Cavitation-Dispersion Method for Copper Cementation from Wastewater by Iron Powder
Abstract
1. Introduction
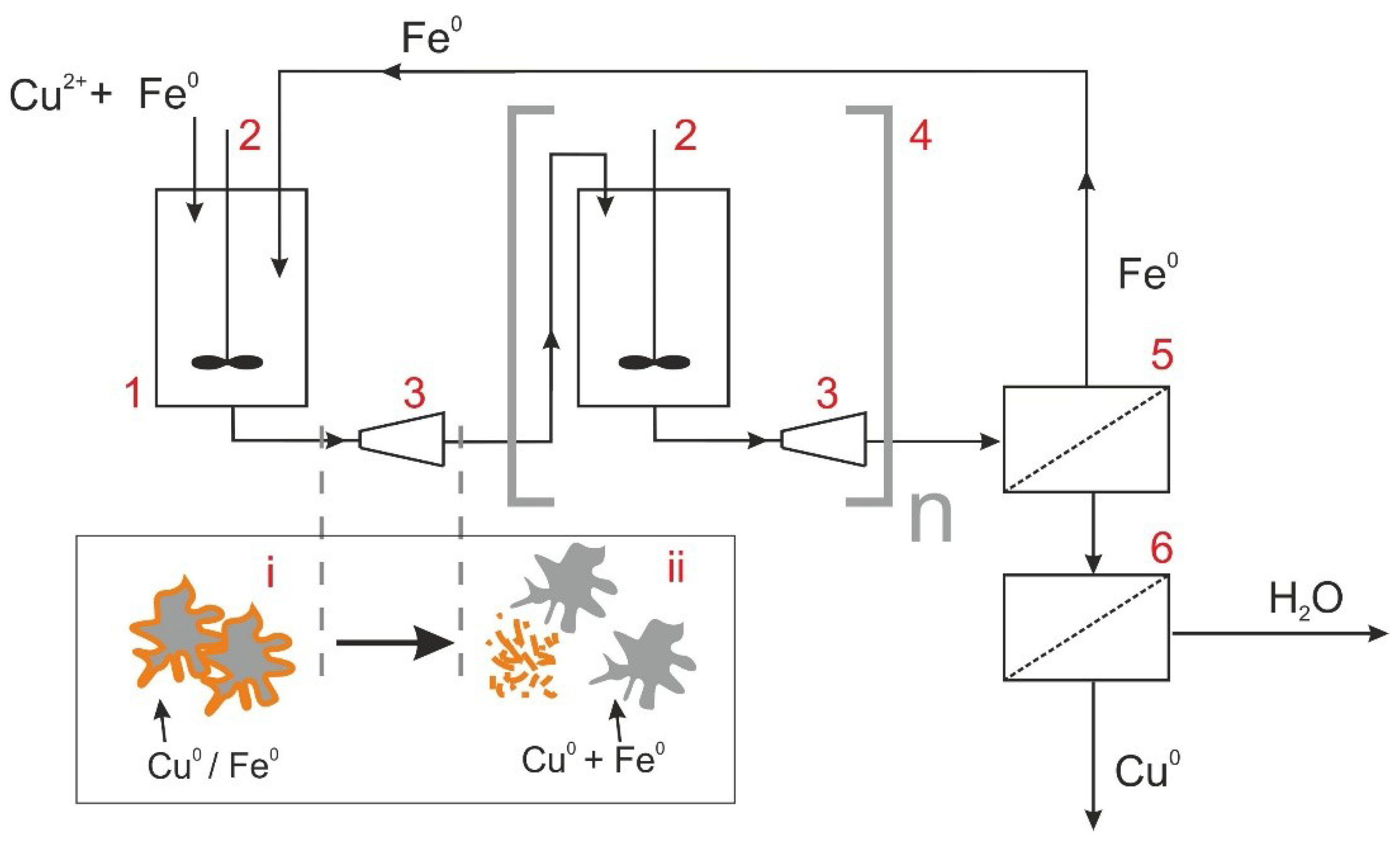
2. Theory
3. Materials and Methods
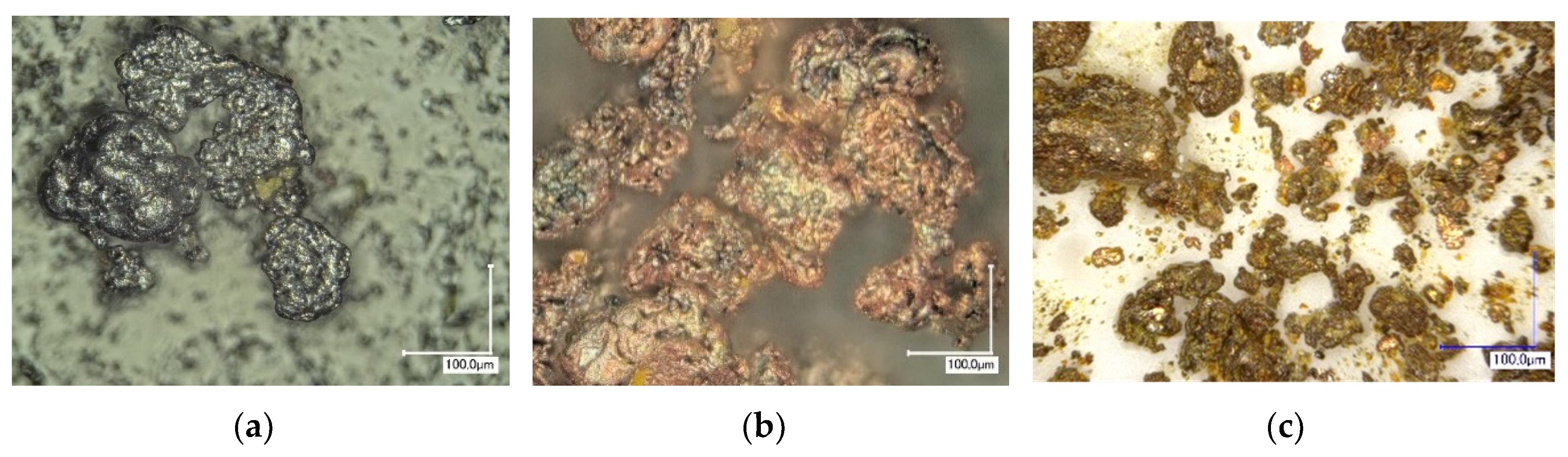
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Al-Saydeh, S.A.; El-Naasa, M.H.; Zaidi, S.J. Copper removal from industrial wastewater: A comprehensive review. J. Ind. Eng. Chem. 2017, 56, 35–44. [Google Scholar] [CrossRef]
- Trakal, L.; Šigut, R.; Šillerová, H.; Faturíková, D.; Komárek, M. Copper removal from aqueous solutions using biochar: Effect of chemical activation. Arab. J. Chem. 2014, 7, 43–52. [Google Scholar] [CrossRef]
- Ruyters, S.; Salaets, P.; Oorts, K.; Smolders, E. Copper toxicity in soils under established vineyards in Europe: A survey. Sci. Total Environ. 2013, 443, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Wang, X.; Khan, A.; Wang, P.; Liu, Y.; Alsaedi, A.; Hayat, T.; Wang, X. Environmental Remediation and Application of Nanoscale Zero-Valent Iron and Its Composites for the Removal of Heavy Metal Ions: A Review. Environ. Sci. Technol. 2016, 50, 7290–7304. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Li, X.; Tian, J.; Yang, J.; Sun, S. Comparative study on the adsorption capacity of raw and modified litchi pericarp for removing Cu(II) from solutions. J. Environ. Manag. 2014, 134, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Various treatment technologies to remove arsenic and mercury from contaminated groundwater: An overview. In Proceedings of the First International Symposium on Southeast Asian Water Environment, Bangkok, Thailand, 24–25 October 2003; pp. 433–440. [Google Scholar]
- Jaishankar, M.; Tsenten, T.; Anbalagan, N.; Mathew, B.B.; Beegegowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.L.; Wang, X.Y.; Zeng, G.M.; Chen, L.; Deng, J.H.; Zhang, X.R.; Niu, Q.Y. Copper (II) removal by pectin–iron oxide magnetic nanocomposite adsorbent. Chem. Eng. J. 2012, 185–186, 100–107. [Google Scholar] [CrossRef]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Aydın, H.; Bulut, Y.; Yerlikaya, Ç. Removal of copper (II) from aqueous solution by adsorption onto low-cost adsorbents. J. Environ. Manag. 2008, 87, 37–45. [Google Scholar] [CrossRef] [PubMed]
- El-Ashtoukhy, E.S.; Amin, N.K.; Abdelwahab, O. Removal of lead (II) and copper (II) from aqueous solution using pomegranate peel as a new adsorbent. Desalination 2008, 223, 162–173. [Google Scholar] [CrossRef]
- Femina Carolin, C.; Senthil Kumar, P.; Saravanan, A.; Janet Joshiba, G.; Naushad, M. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Konsowa, A.H. Intensification of the rate of heavy metal removal from wastewater by cementation. Desalination 2010, 254, 29–34. [Google Scholar] [CrossRef]
- Ahmed, I.M.; El-Nadi, Y.A.; Daoud, J.A. Cementation of copper from spent copper-pickle sulfate solution by zinc ash. Hydrometallurgy 2011, 110, 62–66. [Google Scholar] [CrossRef]
- Gross, F.; Baup, S.; Aurousseau, M. Copper cementation on zinc and iron mixtures: Part 1: Results on rotating disc electrode. Hydrometallurgy 2011, 106, 121–133. [Google Scholar] [CrossRef]
- Nosier, S.A.; Alhamed, Y.A.; Alturaif, H.A. Enhancement of copper cementation using ceramic suspended solids under single phase flow. Sep. Purif. Technol. 2007, 52, 454–460. [Google Scholar] [CrossRef]
- Dil, E.A.; Ghaedi, M.; Asfaram, A. The performance of nanorods materials as adsorbent for removal of azodyes and heavy ions: Application of ultrasound wave, optimization and modeling. Ultrason. Sonochem. 2017, 34, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Kanakaraju, D.; Ravichandar, S.; Chin Lim, Y. Combined effects of adsorption and photocatalysis by hybrid TiO2/ZnO-calcium alginate beads for the removal of copper. J. Environ. Sci. 2017, 55, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.N.; Lin, W.S.; Hou, C.H.; Den, W. Performance of integrated membrane filtration and electrodialysis processes for copper recovery from wafer polishing wastewater. J. Water Proc. Eng. 2014, 4, 149–158. [Google Scholar] [CrossRef]
- Ferrer, O.; Gibert, O.; Cortina, J.L. Reverse osmosis membrane composition, structure and performance. Water Res. 2016, 103, 256–263. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, Y.C.; Wang, Z.X.; Cheng, X.Q.; Xiao, Y.C.; Shao, L. Positively charged nanofiltration membranes via economically mussel-substance-simulated co-deposition for textile wastewater treatment. Chem. Eng. J. 2016, 303, 555–564. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, J.; Sui, M.; Qu, Y.; Ambuchi, J.J.; Wang, H.; Feng, Y. A combined microbial desalination cell and electrodialysis system for copper-containing wastewater treatment and high-salinity-water desalination. J. Hazard. Mater. 2017, 321, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Satyro, S.; Marotta, R.; Clarizia, L.; Di Somma, I.; Vitiello, G.; Dezotti, M.; Pinto, G.; Dantas, R.F.; Andreozzi, R. Removal of EDDS and copper from waters by TiO2 photocatalysis under simulated UV–solar conditions. Chem. Eng. J. 2014, 251, 257–268. [Google Scholar] [CrossRef]
- Ahmed, M.J.K.; Ahmaruzzaman, M. A review on potential usage of industrial waste materials for binding heavy metal ions from aqueous solutions. J. Water Process Eng. 2016, 10, 39–47. [Google Scholar] [CrossRef]
- Ruihua, L.; Lin, Z.; Tao, T.; Bo, L. Phosphorus removal performance of acid mine drainage from wastewater. J. Hazard. Mater. 2011, 190, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.A.H.; Ngo, H.H.; Guo, W.S.; Zhang, J.; Liang, S.; Yue, Q.Y.; Li, Q.; Nguyen, T.V. Applicability of agricultural waste and by-products for adsorptive removal of heavy metals from wastewater. Bioresour. Technol. 2013, 148, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Farooq, U.; Kozinski, J.A.; Khan, M.A.; Athar, M. Biosorption of heavy metal ions using wheat based biosorbents—A review of the recent literature. Bioresour. Technol. 2010, 101, 5043–5053. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Abbas, A.; Al-Amer, A.M.; Laoui, T.; Al-Marri, M.J.; Nasser, M.S.; Khraisheh, M.; Atieh, M.A. Heavy metal removal from aqueous solution by advanced carbon nanotubes: Critical review of adsorption applications. Sep. Purif. Technol. 2016, 157, 141–161. [Google Scholar] [CrossRef]
- Aktas, S. Cementation of rhodium from waste chloride solutions using copper powder. Int. J. Miner. Process 2012, 114–117, 100–105. [Google Scholar] [CrossRef]
- Aktas, S. Rhodium recovery from rhodium-containing waste rinsing water via cementation using zinc powder. Hydrometallurgy 2001, 106, 71–75. [Google Scholar] [CrossRef]
- Wu, L.K.; Xia, J.; Zhang, Y.F.; Li, Y.Y.; Cao, Z.H.; Zheng, G.Q. Effective cementation and removal of arsenic with copper powder in a hydrochloric acid system. RSC Adv. 2016, 6, 70832–70841. [Google Scholar] [CrossRef]
- Demirkiran, N.; Kunkul, A. Recovering of copper with metallic aluminum. Trans. Nonferrous Met. Soc. China 2001, 21, 2778–2782. [Google Scholar] [CrossRef]
- El-Ashtoukhy, E.-S.Z.; Abdel-Aziz, M.H. Removal of copper from aqueous solutions by cementation in a bubble column reactor fitted with horizontal screens. Int. J. Min. Process. 2013, 121, 65–69. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.H. Production of copper powder from wastewater containing CuSO4 and alcoholic additives in a modified stirred tank reactor by cementation. Hydrometallurgy 2011, 1091, 61–167. [Google Scholar] [CrossRef]
- Polyakov, A.; Polyakova, E.; Lazarenko, L. Mixer-Homogenizer. Latvia Patent LV13592 (B), 20 September 2007. [Google Scholar]
- Polyakov, A.; Polyakova, E. Hydrodynamic cavitation homogenizer. Latvia Patent LV15143 (A), 20 July 2016. [Google Scholar]
- Polyakov, A.; Mironovs, V.; Shishkin, A.; Baronins, J. Preparation of Coal-Water Slurries Using a High—Speed Mixer—Disperser. In Proceedings of the 4th International Scientific Conference Civil Engineering’ 13, Jeglava, Latvia, 16–17 May 2013; pp. 77–81. [Google Scholar]
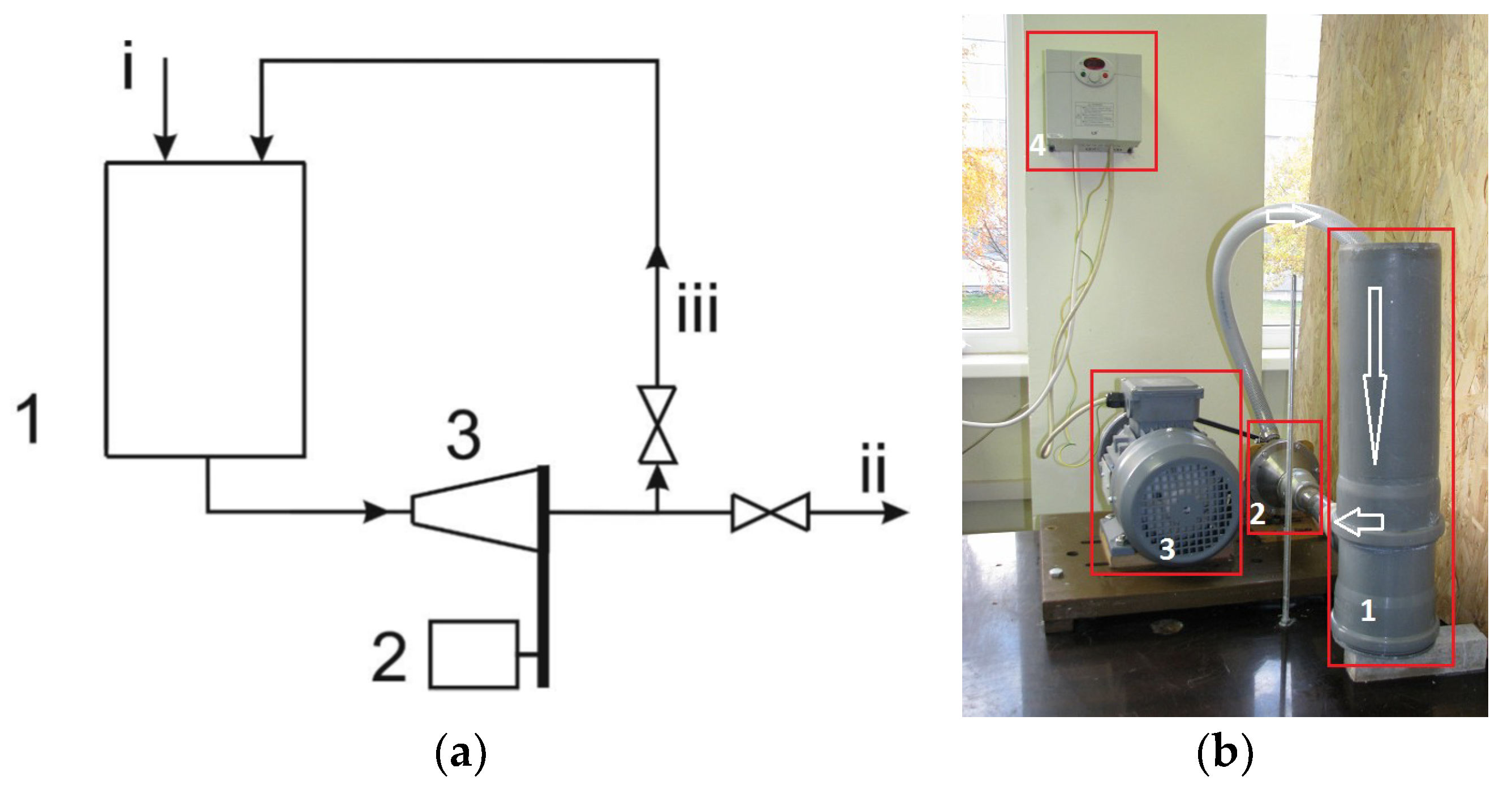
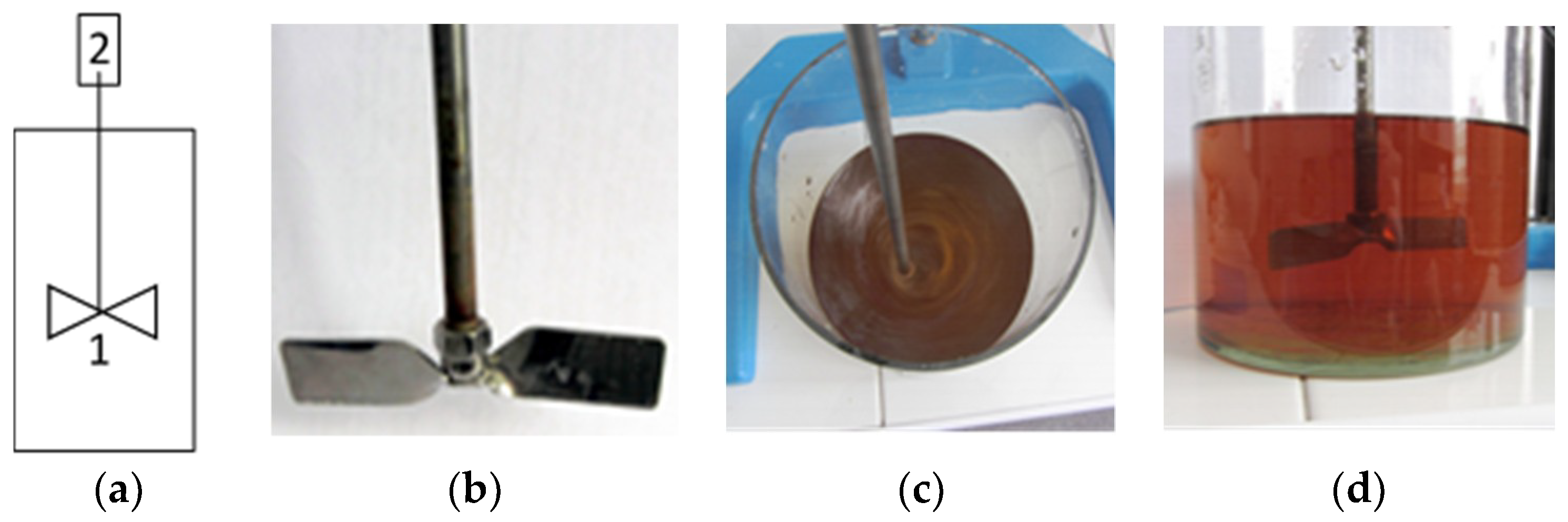

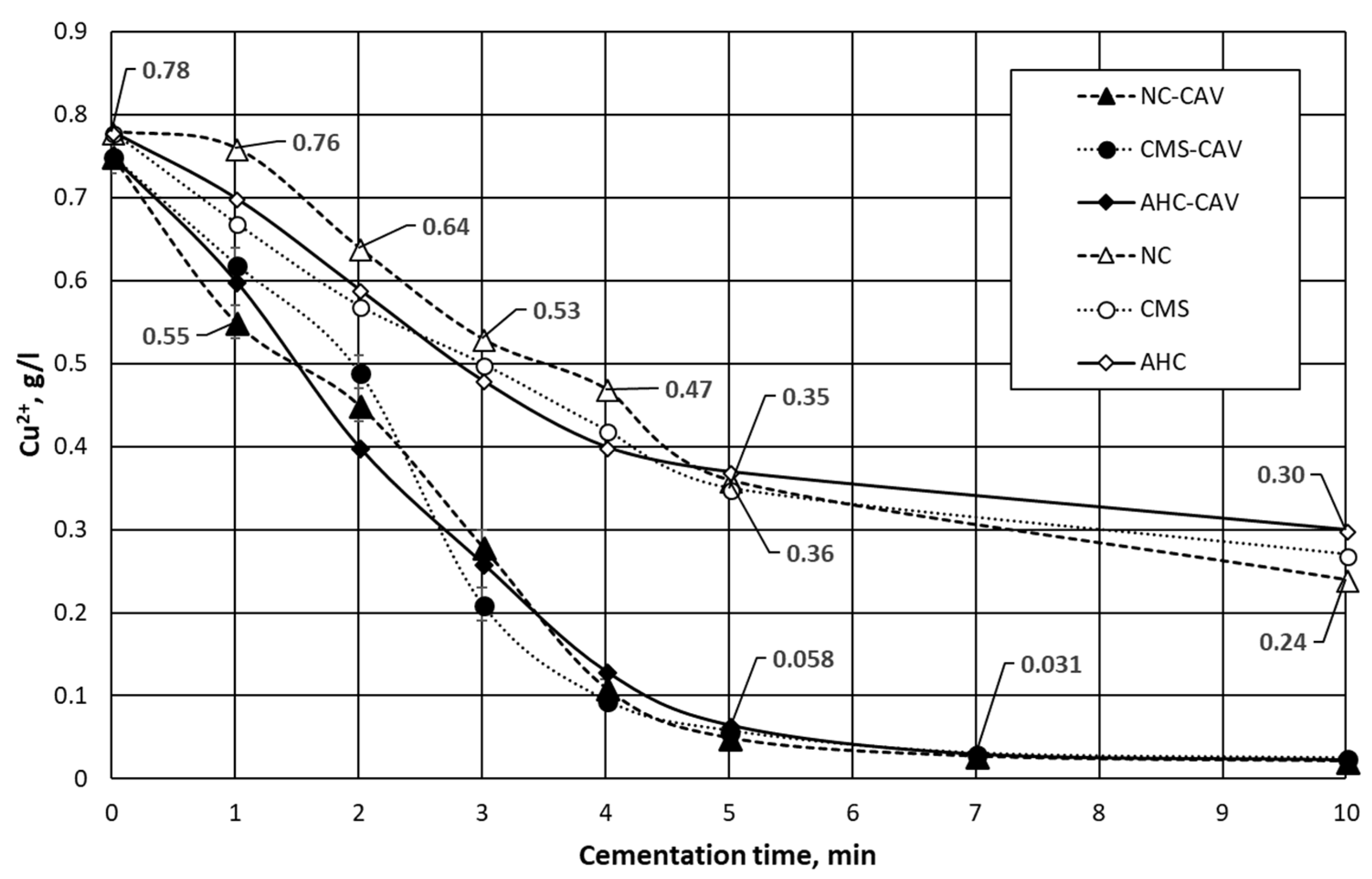
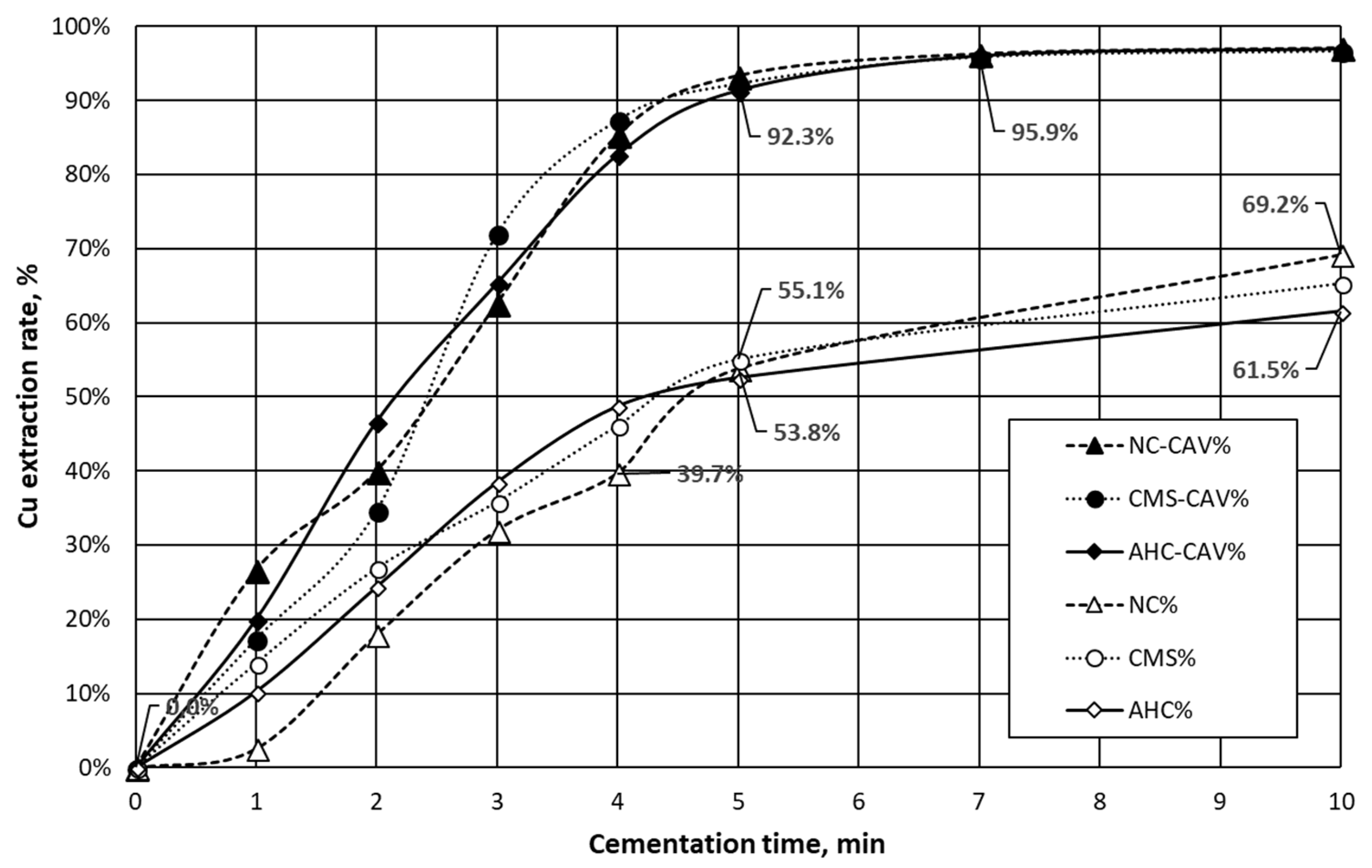
| Parameter | Value | Unit |
|---|---|---|
| pH * | 2.95 | - |
| Copper (Cu) * | 750 ± 10 | mg/L |
| Lead (Pb) | 0.250 | mg/L |
| Sulfate ions (SO42−) | 20.0 | g/L |
| Cyanide ions (CN−) | <0.050 | mg/L |
| Chromium ions (Cr6+) | ≥0.005 | mg/L |
| Nickel (Ni) | ≥4.000 | mg/L |
| Mercury ions (Hg+) | ≥0.005 | mg/L |
| Nitrate ions (NO3−) | <0.010 | mg/L |
| Fe (II+III) * | 6600 ± 100 | mg/L |
| Concentration of other cations: Al3+, Mg2+, Zn2+ | <300 | mg/L |
| * determinate by RTU laboratory. | - | - |
| Parameter | CMS | AHC 100.29 | NC 100.24 |
|---|---|---|---|
| Particle size >150 µm | 0% | 6% | 1% |
| Particle size 150–45 µm | 47% | 70% | 80% |
| Particle size <45 µm | 53% | 24% | 19% |
| Specific surface area, m2/g | 0.67 ± 0.07 | 0.78 ± 0.1 | 0.73 ± 0.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shishkin, A.; Mironovs, V.; Vu, H.; Novak, P.; Baronins, J.; Polyakov, A.; Ozolins, J. Cavitation-Dispersion Method for Copper Cementation from Wastewater by Iron Powder. Metals 2018, 8, 920. https://doi.org/10.3390/met8110920
Shishkin A, Mironovs V, Vu H, Novak P, Baronins J, Polyakov A, Ozolins J. Cavitation-Dispersion Method for Copper Cementation from Wastewater by Iron Powder. Metals. 2018; 8(11):920. https://doi.org/10.3390/met8110920
Chicago/Turabian StyleShishkin, Andrei, Viktors Mironovs, Hong Vu, Pavel Novak, Janis Baronins, Alexandr Polyakov, and Jurijs Ozolins. 2018. "Cavitation-Dispersion Method for Copper Cementation from Wastewater by Iron Powder" Metals 8, no. 11: 920. https://doi.org/10.3390/met8110920
APA StyleShishkin, A., Mironovs, V., Vu, H., Novak, P., Baronins, J., Polyakov, A., & Ozolins, J. (2018). Cavitation-Dispersion Method for Copper Cementation from Wastewater by Iron Powder. Metals, 8(11), 920. https://doi.org/10.3390/met8110920








