Effects of Gd, Y Content on the Microstructure and Mechanical Properties of Mg-Gd-Y-Nd-Zr Alloy
Abstract
:1. Introduction
2. Alloy Design
3. Materials and Methods
4. Results
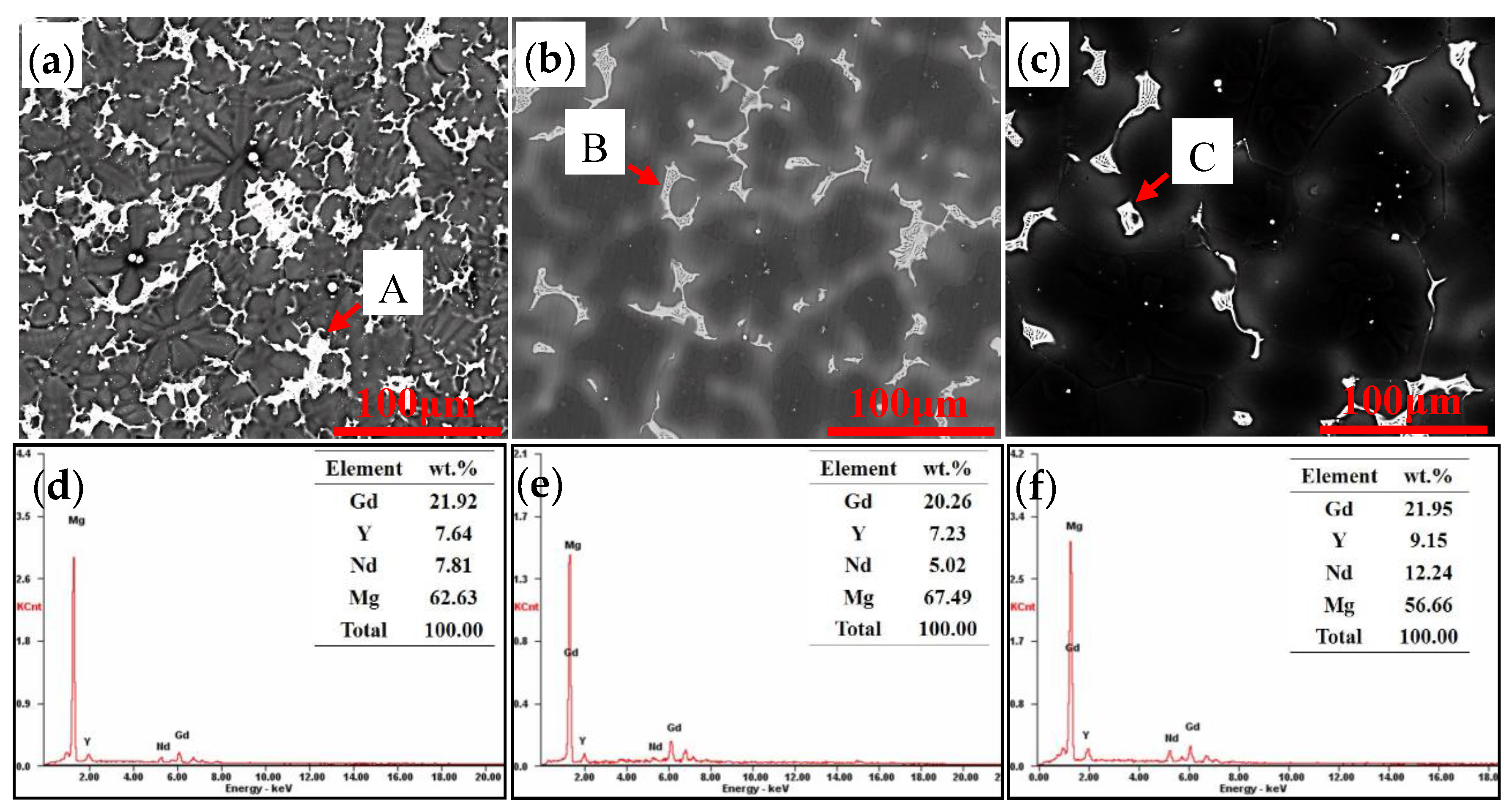
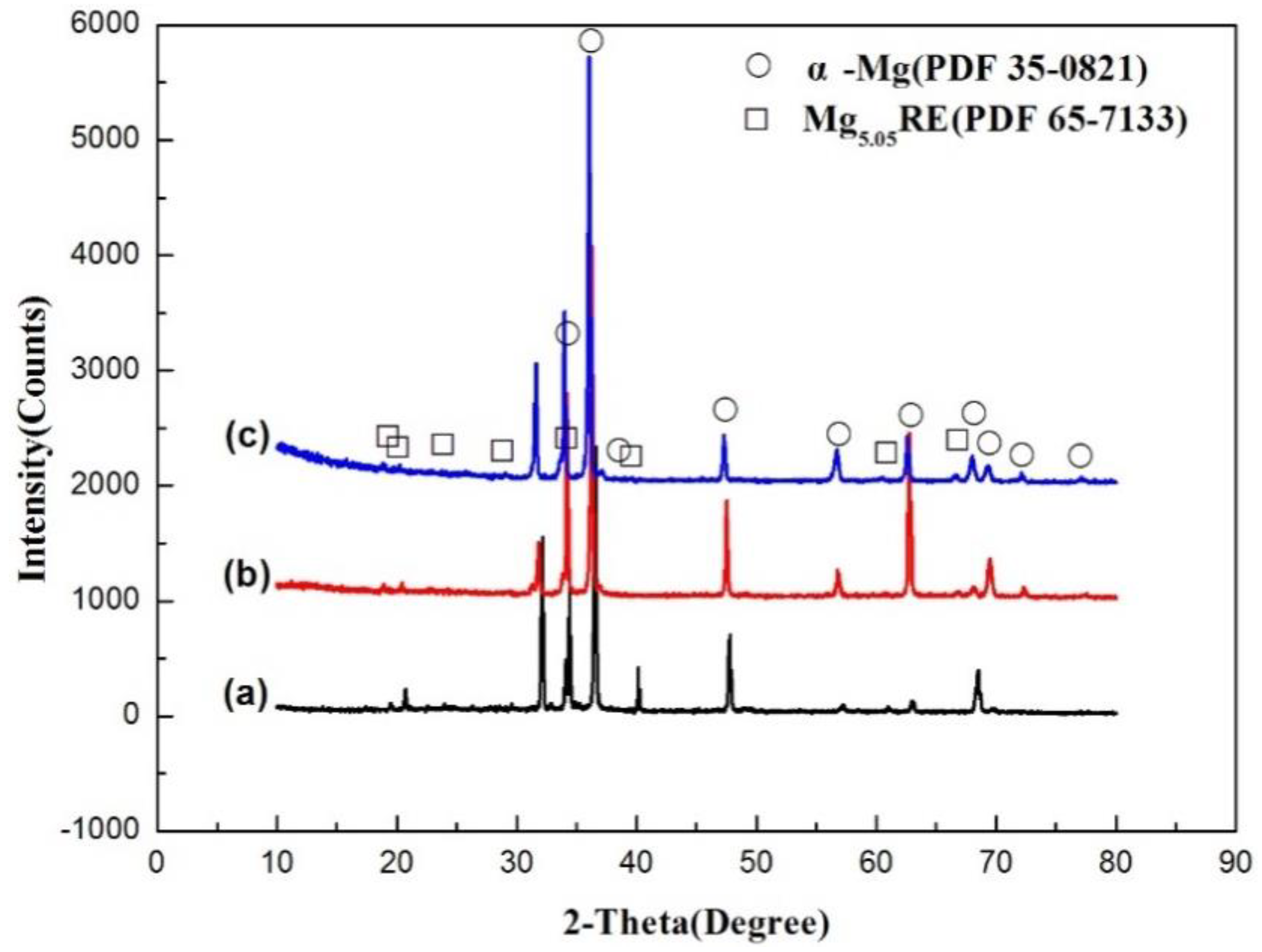
4.1. Microstructure of the As-Cast Sample
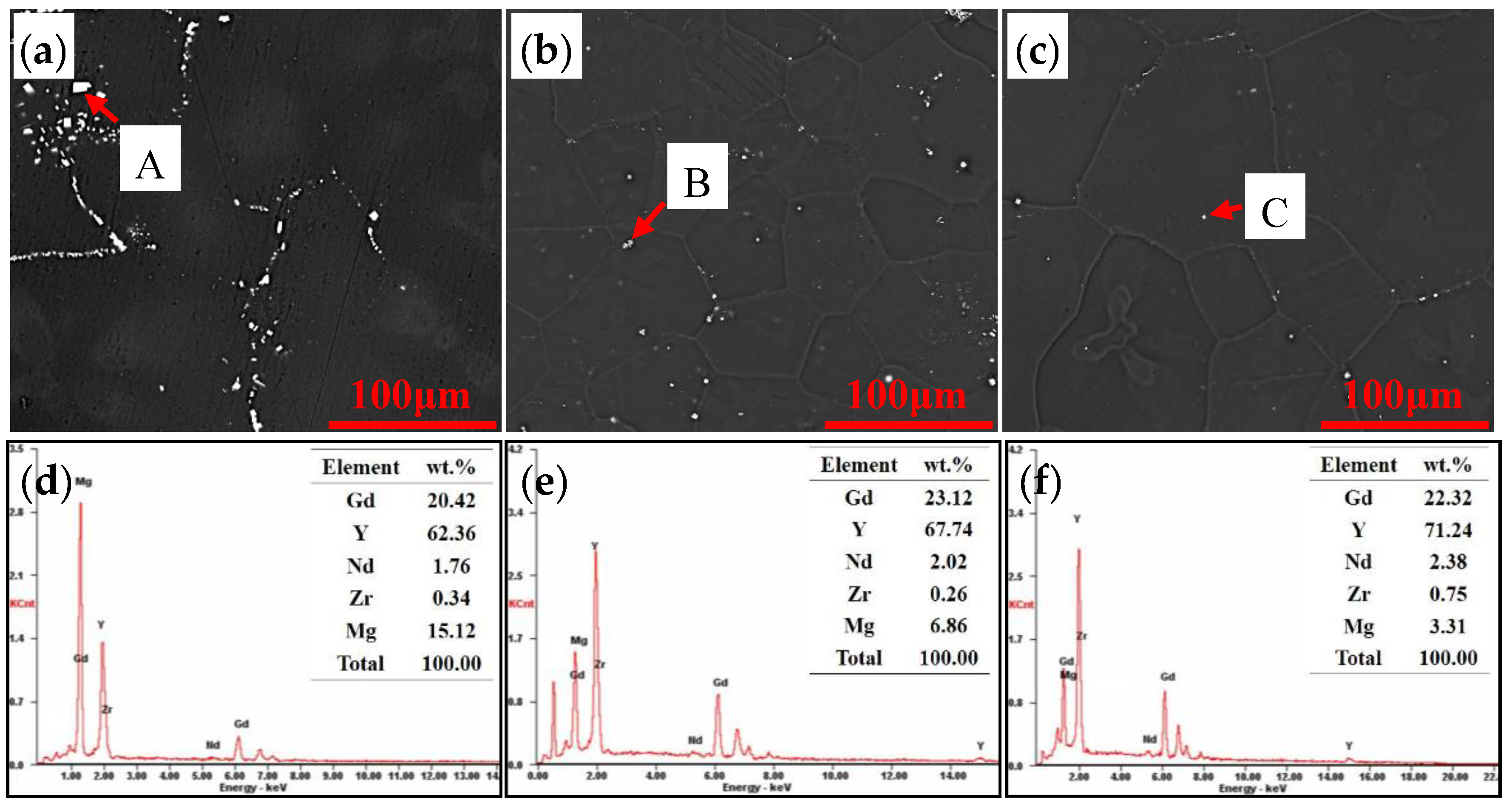
4.2. Microstructure of the Solution Treated Alloys
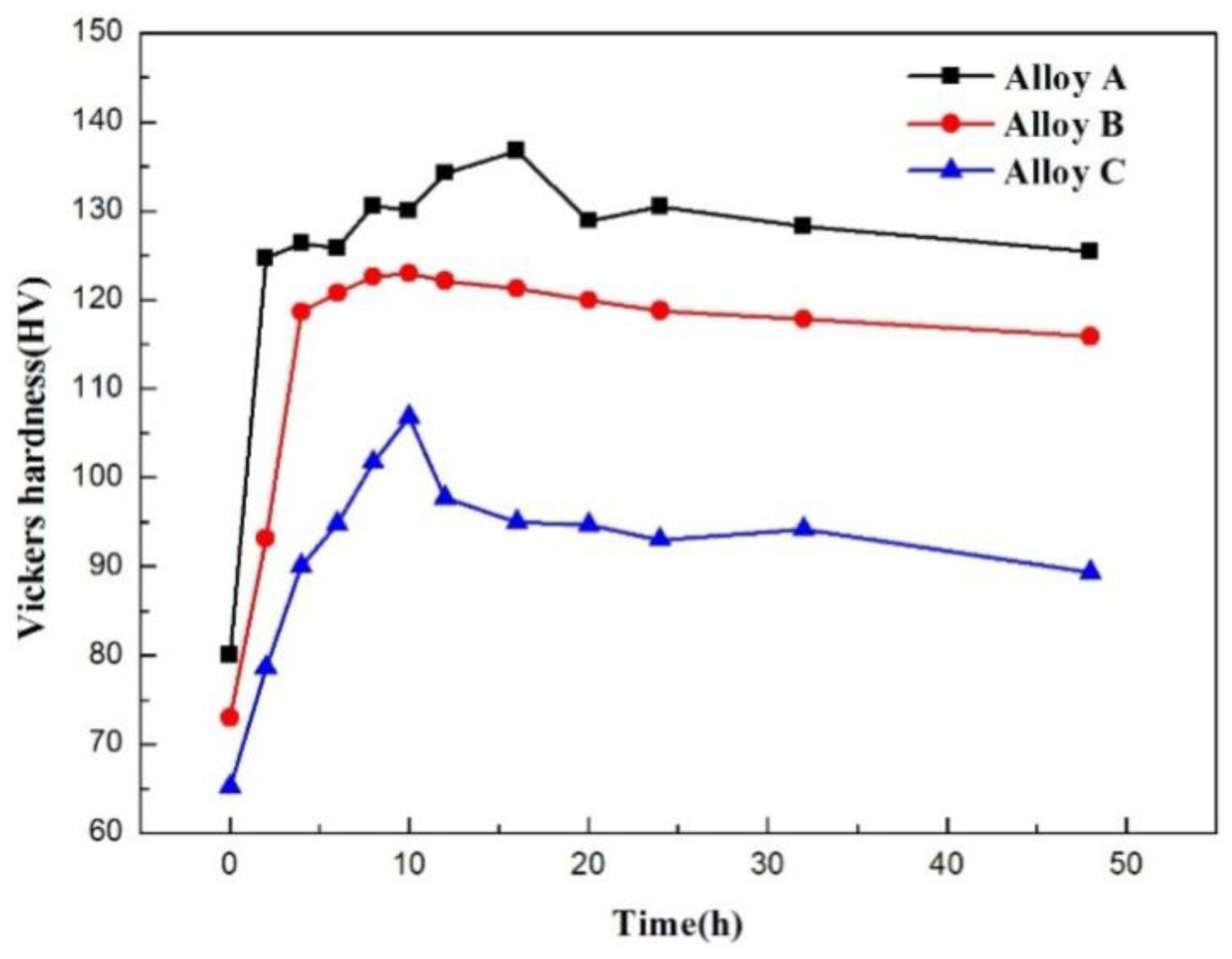
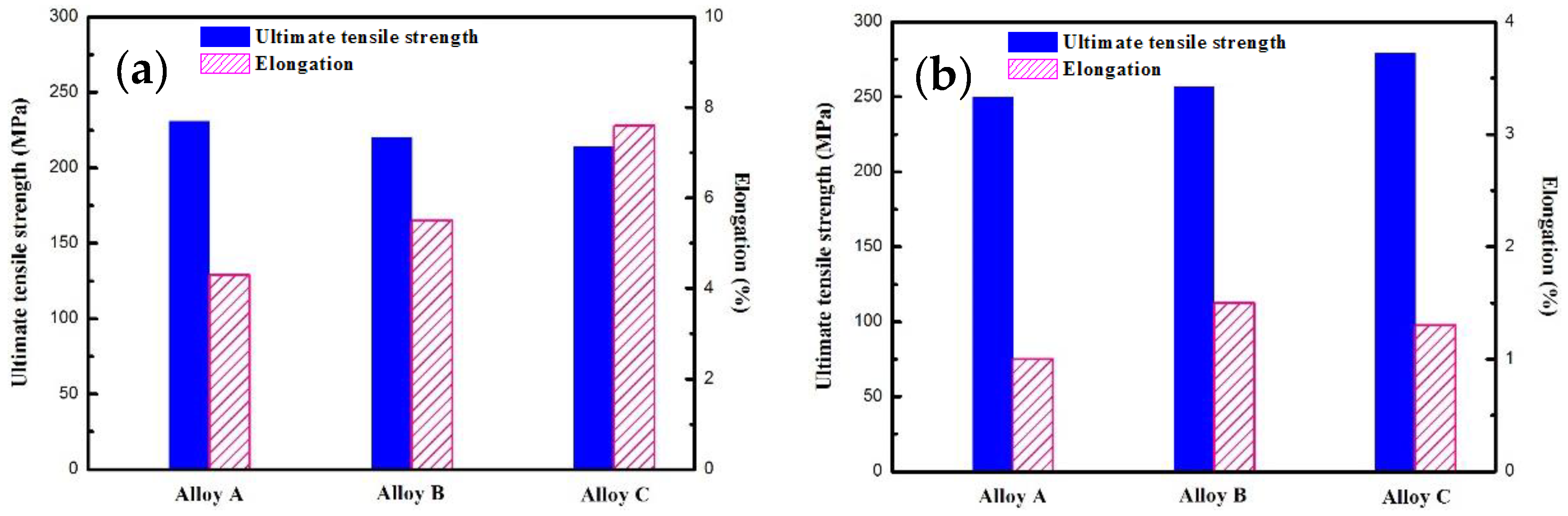
4.3. Mechanical Properties
5. Discussion
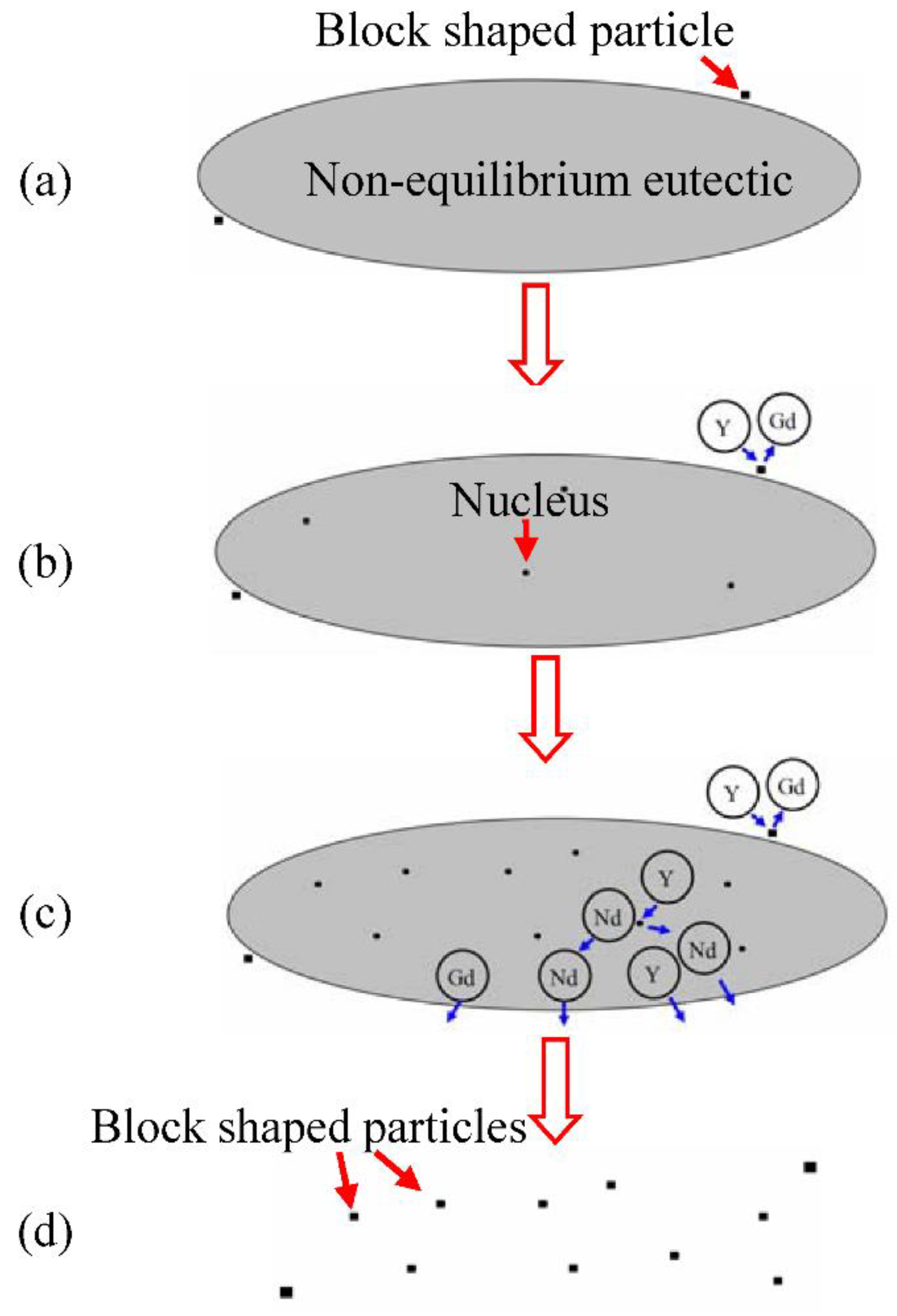
5.1. Formation Process of the Block Shaped Particles
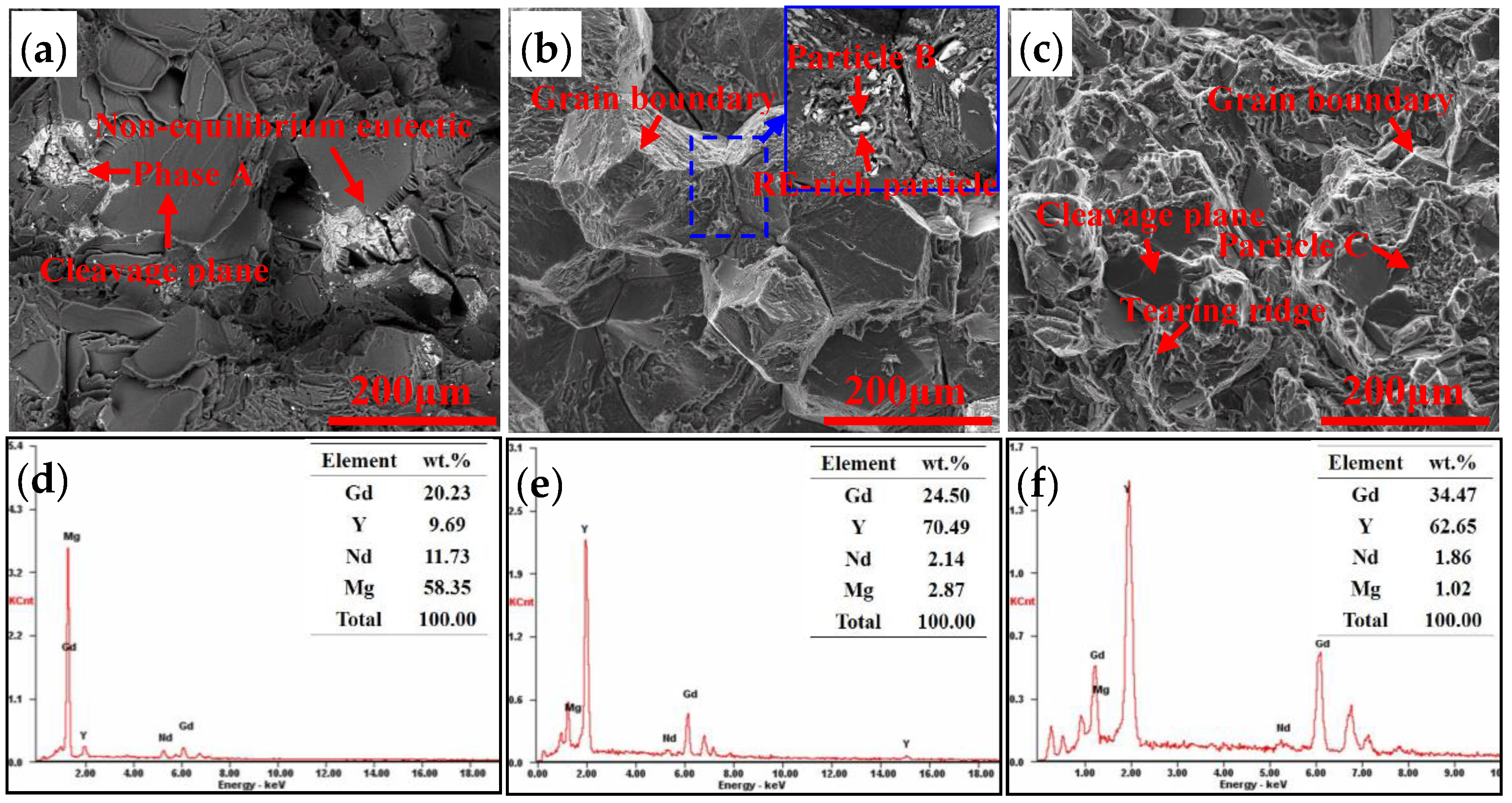
5.2. Effects of Second Phases on Fracture
6. Conclusions
- (1)
- The as-cast ingots of the three alloys are mainly composed of α-Mg and non-equilibrium eutectic Mg5.05RE. The total contents of Gd and Y have no significant effect on the phase composition of the as-cast alloy.
- (2)
- The non-equilibrium eutectics are able to dissolve into the matrix when solution treated at 520 °C, but block shaped particles are left at the grain boundaries and within grains. The particles are RE-rich especially rich in Y element, and the compositions of the particles are independent of the total Gd and Y contents of the alloys while the quantity increases on increasing the total Gd and Y content.
- (3)
- The ultimate tensile strength of the solution treated samples increases as the Gd, Y content increases, while the ultimate tensile strength of the T6 treated samples decreases with increasing Gd, Y content. The increasing strength in solution treated samples is attributed to solid solution strengthening and excellent deformation capacity of the grains, and the decreasing strength in the T6 treated samples is ascribed to the increasing number of block shaped particles and the residual non-equilibrium eutectics, which deteriorate the mechanical properties.
- (4)
- The Mg-5.56Gd-3.38Y-1.11Nd-0.48Zr alloy exhibits the highest strength. The ultimate tensile strength and elongation of the alloy with T6 temper are 280 MPa and 1.3%, respectively. The high strength is attributed to the age hardening behavior and the decrease in block shaped particles.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mordike, B.L.; Ebert, T. Magnesium: Properties—Applications—Potential. Mater. Sci. Eng. A 2001, 302, 37–45. [Google Scholar] [CrossRef]
- Dharmendra, C.; Rao, K.P.; Suresh, K.; Hort, N. Hot deformation behavior and processing map of Mg-3Sn-2Ca-0.4Al-0.4Zn alloy. Metals 2018, 8, 216. [Google Scholar] [CrossRef]
- Liu, H.; Huang, H.; Wang, C.; Ju, J.; Sun, J.P.; Wu, Y.N.; Jiang, J.H.; Ma, A.B. Comparative study of two aging treatments on microstructure and mechanical properties of an ultra-finegrained Mg-10Y-6Gd-1.5Zn-0.5Zr alloy. Metals 2018, 8, 658. [Google Scholar] [CrossRef]
- Saboori, A.; Padovano, E.; Pavese, M.; Dieringa, H.; Badini, C. Effect of solution treatment on precipitation behaviors, age hardening response and creep properties of Elektron21 alloy reinforced by AlN nanoparticles. Materials 2017, 10, 1380. [Google Scholar] [CrossRef] [PubMed]
- Saboori, A.; Padovano, E.; Pavese, M.; Badini, C. Novel magnesium Elektron21-AlN nanocomposites produced by ultrasound-assisted casting; microstructure, thermal and electrical conductivity. Materials 2018, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- He, S.M.; Zeng, X.Q.; Peng, L.M.; Gao, X.; Nie, J.F.; Ding, W.J. Precipitation in a Mg-10Gd-3Y-0.4Zr (wt.%) alloy during isothermal ageing at 250 °C. J. Alloys Compd. 2006, 421, 309–313. [Google Scholar] [CrossRef]
- He, S.M.; Zeng, X.Q.; Peng, L.M.; Gao, X.; Nie, J.F.; Ding, W.J. Microstructure and strengthening mechanism of high strength Mg-10Gd-2Y-0.5Zr alloy. J. Alloys Compd. 2007, 427, 316–323. [Google Scholar] [CrossRef]
- Wang, Q.D.; Chen, J.; Zhao, Z.; He, S.M. Microstructure and super high strength of cast Mg-8.5Gd-2.3Y-1.8Ag-0.4Zr alloy. Mater. Sci. Eng. A 2010, 528, 323–328. [Google Scholar] [CrossRef]
- Jiang, L.K.; Liu, W.C.; Wu, G.H.; Ding, W.J. Effect of chemical composition on the microstructure, tensile properties and fatigue behavior of sand-cast Mg-Gd-Y-Zr alloy. Mater. Sci. Eng. A 2014, 612, 293–301. [Google Scholar] [CrossRef]
- Nodooshan, H.R.J.; Liu, W.C.; Wu, G.H.; Rao, Y.; Zhou, C.X.; He, S.P.; Ding, W.J.; Mahmudi, R. Effect of Gd content on microstructure and mechanical properties of Mg-Gd-Y-Zr alloys under peak-aged condition. Mater. Sci. Eng. A 2014, 615, 79–86. [Google Scholar] [CrossRef]
- Tekumalla, S.; Seetharaman, S.; Almajid, A.; Gupta, M. Mechanical properties of magnesium-rare earth alloy systems: A review. Metals 2015, 5, 1–39. [Google Scholar] [CrossRef]
- Rokhlin, L.L. Magnesium Alloys Containing Rare Earth Metals: Structure and Properties, 1st ed.; Taylor & Francis: London, UK, 2003; pp. 32–45. [Google Scholar]
- Zhang, Y.; Wu, Y.J.; Peng, L.M.; Fu, P.H.; Huang, F.; Ding, W.J. Microstructure evolution and mechanical properties of an ultra-high strength casting Mg-15.6Gd-1.8Ag-0.4Zr alloy. J. Alloys Compd. 2014, 615, 703–711. [Google Scholar] [CrossRef]
- Honma, T.; Ohkubo, T.; Kamado, S.; Hono, K. Effect of Zn additions on the age-hardening of Mg-2.0Gd-1.2Y-0.2Zr alloys. Acta Mater. 2007, 55, 4137–4150. [Google Scholar] [CrossRef]
- Peng, Q.M.; Wang, J.L.; Wu, Y.M.; Meng, J.; Wang, L.M. The effect of La or Ce on ageing response and mechanical properties of cast Mg-Gd-Zr alloys. Mater. Charact. 2008, 59, 435–439. [Google Scholar] [CrossRef]
- Peng, Q.M.; Wang, L.D.; Wu, Y.M.; Wang, L.M. Structure stability and strengthening mechanism of die-cast Mg-Gd-Dy based alloy. J. Alloys Compd. 2009, 469, 587–592. [Google Scholar] [CrossRef]
- Dai, J.C.; Zhu, S.M.; Easton, M.A.; Zhang, M.X.; Qiu, D.; Wu, G.H.; Liu, W.C.; Ding, W.J. Heat treatment, microstructure and mechanical properties of a Mg-Gd-Y alloy grain-refined by Al additions. Mater. Sci. Eng. A 2013, 576, 298–305. [Google Scholar] [CrossRef]
- Zhang, X.G.; Meng, L.G.; Fang, C.F.; Peng, P.; Ja, F.; Hao, H. Effect of Nd on the microstructure and mechanical properties of Mg-8Gd-5Y-2Zn-0.5Zr alloy. Mater. Sci. Eng. A 2013, 586, 19–24. [Google Scholar] [CrossRef]
- Zhang, L.; Gong, M.; Peng, L.M. Microstructure and strengthening mechanism of a thermomechanically treated Mg-10Gd-3Y-1Sn-0.5Zr alloy. Mater. Sci. Eng. A 2013, 565, 262–268. [Google Scholar] [CrossRef]
- Fang, C.F.; Liu, G.X.; Hao, H.; Wen, Z.H.; Zhang, X.G. Effect of Al addition on microstructure, texture and mechanical properties of Mg-5Gd-2.5Y-2Zn alloy. J. Alloys Compd. 2016, 686, 347–355. [Google Scholar] [CrossRef]
- Peng, Z.K.; Zhang, X.M.; Chen, J.M.; Xiao, Y.; Jiang, H. Grain refining mechanism in Mg-9Gd-4Y alloys by zirconium. Mater. Sci. Technol. 2005, 21, 722–726. [Google Scholar] [CrossRef]
- Magnesium Elektron: Service and Innovation in Magnesium. Available online: https://www.magnesium-elektron.com/wp-content/uploads/2016/10/Elektron-WE54_0.pdf (accessed on 31 August 2018).
- Magnesium Elektron: Service and Innovation in Magnesium. Available online: https://www.magnesium-elektron.com/wp-content/uploads/2017/05/Elektron-21.pdf (accessed on 23 September 2018).
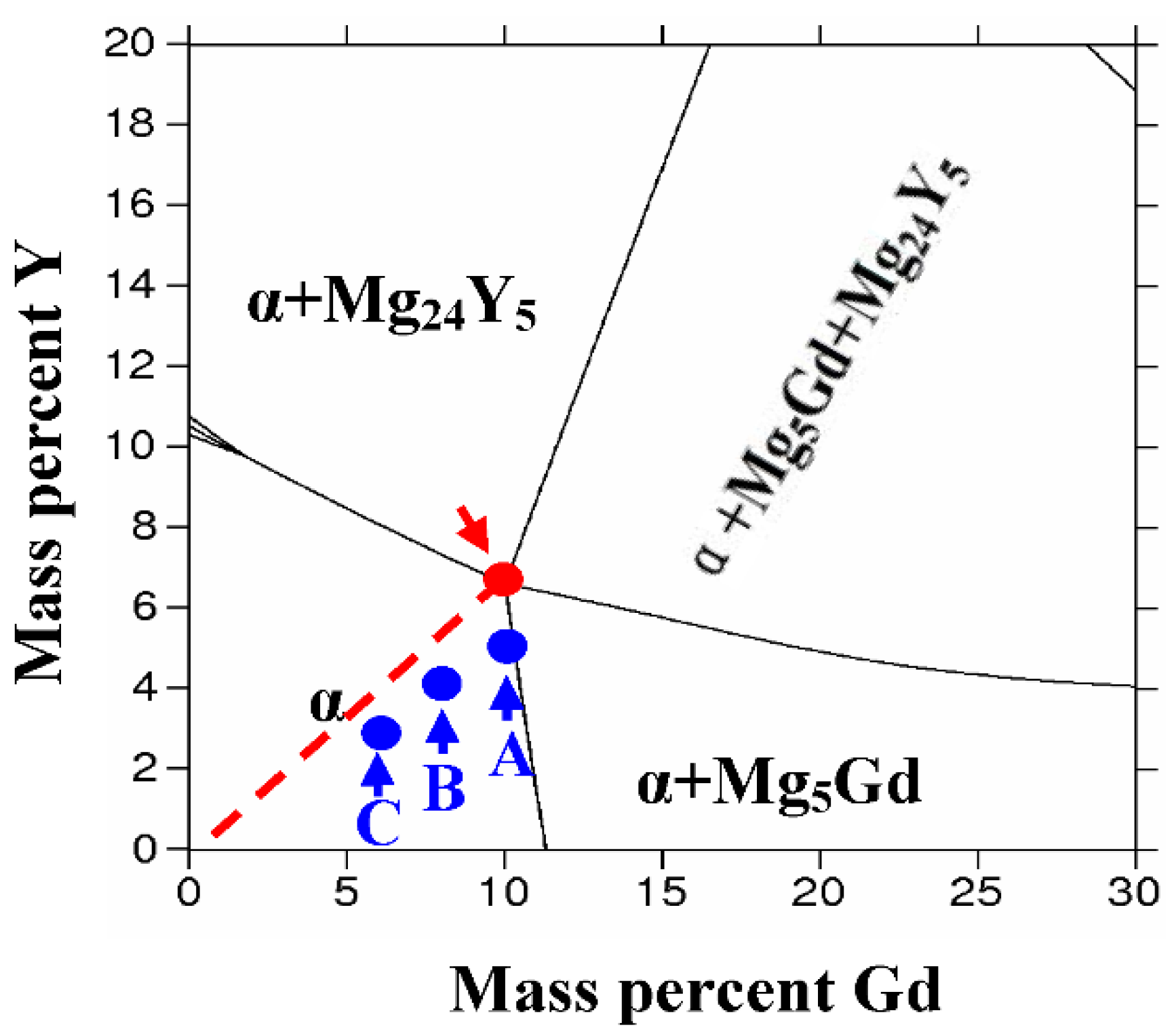

| Alloy Numbers | Element and Composition (wt%) | ||||
|---|---|---|---|---|---|
| Gd | Y | Nd | Zr | Mg | |
| A | 10.16 | 4.31 | 0.97 | 0.43 | Balance |
| B | 7.71 | 3.45 | 1.02 | 0.51 | Balance |
| C | 5.56 | 3.38 | 1.11 | 0.48 | Balance |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, C.; Wu, K.; Liu, W.; Feng, D.; Wang, X.; Miao, G.; Yang, M.; Liu, X.; Li, Q. Effects of Gd, Y Content on the Microstructure and Mechanical Properties of Mg-Gd-Y-Nd-Zr Alloy. Metals 2018, 8, 790. https://doi.org/10.3390/met8100790
Tang C, Wu K, Liu W, Feng D, Wang X, Miao G, Yang M, Liu X, Li Q. Effects of Gd, Y Content on the Microstructure and Mechanical Properties of Mg-Gd-Y-Nd-Zr Alloy. Metals. 2018; 8(10):790. https://doi.org/10.3390/met8100790
Chicago/Turabian StyleTang, Changping, Kai Wu, Wenhui Liu, Di Feng, Xuezhao Wang, Guodong Miao, Maomao Yang, Xiao Liu, and Quan Li. 2018. "Effects of Gd, Y Content on the Microstructure and Mechanical Properties of Mg-Gd-Y-Nd-Zr Alloy" Metals 8, no. 10: 790. https://doi.org/10.3390/met8100790
APA StyleTang, C., Wu, K., Liu, W., Feng, D., Wang, X., Miao, G., Yang, M., Liu, X., & Li, Q. (2018). Effects of Gd, Y Content on the Microstructure and Mechanical Properties of Mg-Gd-Y-Nd-Zr Alloy. Metals, 8(10), 790. https://doi.org/10.3390/met8100790





