Iron Metallurgy via Concentrated Solar Energy
Abstract
1. Introduction
2. Solar Agglomeration of Iron Ore
2.1. Materials and Methods
2.2. Results and Discussion
3. Solar Synthesized Iron
3.1. Theoretical Basis
- Nature of the substances, which is related with the structure and energy of the phases involved in the reaction;
- Concentration of the reagents, which affects the surface chemical process and the transportation rate;
- Temperature (the higher the temperature, the bigger the reaction ratio will be);
- Interphase surface;
- Geometry of the interphase;
- Nature of the interphase;
- Presence and characteristics of the reaction products in the interphase.
3.2. Smelting Reduction of Hematite with Carbon
3.2.1. Materials and Methods
- Power, which is controlled with the shutter (between 725 and 1160 W);
- Displacement speed below the solar beam (0.25 to 0.75 mm/s);
- Ratio iron oxide (III) to carbon (10, 25 and 40% carbon over the stoichiometric).
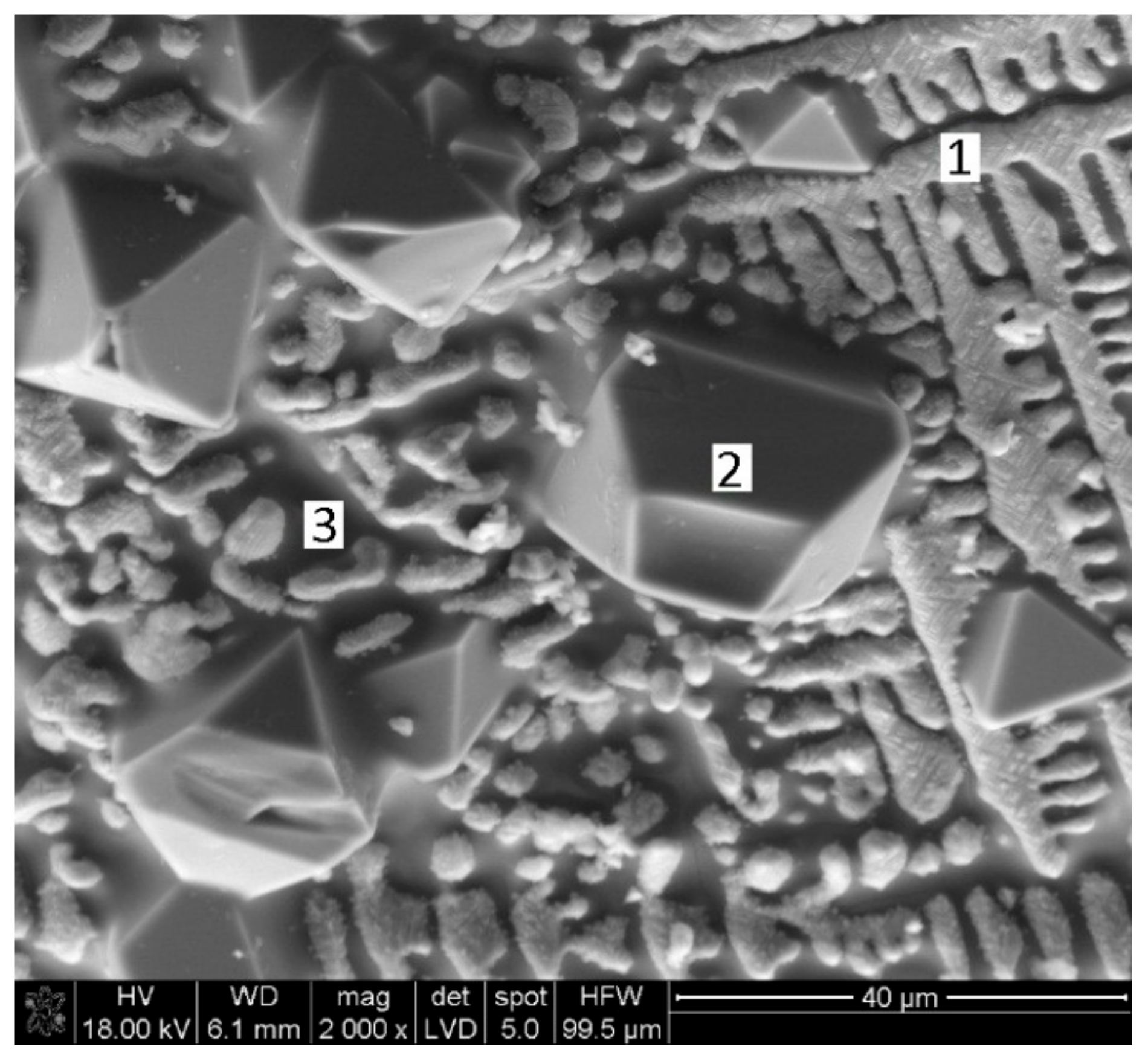
3.2.2. Results and Discussion
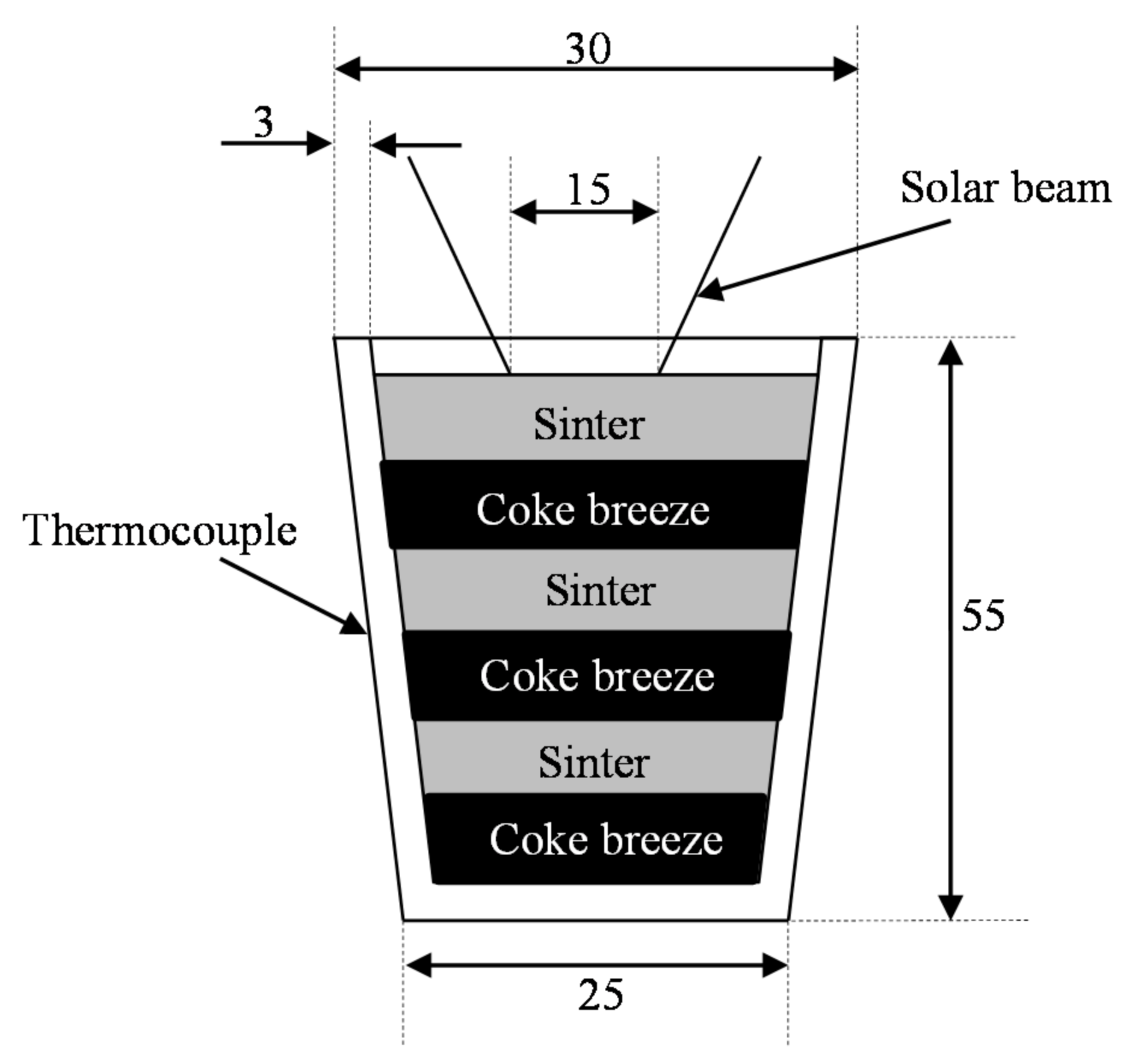
3.3. Reduction of Real Sinter with Coke Breeze
3.3.1. Materials and Methods
3.3.2. Results and Discussion
3.4. Potential Advantages of Solar Energy: Reduction in Both CO2 Emissions and Costs
4. Conclusions
- −
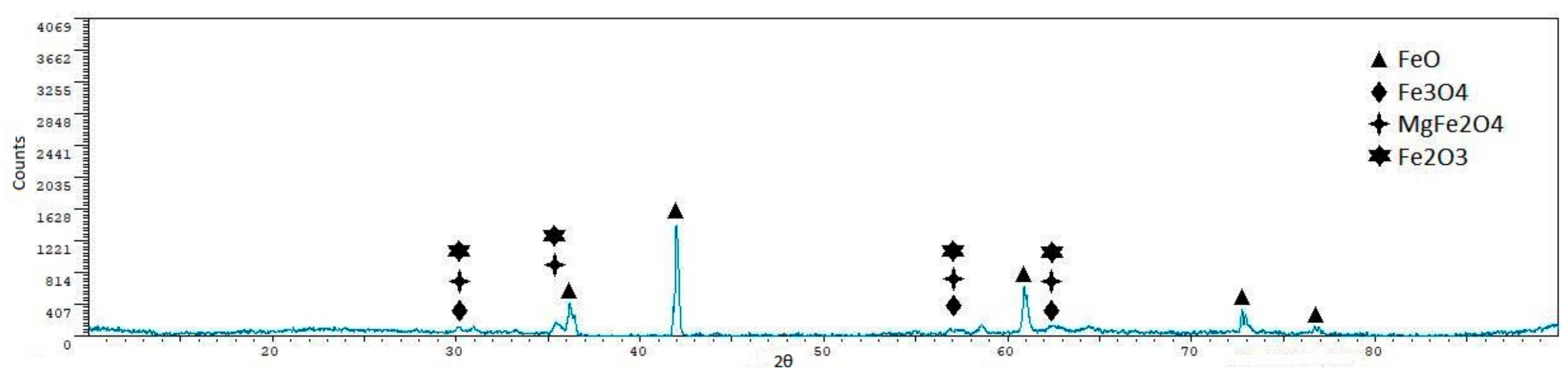
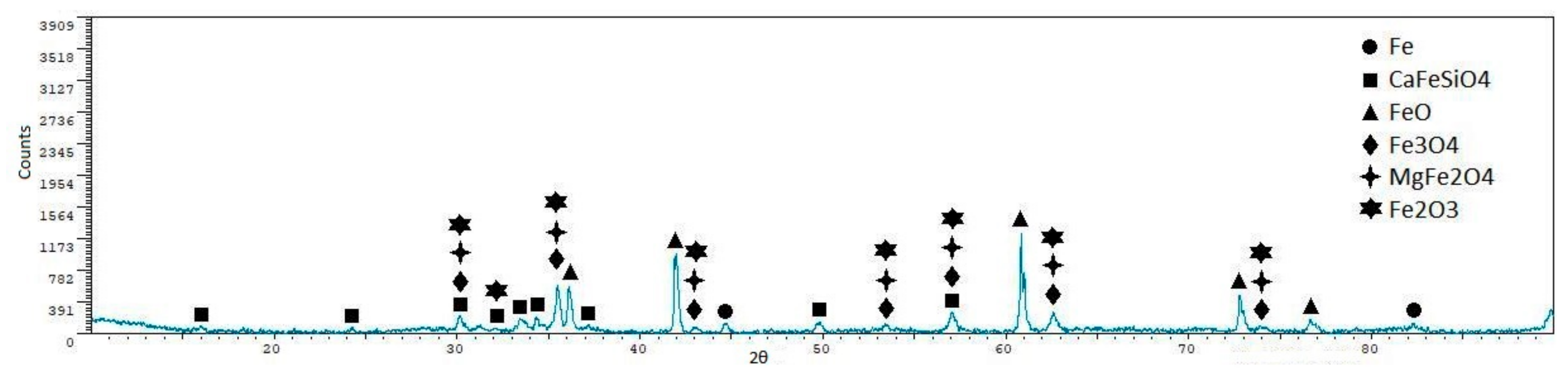
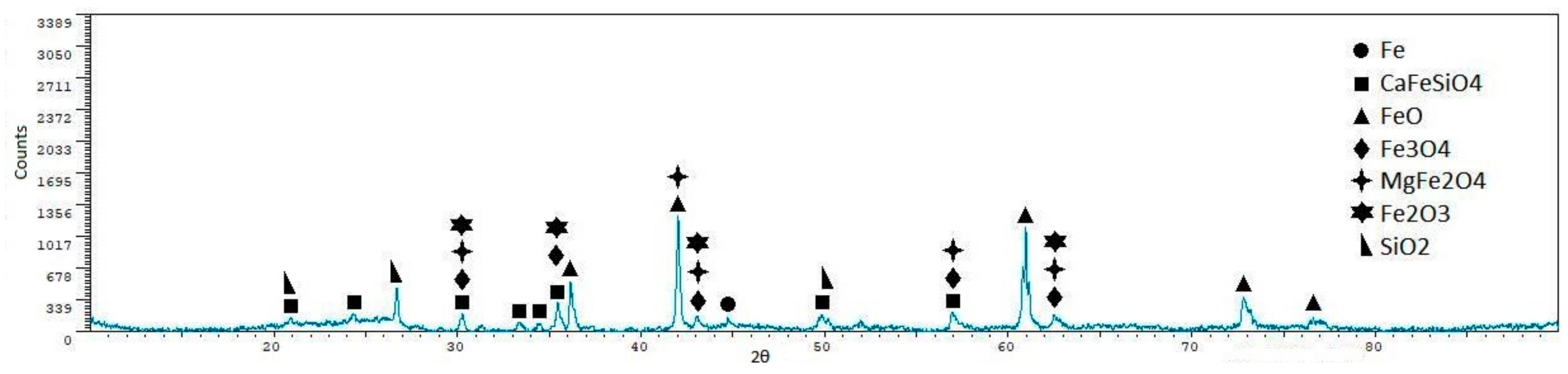
- Iron ore agglomeration: Industrial iron ore mixture and coke breeze were used as raw materials. The reduction of CO2 emissions in the case of the iron ore sintering could be achieved through two routes: Replacing the initializing system, although this would have a limited impact in the reduction of carbon dioxide emissions; and/or reducing/eliminating the coke mixed with the sinter. Experiments performed have shown that the temperatures were so high that the mixture was melted in the upper face, and in this way, the reaction was blocked in a depth of approximately 12–15 mm. Moreover, the lack of a downdraught system impeded the circulation of the gases through the load and, in this way, the progress of the process until the bottom of the crucible. The lack of gas flow also explains the presence of important quantities of wüstite, as it would be oxidized in the presence of air. Reducing/eliminating the coke mixed with the sinter could be achieved by replacing coke with solar energy, but this seems difficult, taking into account that concentrated solar energy is a punctual heat source. The application of concentrated solar energy seems to have limited utility in the sintering process.
- −
- Reduction of iron oxides: Laboratory quality iron oxide (III) and carbon, and industrial iron ore sinter and coke breeze were used as raw materials. The decrease of the carbon dioxide emissions in the reduction of the iron ore with carbonaceous material can be achieved by replacing the part of the coke used to supply heat to the process with solar energy, as coke used to reduce the charge cannot be eliminated. In this way, the carbon dioxide emissions could be significantly reduced. Both iron oxide (III) and carbon and iron ore sinter and coke breeze mixtures were partially reduced, as magnetite and wüstite were detected in all samples, and metallic iron was detected in several samples. The utilization of air atmosphere makes that part of the carbon monoxide pass to the atmosphere without reducing the charge (the formation of carbon monoxide through the Boudouard mechanism is the controlling step). If a reductant or CO2 atmosphere was used, the conversion of the iron oxide (III) into iron would have been higher. The disposition of the sinter and coke in alternative layers when using real materials increased the amount of metallic iron in the final samples, but reduced products were only found in the upper sinter layer. A moving system with two layers (sinter above, coke below) could have given better results.
Author Contributions
Funding
Conflicts of Interest
References
- World Steel Association: World Steel in Figures. 2018. Available online: https://www.worldsteel.org/publications/bookshop/product-details.~World-Steel-in-Figures-2018~PRODUCT~World-Steel-in-Figures-2018~.html (accessed on 24 October 2018).
- Fernández-González, D.; Ruiz-Bustinza, I.; Mochón, J.; González-Gasca, C.; Verdeja, L.F. Iron Ore Sintering: Raw Materials and Granulation. Miner. Process. Extr. Metall. Rev. 2017, 38, 36–46. [Google Scholar] [CrossRef]
- Fernández-González, D.; Ruiz-Bustinza, I.; González-Gasca, C.; Piñuela-Noval, J.; Mochón-Castaños, J.; Sancho-Gorostiaga, J.; Verdeja, L.F. Concentrated solar energy applications in materials science and metallurgy. Sol. Energy 2018, 170, 520–540. [Google Scholar] [CrossRef]
- Fernández-González, D.; Prazuch, J.; Ruiz-Bustinza, I.; González-Gasca, C.; Piñuela-Noval, J.; Verdeja, L.F. Solar synthesis of calcium aluminates. Sol. Energy 2018, 171, 658–666. [Google Scholar] [CrossRef]
- Ruiz-Bustinza, I.; Cañadas, I.; Rodríguez, J.; Mochón, J.; Verdeja, L.F.; García-Carcedo, F.; Vázquez, A. Magnetite Production from Steel Wastes with Concentrated Solar Energy. Steel Res. Int. 2013, 84, 207–217. [Google Scholar] [CrossRef]
- Sibieude, F.; Ducarroir, M.; Tofighi, A.; Ambriz, J. High temperature experiments with a solar furnace: The decomposition of Fe3O4, Mn3O4, CdO. Int. J. Hydrogen Energy 1982, 7, 79–88. [Google Scholar] [CrossRef]
- Steinfeld, A.; Fletcher, E.A. Theoretical and experimental investigation of the carbothermic reduction of Fe2O3 using solar energy. Energy 1991, 16, 1011–1019. [Google Scholar] [CrossRef]
- Steinfeld, A.; Kuhn, P.; Karni, J. High-temperature solar thermochemistry: Production of iron and synthesis gas by Fe3O4-reduction with methane. Energy 1993, 18, 239–249. [Google Scholar] [CrossRef]
- Mochón, J.; Ruiz-Bustinza, I.; Vázquez, A.; Fernández, D.; Ayala, J.M.; Barbés, M.F.; Verdeja, L.F. Transformations in the Iron-Manganese-Oxygen-Carbon System Resulted from Treatment of Solar Energy with High Concentration. Steel Res. Int. 2014, 85, 1469–1476. [Google Scholar] [CrossRef]
- Pardo, N.; Moya, J.A. Prospective scenarios on energy efficiency and CO2 emissions in the European iron & steel industry. Energy 2013, 54, 113–128. [Google Scholar]
- Wang, K.; Wang, C.; Lu, X.; Chen, J. Scenario analysis on CO2 emissions reduction potential in China’s iron and steel industry. Energy Policy 2007, 35, 2320–2335. [Google Scholar] [CrossRef]
- Ariyama, T.; Sato, M. Optimization of ironmaking process for reducing CO2 emissions in the integrated steel works. ISIJ Int. 2006, 46, 1736–1744. [Google Scholar] [CrossRef]
- Naito, M.; Takeda, K.; Matsui, Y. Ironmaking technology for the last 100 years: Deployment to advanced technologies form introduction of technological know-how, and evolution next-generation process. ISIJ Int. 2015, 55, 7–35. [Google Scholar] [CrossRef]
- Bertlin, H. Review coal and coke for blast furnaces. ISIJ Int. 1999, 39, 617–624. [Google Scholar] [CrossRef][Green Version]
- Kirschena, M.; Badr, K.; Pfeifer, H. Influence of direct reduced iron on the energy balance of the electric arc furnace in steel industry. Energy 2011, 36, 6146–6155. [Google Scholar] [CrossRef]
- Pardo, N.; Moya, J.A.; Vatopoulos. Prospective Scenarios on Energy Efficiency and CO2 Emissions in the EU Iron and Steel Industry; European Commission, Joint Research Centre, Institute for Energy and Transport: Petten, The Netherlands, 2012; ISBN 978-92-79-54191-9. [Google Scholar]
- Eisele, T.C.; Kawatra, S.K. A review of binders in iron ore pelletization. Miner. Process. Extr. Metall. Rev. 2003, 24, 1–90. [Google Scholar] [CrossRef]
- Fernández-González, D.; Martín-Duarte, R.; Ruiz-Bustinza, I.; Mochón, J.; González-Gasca, C.; Verdeja, L.F. Optimization of sinter plant operating conditions using advanced multivariate statistics: Intelligent data processing. JOM 2016, 68, 2089–2095. [Google Scholar] [CrossRef]
- Fernández-González, D.; Ruiz-Bustinza, I.; Mochón, J.; González-Gasca, C.; Verdeja, L.F. Iron Ore Sintering: Process. Miner. Process. Extr. Metall. Rev. 2017, 38, 215–227. [Google Scholar] [CrossRef]
- Fernández-González, D.; Ruiz-Bustinza, I.; Mochón, J.; González-Gasca, C.; Verdeja, L.F. Iron Ore Sintering: Quality Indices. Miner. Process. Extr. Metall. Rev. 2017, 38, 254–264. [Google Scholar] [CrossRef]
- Fernández-González, D.; Ruiz-Bustinza, I.; Mochón, J.; González-Gasca, C.; Verdeja, L.F. Iron Ore Sintering: Environment, Automatic and Control Techniques. Miner. Process. Extr. Metall. Rev. 2017, 38, 238–249. [Google Scholar] [CrossRef]
- Fernández-González, D.; Piñuela-Noval, J.; Verdeja, L.F. Iron Ore Agglomeration Technologies. In Iron Ores and Iron Oxide Materials, 1st ed.; Shatokha, V., Ed.; Intech Open: London, UK, 2018; pp. 61–80. ISBN 978-1-78923-320-9. [Google Scholar]
- Ghosh, A.; Chatterjee, A. Ironmaking and Steelmaking; PHI Learning Private Limited: New Dehli, India, 2008; ISBN 978-8120332898. [Google Scholar]
- Hayashi, N.; Komarov, S.; Kasai, E. Heat transfer analysis of the mosaic embedding iron ore sintering (MEBIOS) process. ISIJ Int. 2009, 49, 681–686. [Google Scholar] [CrossRef]
- Lu, L.; Ishiyama, O. Iron ore sintering. In Iron Ore. Minaralogy, Processing and Environmental Sustainability; Wordhead Publishing; Elsevier: Cambridge, UK, 2015; pp. 395–429. ISBN 978-1-78242-159-7. [Google Scholar]
- Webster, N.A.S.; Pownceby, M.I.; Madsen, I.C.; Kimpton, J.A. Silico-ferrite of calcium and aluminium (SFCA) iron ore sinter bonding phases: new insights into their formation during heating and cooling. Metall. Mater. Trans. B 2012, 43B, 1344–1357. [Google Scholar] [CrossRef]
- Ballester, A.; Sancho, J.; Verdeja, L.F. Metalurgia Extractiva. Volumen I. Fundamentos; Síntesis: Madrid, Spain, 2003; ISBN 84-7738-802-4. [Google Scholar]
- Sancho, J.; Verdeja, L.F.; Ballester, A. Metalurgia Extractiva. Volumen II. Procesos de Obtención; Síntesis: Madrid, Spain, 2000; ISBN 84-7738-803-2. [Google Scholar]
- Rao, Y.K. The kinetics of reduction of hematite by carbon. Metall. Trans. 1971, 2, 1439–1447. [Google Scholar]
- Fruehan, R.J. The rate of reduction of iron oxides by carbon. Metall. Trans. B 1977, 8, 279–286. [Google Scholar] [CrossRef]
- Pero-Sanz, J.P.; Fernández-González, D.; Verdeja, L.F. Physical Metallergy of Cast Irons; Springer: Cham, Switzerland, 2018; ISBN 978-3-319-97312-8. [Google Scholar]
- Geerdes, M.; Chaigneau, R.; Kurunov, I.; Lingiardi, O.; Ricketts, J. Modern Blast Furnace Ironmaking an Introduction, 3rd ed.; IOS Press: Amsterdam, The Netherlands, 2015; p. 116. ISBN 978-1-61499-499-2. [Google Scholar]
- Kundak, M.; Lazic, L.; Crnko, J. CO2 emissions in the steel industry. Metalurgija 2009, 3, 193–197. [Google Scholar]
- Meier, A.; Bonaldi, E.; Cella, G.M.; Lipinski, W.; Wuillemin, D.; Palumbo, R. Design and experimental investigation of a horizontal rotary reactor for the solar thermal production of lime. Energy 2004, 29, 811–821. [Google Scholar] [CrossRef]
- Meier, A.; Bonaldi, E.; Cella, G.M.; Lipinski, W. Multitube rotary kiln for the industrial solar production of lime. J. Sol. Energy Eng. 2005, 127, 386–395. [Google Scholar] [CrossRef]
- Meier, A.; Gremaud, N.; Steinfeld, A. Economic evaluation of the industrial solar production of lime. Energy Convers. Manag. 2005, 46, 905–926. [Google Scholar]
- Meier, A.; Bonaldi, E.; Cella, G.M.; Lipinski, W.; Wuillmin, D. Solar chemical reactor technology for industrial production of lime. Sol. Energy 2006, 80, 1355–1362. [Google Scholar] [CrossRef]
- Wieckert, C.; Frommherz, U.; Kräupl, S.; Guillot, E.; Olalde, G.; Epstein, M.; Santén, S.; Osinga, T.; Steinfeld, A. A 300 kW solar chemical pilot plant for the carbothermic production of zinc. J. Sol. Energy Eng. 2006, 129, 190–196. [Google Scholar] [CrossRef]
- Epstein, M.; Olalde, G.; Santén, S.; Steinfeld, A.; Wieckert, C. Towards the industrial solar carbothermal production of zinc. J. Sol. Energy Eng. 2008, 130, 014505. [Google Scholar] [CrossRef]



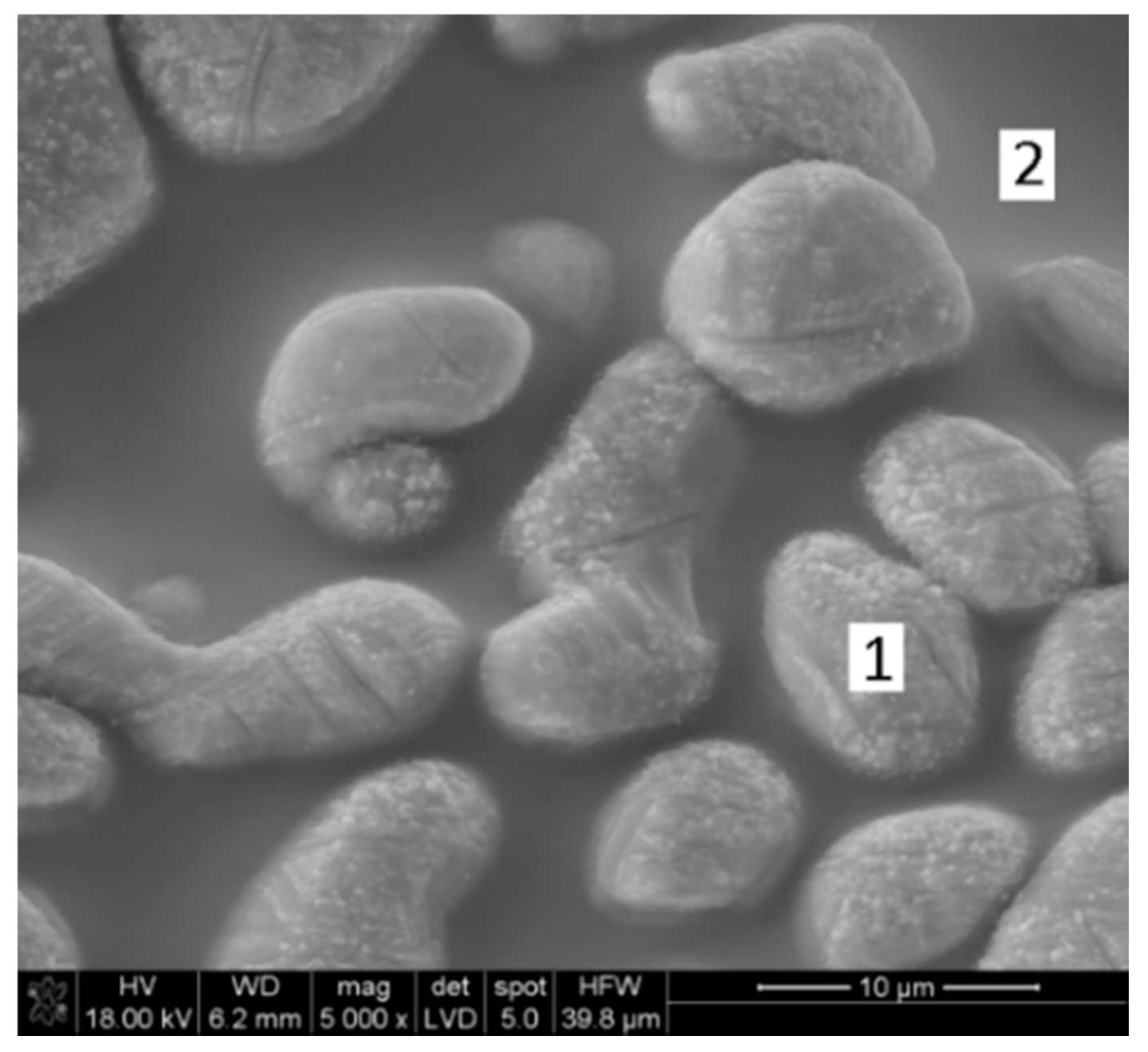
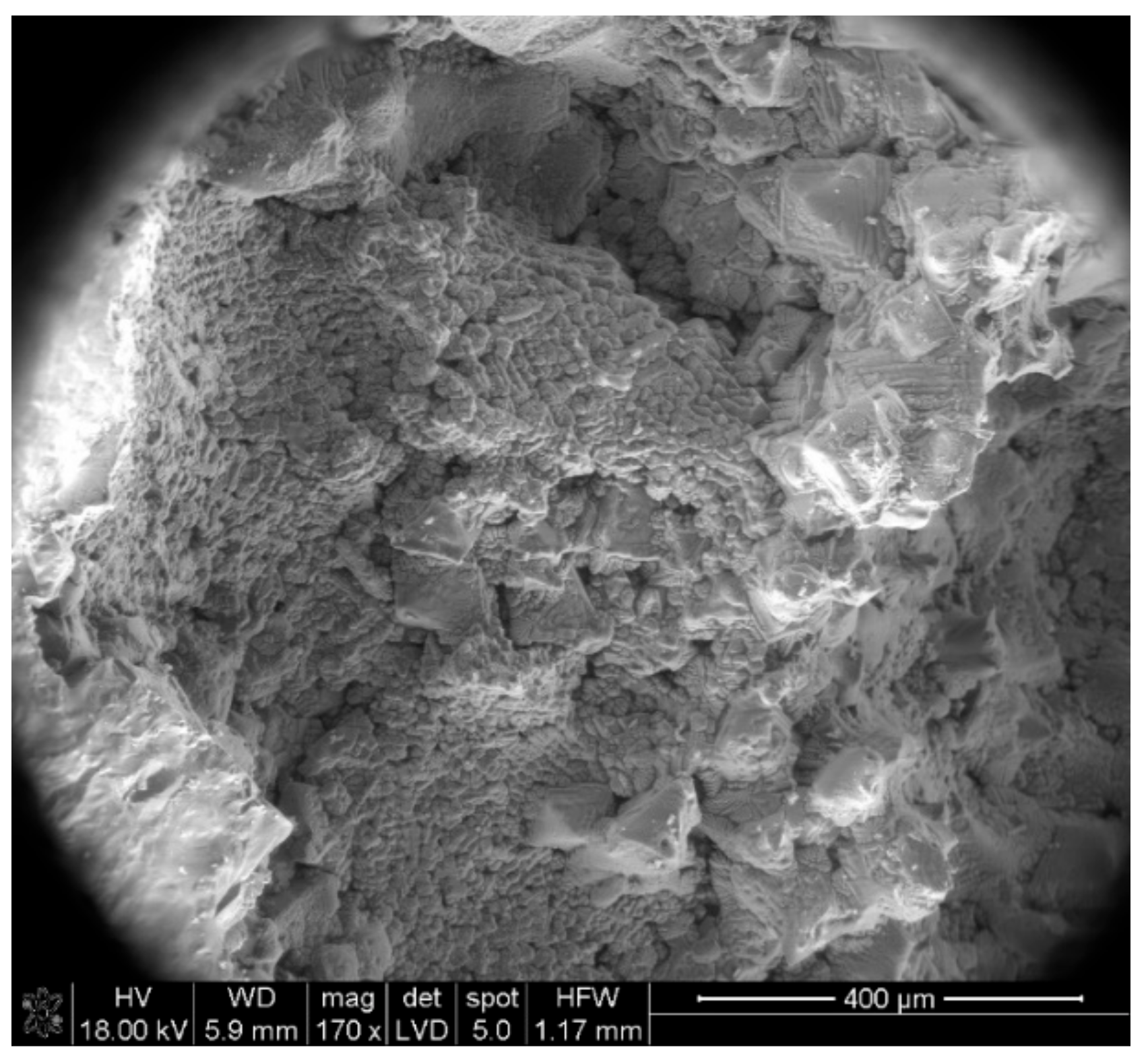
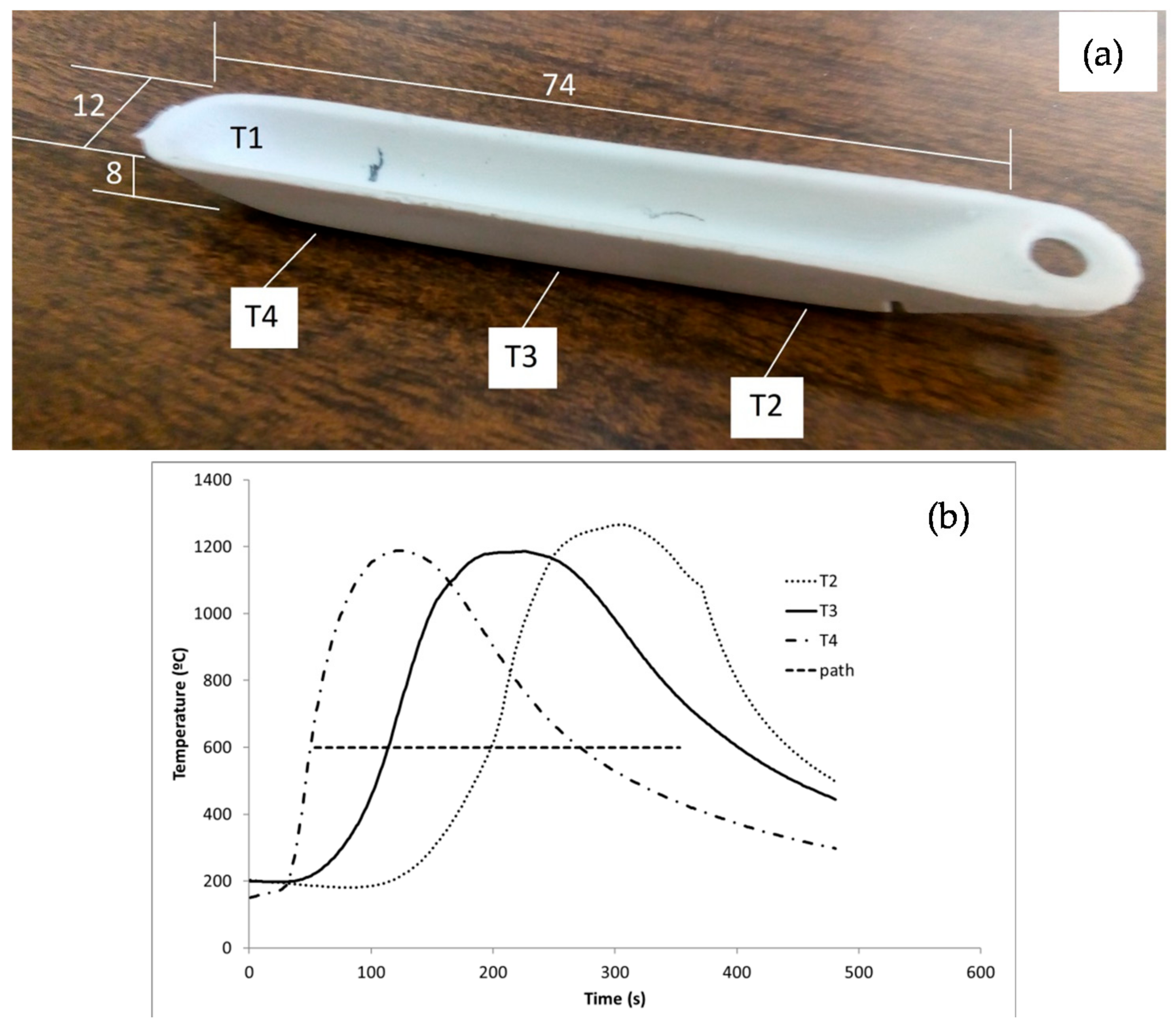

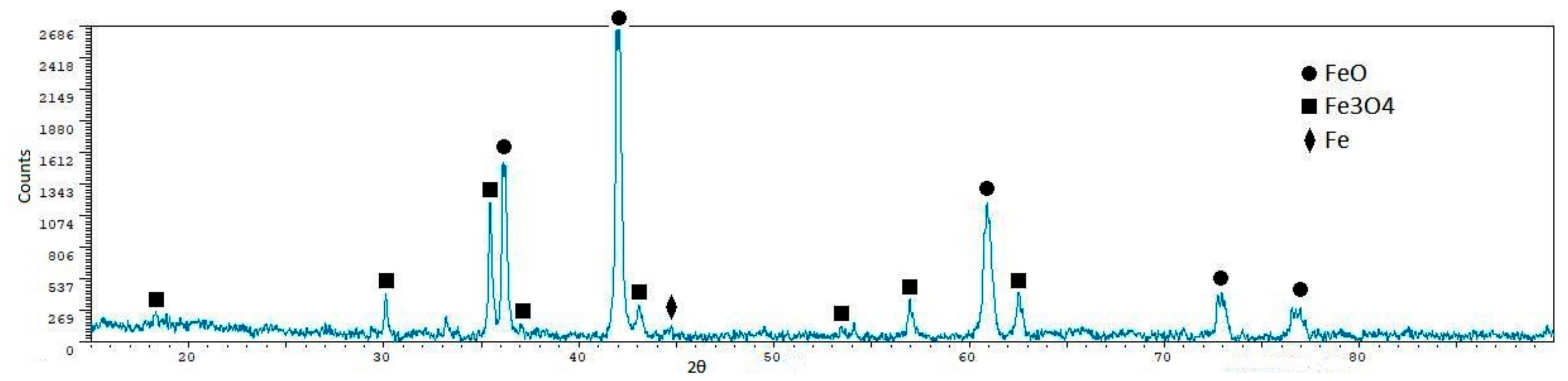
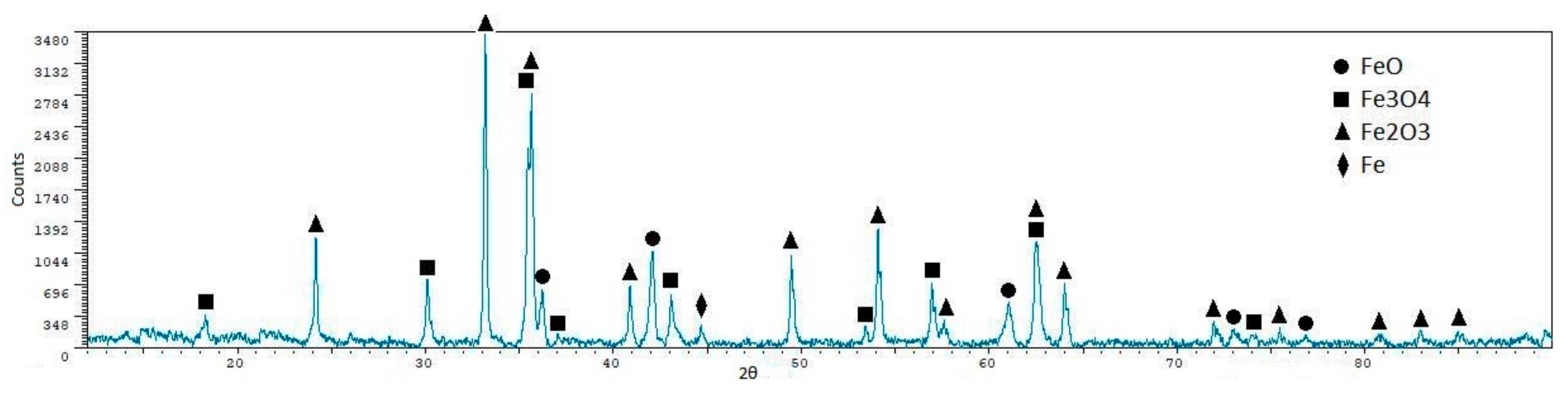
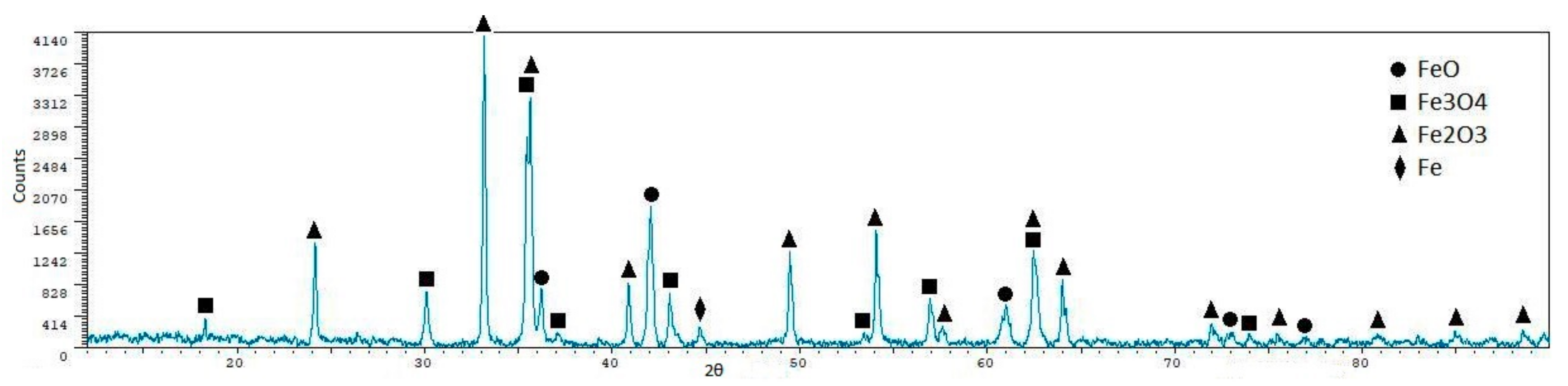
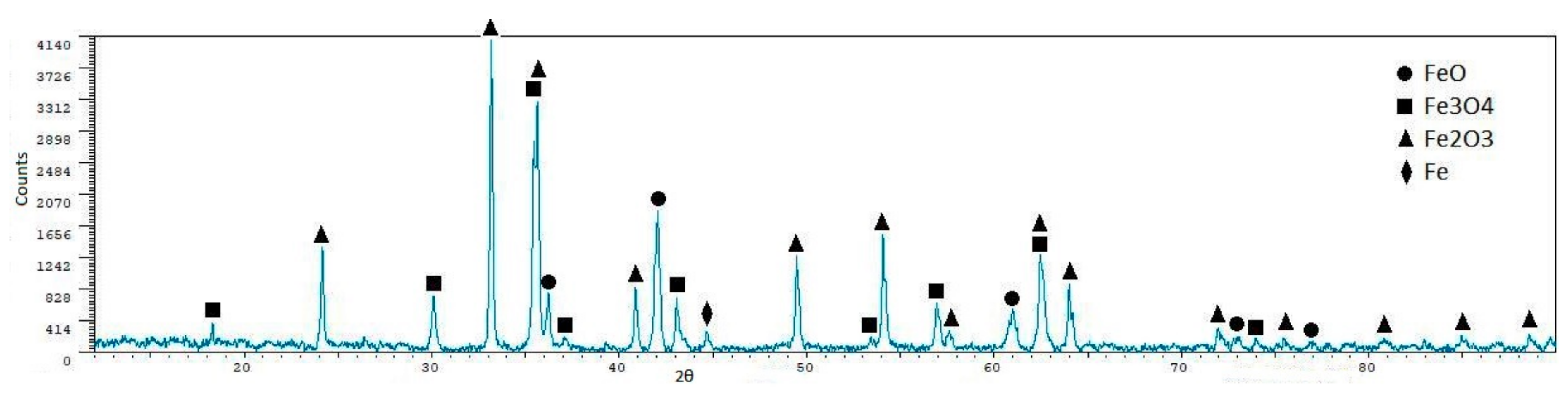

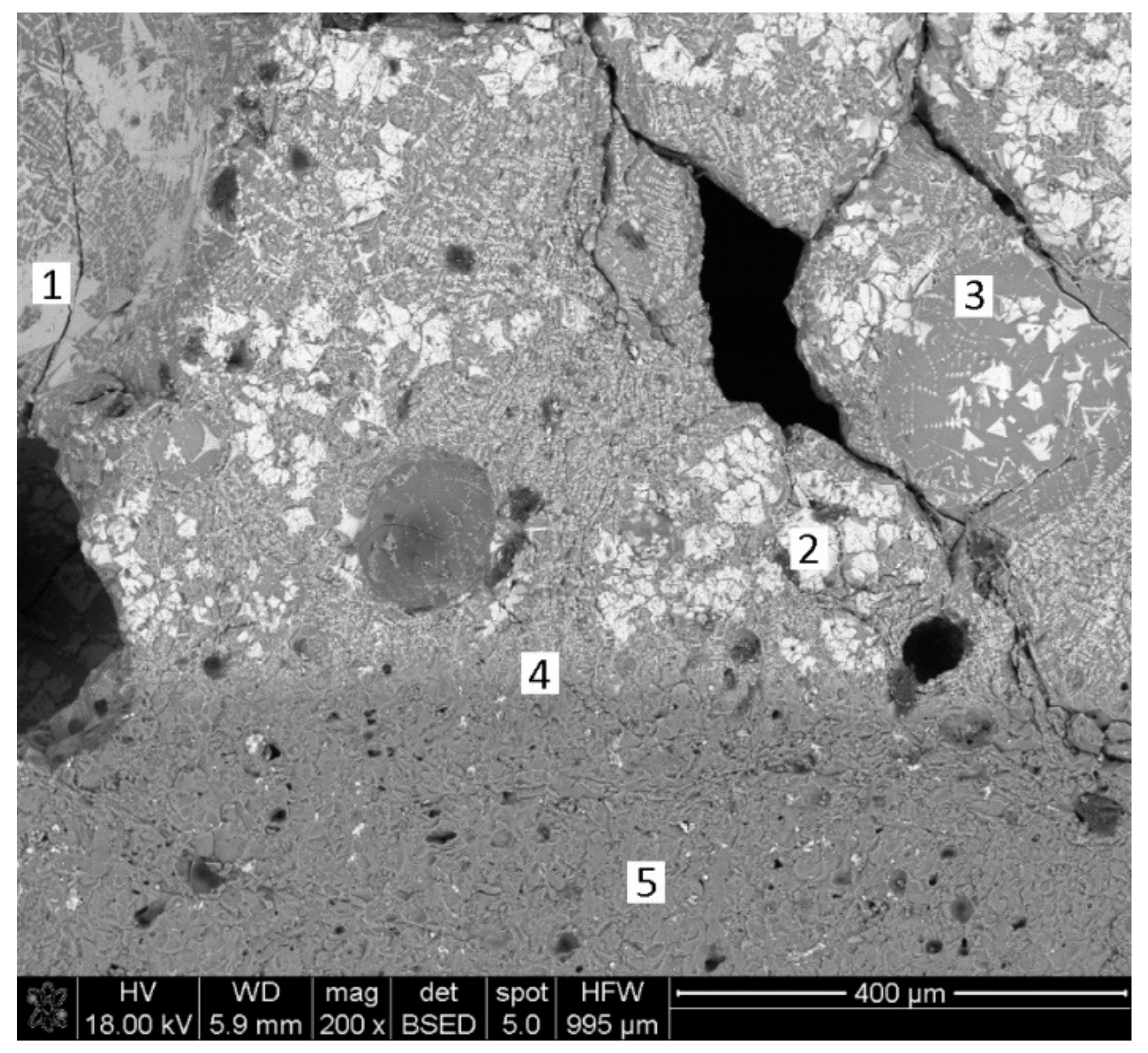


| Phase | Content (mass %) |
|---|---|
| Fe2O3 | 64.09 |
| CaO | 10.20 |
| C | 7.47 |
| SiO2 | 8.64 |
| Al2O3 | 3.57 |
| MgO | 2.87 |
| MnO | 1.99 |
| K2O | 0.53 |
| SO3 | 0.22 |
| Na2O | 0.13 |
| P2O5 | 0.11 |
| TiO2 | 0.10 |
| Cl | 472 ppm |
| Cr2O3 | 127 ppm |
| CuO | - |
| CaO/SiO2 | 1.18 |
| Phase | Content (mass %) |
|---|---|
| H2O | 17.19 |
| Fe Total | 1.18 |
| FeO | 0.00 |
| Fe2O3 | 1.70 |
| CaO | 0.71 |
| SiO2 | 6.58 |
| Al2O3 | 3.23 |
| MgO | 0.19 |
| Celemental | 83.84 |
| H2Ocrystallization (chemically bonded) | 1.92 |
| Balance | 1.83 |
| Sample | Time (min) | Initial Mass (g) | Final Mass (g) | Mass Loss (%) | Average Incident Radiation (W/m2) |
|---|---|---|---|---|---|
| Sint1 | Nondetermined | 22.7 | 16.9 | 25.6 | 564 |
| Sint2 | 12 | 21 | 16.6 | 21.0 | 848 |
| Sint3 | 15 | 22 | 16.8 | 23.6 | 917 |
| Sint4 | 12 | 23 | 17.9 | 22.2 | 881 |
| Sint5 | 20 | 22.7 | 18.1 | 20.3 | 887 |
| Sample | SO1 | T1 | P1 | SO2 | T2 | P2 | SO3 | T3 | P3 | SO4 | T4 | P4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sint1 | 60 | Nondetermined | 472 | - | - | - | - | - | - | - | - | - |
| Sint2 | 51 | 0–3 | 649 | 64 | 3–12 | 814 | - | - | - | - | - | - |
| Sint3 | 25 | 0–1 | 344 | 45 | 1–5 | 619 | 60 | 5–15 | 825 | - | - | - |
| Sint4 | 25 | 0–1 | 330 | 45 | 1–5 | 595 | 70 | 5–12 | 925 | - | - | - |
| Sint5 | 25 | 0–1 | 333 | 40 | 1–5 | 532 | 70 | 5–16.5 | 931 | 90 | 16.5–20 | 1197 |
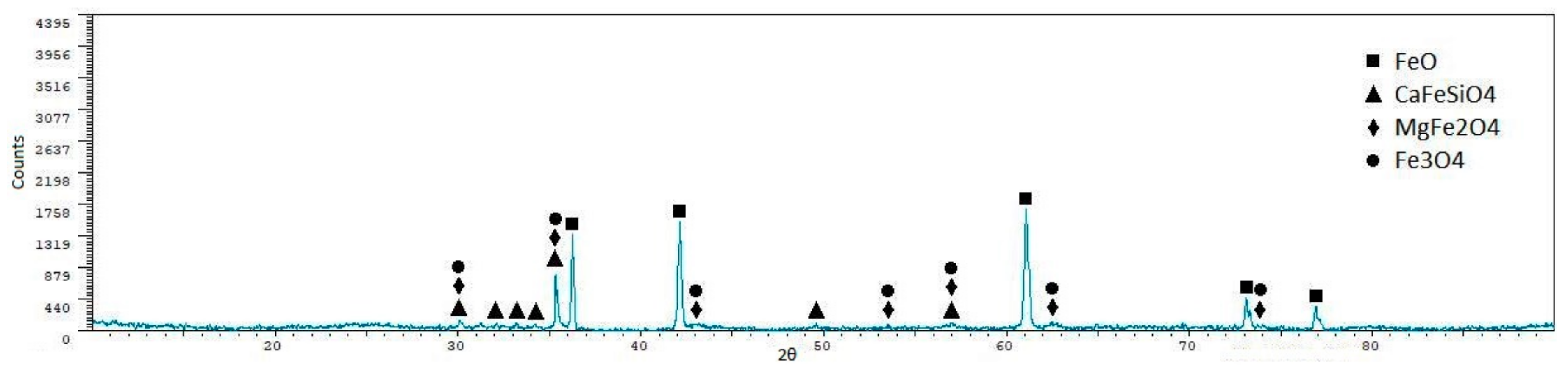
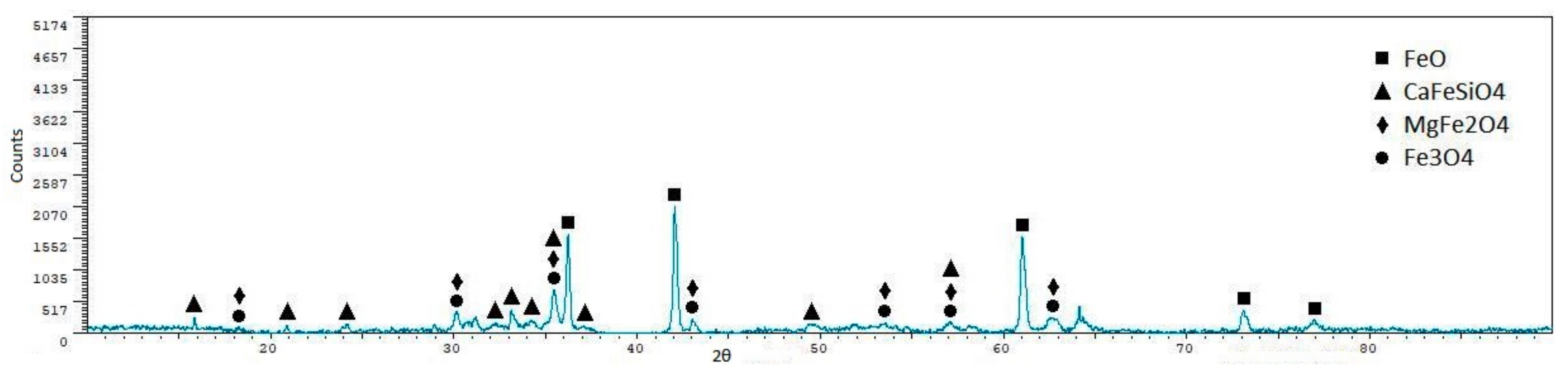
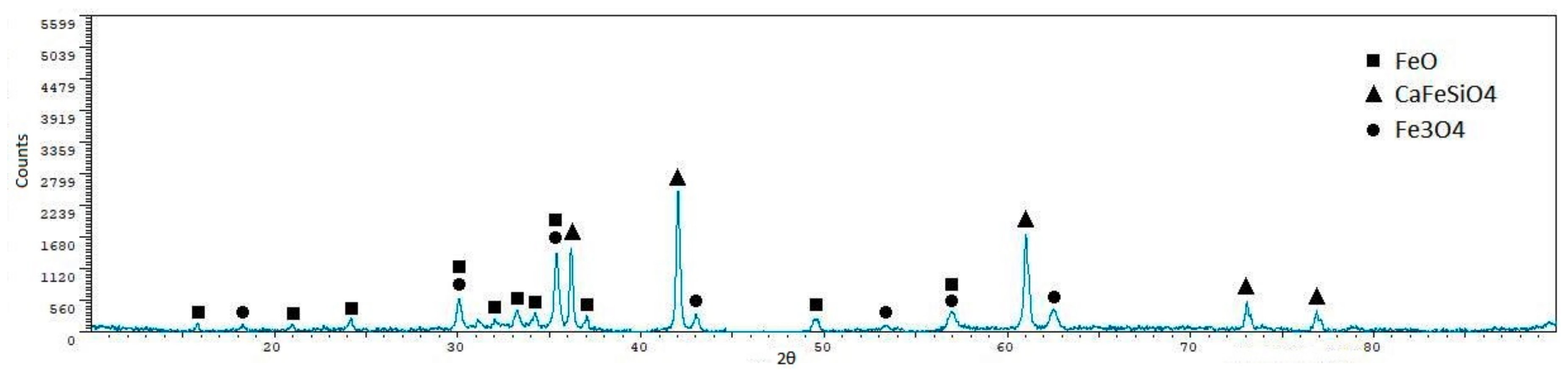
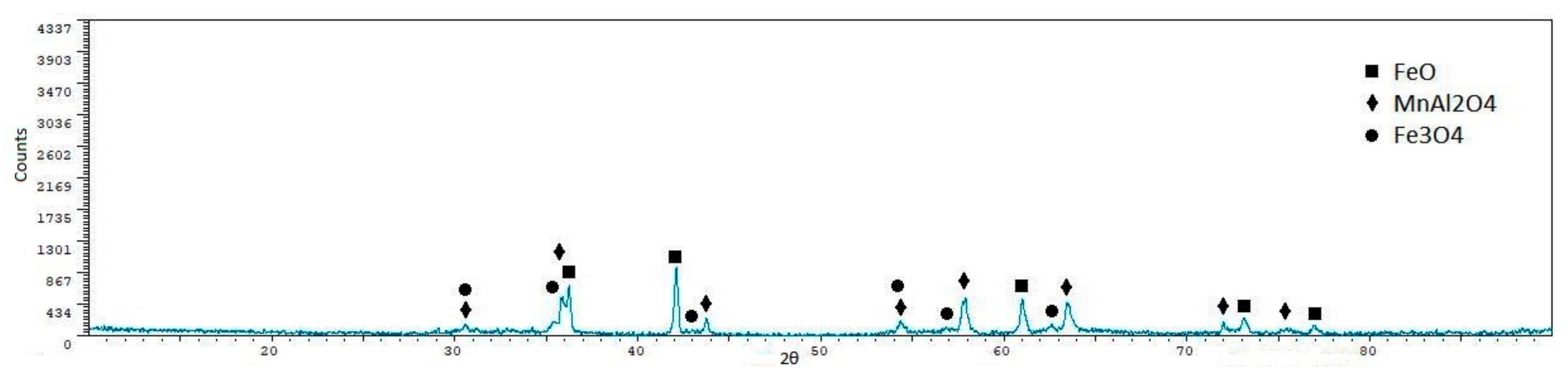
| Phase | Sint1 | Sint2 | Sint3 | Sint4 | Sint5 |
|---|---|---|---|---|---|
| FeO | 56.40 ± 0.7 | 48.90 ± 0.7 | 10.40 ± 1.9 | 57.30 ± 1.2 | 55.30 ± 1.1 |
| CaFeSiO4 | 1.60 ± 1.60 | 7.30 ± 2.3 | 42.60 ± 0.8 | 8.30 ± 3.7 | - |
| MgFe2O4 | 15.10 ± 1.6 | 21.60 ± 1.7 | - | 18.00 ± 3.2 | - |
| Fe3O4 | 22.00 ± 1.4 | 22.10 ± 1.7 | 47.00 ± 0.6 | 16.40 ± 3.3 | 15.10 ± 3.1 |
| MnAl2O4 | - | - | - | - | 26.60 ± 2.3 |
| Element | Point 1 | Point 2 |
|---|---|---|
| Carbon (wt. %) | 1.75 | 2.39 |
| Oxygen (wt. %) | 23.22 | 36.13 |
| Magnesium (wt. %) | 0.98 | 2.46 |
| Aluminum (wt. %) | 0.67 | 5.29 |
| Silicon (wt. %) | 0.78 | 14.55 |
| Phosphorus (wt. %) | - | 1.13 |
| Sulphur (wt. %) | 0.40 | - |
| Potassium (wt. %) | 0.34 | 1.64 |
| Calcium (wt. %) | 1.03 | 9.58 |
| Manganese (wt. %) | 1.59 | 2.59 |
| Iron (wt. %) | 69.24 | 24.25 |
| Sample | Carbon Excess (%) | Initial Mass (g) | Final Mass (g) | Mass Loss (%) |
|---|---|---|---|---|
| E20 P1 | 25 | 2.0273 | - | - |
| E20 P2 | 25 | 1.5173 | - | - |
| E20 P3 | 25 | 2.0484 | - | - |
| E20 P4 | 25 | 1.9949 | - | - |
| E21 P1 | 40 | 1.6758 | - | - |
| E21 P2 | 40 | 1.9317 | - | - |
| E21 P3 | 40 | 1.3882 | - | - |
| E25 P1 | 40 | 1.0984 | 0.8844 | 19.48 |
| E25 P2 | 40 | 1.8509 | 1.4771 | 20.19 |
| E31 P1 | 40 | 1.6830 | 1.4027 | 16.65 |
| E32 P1 | 10 | 0.5147 | - | - |
| E33 P1 | 10 | 1.6956 | 1.4558 | 14.14 |
| E33 P2 | 10 | 1.5092 | 1.2918 | 14.40 |
| E34 P2 | 25 | 1.4814 | 1.2083 | 18.43 |
| E35 P2 | 40 | 1.4801 | 1.2070 | 18.45 |
| Sample | Speed (mm/s) | Incident Radiation (W/m2) | Shutter Opening (%) | Power (W) | Tmax (°C) |
|---|---|---|---|---|---|
| E20 P1 | 0.76 | 927 | 60.1 | 839 | 1367 |
| E20 P2 | 0.60 | 941 | 51.4 | 726 | 1353 |
| E20 P3 | 0.50 | 955 | 51.1 | 732 | 1337 |
| E20 P4 | 0.30 | 968 | 50 | 726 | 1338 |
| E21 P1 | 0.25 | 979 | 50.6 | 743 | 1309 |
| E21 P2 | 0.25 | 995 | 50.6 | 755 | - |
| E21 P3 | 0.25 | 946 | 60 | 854 | - |
| E25 P1 | 0.25 | 1010 | 69.5 | 1053 | 1328 |
| E25 P2 | 0.25 | 1028 | 69.4 | 1073 | 1309 |
| E31 P1 | 0.25 | 957 | 70.1 | 1006 | 1266 |
| E32 P1 | 0.25 | 885 | 70 | 929 | 1084 |
| E33 P1 | 0.25 | 924 | 70.9 | 983 | 1191 |
| E33 P2 | 0.25 | 945 | 75 | 1063 | 1232 |
| E34 P2 | 0.25 | 961 | 80 | 1153 | 1306 |
| E35 P2 | 0.25 | 959 | 79.8 | 1148 | 1266 |
| Sample | Fe2O3 (%) | Fe3O4 (%) | FeO (%) | Fe (%) | Al6Si2O13 (%) |
|---|---|---|---|---|---|
| E20 P1 | - | 37.0 ± 1.4 | 59.5 ± 0.7 | 3.50 ± 2.4 | - |
| E20 P2 | 31.50 ± 0.6 | 47.60 ± 1.2 | 15.30 ± 1.8 | 5.60 ± 2.0 | - |
| E20 P3 | 29.40 ± 0.6 | 47.30 ± 1.0 | 18.60 ± 1.6 | 4.80 ± 1.8 | - |
| E20 P4 | 12.70 ± 1.0 | 63.70 ± 0.5 | 20.20 ± 1.2 | 3.30 ± 1.5 | - |
| E21 P1 | 28.60 ± 0.6 | 60.90 ± 0.9 | 10.50 ± 2.0 | - | - |
| E21 P2 | 18.40 ± 1.0 | 63.10 ± 0.6 | 18.60 ± 1.5 | - | - |
| E21 P3 | (no reaction) | - | - | ||
| E25 P1 | 9.6 ± 2.2 | 64.1 ± 0.7 | 9.7 ± 2.2 | - | 16.6 ± 1.9 |
| E25 P2 | 2.10 ± 0.4 | 93.40 ± 0.2 | - | - | 4.50 ± 0.7 |
| E31 P1 | 5.2 ± 1.0 | 79.8 ± 0.3 | 14.9 ± 0.9 | - | - |
| E32 P1 | 63.6 ± 0.3 | 36.4 ± 0.6 | - | - | - |
| E33 P1 | 12.20 ± 1.1 | 81.20 ± 0.4 | 6.60 ± 1.3 | - | - |
| E33 P2 | 5.90 ± 2.7 | 89.10 ± 0.8 | - | - | 5.90 ± 2.8 |
| E34 P2 | - | 93.80 ± 0.5 | - | - | 6.20 ± 1.6 |
| E35 P2 | 13.20 ± 1.5 | 12.10 ± 1.9 | 6.90 ± 2.0 | - | 67.80 ± 0.6 |
| Element | Point 1 | Point 2 | Point 3 | Point 4 | Point 5 |
|---|---|---|---|---|---|
| Oxygen (wt. %) | 8.90 | 17.40 | 19.03 | 28.67 | 34.28 |
| Aluminum (wt. %) | 3.44 | 12.43 | 13.42 | 18.11 | 23.87 |
| Silicon (wt. %) | 5.53 | 0.63 | 39.40 | 35.73 | 37.30 |
| Potassium (wt. %) | 0.54 | 0.42 | 3.58 | 2.55 | 3.26 |
| Iron (wt. %) | 81.59 | 69.13 | 24.58 | 14.94 | 1.29 |
| Phase | Content |
|---|---|
| Fe2O3 | 69.10% |
| CaO | 10.84% |
| SiO2 | 12.50% |
| Al2O3 | 2.87% |
| MgO | 2.60% |
| MnO | 0.47% |
| K2O | 0.96% |
| Na2O | 0.50% |
| P2O5 | 0.04% |
| TiO2 | 0.07% |
| CaO/SiO2 | 0.87 |
| Sample | CL1 | SL1 | CL2 | SL2 | CL3 | SL3 | CL4 | SL4 | CL5 | SL5 | Initial Mass (g) | Final Mass (g) | Mass Loss (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BF1 | 1.6 | 3.3 | 1.7 | 3 | 1.7 | 3.2 | 3.2 | 3.7 | 4.9 | 15.8 | 42.1 | 33.8 | 19.7 |
| BF2 | 2.5 | 3.2 | 2.8 | 2.6 | 3.2 | 2.9 | - | - | - | - | 17.2 | 14.8 | 14.0 |
| BF3 | 2.5 | 3.2 | 2.9 | 2.7 | 3.4 | 3.2 | - | - | - | - | 17.9 | 15.1 | 15.6 |
| BF4 | 2.7 | 3.2 | 3 | 3 | 3.3 | 3.6 | - | - | - | - | 18.8 | 16.2 | 13.8 |
| BF5 | 3.3 | 3.6 | 2.9 | 3.2 | 3.1 | 4 | - | - | - | - | 20.1 | 17.6 | 12.4 |
| Sample | Time (min) | Incident Radiation (W/m2) | Shutter Opening |
|---|---|---|---|
| BF1 | ≃15 | variable (average 933) | 50–82 |
| BF2 | 15 | 976 | 51 |
| BF3 | 15 | 902 | 50 |
| BF4 | (less than 5 min at >876 W/m2) | 876 | 50 |
| BF5 | 20 | 883 | 20–60 |
| Phase | BF1 | BF2 | BF3 | BF5 |
|---|---|---|---|---|
| FeO | 74.60 ± 0.8 | 53.60 ± 0.8 | 33.50 ± 1.0 | 46.40 ± 0.9 |
| γ-Fe2O3 | 6.30 ± 2.7 | - | 15.00 ± 2.0 | 9.20 ± 2.7 |
| Mg(Fe3+)2O4 | 9.40 ± 2.6 | - | 19.80 ± 1.8 | 8.80 ± 2.8 |
| Fe3O4 | 9.80 ± 2.6 | 13.80 ± 2.2 | 17.60 ± 1.8 | 18.60 ± 2.3 |
| CaFe2+SiO4 | - | 2.90 ± 2.6 | 6.90 ± 2.8 | 4.10 ± 3.1 |
| SiO2 | - | - | - | 6.10 ± 2.0 |
| Fe | - | 29.70 ± 1.7 | 4.50 ± 3.0 | 6.90 ± 3.0 |
| Element | Point 1 | Point 2 | Point 3 |
|---|---|---|---|
| Iron (wt. %) | 84.79 | 36.05 | 81.90 |
| Oxygen (wt. %) | 6.67 | 25.49 | 4.04 |
| Carbon (wt. %) | 2.03 | 1.05 | - |
| Magnesium (wt. %) | 0.19 | 4.38 | 0.33 |
| Aluminum (wt. %) | 1.01 | 26.72 | 2.46 |
| Silicon (wt. %) | 0.29 | 0.88 | 0.68 |
| Manganese (wt. %) | 2.37 | 1.89 | 4.55 |
| Calcium (wt. %) | 1.08 | 1.24 | 3.59 |
| Potassium (wt. %) | 0.40 | 0.55 | 0.33 |
| Phosphorus (wt. %) | 0.06 | 0.28 | - |
| Titanium (wt. %) | 0.71 | 0.80 | 1.10 |
| Vanadium (wt. %) | 0.40 | 0.66 | 1.02 |
| TOTAL | 100 | 100 | 100 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-González, D.; Prazuch, J.; Ruiz-Bustinza, Í.; González-Gasca, C.; Piñuela-Noval, J.; Verdeja González, L.F. Iron Metallurgy via Concentrated Solar Energy. Metals 2018, 8, 873. https://doi.org/10.3390/met8110873
Fernández-González D, Prazuch J, Ruiz-Bustinza Í, González-Gasca C, Piñuela-Noval J, Verdeja González LF. Iron Metallurgy via Concentrated Solar Energy. Metals. 2018; 8(11):873. https://doi.org/10.3390/met8110873
Chicago/Turabian StyleFernández-González, Daniel, Janusz Prazuch, Íñigo Ruiz-Bustinza, Carmen González-Gasca, Juan Piñuela-Noval, and Luis Felipe Verdeja González. 2018. "Iron Metallurgy via Concentrated Solar Energy" Metals 8, no. 11: 873. https://doi.org/10.3390/met8110873
APA StyleFernández-González, D., Prazuch, J., Ruiz-Bustinza, Í., González-Gasca, C., Piñuela-Noval, J., & Verdeja González, L. F. (2018). Iron Metallurgy via Concentrated Solar Energy. Metals, 8(11), 873. https://doi.org/10.3390/met8110873







