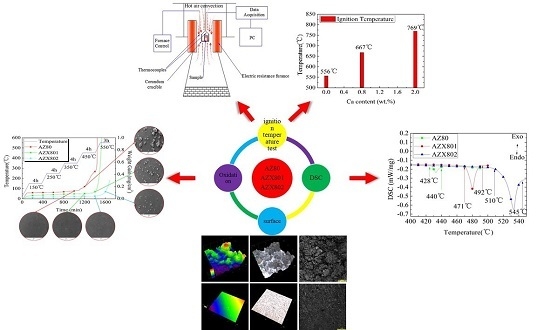
Effect of Ca Additions on Ignition Temperature and Multi-Stage Oxidation Behavior of AZ80
Abstract
:1. Introduction
2. Experiment
2.1. Alloy Preparation
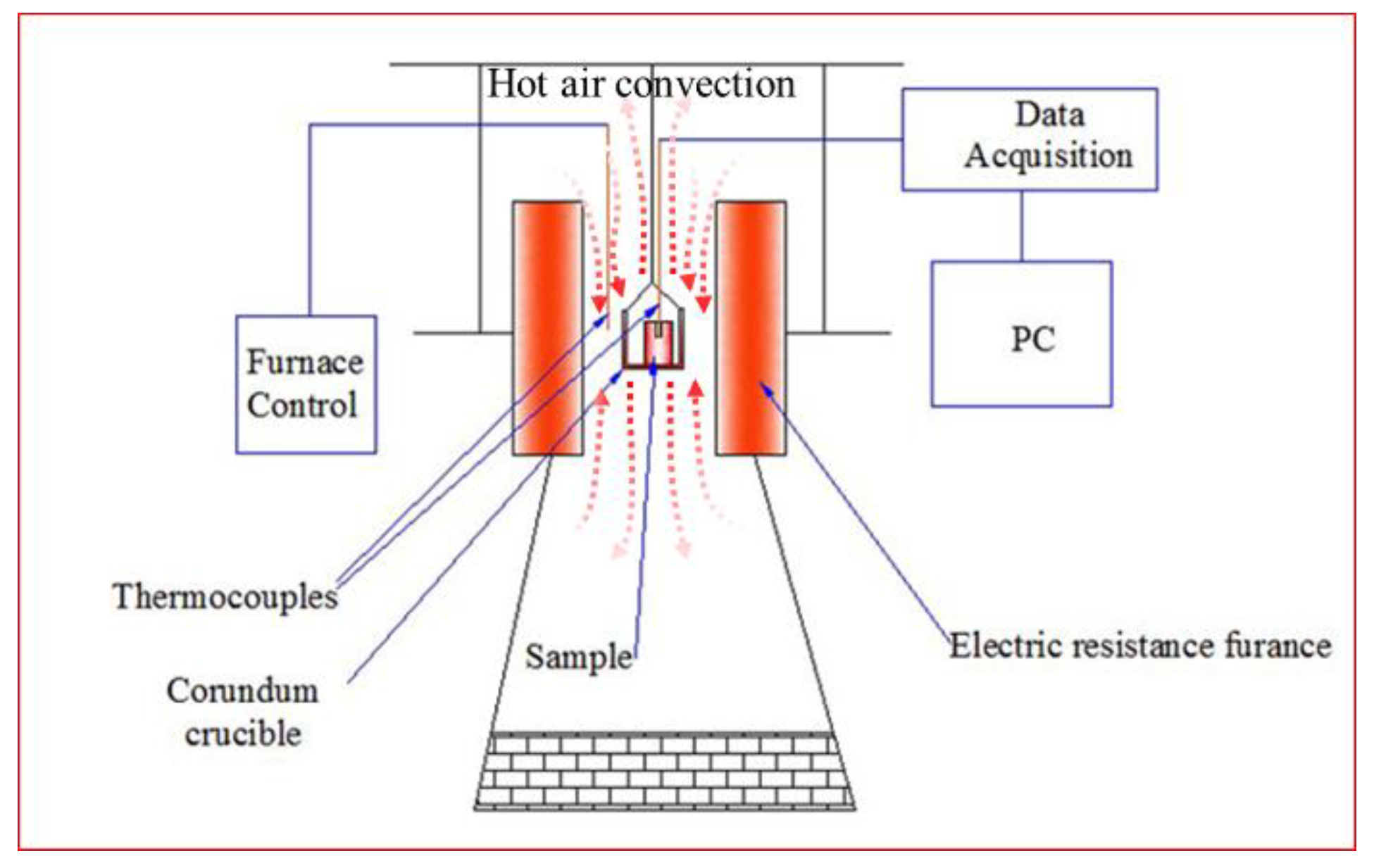
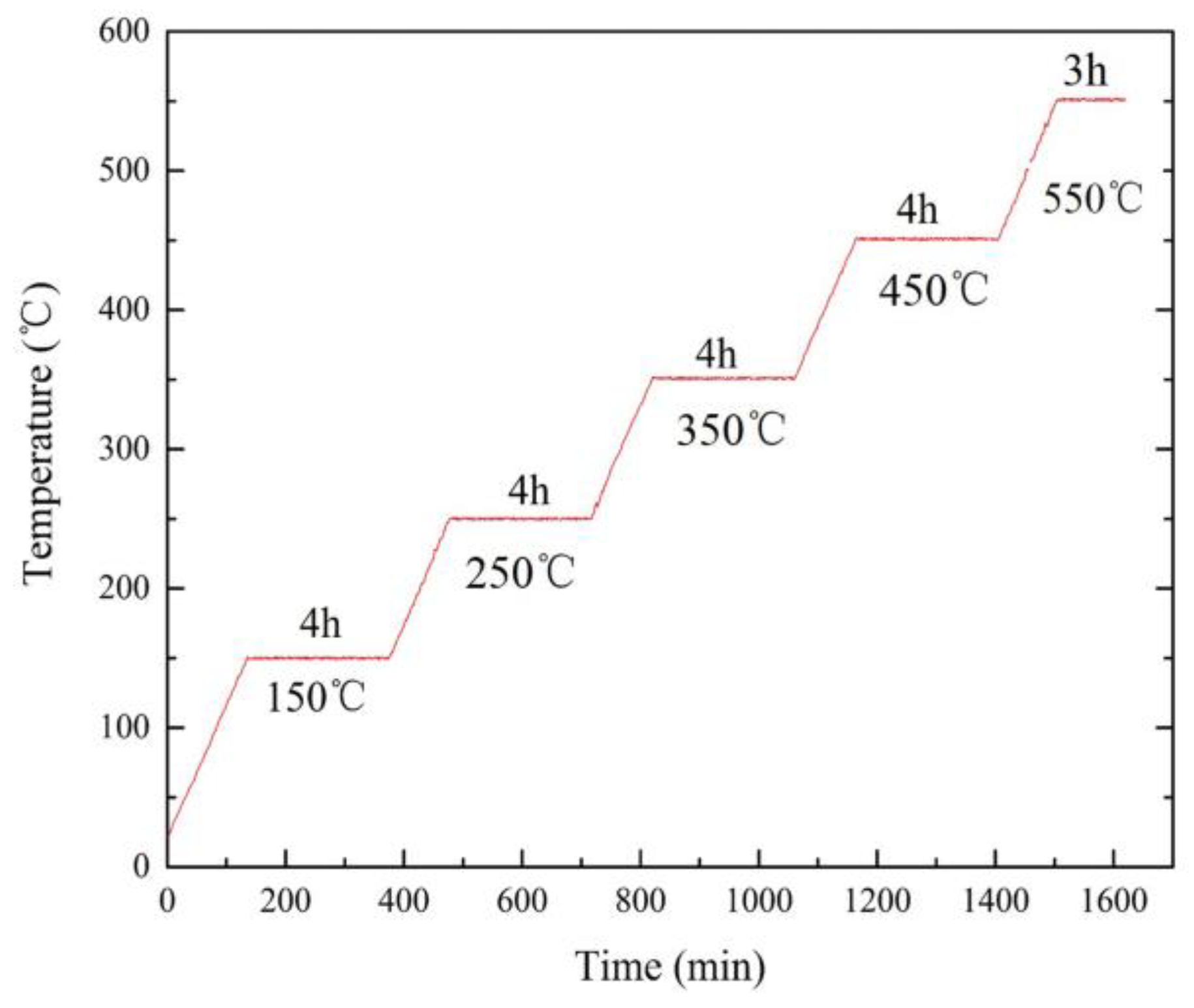
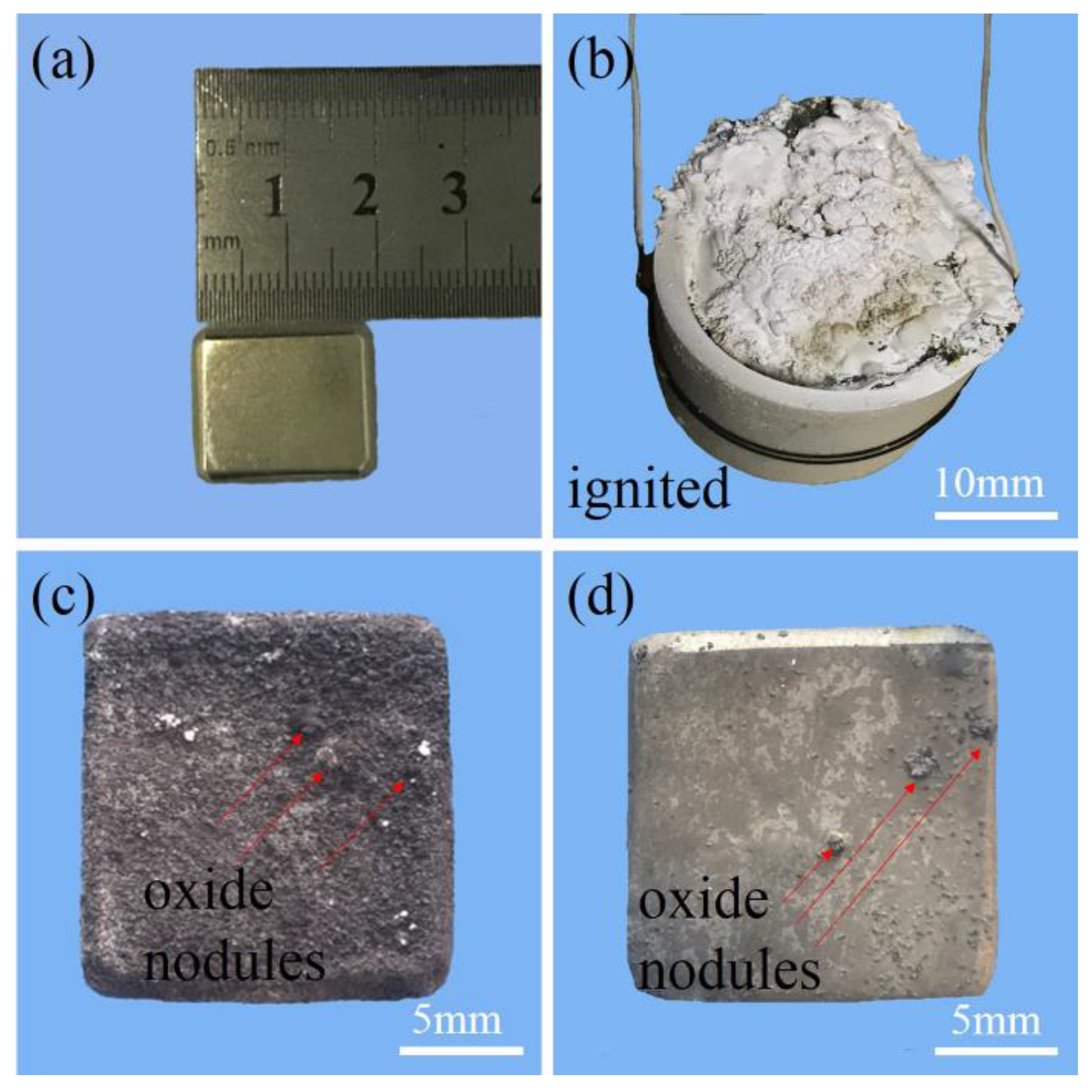
2.2. Oxidation Evaluation
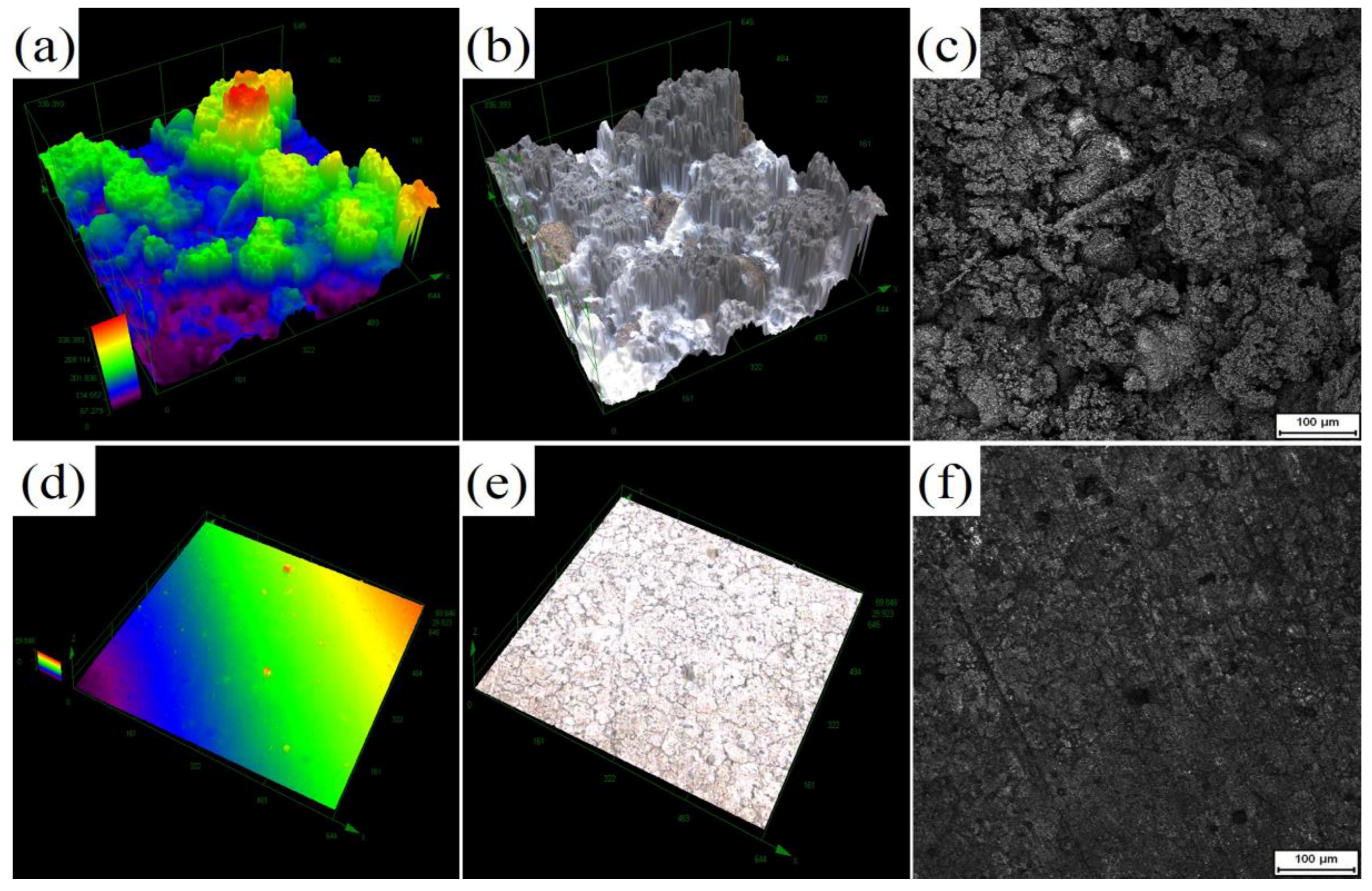
2.3. Microstructure Characterization
3. Results and Discussion
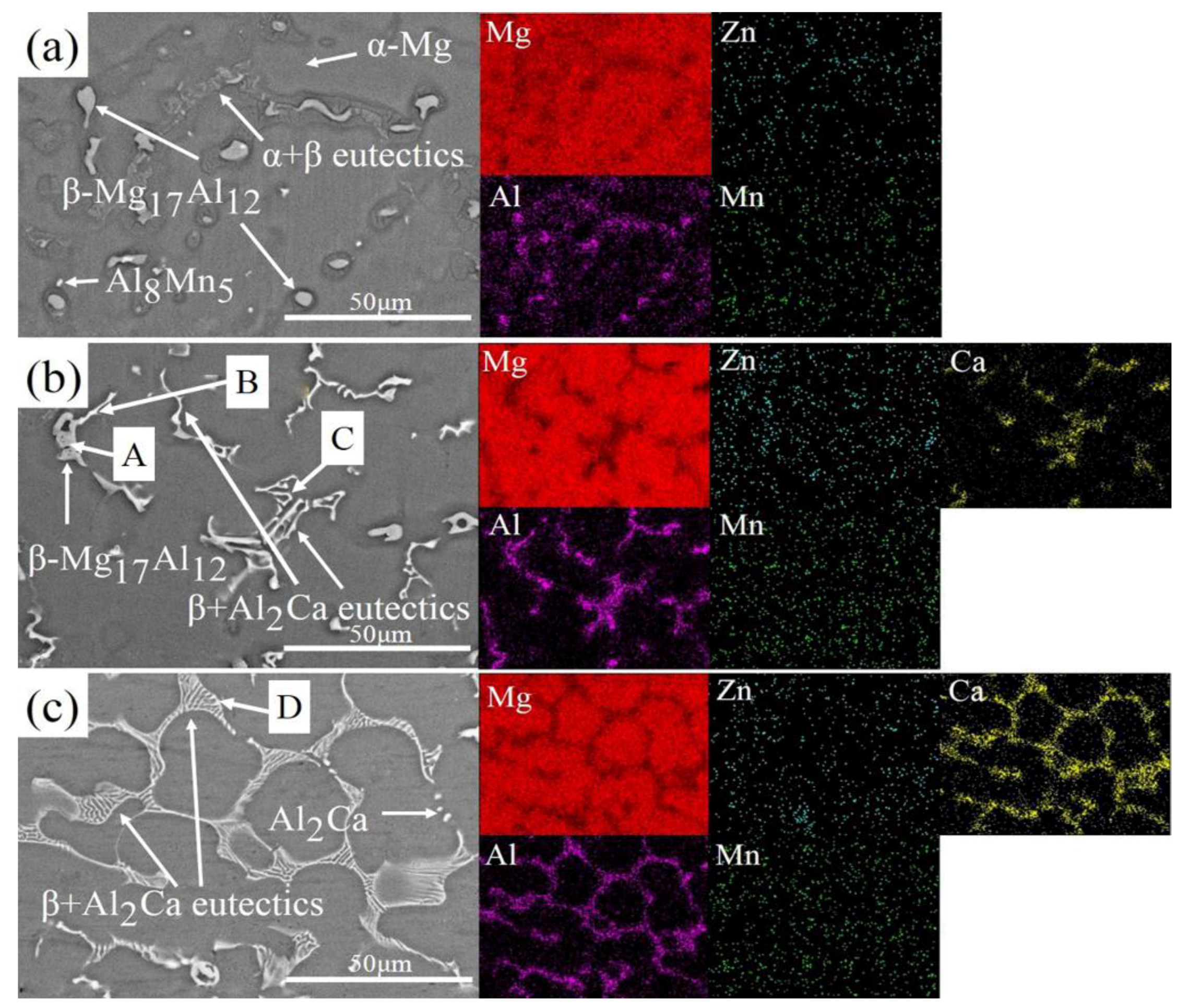
3.1. Microstructure Characterization
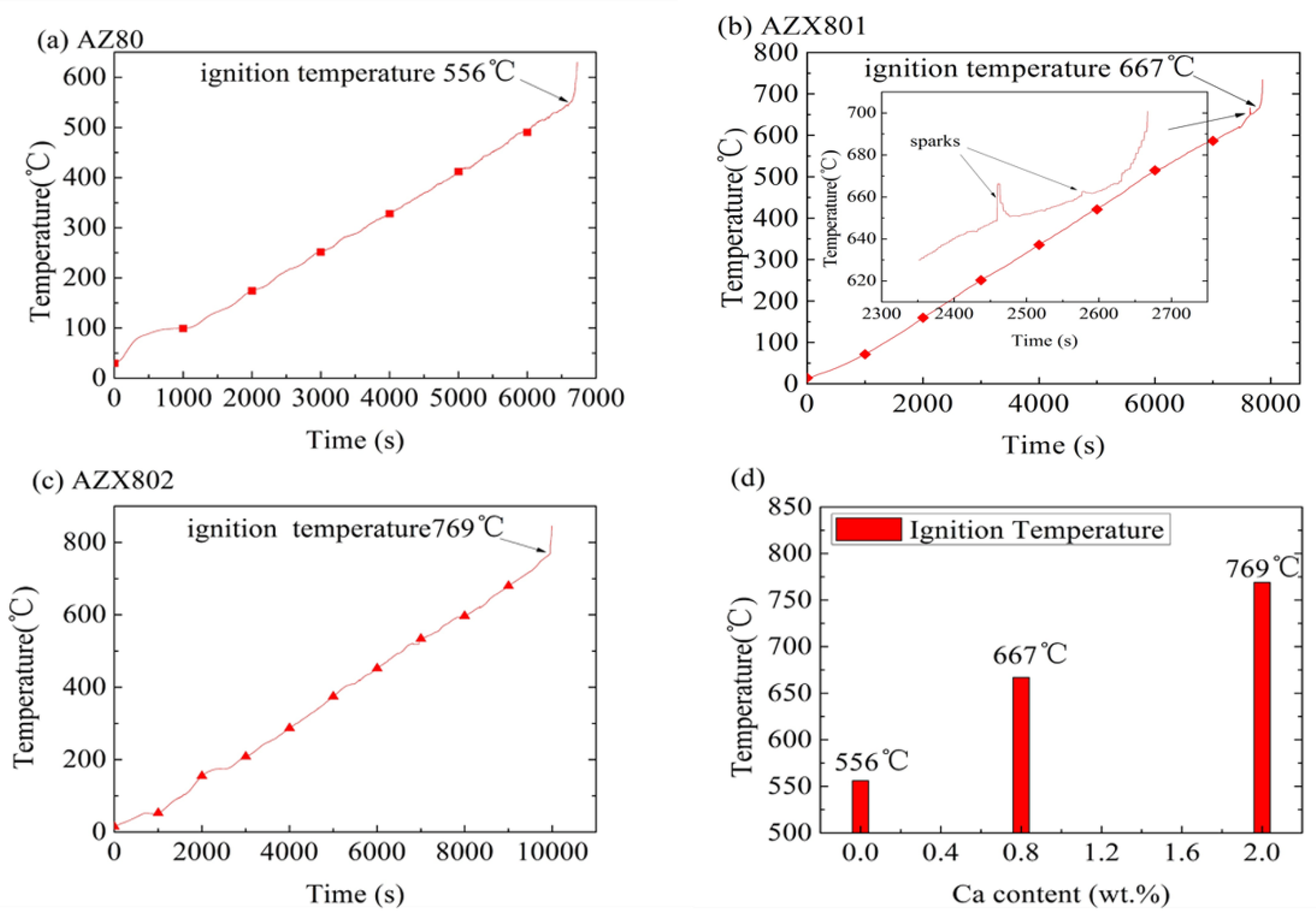
3.2. Ignition Temperature
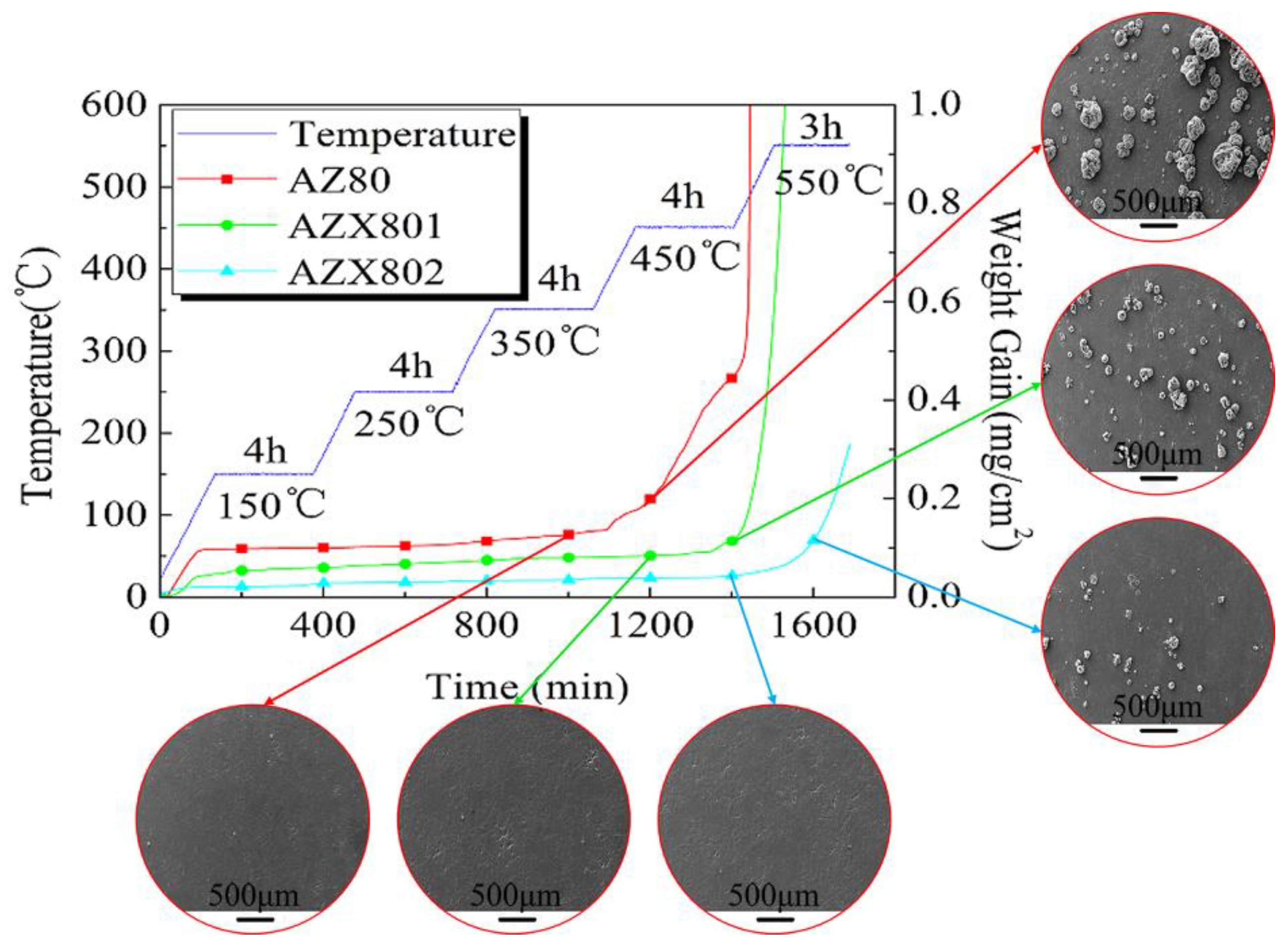
3.3. Multi-Stage Oxidation Behavior
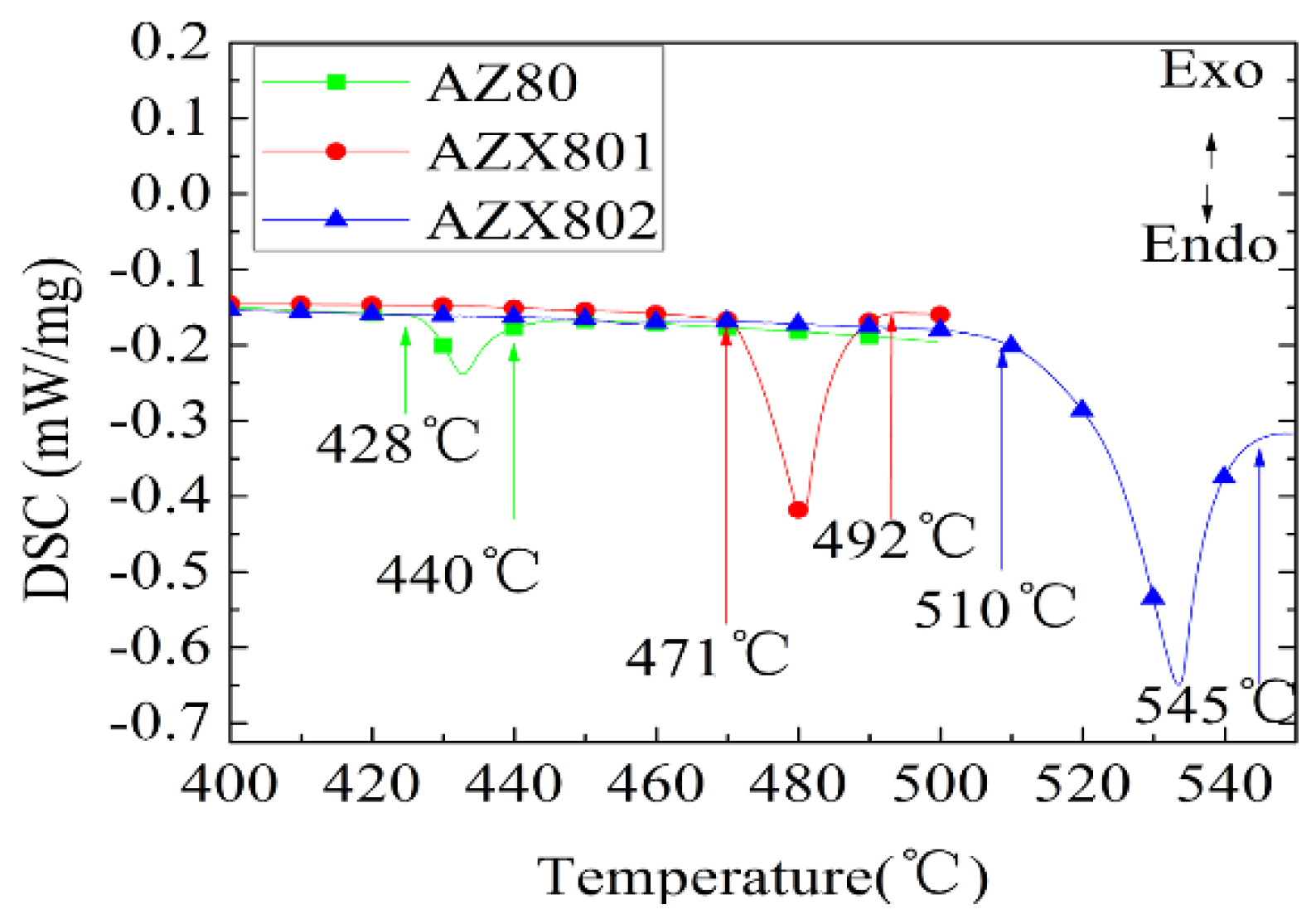
3.4. DSC Analysis
4. Conclusions
- (1)
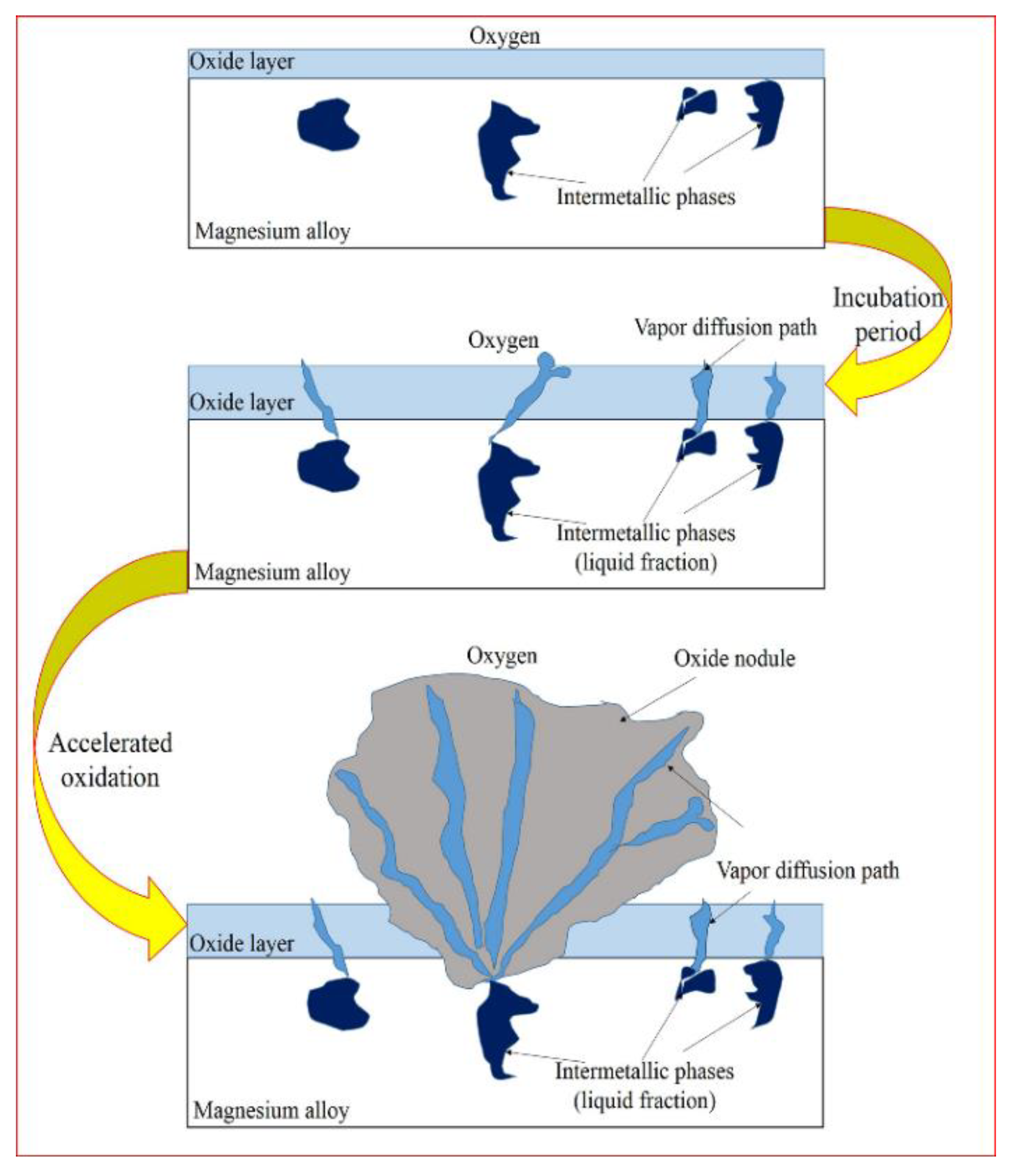
- With the increase of Ca content in AZ80, the divorced eutectic β-Mg17Al12 and α-Mg + β-Mg17Al12 intermetallics, in a lamellar eutectic micro-constituent, are replaced by a chain-shaped phase and network structure, considered as a β-Mg17Al12 + Al2Ca eutectic micro-constituent scattering in a α-Mg matrix. After multi-stage oxidation in air, the chain-shaped phase and network structure are fragmented and replaced by Al2Ca of spherical and rod like structures.
- (2)
- The addition of Ca in AZ80 can remarkably improve ignition point and oxidation resistance by increasing the melting temperature of the secondary phase due to the formation and increase of Al2Ca in alloys.
- (3)
- The incubation periods and beginning of the accelerated oxidation temperatures of AZ80, AZX801, and AZX802 are different during multi-stage oxidation, thus contributing to the different onset melting temperature of the secondary phase. The beginning of the accelerated oxidation of Mg alloys at high temperature is always accompanied by the onset melting of the low melting temperature of the secondary phase and growth of oxide nodule on surface.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Song, G.L.; Atrens, A. Corrosion mechanisms of magnesium alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Jafari, H.; Idris, M.H.; Ourdjini, A.; Payganeh, G. Effect of thermomechanical treatment on microstructure and hardness behavior of AZ63 magnesium alloy. Acta Metall. Sin. Engl. Lett. 2009, 22, 401–407. [Google Scholar] [CrossRef]
- Wei, Y.; Hou, L.; Yang, L.; Xu, B.M.; Kozuka, M.; Ichinose, H. Mechanical properties of die casting components with various thicknesses made of AZ91D alloy. J. Mater. Process. Technol. 2009, 209, 3278–3284. [Google Scholar] [CrossRef]
- Prasad, A.; Shi, Z.; Atrens, A. Influence of Al and Y on the ignition and flammability of Mg alloys. Corros. Sci. 2012, 55, 153–163. [Google Scholar] [CrossRef]
- Micro, P.; Jan, T.; Filippo, B. Mg and its alloys for biomedical applications: Exploring corrosion and its interplay with mechanical failure. Metals 2017, 7, 252. [Google Scholar]
- Aydin, D.S.; Bayindir, Z.; Hoseini, M.; Pekguleryuz, M.O. The high temperature oxidation and ignition behavior of Mg-Nd alloys. Part I: The oxidation of dilute alloys. J. Alloys Compd. 2013, 569, 35–44. [Google Scholar] [CrossRef]
- Zhao, S.; Zhou, H.; Zhou, T.; Zhang, Z.; Lin, P.; Ren, L. The oxidation resistance and ignition temperature of AZ31 magnesium alloy with additions of La2O3 and La. Corros. Sci. 2013, 67, 75–81. [Google Scholar] [CrossRef]
- Aydin, D.S.; Bayindir, Z.; Pekguleryuz, M.O. The high temperature oxidation behavior of Mg-Nd alloys. Part II: The effect of the two-phase microstructure on the on-set of oxidation and on oxide morphology. J. Alloys Compd. 2014, 584, 558–565. [Google Scholar] [CrossRef]
- Ravi Kumar, N.V.; Blandin, J.J.; Suéry, M.; Grosjean, E. Effect of alloying elements on the ignition resistance of magnesium alloys. Scr. Mater. 2003, 49, 225–230. [Google Scholar] [CrossRef]
- Feliu, S.; Samaniego, A.; Barranco, V.; El-Hadad, A.A.; Llorente, I.; Serra, C.; Galván, J.C. A study on the relationships between corrosion properties and chemistry of thermally oxidized surface films formed on polished commercial magnesium alloys AZ31 and AZ61. Appl. Surf. Sci. 2014, 295, 219–230. [Google Scholar] [CrossRef]
- Gobara, M.; Shamekh, M.; Akid, R. Improving the corrosion resistance of AZ91D magnesium alloy through reinforcement with titanium carbides and borides. J. Magn. Alloys 2015, 3, 112–120. [Google Scholar] [CrossRef]
- Mirak, A.R.; Davidson, C.J.; Taylor, J.A. Characterization of fresh surface films formed on molten Mg–Nd alloy protected by different atmospheres. Appl. Surf. Sci. 2014, 301, 91–98. [Google Scholar] [CrossRef]
- Kim, Y.M.; Yim, C.D.; Kim, H.S.; You, B.S. Key factor influencing the ignition resistance of magnesium alloys at elevated temperatures. Scr. Mater. 2011, 65, 958–961. [Google Scholar] [CrossRef]
- Li, W.; Zhou, H.; Zhou, W.; Li, W.P.; Wang, M.X. Effect of cooling rate on ignition point of AZ91D-0.98 wt.% Ce magnesium alloy. Mater. Lett. 2007, 61, 2772–2774. [Google Scholar] [CrossRef]
- Czerwinski, F. Controlling the ignition and flammability of magnesium for aerospace applications. Corros. Sci. 2014, 86, 1–16. [Google Scholar] [CrossRef]
- Lee, D.B. High temperature oxidation of AZ31+0.3 wt.% Ca and AZ31+0.3 wt.% CaO magnesium alloys. Corros. Sci. 2013, 70, 243–251. [Google Scholar] [CrossRef]
- Tekumalla, S.; Gupta, M. An insight into ignition factors and mechanisms of magnesium based materials: A review. Mater. Des. 2017, 113, 84–98. [Google Scholar] [CrossRef]
- Fan, J.F.; Yang, C.L.; Han, G.; Fang, S.; Yang, W.D.; Xu, B.S. Oxidation behavior of ignition-proof magnesium alloys with rare earth addition. J. Alloys Compd. 2011, 509, 2137–2142. [Google Scholar] [CrossRef]
- Liu, C.; Lu, S.; Fu, Y.; Zhang, H. Flammability and the oxidation kinetics of the magnesium alloys AZ31, WE43, and ZE10. Corros. Sci. 2015, 100, 177–185. [Google Scholar] [CrossRef]
- Allameh, S.H.; Emamy, M.; Maleki, E.; Pourbahari, B. Effect of microstructural refinement on tensile properties of AZ80 magnesium alloy via Ca addition and extrusion process. Procedia Mater. Sci. 2015, 11, 89–94. [Google Scholar] [CrossRef]
- Min, X.G.; Du, W.W.; Xue, F.; Sun, Y.S. Analysis of EET on Ca increasing the melting point of Mg17Al12 phase. Chin. Sci. Bull. 2002, 47, 1082–1086. [Google Scholar] [CrossRef]
- Liang, S.M.; Chen, R.S.; Blandin, J.J.; Suery, M.; Han, E.H. Thermal analysis and solidification pathways of Mg–Al–Ca system alloys. Mater. Sci. Eng. 2008, 480, 365–372. [Google Scholar] [CrossRef]
- Shi, L.L.; Xu, J.; Ma, E. Mg-Al-Ca In-Situ composites with a refined eutectic structure and their compressive properties. Metall. Mater. Trans. 2008, 39, 1225–1235. [Google Scholar] [CrossRef]
- Suzuki, A.; Saddock, N.D.; Jones, J.W.; Pollock, T.M. Solidification paths and eutectic intermetallic phases in Mg–Al–Ca ternary alloys. Acta Mater. 2005, 53, 2823–2834. [Google Scholar] [CrossRef]
- Wang, Z.J.; Xu, Y.; Zhu, J.; Li, S.L. Effect of homogenizing heat treatment on microstructure and hardness of AZ80–2.0Ca magnesium alloy. Spec. Cast. Nonferrous Alloys 2012, 32, 500–504. [Google Scholar]
- Bao, L.; Zhang, Z.; Le, Q.; Zhang, S.; Cui, J. Corrosion behavior and mechanism of Mg-Y-Zn-Zr alloys with various Y/Zn mole ratios. J. Alloys Compd. 2017, 712, 15–23. [Google Scholar] [CrossRef]
- Sakamoto, M.; Akiyama, S.; Ogi, K. Suppression of ignition and burning of molten Mg alloys by Ca bearing stable oxide film. J. Mater. Sci. Lett. 1997, 16, 1048–1050. [Google Scholar] [CrossRef]
- Czerwinski, F. The oxidation behavior of an AZ91D magnesium alloy at high temperatures. Acta Mater. 2002, 50, 2639–2654. [Google Scholar] [CrossRef]
- Czerwinski, F. Overcoming barriers of magnesium ignition and flammability. Adv. Mater. Process. 2014, 172, 28–31. [Google Scholar]
- Tan, Q.; Atrens, A.; Mo, N.; Zhang, M.X. Oxidation of magnesium alloys at elevated temperatures in air: A review. Corros. Sci. 2016, 112, 734–759. [Google Scholar] [CrossRef]
- Tan, Q.Y.; Mo, N.; Jiang, B.; Pan, F.S.; Atrens, A.; Zhang, M.X. Oxidation resistance of Mg-9Al-1Zn alloys micro-alloyed with Be. Scr. Mater. 2016, 115, 38–41. [Google Scholar] [CrossRef]
- Czerwinski, F. The reactive element effect on high-temperature oxidation of magnesium. Int. Mater. Rev. 2015, 60, 264–296. [Google Scholar] [CrossRef]
- Czerwinski, F. Oxidation characteristics of magnesium alloys. JOM 2012, 64, 1477–1483. [Google Scholar] [CrossRef]
- Czerwinski, F. Factors affecting the oxidation nature of magnesium alloys. JOM 2004, 56, 29–31. [Google Scholar] [CrossRef]
- Medved, J.; Mrvar, P.; Vončina, M. Oxidation resistance of cast magnesium alloys. Oxid. Met. 2009, 71, 257–270. [Google Scholar] [CrossRef]
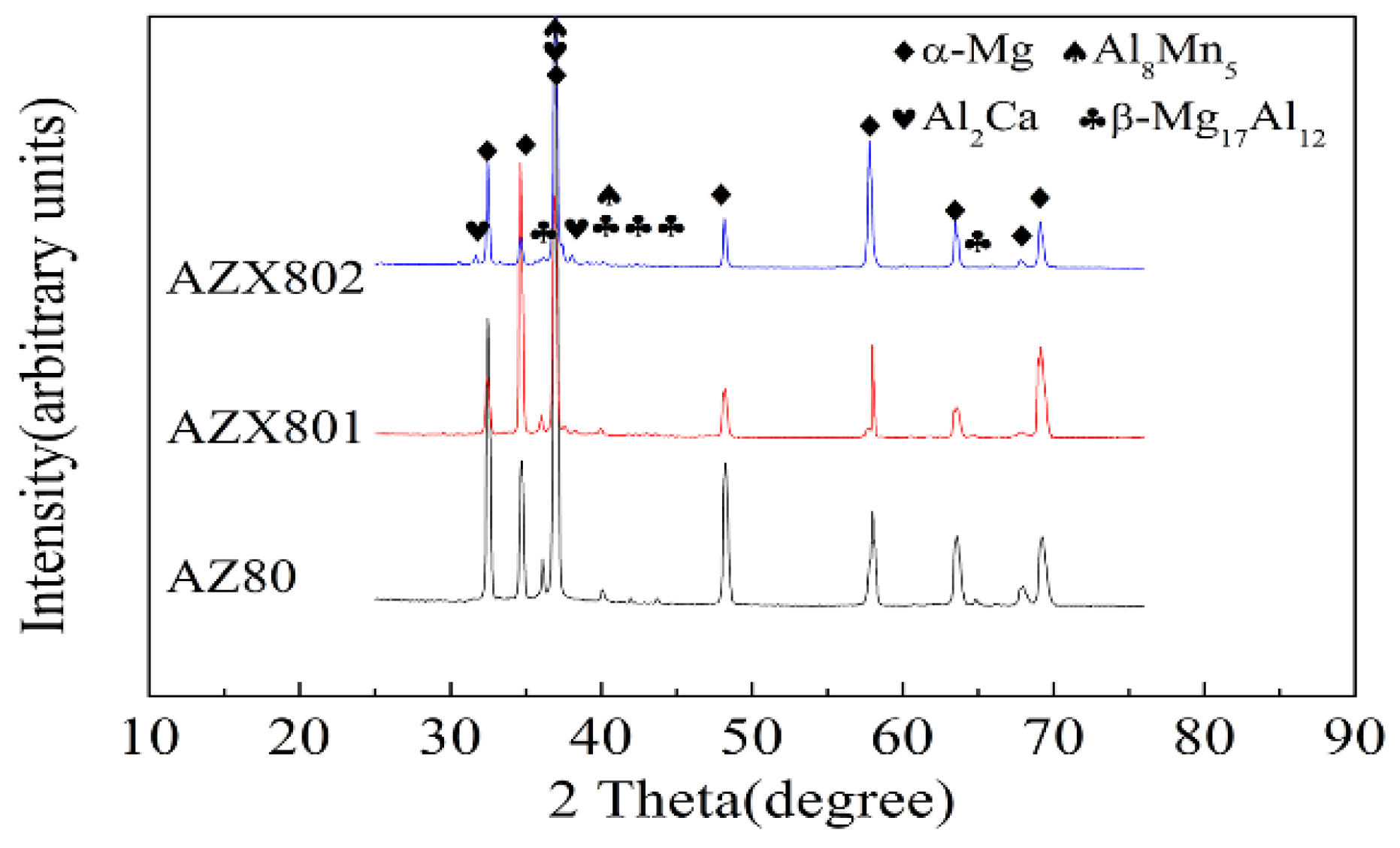
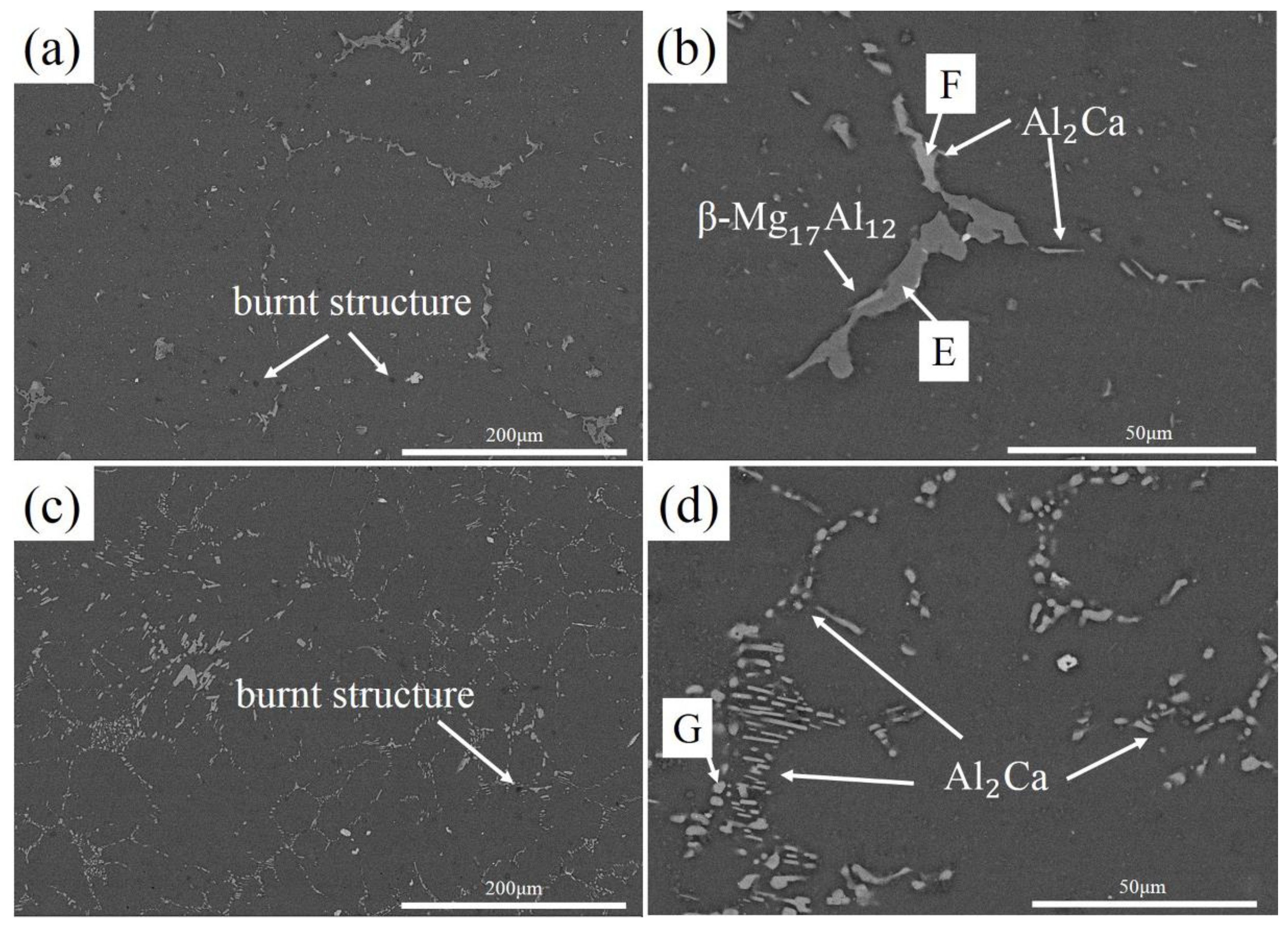
| Alloy | Al | Zn | Mn | Ca | Others | Mg |
|---|---|---|---|---|---|---|
| AZ80 | 8.23 | 0.56 | 0.33 | - | <0.1 | Bal. |
| AZX801 | 8.19 | 0.61 | 0.28 | 0.83 | <0.1 | Bal. |
| AZX802 | 8.21 | 0.59 | 0.35 | 1.97 | <0.1 | Bal. |
| Zone | Mg (at.%) | Al (at.%) | Ca (at.%) | Al/Ca | Phase |
|---|---|---|---|---|---|
| A | 74.67 | 23.27 | 2.27 | - | Mg17Al12 |
| B | 59.62 | 30.3 | 10.09 | 3.00 | Mg17Al12 + Al2Ca |
| C | 58.63 | 29.93 | 11.44 | 2.62 | Mg17Al12 + Al2Ca |
| D | 59.64 | 28.5 | 11.85 | 2.41 | Mg17Al12 + Al2Ca |
| E | 60.26 | 38.33 | 1.41 | - | Mg17Al12 |
| F | 9.78 | 63.38 | 26.85 | 2.36 | Al2Ca |
| G | 53.24 | 33.40 | 13.36 | 2.50 | Al2Ca |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.; Lan, Q.; Wang, A.; Le, Q.; Yang, F.; Li, X. Effect of Ca Additions on Ignition Temperature and Multi-Stage Oxidation Behavior of AZ80. Metals 2018, 8, 766. https://doi.org/10.3390/met8100766
Cheng C, Lan Q, Wang A, Le Q, Yang F, Li X. Effect of Ca Additions on Ignition Temperature and Multi-Stage Oxidation Behavior of AZ80. Metals. 2018; 8(10):766. https://doi.org/10.3390/met8100766
Chicago/Turabian StyleCheng, Chunlong, Qing Lan, An Wang, Qichi Le, Fan Yang, and Xiaoqiang Li. 2018. "Effect of Ca Additions on Ignition Temperature and Multi-Stage Oxidation Behavior of AZ80" Metals 8, no. 10: 766. https://doi.org/10.3390/met8100766
APA StyleCheng, C., Lan, Q., Wang, A., Le, Q., Yang, F., & Li, X. (2018). Effect of Ca Additions on Ignition Temperature and Multi-Stage Oxidation Behavior of AZ80. Metals, 8(10), 766. https://doi.org/10.3390/met8100766