Dealloying of Cu-Mg-Ca Alloys
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
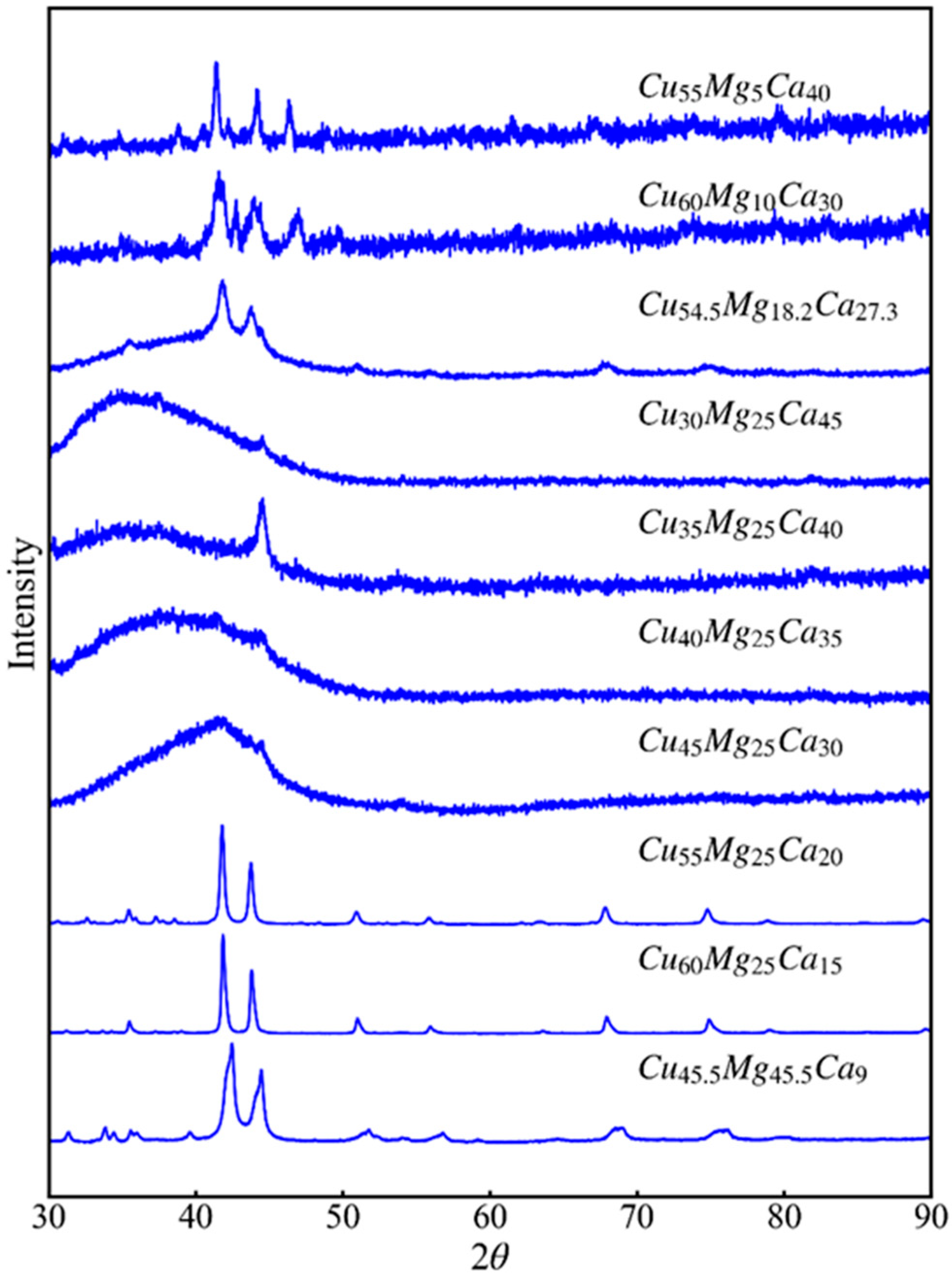
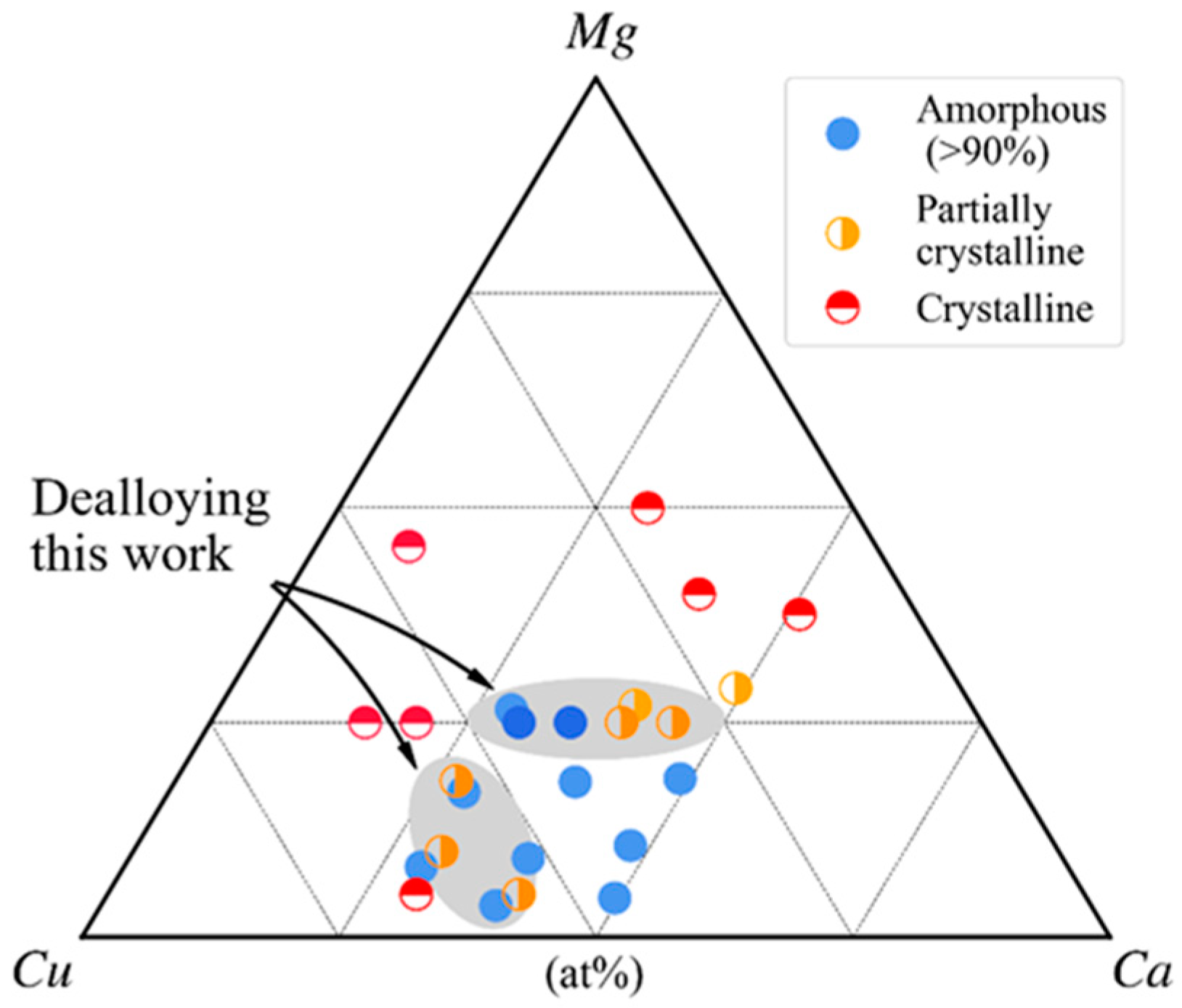
3.1. Glass-Forming Ability and Intermetallic Phases of Cu-Mg-Ca Alloys
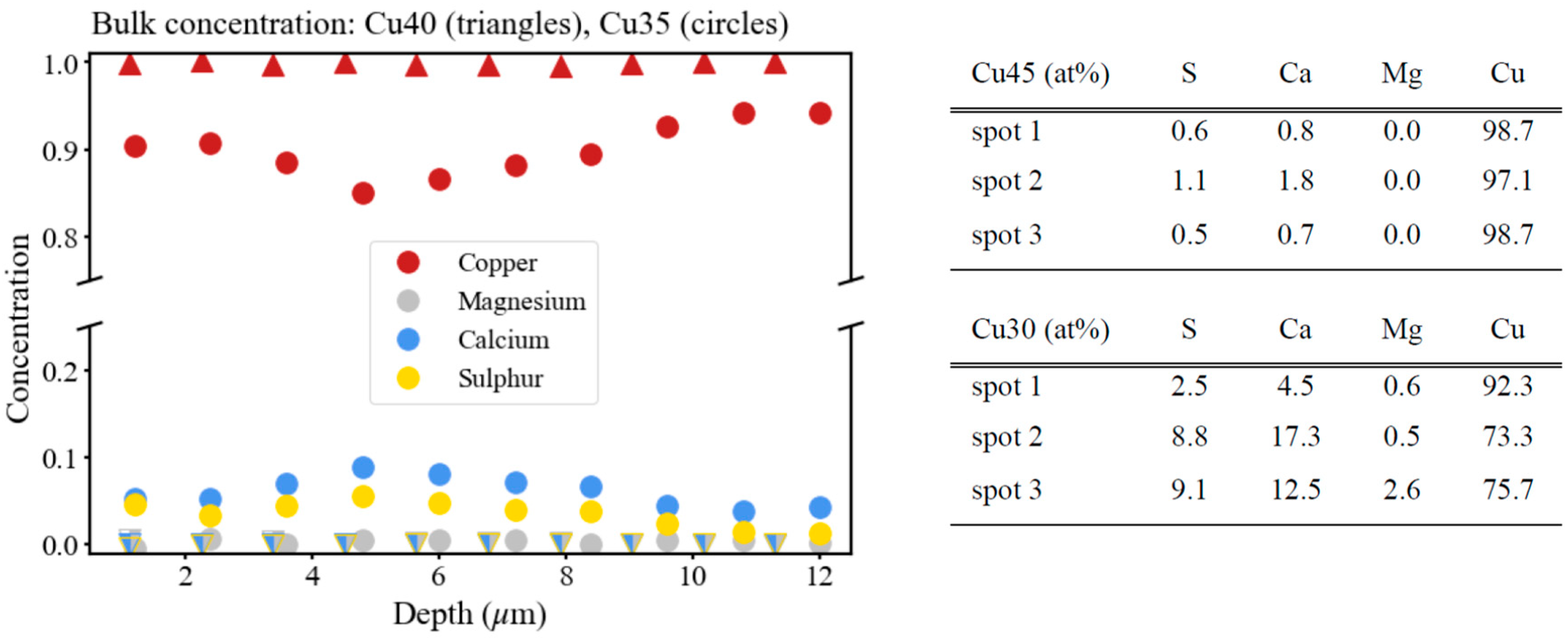
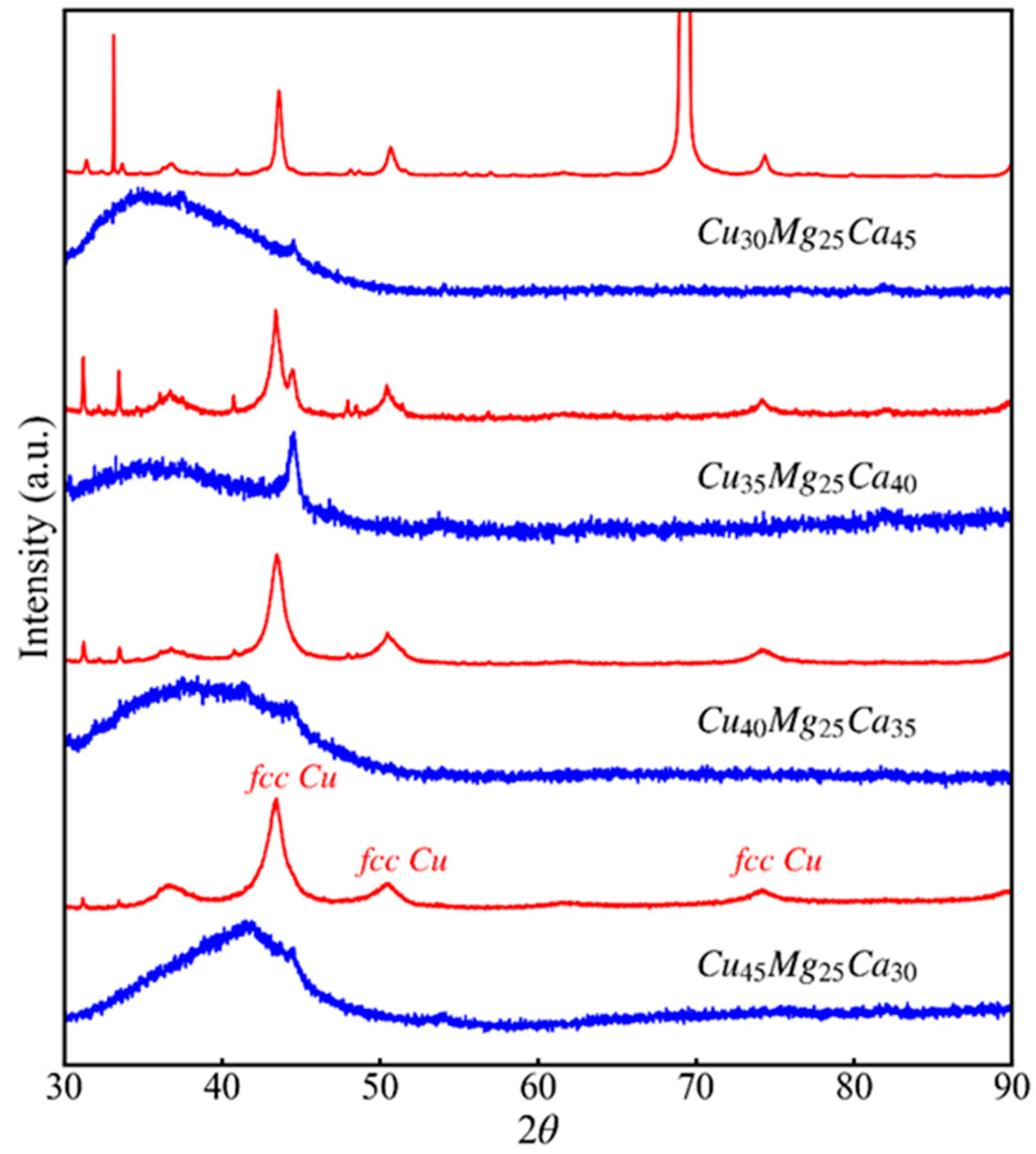
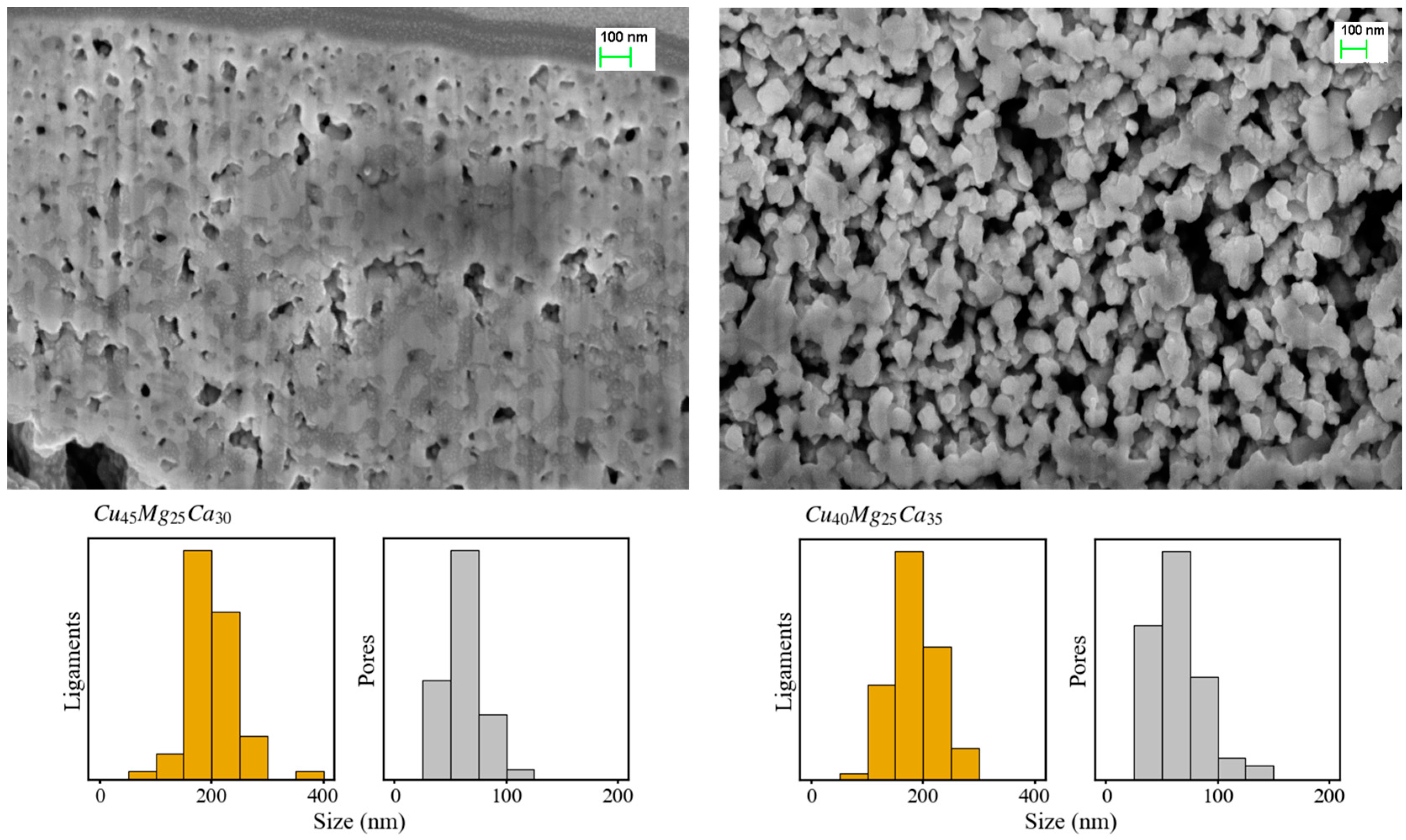
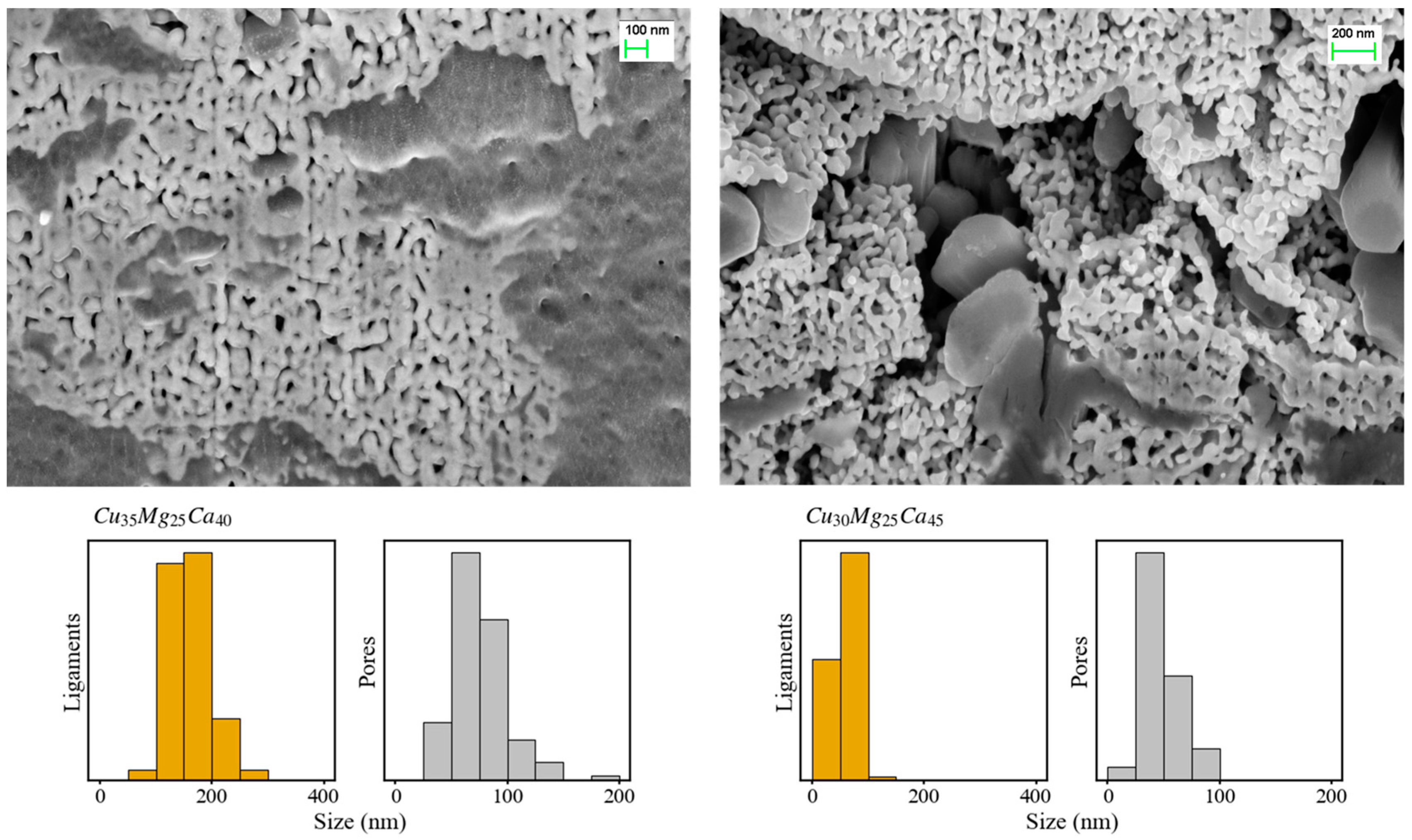
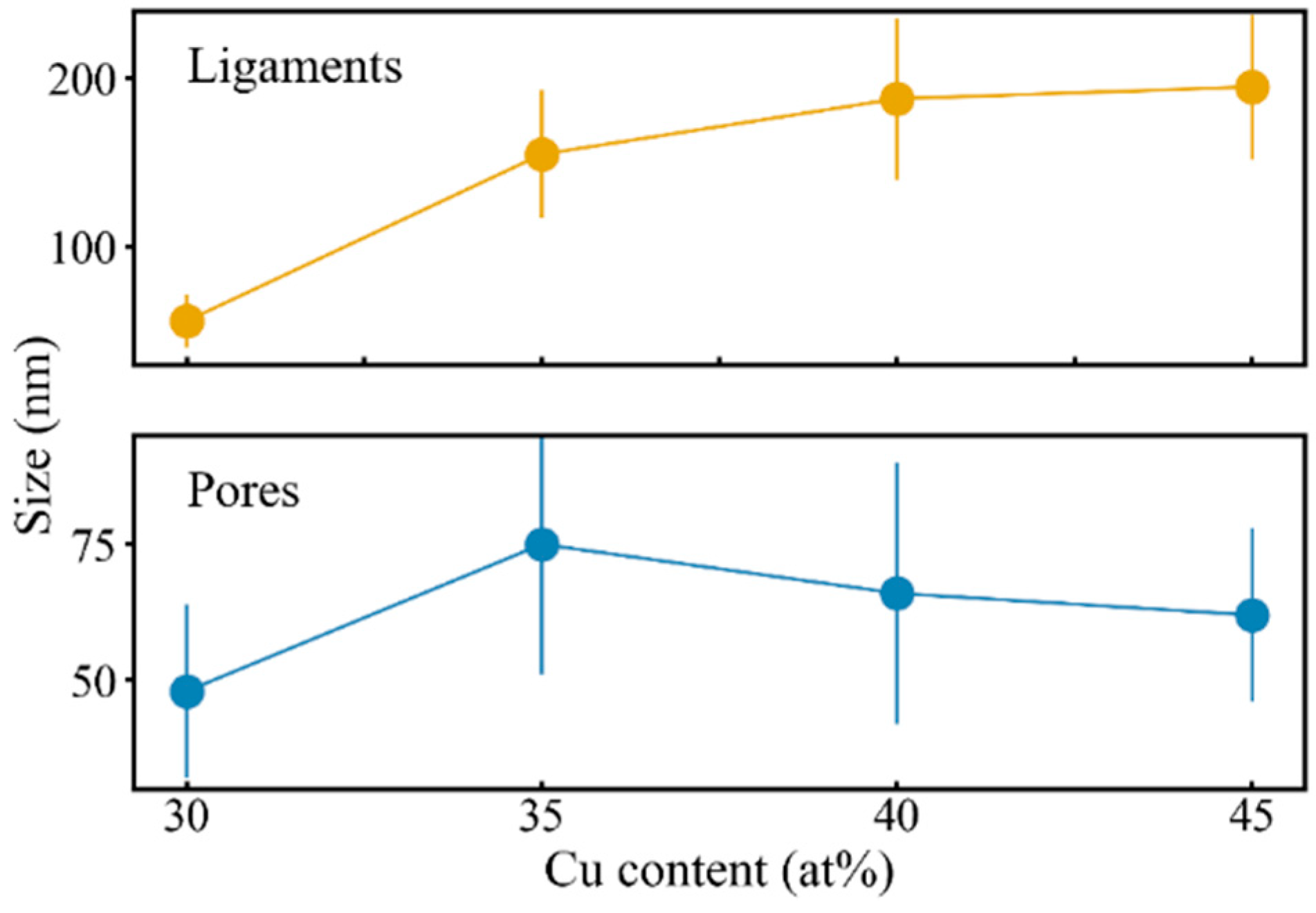
3.2. Dealloying of Series 2 in 0.04 M H2SO4
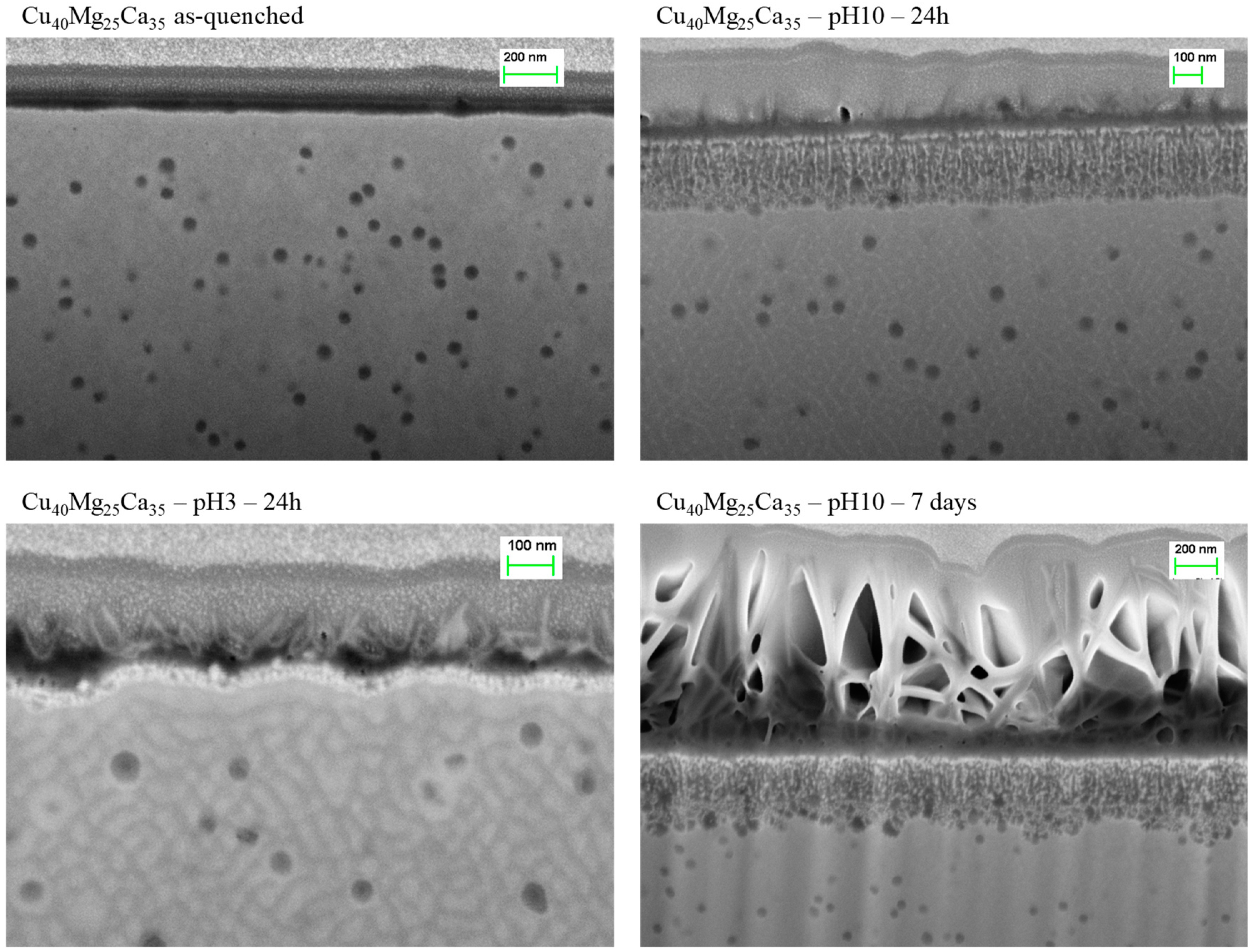
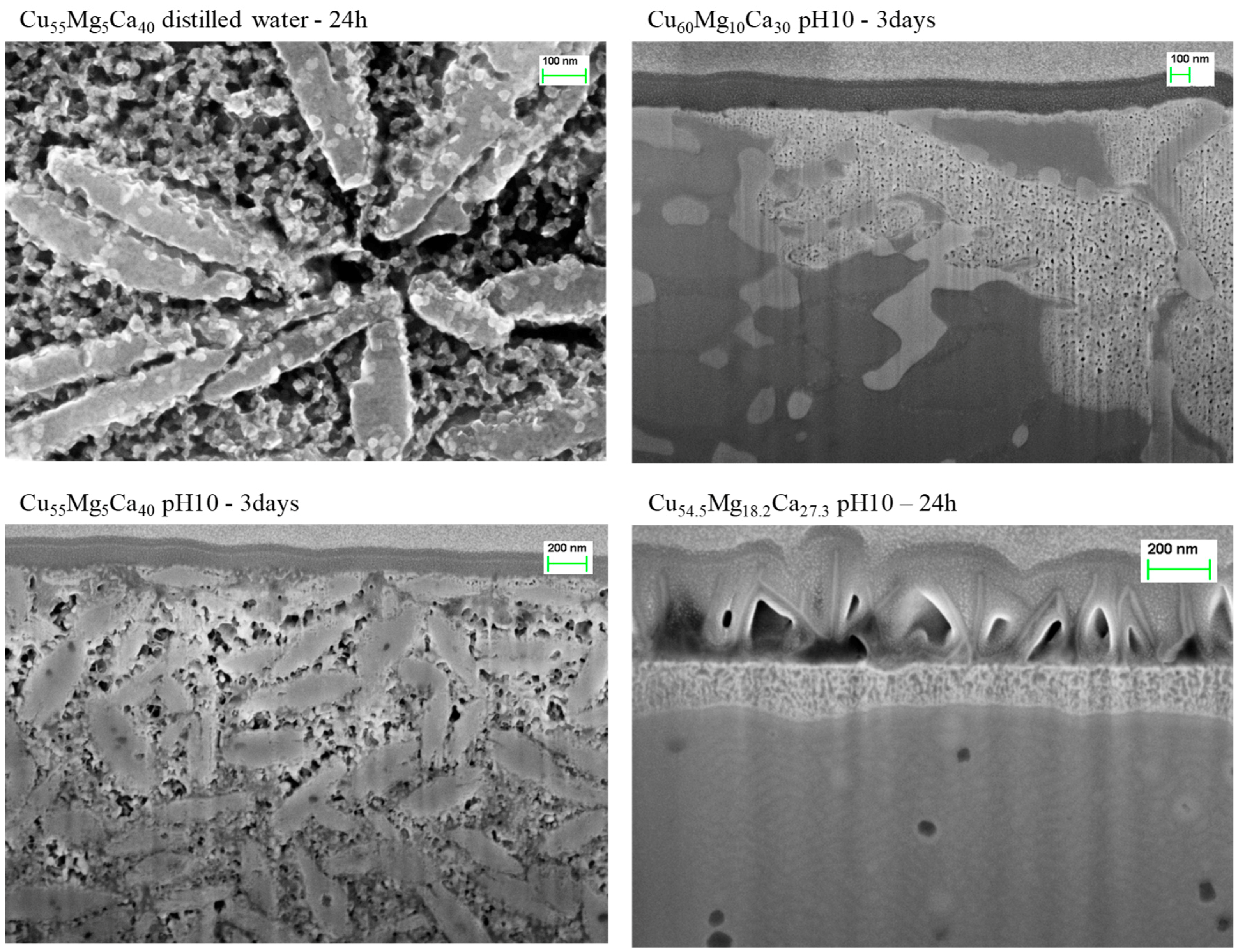
3.3. Dealloying in Distilled Water, 1 M HCl and 0.1 M NaOH Solutions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Note
- Erlebacher, J.; Aziz, M.J.; Karma, A.; Dimitrov, N.; Sieradzki, K. Evolution of nanoporosity in dealloying. Nature 2001, 410, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Erlebacher, J. An Atomistic Description of Dealloying. J. Electrochem. Soc. 2004, 151, C614. [Google Scholar] [CrossRef]
- Bond, G.C.; Thompson, D.T. Catalysis by Gold. Catal. Rev. 1999, 41, 319–388. [Google Scholar] [CrossRef]
- Chen, L.Y.; Yu, J.S.; Fujita, T.; Chen, M.W. Nanoporous copper with tunable nanoporosity for SERS applications. Adv. Funct. Mater. 2009, 19, 1221–1226. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, M. Nanoporous Metals for Catalytic and Optical Applications. MRS Bull. 2009, 34, 569–576. [Google Scholar] [CrossRef]
- Hakamada, M.; Nakano, H.; Furukawa, T.; Takahashi, M.; Mabuchi, M. Hydrogen storage properties of nanoporous palladium fabricated by dealloying. J. Phys. Chem. C 2010, 114, 868–873. [Google Scholar] [CrossRef]
- Pikul, J.H.; Zhang, G.H.; Cho, J.; Braun, P.V.; King, W.P. High-power lithium ion microbatteries from interdigitated three-dimensional bicontinuous nanoporous electrodes. Nat. Commun. 2013, 4, 1732. [Google Scholar] [CrossRef] [PubMed]
- Kramer, D.; Viswanath, R.N.; Weissmüller, J. Surface-Stress Induced Macroscopic Bending of Nanoporous Gold Cantilevers. Nano Lett. 2004, 4, 793–796. [Google Scholar] [CrossRef]
- Mangipudi, K.R.; Epler, E.; Volkert, C.A. Morphological similarity and structure-dependent scaling laws of nanoporous gold from different synthesis methods. Acta Mater. 2017, 140, 337–343. [Google Scholar] [CrossRef]
- Xue, Y.; Markmann, J.; Duan, H.; Weissmüller, J.; Huber, P. Switchable imbibition in nanoporous gold. Nat. Commun. 2014, 5, 4237. [Google Scholar] [CrossRef] [PubMed]
- Masuda, H.; Fukuda, K. Ordered Metal Nanohole Arrays Made by a Two-Step Replication of Honeycomb Structures of Anodic Alumina. Science 1995, 268, 1466–1468. [Google Scholar] [CrossRef] [PubMed]
- Wadley, H.N.G. Cellular Metals Manufacturing. Adv. Eng. Mater. 2002, 4, 726–733. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Qi, Z.; Zhang, W.; Qin, J.; Frenzel, J. Generalized fabrication of nanoporous metals (Au, Pd, Pt, Ag, and Cu) through chemical dealloying. J. Phys. Chem. C 2009, 113, 12629–12636. [Google Scholar] [CrossRef]
- Pugh, D.V.; Dursun, A.; Corcoran, S.G. Electrochemical and Morphological Characterization of Pt–Cu Dealloying. J. Electrochem. Soc. 2005, 152, B455. [Google Scholar] [CrossRef]
- Qian, L.H.; Chen, M.W. Ultrafine nanoporous gold by low-temperature dealloying and kinetics of nanopore formation. Appl. Phys. Lett. 2007, 91, 2005–2008. [Google Scholar] [CrossRef]
- Snyder, J.; Asanithi, P.; Dalton, A.B.; Erlebacher, J. Stabilized nanoporous metals by dealloying ternary alloy precursors. Adv. Mater. 2008, 20, 4883–4886. [Google Scholar] [CrossRef]
- Smith, A.J.; Trimm, D.L. The Preparation of Skeletal Catalysts. Annu. Rev. Mater. Res. 2005, 35, 127–142. [Google Scholar] [CrossRef]
- Min, U.-S.; Li, J.C.M. The microstructure and dealloying kinetics of a Cu-Mn alloy. J. Mater. Res. 1994, 9, 2878–2883. [Google Scholar] [CrossRef]
- Lu, H.B.; Li, Y.; Wang, F.H. Synthesis of porous copper from nanocrystalline two-phase Cu-Zr film by dealloying. Scr. Mater. 2007, 56, 165–168. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, S.; Li, N.; Zheng, J.; An, S.; Xing, Y. Monolithic Nanoporous Copper ribbons from Mg-Cu Alloys with Copper Contents below 33 at.%: Fabrication, Structure Evolution and Coarsening Behavior along the Thickness Direction. Int. J. Electrochem. Sci. 2011, 6, 5445–5461. [Google Scholar]
- Yu, J.; Ding, Y.; Xu, C.; Inoue, A.; Sakurai, T.; Chen, M. Nanoporous Metals by Dealloying Multicomponent Metallic Glasses. Chem. Mater. 2008, 20, 4548–4550. [Google Scholar] [CrossRef]
- Lang, X.Y.; Guo, H.; Chen, L.Y.; Kudo, A.; Yu, J.S.; Zhang, W.; Inoue, A.; Chen, M.W. Novel Nanoporous Au−Pd Alloy with High Catalytic Activity and Excellent Electrochemical Stability. J. Phys. Chem. C 2010, 114, 2600–2603. [Google Scholar] [CrossRef]
- Luo, X.; Li, R.; Liu, Z.; Huang, L.; Shi, M.; Xu, T.; Zhang, T. Three-dimensional nanoporous copper with high surface area by dealloying Mg–Cu–Y metallic glasses. Mater. Lett. 2012, 76, 96–99. [Google Scholar] [CrossRef]
- Luo, X.; Li, R.; Huang, L.; Zhang, T. Nucleation and growth of nanoporous copper ligaments during electrochemical dealloying of Mg-based metallic glasses. Corros. Sci. 2013, 67, 100–108. [Google Scholar] [CrossRef]
- Jin, Y.; Li, R.; Zhang, T. Formation of nanoporous silver by dealloying Ca-Ag metallic glasses in water. Intermetallics 2015, 67, 166–170. [Google Scholar] [CrossRef]
- St.Amand, R.; Giessen, B.C. Easy glass formation in simple metal alloys: Amorphous metals containing calcium and strontium. Scr. Metall. 1978, 12, 1021–1026. [Google Scholar] [CrossRef]
- De Tendler, R.H.; Kovacs, J.A.; Alonso, J.A. Theoretical calculation of the amorphous alloy range of the Mg-Cu system. J. Mater. Sci. 1992, 27, 4935–4939. [Google Scholar] [CrossRef]
- Inoue, A.; Masumoto, T. Mg-based amorphous alloys. Mater. Sci. Eng. A 1993, 173, 1–8. [Google Scholar] [CrossRef]
- Somoza, J.A.; Gallego, L.J.; Rey, C.; Rozenberg, S.; Arcondo, B.; Sirkin, H.; De Tendler, R.H.; Kovacs, J.A.; Alonso, J.A. An experimental and theoretical study of the glass-forming region of the Mg-Cu-Sn system. J. Mater. Sci. 1995, 30, 40–46. [Google Scholar] [CrossRef]
- Li, R.; Liu, X.J.; Wang, H.; Wu, Y.; Chu, X.M.; Lu, Z.P. Nanoporous silver with tunable pore characteristics and superior surface enhanced Raman scattering. Corros. Sci. 2014, 84, 159–164. [Google Scholar] [CrossRef]
- Li, R.; Liu, X.J.; Wang, H.; Zhou, D.Q.; Wu, Y.; Lu, Z.P. Formation mechanism and characterization of nanoporous silver with tunable porosity and promising capacitive performance by chemical dealloying of glassy precursor. Acta Mater. 2016, 105, 367–377. [Google Scholar] [CrossRef]
- Senkov, O.N.; Miracle, D.B.; Keppens, V.; Liaw, P.K. Development and Characterization of Low-Density Ca-Based Bulk Metallic Glasses: An Overview. Metall. Mater. Trans. A 2007, 39, 1888–1900. [Google Scholar] [CrossRef]
- Li, H.F.; Zheng, Y.F. Recent advances in bulk metallic glasses for biomedical applications. Acta Biomater. 2016, 36, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Senkov, O.N.; Scott, J.M. Specific criteria for selection of alloy compositions for bulk metallic glasses. Scr. Mater. 2004, 50, 449–452. [Google Scholar] [CrossRef]
- Senkov, O.N.; Scott, J.M.; Miracle, D.B. Composition range and glass forming ability of ternary Ca-Mg-Cu bulk metallic glasses. J. Alloys Compd. 2006, 424, 394–399. [Google Scholar] [CrossRef]
- Smith, D. Penn State University, University Park, PA, USA. ICDD Grant-in-Aid, 1973.
- Hanawalt, J.D.; Rinn, H.W.; Frevel, L.K. Chemical analysis by x-ray diffraction-Classification and use of x-ray diffraction patterns. Ind. Eng. Chem. Ed. 1938, 10, 457–512. [Google Scholar] [CrossRef]
- Ohba, T.; Kitano, Y.; Komura, Y. The Charge-Density Study of the Laves Phases, MgZn2 and MgCu2. Acta Crystallogr. Sect. C-CRYSTAL Struct. Commun. 1984, 40, 1–5. [Google Scholar] [CrossRef]
- Zhang, Y.; Greer, A.L.; Pineda, E. Fragility and glass-forming ability of the Ca–Mg–Cu system. J. Alloys Compd. 2007, 434–435, 145–148. [Google Scholar]
- Luo, X.; Li, R.; Zong, J.; Zhang, Y.; Li, H.; Zhang, T. Enhanced degradation of azo dye by nanoporous-copper-decorated Mg–Cu–Y metallic glass powder through dealloying pretreatment. Appl. Surf. Sci. 2014, 305, 314–320. [Google Scholar] [CrossRef]
- Graf, M.; Ngô, B.-N.D.; Weissmüller, J.; Markmann, J. X-ray studies of nanoporous gold: Powder diffraction by large crystals with small holes. Phys. Rev. Mater. 2017, 1. [Google Scholar] [CrossRef]
- Dan, Z.; Qin, F.; Yamaura, S.; Xie, G.; Makino, A.; Hara, N. Refinement of Nanoporous Copper by Dealloying MgCuY Amorphous Alloys in Sulfuric Acids Containing Polyvinylpyrrolidone. J. Electrochem. Soc. 2014, 161, C120–C125. [Google Scholar] [CrossRef]
- Dan, Z.; Qin, F.; Sugawara, Y.; Muto, I.; Hara, N. Fabrication of nanoporous copper by dealloying amorphous binary Ti-Cu alloys in hydrofluoric acid solutions. Intermetallics 2012, 29, 14–20. [Google Scholar] [CrossRef]
- Dan, Z.; Qin, F.; Makino, A.; Sugawara, Y.; Muto, I.; Hara, N. Fabrication of nanoporous copper by dealloying of amorphous Ti-Cu-Ag alloys. J. Alloys Compd. 2014, 586, S134–S138. [Google Scholar] [CrossRef]
- Ding, Y.; Kim, Y.-J.; Erlebacher, J. Nanoporous Gold Leaf: “Ancient Technology”/Advanced Material. Adv. Mater. 2004, 16, 1897–1900. [Google Scholar] [CrossRef]
- Pickering, H.W.; Wagner, C. Electrolytic Dissolution of Binary Alloys Containing a Noble Metal. J. Electrochem. Soc. 1967, 114, 698–706. [Google Scholar] [CrossRef]
- Moreira, A.H.; Benedetti, A.V.; Cabot, P.L.; Sumodjo, P.T.A. Electrochemical behaviour of copper electrode in concentrated sulfuric acid solutions. Electrochim. Acta 1993, 38, 981–987. [Google Scholar] [CrossRef]
- Ma, H.; Chen, S.; Yin, B.; Zhao, S.; Liu, X. Impedance spectroscopic study of corrosion inhibition of copper by surfactants in the acidic solutions. Corros. Sci. 2003, 45, 867–882. [Google Scholar] [CrossRef]
- Smith, A.J.; Tran, T.; Wainwright, M.S. Kinetics and mechanism of the preparation of Raney® copper. J. Appl. Electrochem. 1999, 29, 1085–1094. [Google Scholar] [CrossRef]
- Parida, S.; Kramer, D.; Volkert, C.A.; Rösner, H.; Erlebacher, J.; Weissmüller, J. Volume change during the formation of nanoporous gold by dealloying. Phys. Rev. Lett. 2006, 97, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Aburada, T.; Fitz-Gerald, J.M.; Scully, J.R. Synthesis of nanoporous copper by dealloying of Al-Cu-Mg amorphous alloys in acidic solution: The effect of nickel. Corros. Sci. 2011, 53, 1627–1632. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Mbarek, W.; Pineda, E.; Escoda, L.; Suñol, J.J.; Khitouni, M. Dealloying of Cu-Mg-Ca Alloys. Metals 2018, 8, 919. https://doi.org/10.3390/met8110919
Ben Mbarek W, Pineda E, Escoda L, Suñol JJ, Khitouni M. Dealloying of Cu-Mg-Ca Alloys. Metals. 2018; 8(11):919. https://doi.org/10.3390/met8110919
Chicago/Turabian StyleBen Mbarek, Wael, Eloi Pineda, Lluïsa Escoda, Joan Josep Suñol, and Mohamed Khitouni. 2018. "Dealloying of Cu-Mg-Ca Alloys" Metals 8, no. 11: 919. https://doi.org/10.3390/met8110919
APA StyleBen Mbarek, W., Pineda, E., Escoda, L., Suñol, J. J., & Khitouni, M. (2018). Dealloying of Cu-Mg-Ca Alloys. Metals, 8(11), 919. https://doi.org/10.3390/met8110919








