Scandium Recovery from an Ammonium Fluoride Strip Liquor by Anti-Solvent Crystallization
Abstract
1. Introduction—Previous Studies and State of the Art
2. Experimental Procedure
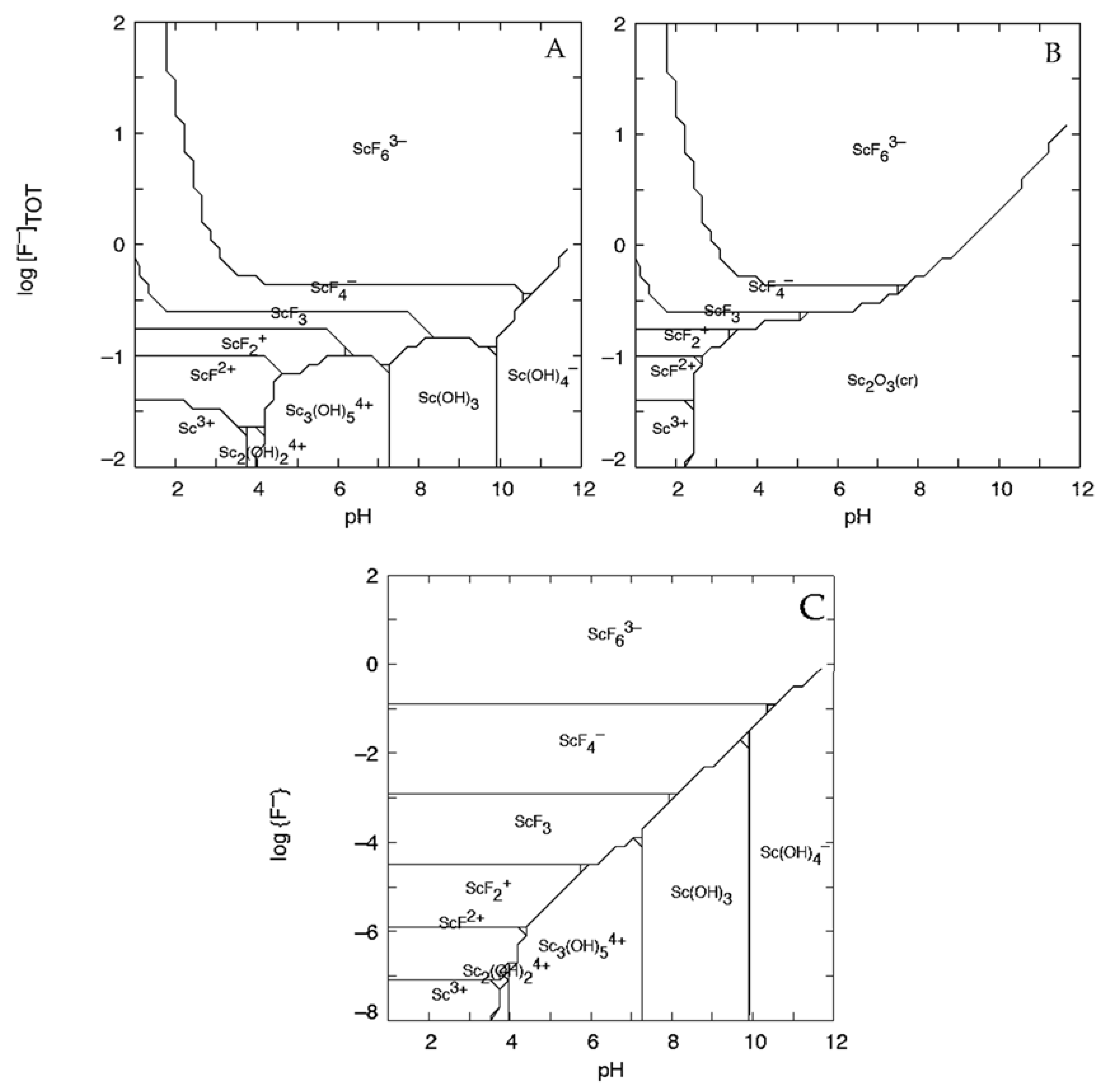
2.1. Thermodynamic Modeling
2.2. Experimental Procedure
3. Results and Discussion
3.1. Thermodynamic Model
3.2. Strip Liquor Preparation
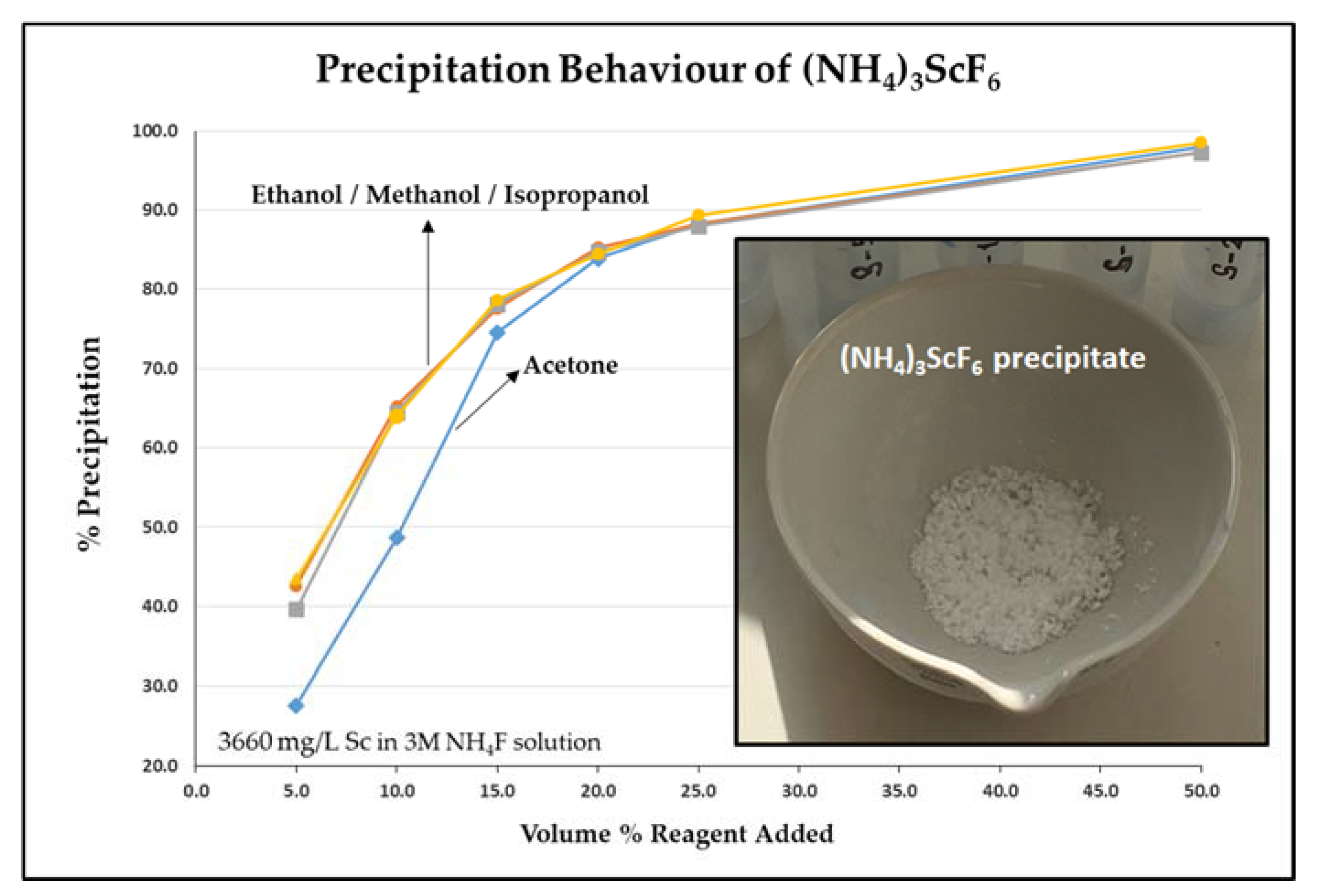
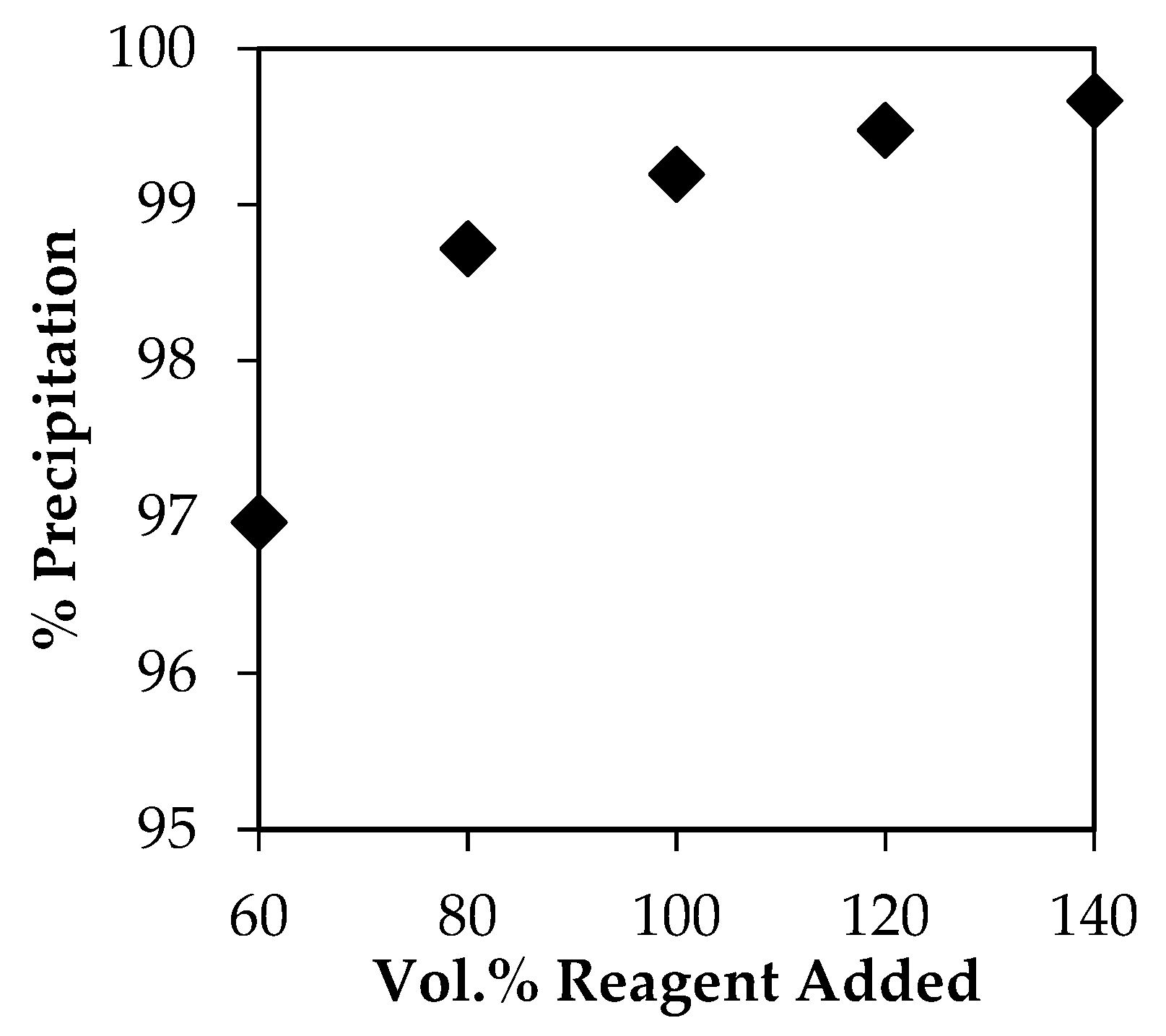
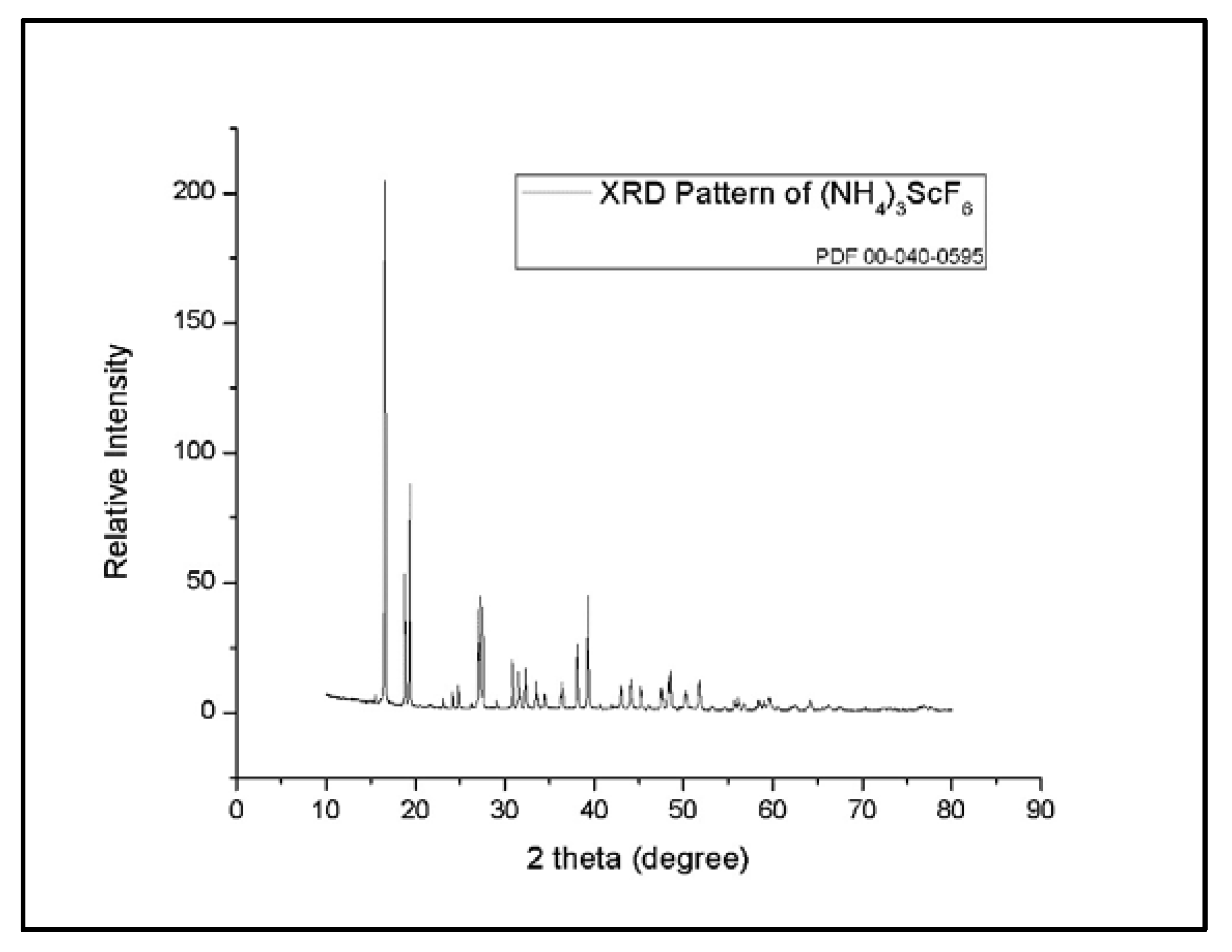
3.3. Anti-Solvent Crystallization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions on the 2017 List of Critical Raw Materials for the EU; European Commission: Brussels, Belgium, 2017. [Google Scholar]
- Magyar, M.J.; Petty, T.R. Draft list of critical minerals. Fed. Regist. 2018, 83, 33. [Google Scholar]
- Binnemans, K.; Jones, P.T.; Müller, T.; Yurramendi, L. Rare Earths and the Balance Problem: How to Deal with Changing Markets? J. Sustain. Metall. 2018, 4, 126–146. [Google Scholar] [CrossRef]
- Laguna-Bercero, M.A.; Kinadjan, N.; Sayers, R.; El Shinawi, H.; Greaves, C.; Skinner, S.J. Performance of La2-xSrxCo0.5Ni0.5O4±δ as an Oxygen Electrode for Solid Oxide Reversible Cells. Fuel Cells 2011, 11, 102–107. [Google Scholar] [CrossRef]
- Boulon, G. Fifty years of advances in solid-state laser materials. Opt. Mater. 2012, 34, 499–512. [Google Scholar] [CrossRef]
- Xia, Z.; Liu, Q. Progress in discovery and structural design of color conversion phosphors for LEDs. Prog. Mater. Sci. 2016, 84, 59–117. [Google Scholar] [CrossRef]
- Riva, S.; Yusenko, K.V.; Lavery, N.P.; Jarvis, D.J.; Brown, S.G.R. The scandium effect in multicomponent alloys. Int. Mater. Rev. 2016, 61, 203–228. [Google Scholar] [CrossRef]
- Wang, W.; Pranolo, Y.; Cheng, C.Y. Metallurgical processes for scandium recovery from various resources: A review. Hydrometallurgy 2011, 108, 100–108. [Google Scholar] [CrossRef]
- Smirnov, D.I.; Molchanova, T.V. The investigation of sulphuric acid sorption recovery of scandium and uranium from the red mud of alumina production. Hydrometallurgy 1997, 45, 249–259. [Google Scholar] [CrossRef]
- Kaya, Ş.; Dittrich, C.; Stopic, S.; Friedrich, B. Concentration and Separation of Scandium from Ni Laterite Ore Processing Streams. Metals 2017, 7, 557. [Google Scholar] [CrossRef]
- Li, D.; Wang, C. Solvent extraction of Scandium(III) by Cyanex 923 and Cyanex 925. Hydrometallurgy 1998, 48, 301–312. [Google Scholar] [CrossRef]
- Hatzilyberis, K.; Lymperopoulou, T.; Tsakanika, L.A.; Ochsenkühn, K.M.; Georgiou, P.; Defteraios, N.; Tsopelas, F.; Ochsenkühn-Petropoulou, M. Process Design Aspects for Scandium-Selective Leaching of Bauxite Residue with Sulfuric Acid. Minerals 2018, 8, 79. [Google Scholar] [CrossRef]
- Lash, L.D.; Ross, J.R. Vitro chemical recovers costly scandium from uranium solutions. Min. Eng. 1961, 220, 967. [Google Scholar]
- Xu, S.Q.; Li, S.Q. Review of the extractive metallurgy of scandium in China (1978–1991). Hydrometallurgy 1996, 42, 337–343. [Google Scholar]
- Martinez, A.M.; Osen, K.S.; Gudbrandsen, H.; Sommerseth, C.; Wang, Z.; Darell, O. Direct Method for Producing Scandium Metal and Scandium-Aluminium Intermetallic Compounds from the Oxides. In Light Metals 2018; Martin, O., Ed.; The Minerals, Metals and Materials Society: Pittsburgh, PA, USA, 2018; pp. 1559–1564. [Google Scholar]
- Gambogi, J. Mineral Commodity Summaries: Scandium; Document No. (703) 648-7718; U.S. Geological Survey: Reston, VA, USA, 2018.
- Moldoveanu, G.A.; Demopoulos, G.P. Organic solvent-assisted crystallization of inorganic salts from acidic media. J. Chem. Technol. Biotechnol. 2015, 90, 686–692. [Google Scholar] [CrossRef]
- Sviridova, T.A.; Sokolova, Y.V. Pirozhenko, K.Y. Crystal structure of (NH4)5Sc3F14. Crystallogr. Rep. 2013, 58, 220–225. [Google Scholar] [CrossRef]
- Sokolova, Y.V.; Cherepanin, R.N. Preparation and examination of the properties of complex scandium fluorides. Russ. J. Appl. Chem. 2011, 84, 1319–1323. [Google Scholar] [CrossRef]
- Mioduski, T.; Gumiński, C.; Zeng, D. IUPAC-NIST Solubility Data Series. 100. Rare Earth Metal Fluorides in Water and Aqueous Systems. Part 1. Scandium Group (Sc, Y, La). J. Phys. Chem. Ref. Data 2014, 43, 013105. [Google Scholar] [CrossRef]
- Puigdomenech, I. Windows software for the graphical presentation of chemical speciation. In Proceedings of the 219th American Chemical Society National Meeting, San Francisco, CA, USA, 26–30 March 2000; American Chemical Society: Washington, DC, USA, 2000. [Google Scholar]
- Eriksson, G. An algorithm for the computation of aqueous multicomponent, multiphase equilibria. Anal. Chim. Acta 1979, 112, 375–383. [Google Scholar] [CrossRef]
- Ingri, N.; Kakolowicz, W.; Sillén, L.G.; Warnqvist, B. Errata: High-speed computers as a supplement to graphical methods—V: Haltafall, a general program for calculating the composition of equilibrium mixtures. Talanta 1968, 15, xi–xii. [Google Scholar]
- Ingri, N.; Kakolowicz, W.; Sillén, L.G.; Warnqvist, B. High-speed computers as a supplement to graphical methods—V: Haltafall, a general program for calculating the composition of equilibrium mixtures. Talanta 1967, 14, 1261–1286. [Google Scholar] [CrossRef]
- Constable, E.C. Scandium. Coord. Chem. Rev. 1984, 57, 229–236. [Google Scholar] [CrossRef]
- Kury, J.W.; Paul, A.D.; Hepler, L.G.; Connick, R.E. The Fluoride Complexing of Scandium(III) in Aqueous Solution: Free Energies, Heats and Entropies. J. Am. Chem. Soc. 1959, 81, 4185–4189. [Google Scholar] [CrossRef]
- Baes, C.F.; Mesmer, R.E. The Hydrolysis of Cations; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 1976. [Google Scholar]
- Burgess, D. Standard Reference Data NIST46—NIST Critically Selected Stability Constants of Metal Complexes: Version 8.0; NIST: Gaithersburg, MD, USA, 2013. [Google Scholar]
- Itoh, H.; Hachiya, H.; Tsuchiya, M.; Suzuki, Y.; Asano, Y. Determination of solubility products of rare earth fluorides by fluoride ion-selective electrode. Bull. Chem. Soc. Jpn. 1984, 57, 1689–1690. [Google Scholar] [CrossRef]
- Wood, S.A.; Samson, I.M. The aqueous geochemistry of gallium, germanium, indium and scandium. Ore Geol. Rev. 2006, 28, 57–102. [Google Scholar] [CrossRef]
- Mackay, K.M.; Mackay, R.A.; Henderson, W. Introduction to Modern Inorganic Chemistry, 6th ed.; Nelson Thornes Ltd.: Cheltenham, UK, 2002. [Google Scholar]
- Watanabe, M.; Nishimura, S. Process for producing fluorides of metals. U.S. Patent 4,741,893, 3 May 1988. [Google Scholar]
- Stevenson, P.C.; Nervik, W.E. The Radiochemistry of the Rare Earths, Scandium, Yttrium and Actinium; USAEC Technical Information Center: Oak Ridge, TN, USA, 1961. [Google Scholar]
- Vickery, R.C. Some Reactions of Scandium. J. Chem. Soc. 1956, 3113–3120. [Google Scholar] [CrossRef]
- Ouellette, R.J.; Rawn, J.D. Principles of Organic Chemistry; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Hansen, C.M. The Three Dimensional Solubility Parameter and Solvent Diffusion Coefficient. Their Importance in Surface Coating Formulation. Ph.D. Thesis, Polytechnic Lrereanstalt, Danmarks Tekniske Højskole, Copenhagen, Denmark, August 1967. [Google Scholar]
- Peters, E.; Kaya, Ş.; Dittrich, C.; Forsberg, K. Recovery of scandium by crystallization techniques. In Proceedings of the 2nd International Bauxite Residue Valorization and Best Practices Conference, Athens, Greece, 7–10 May 2018; pp. 401–408. [Google Scholar]
- Rakov, E.G.; Mel’nichenko, E.I. The Properties and Reactions of Ammonium Fluorides. Russ. Chem. Rev. 1984, 53, 851–869. [Google Scholar] [CrossRef]

| Complex | pKn | Ref. | Complex | pKn | Ref. |
|---|---|---|---|---|---|
| ScF2+ (aq) | 7.08 | [26] | ScO(OH) | −9.4 | [27] |
| ScF2+ (aq) | 12.89 | Sc(OH)3 | −29.7 | ||
| ScF30 (aq) | 17.36 | Sc(OH)2+ (aq) | −4.3 | ||
| ScF4− (aq) | 20.21 | Sc(OH)2+ (aq) | −9.7 | ||
| ScF63− (aq) | 22.00 * | Sc(OH)3 (aq) | −16.1 | ||
| Sc2F33+ (aq) | 20.7 | [28] | Sc(OH)4− (aq) | −26 | |
| ScF3 (s) | −11.5 | [29] | Sc2(OH)24+ (aq) | −6 | |
| Sc2O3 (s) | −36.3 | [27] | Sc3(OH)54+ (aq) | −16.34 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaya, Ş.; Peters, E.M.; Forsberg, K.; Dittrich, C.; Stopic, S.; Friedrich, B. Scandium Recovery from an Ammonium Fluoride Strip Liquor by Anti-Solvent Crystallization. Metals 2018, 8, 767. https://doi.org/10.3390/met8100767
Kaya Ş, Peters EM, Forsberg K, Dittrich C, Stopic S, Friedrich B. Scandium Recovery from an Ammonium Fluoride Strip Liquor by Anti-Solvent Crystallization. Metals. 2018; 8(10):767. https://doi.org/10.3390/met8100767
Chicago/Turabian StyleKaya, Şerif, Edward Michael Peters, Kerstin Forsberg, Carsten Dittrich, Srecko Stopic, and Bernd Friedrich. 2018. "Scandium Recovery from an Ammonium Fluoride Strip Liquor by Anti-Solvent Crystallization" Metals 8, no. 10: 767. https://doi.org/10.3390/met8100767
APA StyleKaya, Ş., Peters, E. M., Forsberg, K., Dittrich, C., Stopic, S., & Friedrich, B. (2018). Scandium Recovery from an Ammonium Fluoride Strip Liquor by Anti-Solvent Crystallization. Metals, 8(10), 767. https://doi.org/10.3390/met8100767








