Effects of Ag, Nd, and Yb on the Microstructures and Mechanical Properties of Mg‒Zn‒Ca Metallic Glasses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Composition Design of the Experimental Alloys
2.2. Material Preparation
2.3. Microstructure and Mechanical Properties
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zong, X.M.; Zhang, J.S.; Liu, W.; Zhang, Y.T.; You, Z.Y.; Xu, C.X. Corrosion behaviors of long-period stacking ordered structure in Mg alloys used in biomaterials: A review. Adv. Eng. Mater. 2018, 20, 1800017. [Google Scholar] [CrossRef]
- Xiong, H.Q.; Liang, Z.F.; Wang, Z.F.; Qin, C.L.; Zhao, W.M.; Yu, H. Mechanical properties and degradation behavior of Mg(100-7x)Zn6xYx(x = 0.2, 0.4, 0.6, 0.8) alloys. Metals 2018, 8, 261. [Google Scholar] [CrossRef]
- He, M.F.; Wang, H.; Zhou, K.G.; Pan, D.; Liu, F. Effects of Li addition on the corrosion behaviour and biocompatibility of Mg(Li)–Zn–Ca metallic glasses. J. Mater. Sci. 2018, 53, 9928–9942. [Google Scholar]
- Baulin, O.; Fabrègue, D.; Kato, H.; Liens, A.; Wada, T.; Pelletier, J.M. A new, toxic element-free Mg-based metallic glass for biomedical applications. J. Non-Cryst. Solids 2018, 481, 397–402. [Google Scholar] [CrossRef]
- Jia, H.L.; Wang, G.Y.; Chen, S.Y.; Gao, Y.F.; Li, W.D.; Liaw, P.K. Fatigue and fracture behavior of bulk metallic glasses and their composites. Prog. Mater. Sci. 2018, 98, 168–248. [Google Scholar] [CrossRef]
- Liu, J.; Fu, Y.; Tang, Y.; Wang, X.D.; Cao, Q.P.; Zhang, D.X.; Jiang, J.Z. Thickness dependent structural evolution in Mg-Zn-Ca thin film metallic glasses. J. Alloy. Compd. 2018, 742, 524–535. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Zberg, B.; Uggowitzer, P.J.; Löffler, J.F. MgZnCa glasses without clinically observable hydrogen evolution for biodegradable implants. Nat. Mater. 2009, 8, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Ma, E.; Xu, J. The glass window of opportunities. Nat. Mater. 2009, 8, 855–857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Feng, W.; Wang, X.Y.; Yang, S. Fabrication of Mg65Zn30Ca5 amorphous coating by laser remelting. J. Non-Cryst. Solids 2018, 500, 205–209. [Google Scholar] [CrossRef]
- Ramya, M.; Sarwat, S.G.; Udhayabanu, V.; Subramanian, S.; Raj, B.; Ravi, K.R. Role of partially amorphous structure and alloying elements on the corrosion behavior of Mg–Zn–Ca bulk metallic glass for biomedical applications. Mater. Des. 2015, 86, 829–835. [Google Scholar] [CrossRef]
- Gu, X.; Shiflet, G.J.; Guo, F.Q.; Poon, S.J. Mg–Ca–Zn bulk metallic glasses with high strength and significant ductility. J. Mater. Res. 2005, 20, 1935–1938. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Ma, E.; Xu, E. Reliability of compressive fracture strength of Mg–Zn–Ca bulk metallic glasses: Flaw sensitivity and Weibull statistics. Scr. Mater. 2008, 58, 496–499. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Zhao, X. Structural relaxation and its influence on the elastic properties and notch toughness of Mg–Zn–Ca bulk metallic glass. J. Alloys Compd. 2012, 515, 154–160. [Google Scholar] [CrossRef]
- Li, H.F.; Pang, S.J.; Liu, Y.; Sun, L.L.; Liaw, P.K.; Zhang, T. Biodegradable Mg–Zn–Ca–Sr bulk metallic glasses with enhanced corrosion performance for biomedical applications. Mater. Des. 2015, 67, 9–19. [Google Scholar] [CrossRef]
- Li, H.F.; Pang, S.J.; Liu, Y.; Liaw, P.K.; Zhang, T. In vitro investigation of Mg–Zn–Ca–Ag bulk metallic glasses for biomedical applications. J. Non-Crystal. Solids 2015, 427, 134–138. [Google Scholar] [CrossRef]
- Wang, J.F.; Li, Y.; Huang, S.; Wei, Y.Y.; Xi, X.F.; Cai, K.Y.; Pan, F.S. Effects of Y on the microstructure, mechanical and bio-corrosion properties of Mg-Zn-Ca bulk metallic glass. J. Mater. Sci. Technol. 2014, 30, 1255–1261. [Google Scholar] [CrossRef]
- Tokunaga, T.; Ohno, M.; Matsuura, K. Coatings on Mg alloys and their mechanical properties: A review. J. Mater. Sci. Technol. 2018, 34, 1119–1126. [Google Scholar] [CrossRef]
- Jiang, S.; Cai, S.; Zhang, F.Y.; Xu, P.; Ling, R.; Li, Y.; Jiang, Y.Y.; Xu, G.H. Synthesis and characterization of magnesium phytic acid/apatite composite coating on AZ31 Mg alloy by microwave assisted treatment. Mater. Sci. Eng. C 2018, 91, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Ge, Y.F.; Liu, G.L.; Gao, F.; Li, P.Y. Investigation of tribological properties of micro-arc oxidation ceramic coating on Mg alloy under dry sliding condition. Ceram. Int. 2018, 44, 16164–16172. [Google Scholar] [CrossRef]
- Zhu, H.K.; Zhao, T.; Wei, Q.P.; Liu, N.; Ma, L.; Hu, Z.Q.; Wang, Y.J.; Yu, Z.M. Corrosion resistance improvement of Mg alloy AZ31 by combining bilayer amorphous DLC:H/SiNx film with N+ ions implantation. J. Alloy. Compd. 2018, 762, 171–183. [Google Scholar] [CrossRef]
- Wang, X.; Li, L.X.; Xie, Z.H.; Yu, G. Duplex coating combining layered double hydroxide and 8-quinolinol layers on Mg alloy for corrosion protection. Electrochim. Acta 2018, 283, 1845–1857. [Google Scholar] [CrossRef]
- Yu, H.J.; Wang, J.Q.; Shi, X.T.; Louzguine-Luzgin, D.V.; Wu, H.K.; Perepezko, J.H. Ductile biodegradable Mg-based metallic glasses with excellent biocompatibility. Adv. Func. Mater. 2013, 23, 4793–4800. [Google Scholar] [CrossRef]
- Qin, F.X.; Xie, G.Q.; Dan, Z.H.; Zhu, S.L.; Seki, I. Corrosion behavior and mechanical properties of Mg-Zn-Ca amorphous alloys. Intermetallics 2013, 42, 9–13. [Google Scholar] [CrossRef]
- Wang, J.F.; Wei, Y.Y.; Guo, S.F.; Huang, S.; Zhou, X.E.; Pan, F.S. The Y-doped MgZnCa alloys with ultrahigh specific strength and good corrosion resistance in simulated body fluid. Mater. Lett. 2012, 81, 112–114. [Google Scholar] [CrossRef]
- Chen, S.S.; Tu, J.X.; Hu, Q.; Xiong, X.B.; Wu, J.J.; Zou, J.Z.; Zeng, X.R. Corrosion resistance and in vitro bioactivity of Si-containing coating prepared on a biodegradable Mg-Zn-Ca bulk metallic glass by micro-arc oxidation. J. Non-Cryst. Solids 2017, 456, 125–131. [Google Scholar] [CrossRef]
- Monfared, A.; Ghaee, A.; Ebrahimi-Barough, S. Preparation and characterization of crystallized and relaxed amorphous Mg-Zn-Ca alloy ribbons for nerve regeneration application. J. Non-Cryst. Solids 2018, 489, 71–76. [Google Scholar] [CrossRef]
- Matias, T.B.; Roche, V.; Nogueira, R.P.; Asato, G.H.; Kiminami, C.S.; Bolfarini, C.; Botta, W.J.; Jorge, A.M., Jr. Mg-Zn-Ca amorphous alloys for application as temporary implant: Effect of Zn content on the mechanical and corrosion properties. Mater. Des. 2016, 110, 188–195. [Google Scholar] [CrossRef]
- Wong, P.C.; Tsai, P.H.; Li, T.H.; Cheng, C.K.; Jang, J.S.C.; Huang, J.C. Degradation behavior and mechanical strength of Mg-Zn-Ca bulk metallic glass composites with Ti particles as biodegradable materials. J. Alloys Compd. 2017, 699, 914–920. [Google Scholar] [CrossRef]
- Nowosielski, R.; Cesarz-Andraczke, K. Impact of Zn and Ca on dissolution rate, mechanical properties and GFA of resorbable Mg–Zn–Ca metallic glasses. Arch. Civ. Mech. Eng. 2018, 18, 1–11. [Google Scholar] [CrossRef]
- Wong, P.C.; Lee, T.H.; Tsai, P.H.; Cheng, C.K.; Li, C.; Jang, J.S.C.; Huang, J.C. Enhanced mechanical properties of MgZnCa bulk metallic glass composites with Ti-particle dispersion. Metals 2016, 6, 116. [Google Scholar] [CrossRef]
- Qin, F.X.; Ji, C.; Dan, Z.H.; Xie, G.Q.; Wang, H.; Yamaura, S.I.; Niinomi, M.; Li, Y.D. Corrosion behavior of MgZnCa bulk amorphous alloys fabricated by spark plasma sintering. Acta Metall. Sin. 2016, 29, 793–799. [Google Scholar] [CrossRef]
- Ramya, M.; Sarwat, S.G.; Udhayabanu, V.; Raj, B.; Ravi, K.R. Exploring Mg-Zn-Ca-based bulk metallic glasses for biomedical applications based on thermodynamic approach. Metall. Mat. Trans. A 2015, 46, 5962–5971. [Google Scholar] [CrossRef]
- Wang, J.L.; Wan, Y.; Ma, Z.J.; Guo, Y.C.; Yang, Z.; Wang, P.; Li, J.P. Glass-forming ability and corrosion performance of Mn-doped Mg–Zn–Ca amorphous alloys for biomedical applications. Rare Met. 2018, 37, 579–586. [Google Scholar] [CrossRef]
- Wang, Z.F.; Zhao, W.M.; Qin, C.L.; Cui, Y.; Fan, S.L.; Jia, J.Q. Direct preparation of nano-quasicrystals via a water-cooled wedge-shaped copper mould. J. Nanomater. 2012, 2012, 708240. [Google Scholar] [CrossRef]
- Wang, Z.F.; Zhao, W.M.; Li, H.P.; Ding, J.; Li, Y.Y.; Liang, C.Y. Effect of titanium, antimony, cerium and carbon nanotubes on the morphology and microhardness of Mg-based icosahedral quasicrystal phase. J. Mater. Sci. Technol. 2010, 26, 27–32. [Google Scholar] [CrossRef]
- Wang, Z.F.; Zhao, W.M.; Hur, B.Y.; Huang, C.Y.; Yu, C.Q. Effects of the fourth component and undercooling on morphology of primary Mg-Zn-Y icosahedral quasicrystal phase under normal casting conditions. China Foundry 2009, 6, 293–299. [Google Scholar]
- Manne, B.; Bontha, S.; Ramesh, M.R.; Krishna, M.; Balla, V.K. Solid state amorphization of Mg-Zn-Ca system via mechanical alloying and characterization. Adv. Powder Technol. 2017, 28, 223–229. [Google Scholar] [CrossRef]
- Dai, C.; Forbes, S. On the phase transformation of Ag-41.53Mg (at.%)—An in situ XRD study. Vacuum 2014, 109, 124–126. [Google Scholar] [CrossRef]
- Kulik, J.; Takeda, S.; de Fontaine, D. Long period superstructures in Ag3Mg. Acta Metall. 1987, 35, 1137–1147. [Google Scholar] [CrossRef]

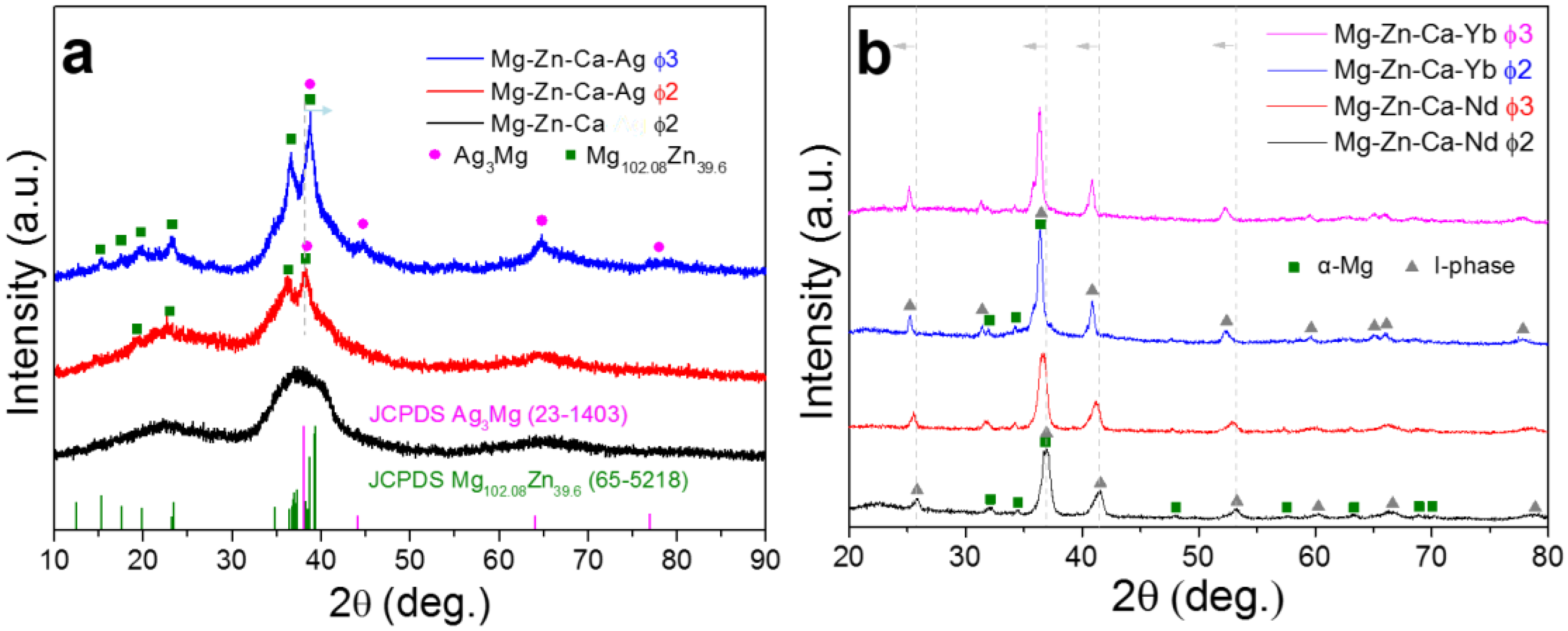
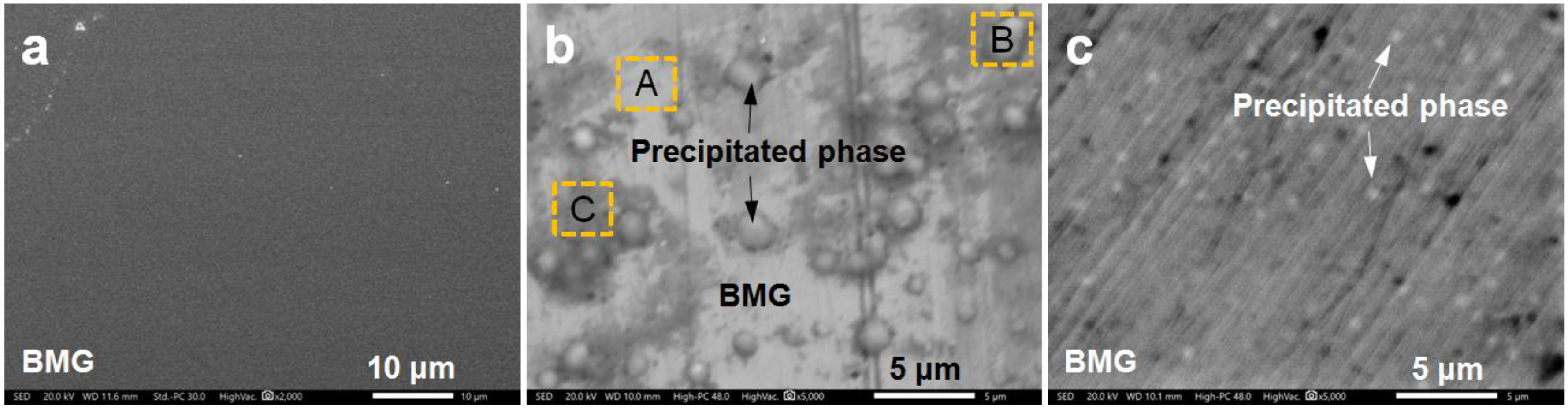

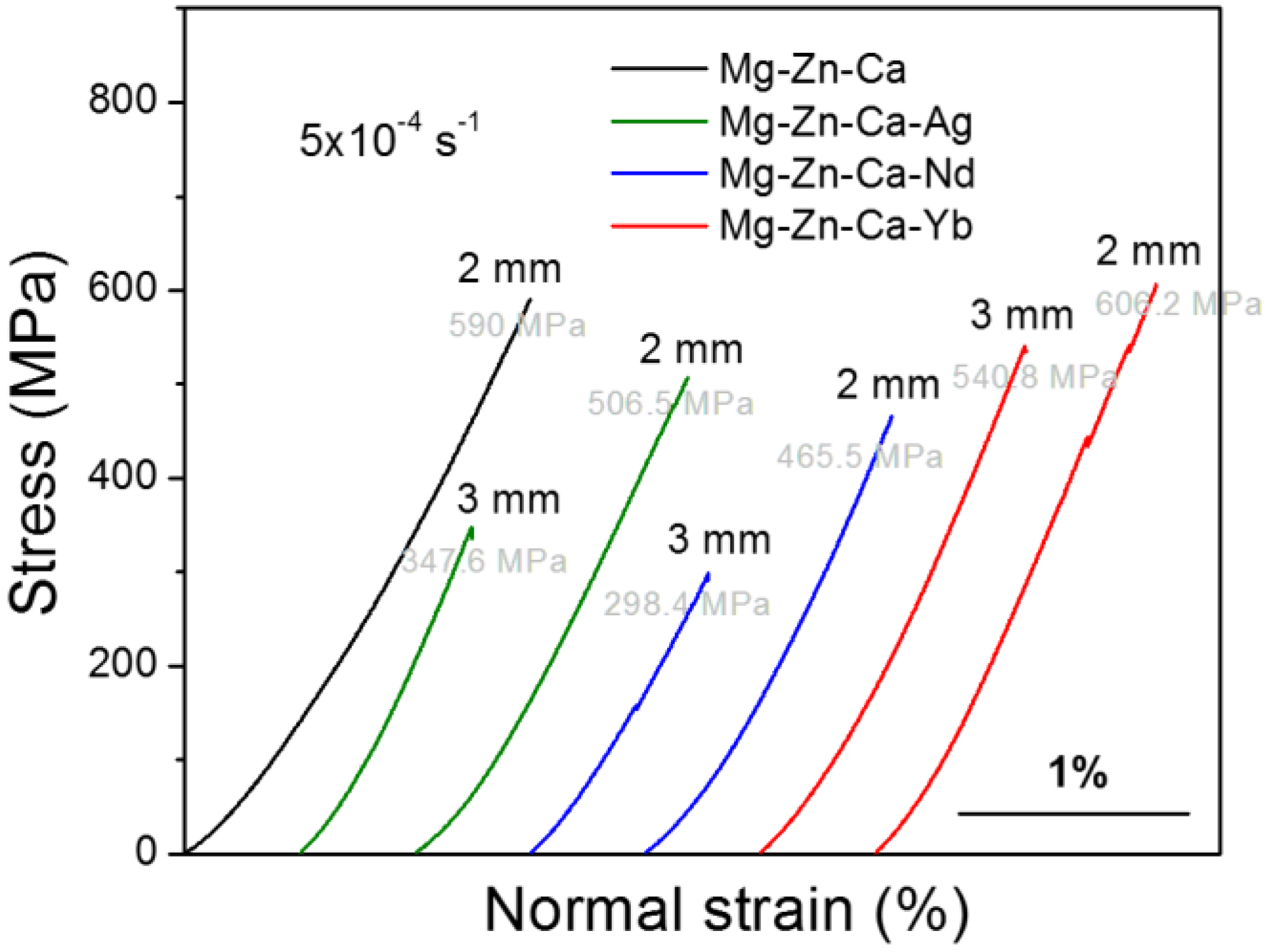
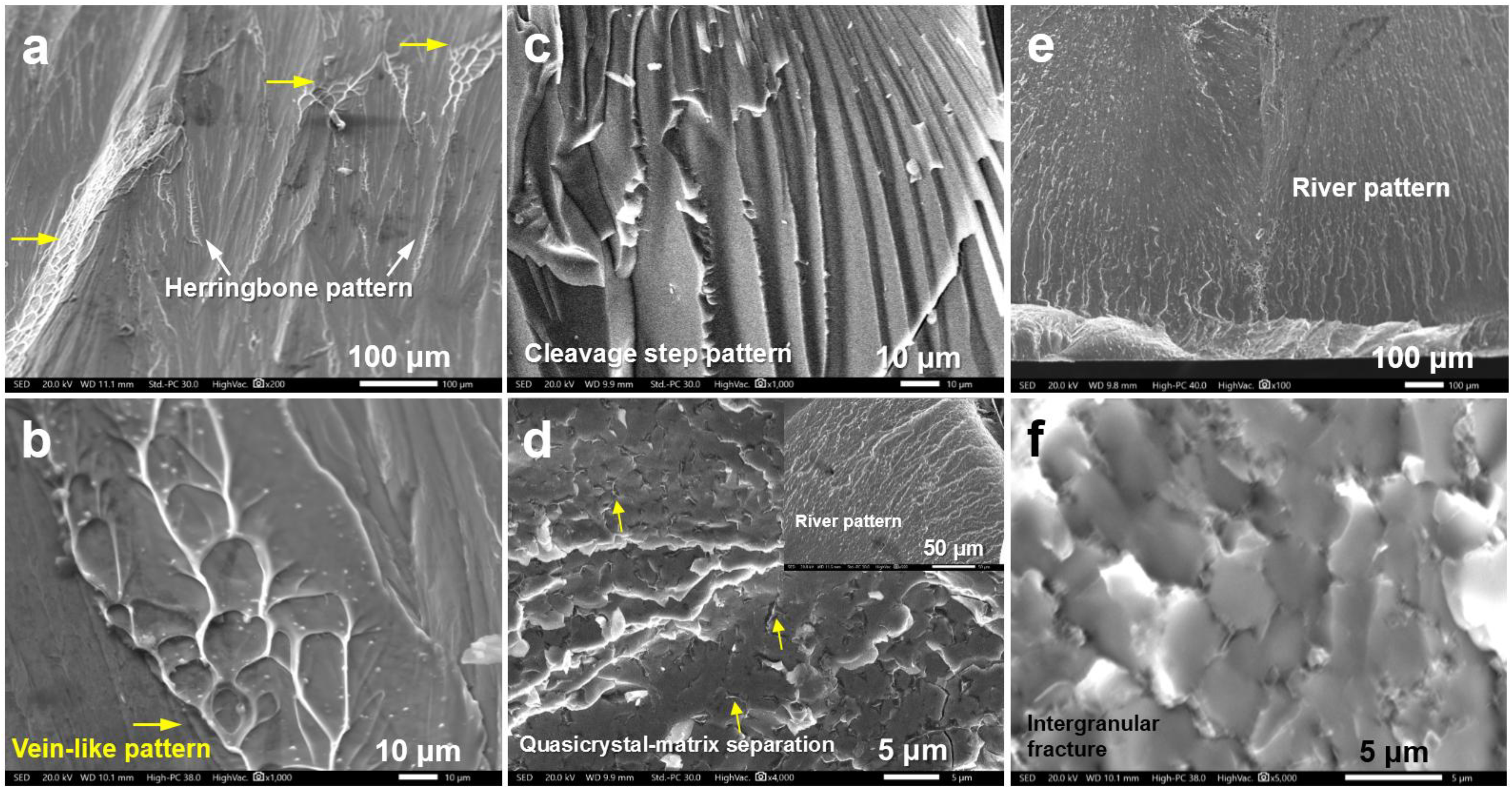
| Alloy | Mg | Zn | Ca | Ag | Nd | Yb |
|---|---|---|---|---|---|---|
| Mg‒Zn‒Ca | 62.9 | 32.3 | 4.8 | - | - | - |
| Mg‒Zn‒Ca‒Ag | 63 | 30.2 | 4.5 | 2.3 | - | - |
| Mg‒Zn‒Ca‒Nd | 59.8 | 33.1 | 4.7 | - | 2.4 | - |
| Mg‒Zn‒Ca‒Yb | 59.3 | 32.4 | 4.8 | - | - | 3.5 |
| Testing Area | Mg | Zn | Ca | Ag |
|---|---|---|---|---|
| Area A | 63.2 | 30.3 | 4.3 | 2.2 |
| Area B | 71.1 | 27.2 | 1.1 | 0.6 |
| Area C | 49.3 | 31.1 | 5.5 | 14.1 |
| Testing Area | Mg | Zn | Ca | Yb |
|---|---|---|---|---|
| Area A | 56.9 | 33.8 | 5.4 | 3.9 |
| Area B | 61.7 | 30.7 | 4.4 | 3.2 |
| Alloy (at %) | Diameter (mm) | Compressive Strength (MPa) | Quasicrystal Phases | |
|---|---|---|---|---|
| Size (μm) | Volume Fraction (%) | |||
| Mg62.9Zn32.3Ca4.8 | 2 | 590 ± 5.1 | - | - |
| Mg63Zn30.2Ca4.5Ag2.3 | 3 | 347.6 ± 8.2 | - | - |
| 2 | 506.5 ± 7.5 | - | - | |
| Mg59.8Zn33.1Ca4.7Nd2.4 | 3 | 298.4 ± 9.3 | ~8.5 | 45 ± 3 |
| 2 | 465.5 ± 6.4 | ~4.5 | 67 ± 4 | |
| Mg59.3Zn32.4Ca4.8Yb3.5 | 3 | 540.8 ± 5.2 | ~8 | 44 ± 5 |
| 2 | 606.2 ± 4.9 | ~3.5 | 79 ± 3 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Z.; Yang, L.; Li, Y.; Wang, X.; Qin, C.; Zhao, W.; Yu, H.; Wang, Z. Effects of Ag, Nd, and Yb on the Microstructures and Mechanical Properties of Mg‒Zn‒Ca Metallic Glasses. Metals 2018, 8, 856. https://doi.org/10.3390/met8100856
Liang Z, Yang L, Li Y, Wang X, Qin C, Zhao W, Yu H, Wang Z. Effects of Ag, Nd, and Yb on the Microstructures and Mechanical Properties of Mg‒Zn‒Ca Metallic Glasses. Metals. 2018; 8(10):856. https://doi.org/10.3390/met8100856
Chicago/Turabian StyleLiang, Zhuofan, Lianzan Yang, Yongyan Li, Xi Wang, Chunling Qin, Weimin Zhao, Hui Yu, and Zhifeng Wang. 2018. "Effects of Ag, Nd, and Yb on the Microstructures and Mechanical Properties of Mg‒Zn‒Ca Metallic Glasses" Metals 8, no. 10: 856. https://doi.org/10.3390/met8100856
APA StyleLiang, Z., Yang, L., Li, Y., Wang, X., Qin, C., Zhao, W., Yu, H., & Wang, Z. (2018). Effects of Ag, Nd, and Yb on the Microstructures and Mechanical Properties of Mg‒Zn‒Ca Metallic Glasses. Metals, 8(10), 856. https://doi.org/10.3390/met8100856






