Recycling the GaN Waste from LED Industry by Pressurized Leaching Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Reagents and Instruments
2.2. Leaching
3. Results
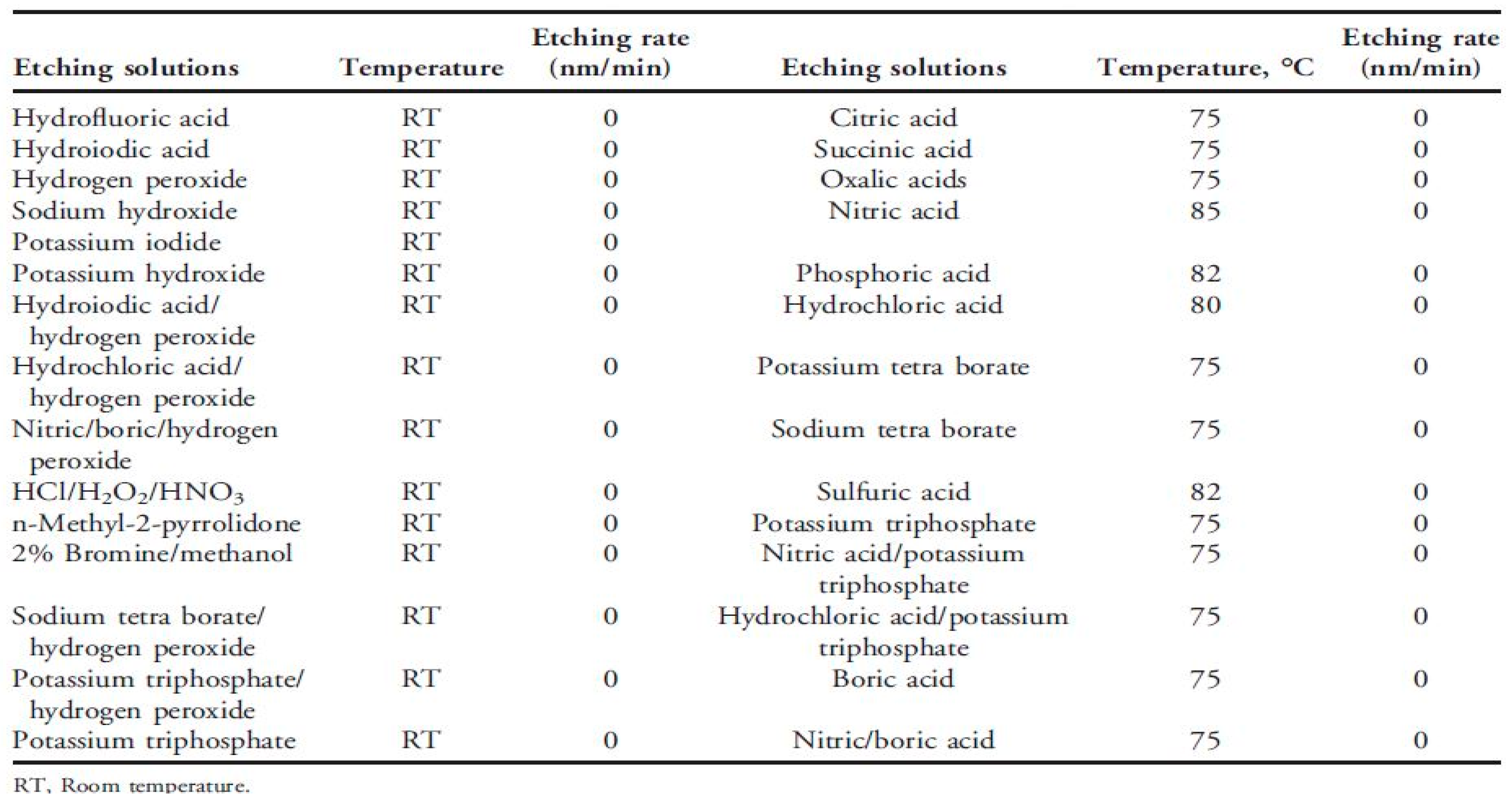
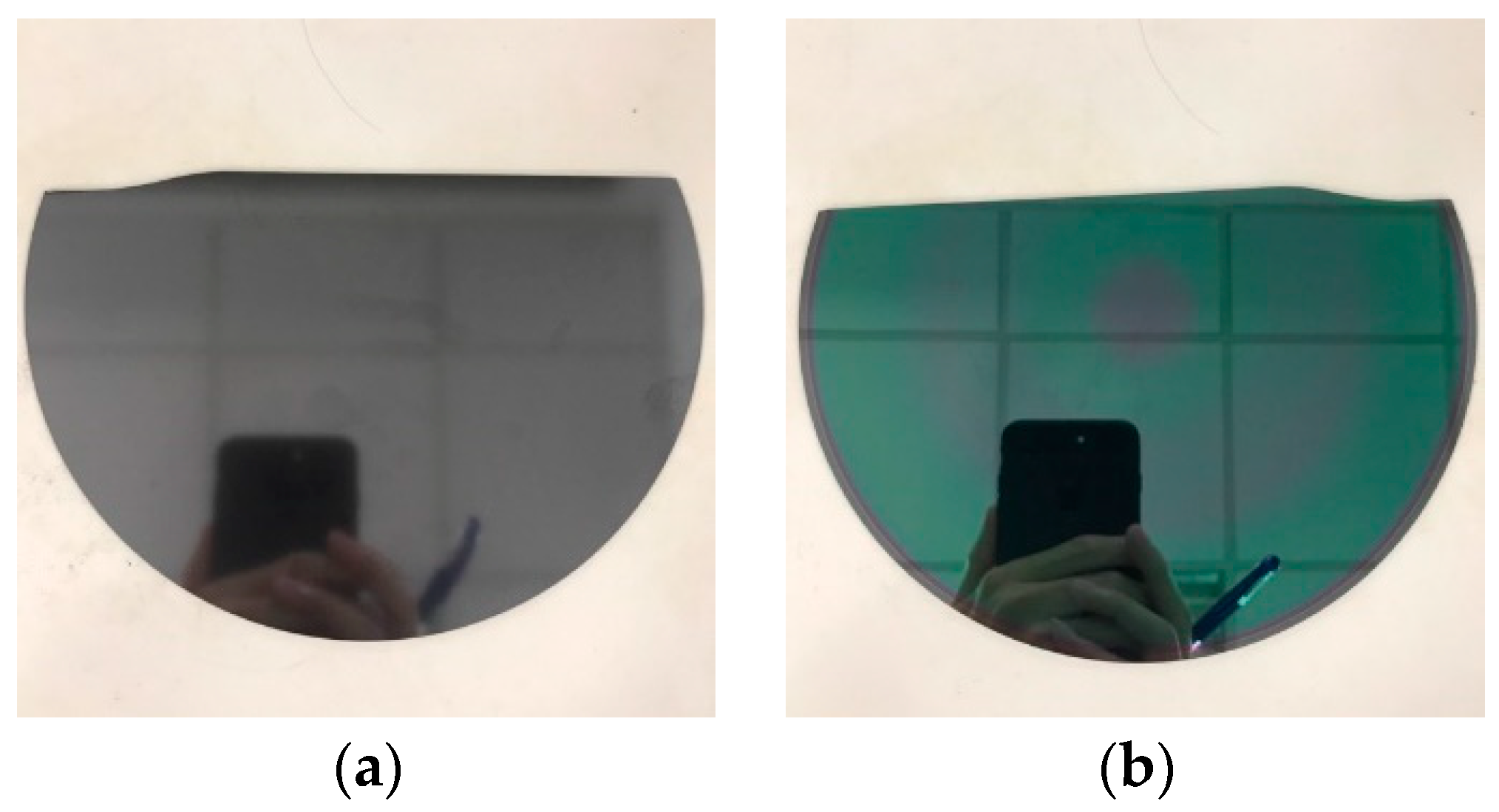
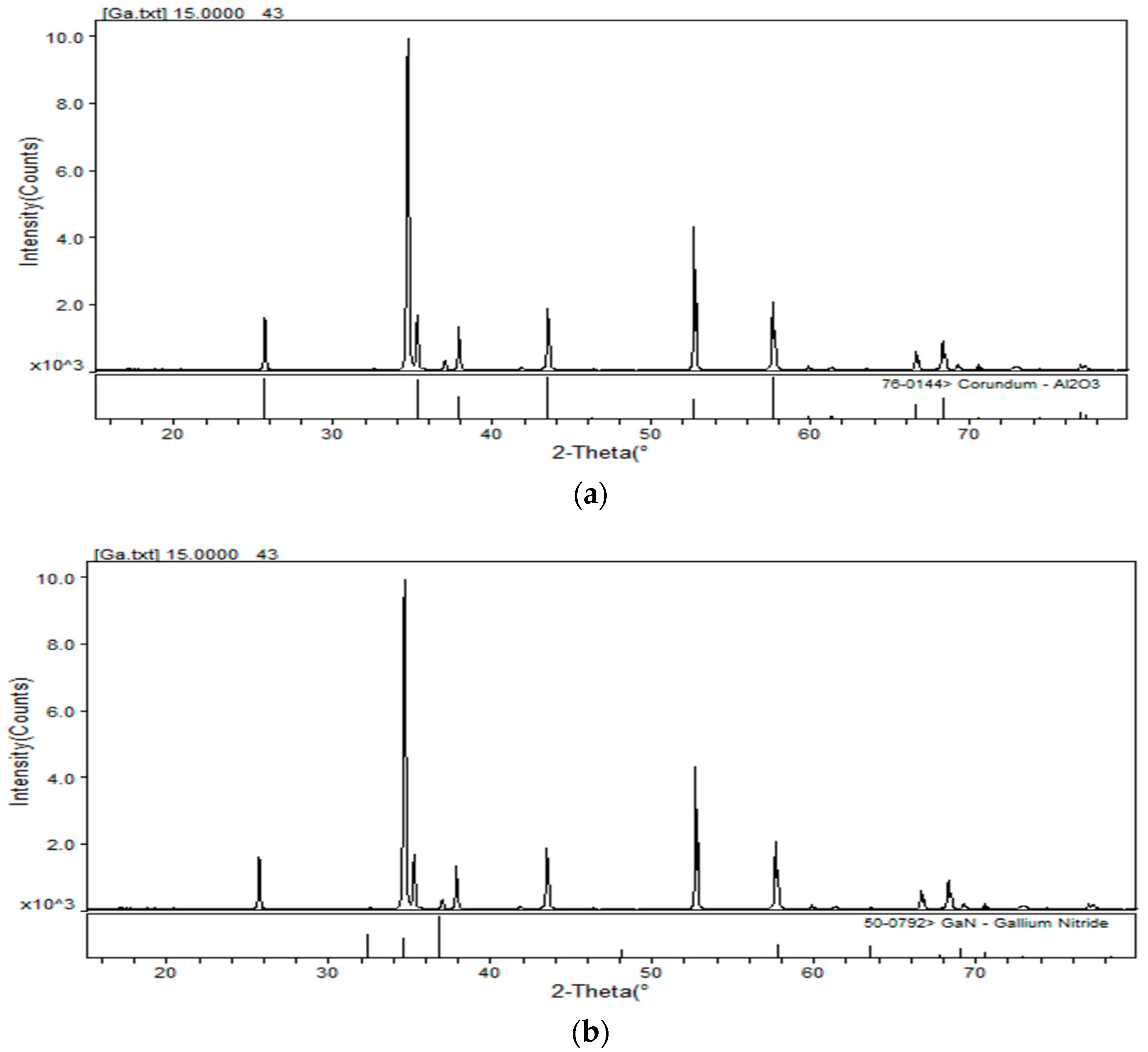
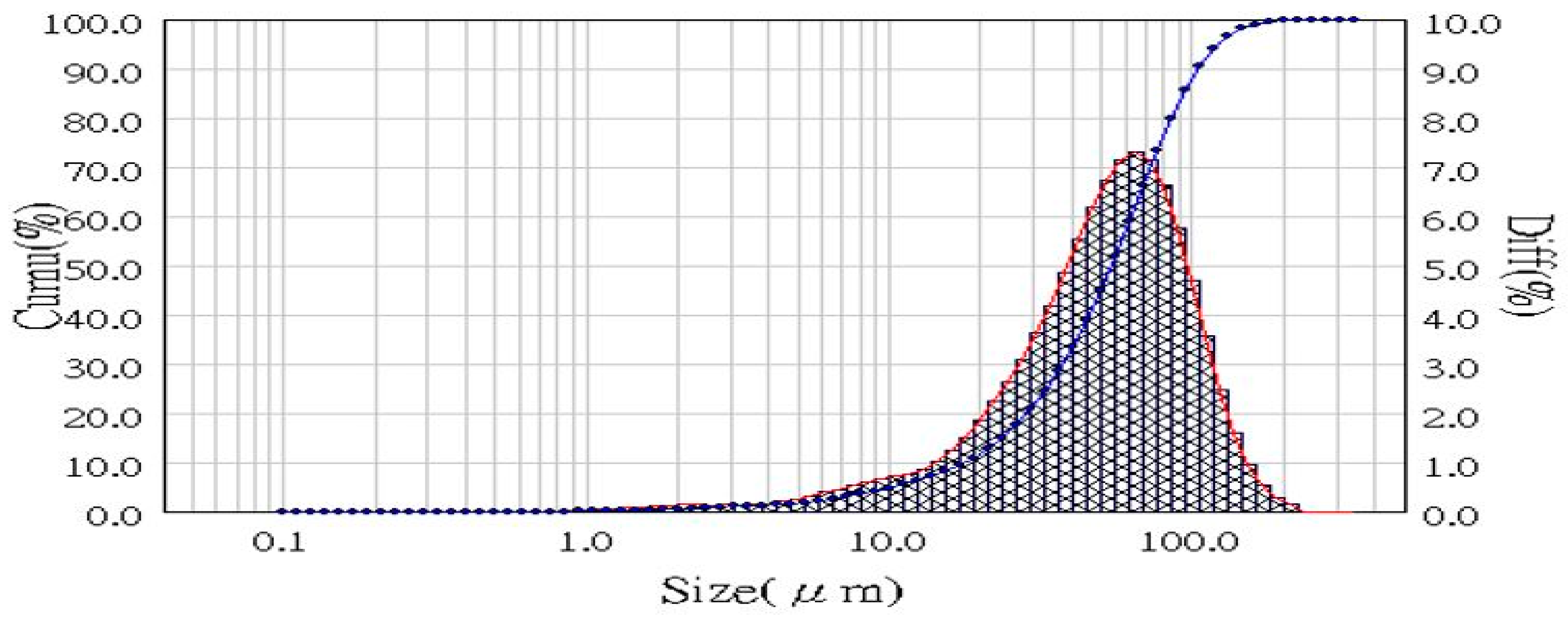
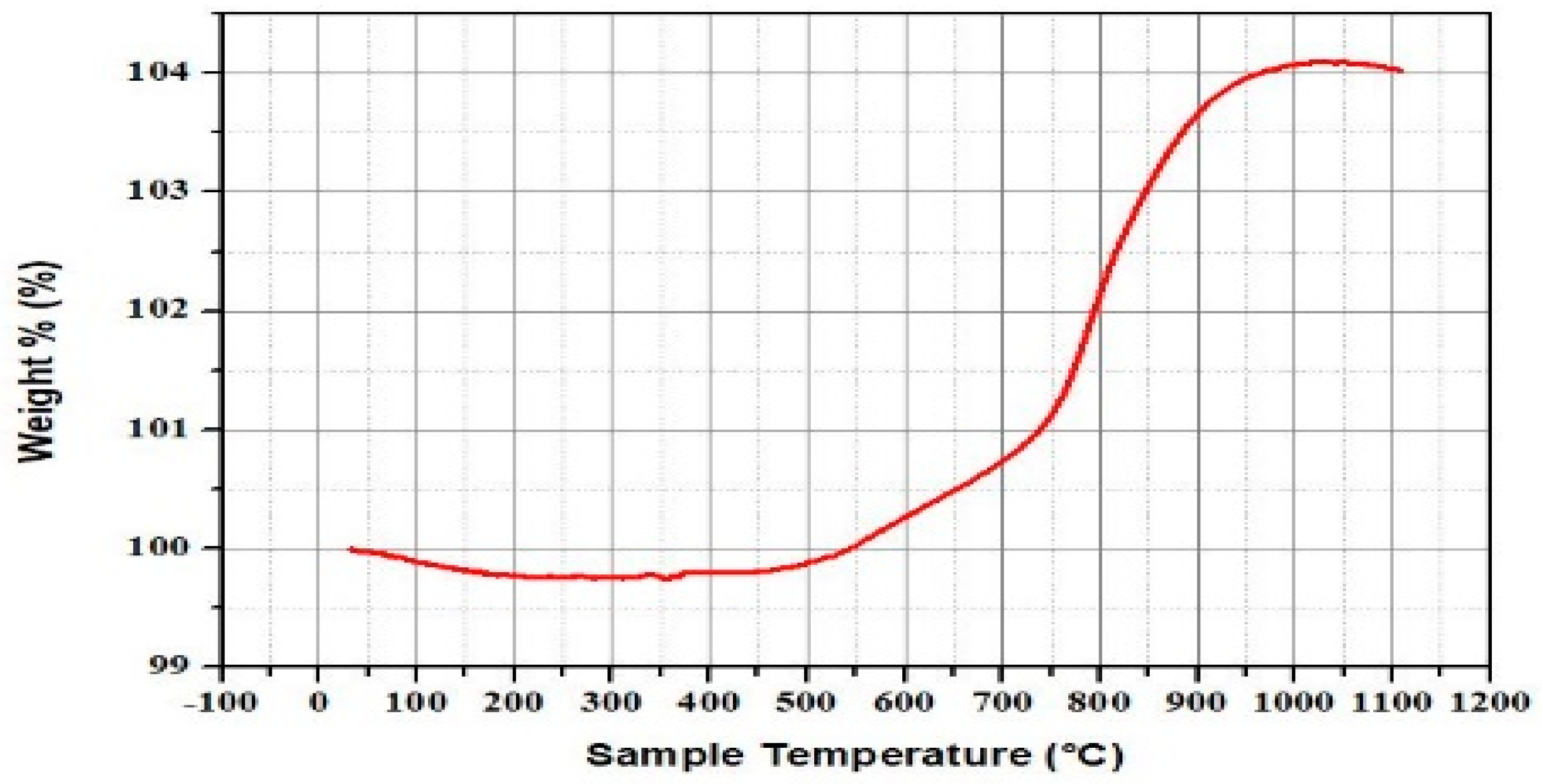
3.1. Waste Characteristic Analysis
3.2. Leaching under Atmospheric-Pressure
3.3. Alkaline-Roasting Leaching
3.4. Pressurized Leaching
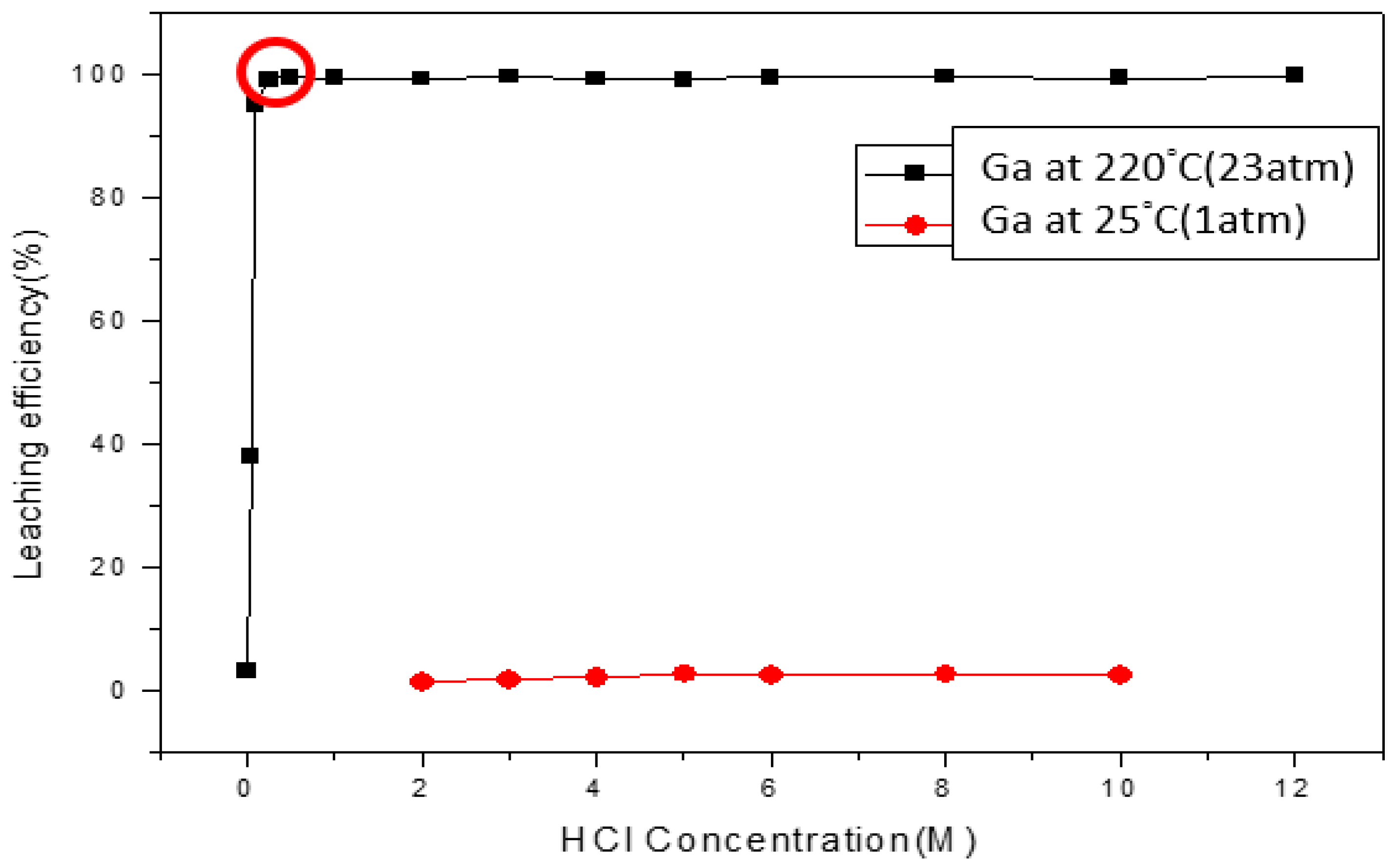
3.4.1. Effect of Concentration and Pressure
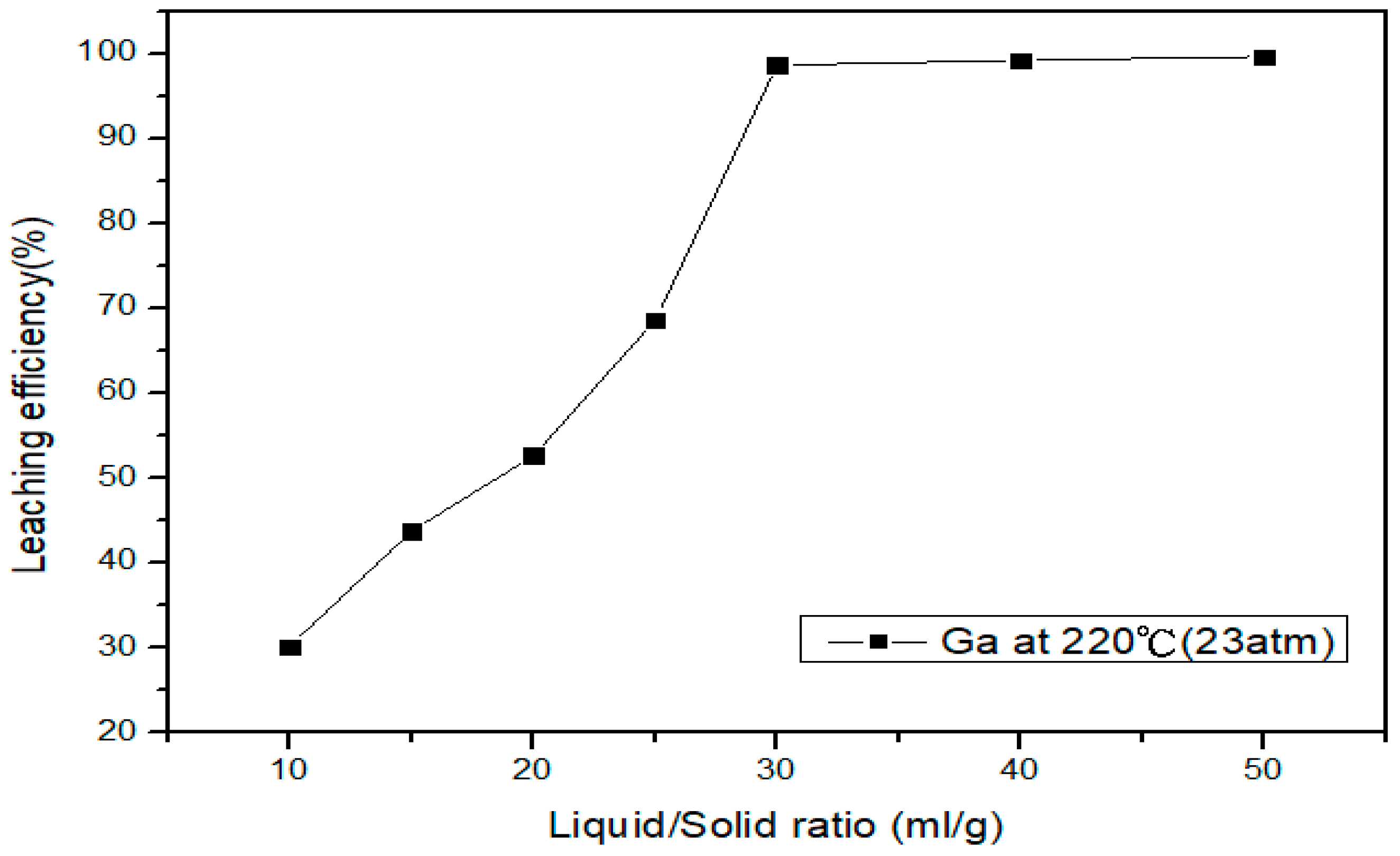
3.4.2. Effect of Liquid-Solid Ratio
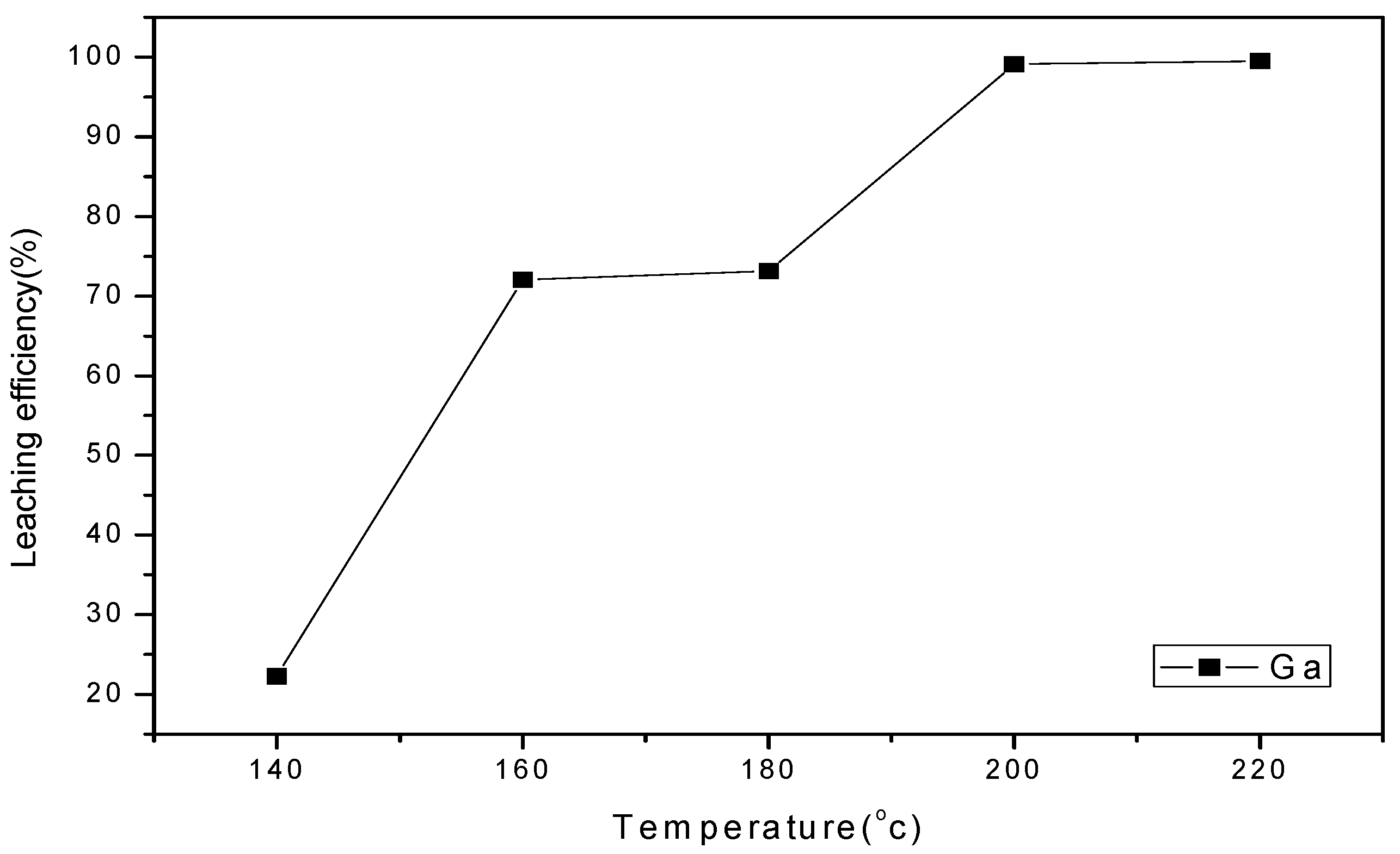
3.4.3. Effect of Temperature
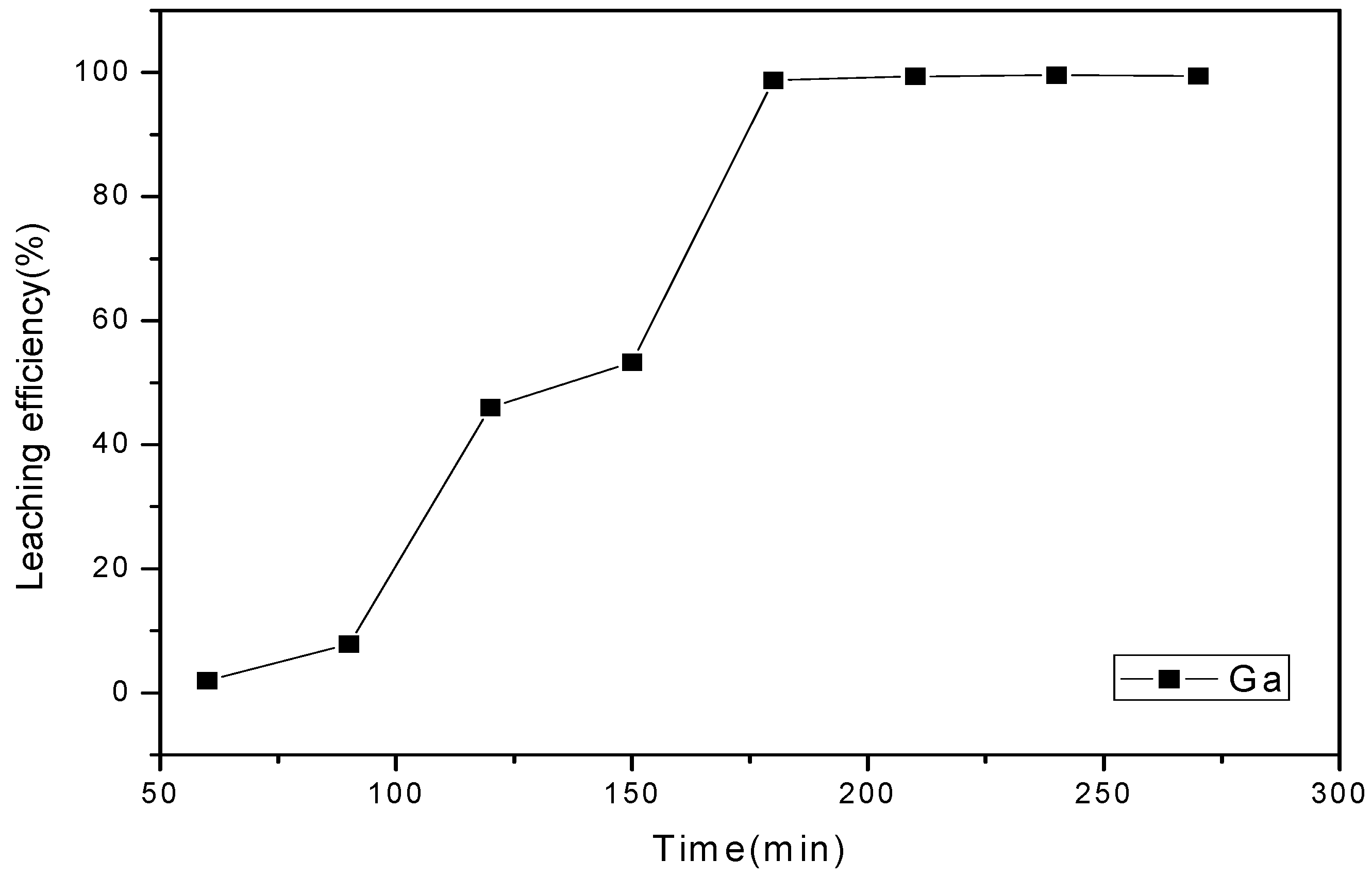
3.4.4. Effect of Reaction Time
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kinoshita, T.; Ishigaki, Y.; Shibata, N.; Yamaguchi, K.; Akita, S.; Kitagawa, S.; Kondou, H.; Nii, S. Selective recovery of gallium with continuous counter-current foam separation and its application to leaching solution of zinc refinery residues. Separ. Purif. Technol. 2011, 78, 181–188. [Google Scholar] [CrossRef]
- Graedel, T.; Allwood, J.; Birat, J.; Buchert, M.; Hagelüken, C.; Reck, B.K.; Sibley, S.F.; Sonnemann, G. What Do We Know About Metal Recycling Rates? J. Ind. Ecol. 2011, 15, 355–366. [Google Scholar] [CrossRef]
- Elshkaki, A.; Graedel, T.; Ciacci, L.; Reck, B.K. Resource Demand Scenarios for the Major Metals. Environ. Sci. Technol. 2018, 52, 2491–2497. [Google Scholar] [CrossRef] [PubMed]
- Facts about Gallium. Live Science. Available online: https://www.livescience.com/29476-gallium.html (accessed on 8 April 2017).
- Pubs.usgs.gov. 2012. Available online: https://pubs.usgs.gov/pp/1802/h/pp1802h.pdf (accessed on 12 September 2018).
- Minerals.usgs.gov. Available online: https://minerals.usgs.gov/minerals/pubs/mcs/2017/mcs2017.pdf (accessed on 12 September 2018).
- Alonso, E.; Sherman, A.; Wallington, T.J.; Everson, M.P.; Field, F.R.; Roth, R.; Kirchain, R.E. Correction to Evaluating Rare Earth Element Availability: A Case with Revolutionary Demand from Clean Technologies. Environ. Sci. Technol. 2012, 46, 4684. [Google Scholar] [CrossRef]
- Pearton, S.; Kuo, C. GaN and Related Materials for Device Applications. MRS Bull. 1997, 22, 17–21. [Google Scholar] [CrossRef]
- Morkoc, H. Nitride Semiconductors and Devices; Springer: Berlin, Germany, 1999. [Google Scholar]
- Minerals.usgs.gov. 2013. Available online: https://minerals.usgs.gov/minerals/pubs/country/2013/myb3-2013-hu.xls (accessed on 12 May 2018).
- Minerals.usgs.gov. 2017. Available online: https://minerals.usgs.gov/minerals/pubs/commodity/gallium/mcs-2017-galli.pdf (accessed on 14 April 2018).
- Marketsandmarkets.com. Gallium Nitride Semiconductor Device Market by Device Type & Application—Global Forecast to 2023 | MarketsandMarkets. Available online: https://www.marketsandmarkets.com/Market-Reports/gallium-nitride-gan-semiconductor-materials-devices-market-698.html (accessed on 8 May 2017).
- Marketsandmarkets.com. Gallium Nitride Semiconductor Device Market worth 22.47 Billion USD by 2023. Available online: https://www.marketsandmarkets.com/PressReleases/gallium-nitride-semiconductor.asp (accessed on 13 September 2017).
- Semiconductortoday.com. The Lighthouse Worldwide Solutions Blog. Available online: http://www.semiconductortoday.com/news_items/2012/JULY/MANDM_110712.html (accessed on 8 June 2017).
- Chiou, Y.; Chen, C.; Chang, S.J.; Chen, C.H. GaN metal–semiconductor interface and its applications in GaN and InGaN metal–semiconductor–metal photodetectors. IEE Proc. Optoelectron. 2003, 150, 115–118. [Google Scholar] [CrossRef]
- Zhuang, D.; Edgar, J. Wet etching of GaN, AlN, and SiC: A review. Mater. Sci. Eng. R Rep. 2005, 48, 1–46. [Google Scholar] [CrossRef]
- Moskalyk, R. Gallium: The backbone of the electronics industry. Miner. Eng. 2003, 16, 921–929. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhao, Y.; Yang, X.; Li, W.; Wei, Z.; Xiao, J.; Leung, S.F.; Lin, Q.; Wu, H.; Zhang, Y. Three-dimensional metal/oxide nanocone arrays for high-performance electrochemical pseudocapacitors. Nanoscale 2014, 6, 3626–3631. [Google Scholar] [CrossRef] [PubMed]
- Mihaylov, I.; Distin, P. Solvent Extraction of Gallium with D2EHPA from Acidic Sulphate Solutions—Equilibria and Complexation. Can. Metall. Q. 1993, 32, 21–30. [Google Scholar] [CrossRef]
- Fang, Z.; Gesser, H. Recovery of gallium from coal fly ash. Hydrometallurgy 1996, 41, 187–200. [Google Scholar] [CrossRef]
- Xu, K.; Deng, T.; Liu, J.; Peng, W. Study on the recovery of gallium from phosphorus flue dust by leaching with spent sulfuric acid solution and precipitation. Hydrometallurgy 2007, 86, 172–177. [Google Scholar] [CrossRef]
- Lee, H.; Nam, C. A study on the extraction of gallium from gallium arsenide scrap. Hydrometallurgy 1998, 49, 125–133. [Google Scholar] [CrossRef]
- Wu, X.; Wu, S.; Qin, W.; Ma, X.; Niu, Y.; Lai, S.; Yang, C.; Jiao, F.; Ren, L. Reductive leaching of gallium from zinc residue. Hydrometallurgy 2012, 113–114, 195–199. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, L. Recovery Method of Copper Indium Gallium Selenide Thin-Film Solar Panel. Patent CN103184338A, 29 December 2011. [Google Scholar]
- Li, Y.; Liu, Z. Properties of Gallium Phosphide Thick Films Prepared on Zinc Sulfide Substrates by Radio-Frequency Magnetron Sputtering. J. Mater. Sci. Technol. 2010, 26, 93–96. [Google Scholar] [CrossRef]
- Tanaka, A.; Hirata, M.; Koga, K.; Nakano, M.; Omae, K.; Kiyohara, Y. Pulmonary Toxicity of Indium Tin Oxide and Copper Indium Gallium Diselenide. MRS Proc. 2012, 1469. [Google Scholar] [CrossRef]
- Koo, S.; Ju, C. Preparation of indium oxide from waste indium tin oxide targets by oxalic acid. Korean J. Chem. Eng. 2017, 35, 251–256. [Google Scholar] [CrossRef]
- Swain, B.; Mishra, C.; Kang, L.; Park, K.-S.; Lee, C.G.; Hong, H.S.; Park, J.J. Recycling of metal-organic chemical vapor deposition waste of GaN based power device and LED industry by acidic leaching: Process optimization and kinetics study. J. Power Sources 2015, 281, 265–271. [Google Scholar] [CrossRef]
- Swain, B.; Mishra, C.; Kang, L.; Park, K.-S.; Lee, C.G.; Hong, H.S. Recycling process for recovery of gallium from GaN an e-waste of LED industry through ball milling, annealing and leaching. Environ. Res. 2015, 138, 401–408. [Google Scholar] [CrossRef] [PubMed]

| Element | Al2O3 | Fe2O3 | Ga | SiO2 | CrO3 | N |
|---|---|---|---|---|---|---|
| Content (%) | 89.9 | 3.39 | 3.38 | 1.91 | 0.96 | 0.45 |
| Temperature | HNO3 | H2SO4 | HCl | HF |
|---|---|---|---|---|
| 25 °C | 0.17 | 1.04 | 3.16 | 1.13 |
| 90 °C | 1.36 | 3.28 | 8.9 | 2.73 |
| Acid Agent/Alkali Agent | LiBO2 | NaOH | Na2CO3 |
|---|---|---|---|
| H2O | 17.3 | 24.5 | 21.6 |
| HNO3 | 36.6 | 45.2 | 41.7 |
| H2SO4 | 37.8 | 47.5 | 43.7 |
| HCl | 52.9 | 73.3 | 62.4 |
| HF | 34.4 | 52.5 | 43.1 |
| Material | [HCl] | Liquid-Solid Ratio | Temperature | Leaching Time |
|---|---|---|---|---|
| GaN waste | 0.25 M | 30 mL/g | 200 °C (15 atm) | 180 min |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.-S.; Hsu, L.-L.; Wang, L.-P. Recycling the GaN Waste from LED Industry by Pressurized Leaching Method. Metals 2018, 8, 861. https://doi.org/10.3390/met8100861
Chen W-S, Hsu L-L, Wang L-P. Recycling the GaN Waste from LED Industry by Pressurized Leaching Method. Metals. 2018; 8(10):861. https://doi.org/10.3390/met8100861
Chicago/Turabian StyleChen, Wei-Sheng, Li-Lin Hsu, and Li-Pang Wang. 2018. "Recycling the GaN Waste from LED Industry by Pressurized Leaching Method" Metals 8, no. 10: 861. https://doi.org/10.3390/met8100861
APA StyleChen, W.-S., Hsu, L.-L., & Wang, L.-P. (2018). Recycling the GaN Waste from LED Industry by Pressurized Leaching Method. Metals, 8(10), 861. https://doi.org/10.3390/met8100861








