Co2(Ti,Ta)(c)/CoTi | 20.6 | 12.8 | 12.3 | 34.0 | — | — |
| Co20Ti75Ta5 | 8 h | Liquid/β(Ti,Ta) | 4.0 | 73.9 | 7.3 | 82.7 | — | — |
| Co42Ti3Ta55 | 35 days | Co6Ta7/CoTa2 | 52.9 | 3.0 | 63.1 | 1.6 | — | — |
| Co30Ti40Ta30 | 35 days | CoTi/β(Ti,Ta) | 3.9 | 48.5 | 66.7 | 28.9 | — | — |
| Alloys (at.%) | Annealed Time | Equilibria | Composition (at.%) | |||||
|---|---|---|---|---|---|---|---|---|
| Phase 1/Phase 2/Phase 3 | Phase 1 | Phase 2 | Phase 3 | |||||
| Ta | Ti | Ta | Ti | Ta | Ti | |||
| Co85Ti13Ta2 | 8 h | (αCo)/Liquid | 1.2 | 11.4 | 2.1 | 17.2 | — | — |
| Co85Ti2Ta13 | 25 days | (αCo)/Co2(Ti,Ta) (h) | 4.7 | 1.2 | 22.5 | 1.0 | — | — |
| Co80Ti9Ta11 | 25 days | (αCo)/Co2(Ti,Ta)(h) | 3.2 | 7.2 | 16.5 | 8.6 | — | — |
| Co71Ti28Ta1 | 8 h | Liquid/Co2(Ti,Ta)(h) | 0.1 | 23.2 | 0.9 | 30.2 | — | — |
| Co74Ti16Ta10 | 8 h | Liquid/Co2(Ti,Ta)(h) | 3.1 | 18.5 | 9.9 | 17.4 | — | — |
| Co60Ti2Ta38 | 25 days | Co2(Ti,Ta)(c)/Co6Ta7 | 32.5 | 1.0 | 44.4 | 0.8 | — | — |
| Co55Ti15Ta30 | 25 days | Co2(Ti,Ta)(c)/Co6Ta7/CoTi | 30.4 | 7.5 | 40.7 | 5.4 | 26.0 | 22.0 |
| Co60Ti32Ta8 | 25 days | Co2(Ti,Ta)(c)/CoTi | 10.3 | 20.3 | 5.9 | 37.7 | — | — |
| Co45Ti15Ta40 | 25 days | CoTi/Co6Ta7/CoTa2 | 25.2 | 24.1 | 45.1 | 7.0 | 60.3 | 3.8 |
| Co30Ti50Ta20 | 8 h | CoTi/Liquid/β(Ti,Ta) | 5.5 | 46.5 | 7.7 | 63.2 | 49.5 | 43.5 |
| Co40Ti58Ta2 | 8 h | CoTi/Liquid | 1.0 | 49.8 | 2.7 | 67.6 | — | — |
| Co14Ti84Ta2 | 8 h | Liquid/β(Ti,Ta) | 1.5 | 84.5 | 2.5 | 89.2 | — | — |
| Co80Ti15Ta5 | 8 h | (αCo)/Liquid/Co2(Ti,Ta)(h) | 2.1 | 11.7 | 3.8 | 16.0 | 10.0 | 16.2 |
| Co60Ti20Ta20 | 25 days | Co2(Ti,Ta)(c)/CoTi | 19.1 | 14.2 | 12.3 | 32.5 | — | — |
| Co75Ti6Ta19 | 25 days | (αCo)/Co2(Ti,Ta)(h) | 3.9 | 4.4 | 19.2 | 4.7 | — | — |
| Co42Ti3Ta55 | 25 days | Co6Ta7/CoTa2 | 52.9 | 3.0 | 63.1 | 1.6 | — | — |
| Co30Ti40Ta30 | 25 days | CoTi/β(Ti,Ta) | 6.6 | 44.7 | 74.2 | 23.2 | — | — |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Zhang, X.; Li, L.; Pan, Y.; Chen, Y.; Yang, S.; Lu, Y.; Han, J.; Liu, X. Phase Equilibria of the Co-Ti-Ta Ternary System. Metals 2018, 8, 958. https://doi.org/10.3390/met8110958
Wang C, Zhang X, Li L, Pan Y, Chen Y, Yang S, Lu Y, Han J, Liu X. Phase Equilibria of the Co-Ti-Ta Ternary System. Metals. 2018; 8(11):958. https://doi.org/10.3390/met8110958
Chicago/Turabian StyleWang, Cuiping, Xianjie Zhang, Lingling Li, Yunwei Pan, Yuechao Chen, Shuiyuan Yang, Yong Lu, Jiajia Han, and Xingjun Liu. 2018. "Phase Equilibria of the Co-Ti-Ta Ternary System" Metals 8, no. 11: 958. https://doi.org/10.3390/met8110958
APA StyleWang, C., Zhang, X., Li, L., Pan, Y., Chen, Y., Yang, S., Lu, Y., Han, J., & Liu, X. (2018). Phase Equilibria of the Co-Ti-Ta Ternary System. Metals, 8(11), 958. https://doi.org/10.3390/met8110958