The Co-B Amorphous Alloy: A High Capacity Anode Material for an Alkaline Rechargeable Battery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
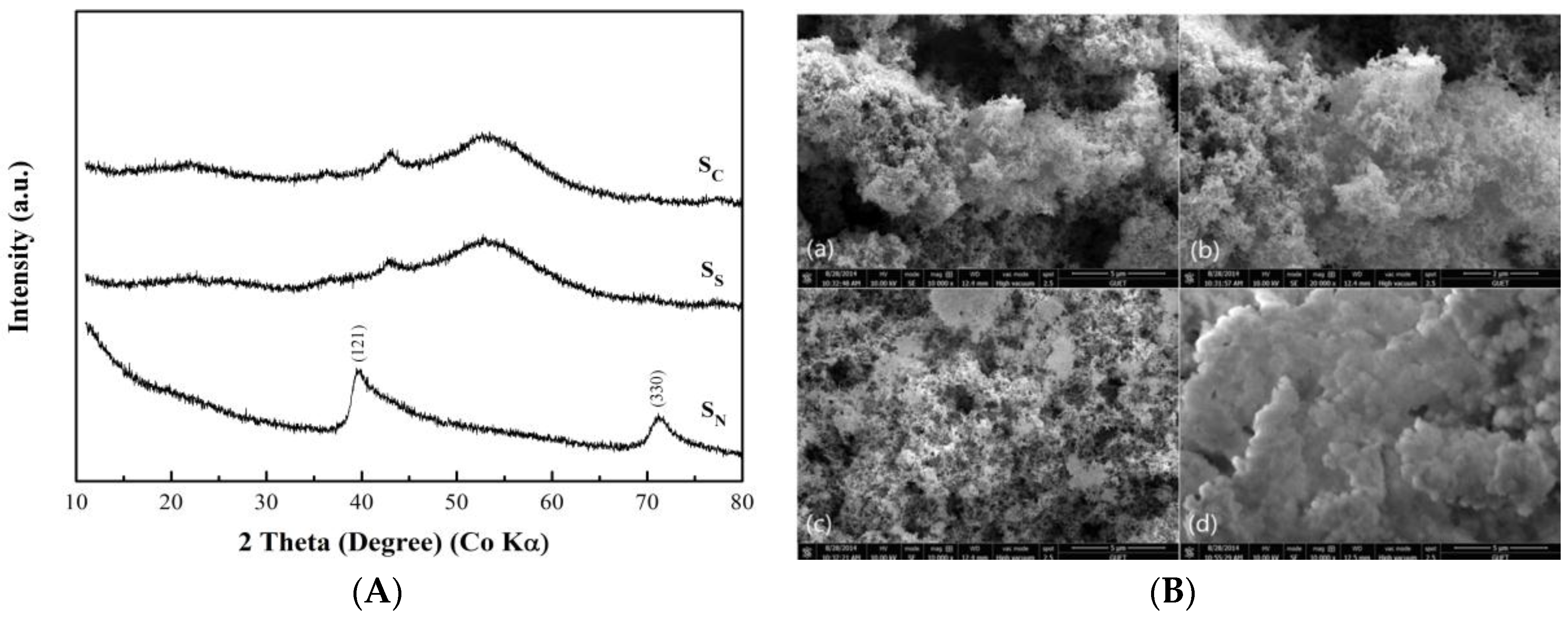
2.2. Structural and Morphological Characterization
2.3. Electrochemical Measurements
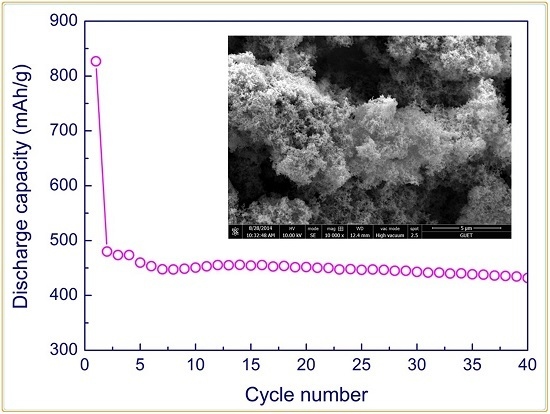
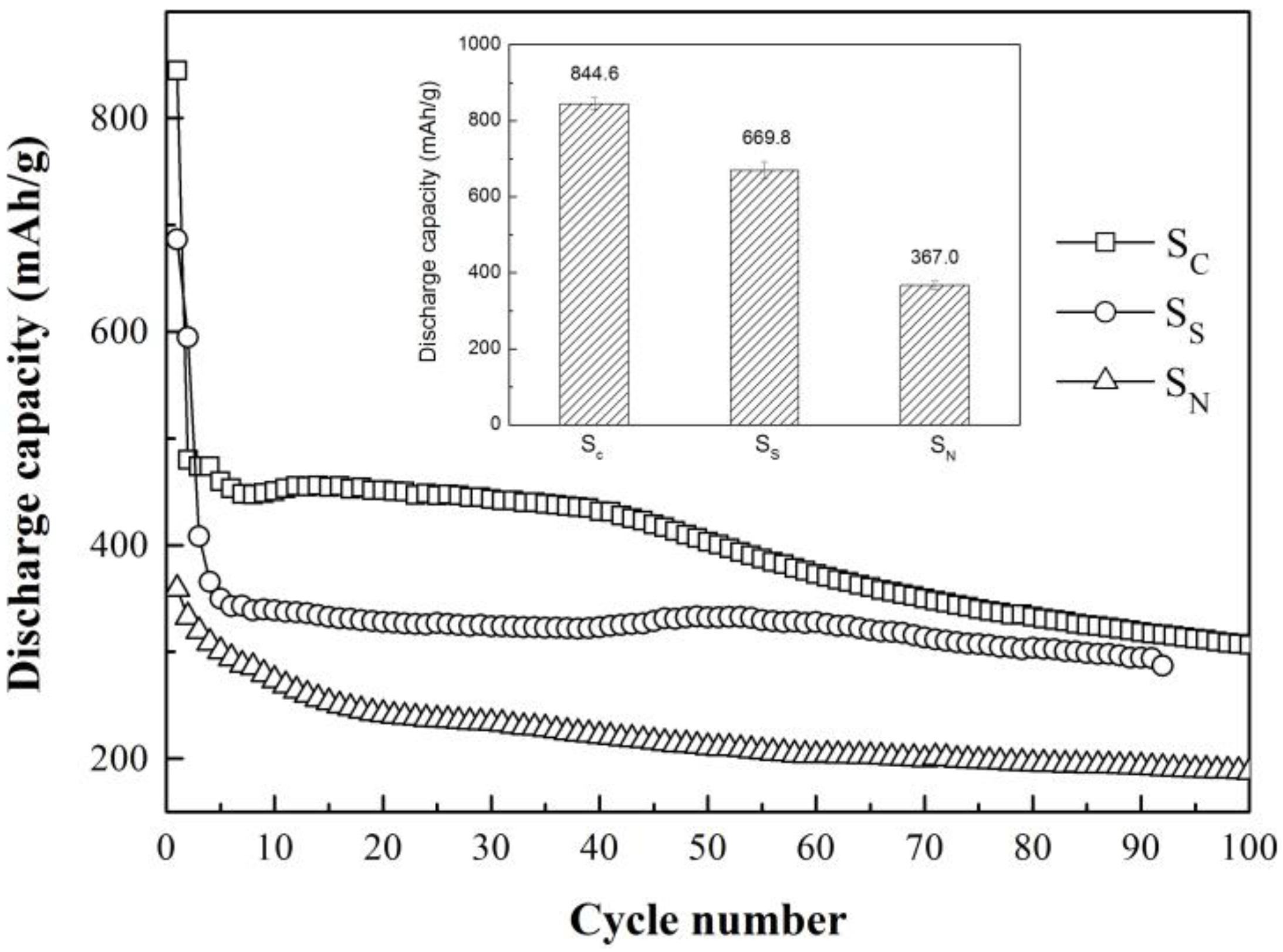
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tliha, M.; Khaldi, C.; Boussami, S.; Fenineche, N.; El-Kedim, O.; Mathlouthi, H.; Lamloumi, J. Kinetic and thermodynamic studies of hydrogen storage alloys as negative electrode materials for Ni/MH batteries: A review. J. Solid State Electrochem. 2014, 18, 577–593. [Google Scholar] [CrossRef]
- Huang, J.L.; Qiu, S.J.; Chu, H.L.; Zou, Y.J.; Xiang, C.L.; Zhang, H.Z.; Xu, F.; Sun, L.X.; Ouyang, L.Z.; Zhou, H.Y. Enhancement of the electrochemical properties of rare earth-based alloy by doping with CoZnB alloy. Int. J. Hydrog. Energy 2015, 40, 14173–14178. [Google Scholar] [CrossRef]
- Liu, Y.F.; Pan, H.G.; Gao, M.X.; Wang, Q.D. Advanced hydrogen storage alloys for Ni/MH rechargeable batteries. J. Mater. Chem. 2011, 21, 4743–4755. [Google Scholar] [CrossRef]
- Qiu, S.J.; Huang, J.L.; Chu, H.L.; Zou, Y.J.; Xiang, C.L.; Zhang, H.Z.; Xu, F.; Sun, L.X.; Zhou, H.Y. Influence of boron introduction on structure and electrochemical hydrogen storage properties of Ti–V-based alloys. J. Alloy. Compd. 2015, 648, 320–325. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Li, J.J.; Yao, Y.; Ma, L.Q.; Shen, X.D. Electrochemical hydrogen storage properties of a non-equilibrium Ti2Ni alloy. RSC Adv. 2012, 2, 2149–2153. [Google Scholar] [CrossRef]
- Qiu, S.J.; Huang, J.L.; Chu, H.L.; Zou, Y.J.; Xiang, C.L.; Zhang, H.Z.; Xu, F.; Sun, L.X.; Ouyang, L.Z.; Zhou, H.Y. Influence of Zr addition on structure and performance of rare earth Mg-based alloys as anodes in Ni/MH battery. Metals 2015, 5, 565–577. [Google Scholar] [CrossRef]
- Zhuang, X.Y.; Zhang, Y.; Zhu, Y.F.; Qu, Y.; Zhan, L.Y.; Wan, N.; Cheng, H.H.; Guo, X.L.; Chen, J.; Wang, Z.M.; et al. The effects of Pd and/or Zr additives on the structures and cyclic stabilities of Mg50Ni50-based electrode alloys. Int. J. Hydrog. Energy 2015, 40, 2768–2774. [Google Scholar] [CrossRef]
- Dymek, M.; Bala, H.; Hackemer, A.; Drulis, H. Hydrogenation and electrochemical corrosion properties of LaNi4.5Co0.5 alloy doped with aluminum. Solid State Ionics 2015, 271, 116–120. [Google Scholar] [CrossRef]
- Wang, Y.D.; Ai, X.P.; Yang, H.X. Electrochemical hydrogen storage behaviors of ultrafine amorphous Co−B alloy particles. Chem. Mater. 2004, 16, 5194–5197. [Google Scholar] [CrossRef]
- Qiu, S.J.; Huang, J.L.; Shen, F.H.; Pang, R.; Chu, H.L.; Zou, Y.J.; Xiang, C.L.; Xu, F.; Du, Y.; Wang, J.C.; et al. Ternary Co–Ni–B amorphous alloy with a superior electrochemical performance in a wide temperature range. Int. J. Hydrog. Energy 2016, 41, 3955–3960. [Google Scholar] [CrossRef]
- Qiu, S.J.; Huang, J.L.; Shen, F.H.; Pang, R.; Chu, H.L.; Zou, Y.J.; Xiang, C.L.; Yan, E.H.; Xu, F.; Sun, L.X.; et al. Enhancement of the electrochemical performance of CoB amorphous alloy through the addition of A2B7-type alloy. Int. J. Hydrog. Energy 2016, 41, 16142–16147. [Google Scholar]
- Gao, X.P.; Yang, H.X. Multi-electron reaction materials for high energy density batteries. Energy Environ. Sci. 2010, 3, 174–189. [Google Scholar]
- Zhao, X.Y.; Ma, L.Q.; Yao, Y.; Yang, M.; Ding, Y.; Shen, X.D. Electrochemical energy storage of Co powders in alkaline electrolyte. Electrochim. Acta 2010, 55, 1169–1174. [Google Scholar] [CrossRef]
- He, G.H.; Jiao, L.F.; Yuan, H.T.; Zhang, Y.Y.; Zhang, Y.H.; Wang, Y.J. Effect of synthesis method on the structure and electrochemical behaviour of Co–Si particles. Int. J. Hydrog. Energy 2007, 32, 3416–3419. [Google Scholar] [CrossRef]
- Lu, D.S.; Li, W.S.; Xiao, F.M.; Tang, R.H. Drastically enhanced cycle life of Co-B alloy electrode by 8-hydroxyquinoline at elevated temperature. Electrochem. Commun. 2010, 12, 362–366. [Google Scholar] [CrossRef]
- Wang, Q.H.; Jiao, L.F.; Du, H.M.; Huan, Q.N.; Peng, W.X.; Song, D.W.; Wang, Y.J.; Yuan, H.T. Chainlike structures assembled by Co hierarchitectures: Synthesis and electrochemical properties as negative materials for alkaline secondary batteries. J. Mater. Chem. 2011, 21, 14159–14162. [Google Scholar] [CrossRef]
- Wang, Q.H.; Jiao, L.F.; Du, H.M.; Huan, Q.N.; Peng, W.X.; Song, D.W.; Wang, Y.J.; Yuan, H.T. Novel flower-like CoS hierarchitectures: One-pot synthesis and electrochemical properties. J. Mater. Chem. 2011, 21, 327–329. [Google Scholar] [CrossRef]
- Cao, Y.L.; Zhou, W.C.; Li, X.Y.; Ai, X.P.; Gao, Y.P.; Yang, H.X. Electrochemical hydrogen storage behaviors of ultrafine Co–P particles prepared by direct ball-milling method. Electrochim. Acta 2006, 51, 4285–4290. [Google Scholar] [CrossRef]
- Lu, D.S.; Li, W.S.; Tan, C.L.; Zeng, R.H. Investigation of Co-B–S system as anode material for secondary alkaline battery. Electrochim. Acta 2009, 55, 171–177. [Google Scholar] [CrossRef]
- Tong, D.G.; Wang, D.; Chu, W.; Sun, J.H.; Wu, P. Cobalt–boron amorphous alloy prepared in water/cetyl-trimethyl-ammonium bromide/n-hexanol microemulsion as anode for alkaline secondary batteries. Electrochim. Acta 2010, 55, 2299–2305. [Google Scholar] [CrossRef]
- Wu, C.; Pang, C.H.; Wu, F.; Bai, Y.; Chen, C.; Zhong, Y. Effects of the pre-freezing time on the structures and electrochemical performances of Co-B alloys. Adv. Mater. Res. 2012, 391, 1085–1089. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Li, J.J.; Yao, Y.; Ma, L.Q.; Shen, X.D. Electrochemical redox mechanism of Co-B-H anode material and its optimization by a novel electrolyte additive. RSC Adv. 2013, 3, 1327–1331. [Google Scholar] [CrossRef]
- Patel, N.; Miotello, A. Progress in Co-B related catalyst for hydrogen production by hydrolysis of boron-hydrides: A review and the perspectives to substitute noble metals. Int. J. Hydrog. Energy 2015, 40, 1429–1464. [Google Scholar] [CrossRef]
- Chen, Y. Chemical preparation and characterization of metal–metalloid ultrafine amorphous alloy particles. Catal. Today 1998, 44, 3–16. [Google Scholar] [CrossRef]
- Zhang, X.X.; Gu, G.; Huang, H.J.; Yang, S.H.; Du, Y.W. Self-assembled Co3(BO3)2/surfactant nanostructured multilayers. J. Phys. Condens. Matter 2001, 13, 3913. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Liu, Y.N.; Yan, W. Preparation of porous (Ni,Co)3(BO3)2/Ni(OH)2 nanosheet networks as pseudocapacitor materials with superior performance. J. Mater. Chem. A 2014, 2, 5903–5909. [Google Scholar] [CrossRef]
- Zou, Y.J.; Cheng, J.; Wang, Q.Y.; Xiang, C.L.; Chu, H.L.; Qiu, S.J.; Zhang, H.Z.; Xu, F.; Liu, S.S.; Tang, C.Y.; et al. Cobalt–boron/nickel–boron nanocomposite with improved catalytic performance for the hydrolysis of ammonia borane. Int. J. Hydrog. Energy 2015, 40, 13423–13430. [Google Scholar] [CrossRef]
- Minakshi, M. Examining manganese dioxide electrode in KOH electrolyte using TEM technique. J. Electroanal. Chem. 2008, 616, 99–106. [Google Scholar] [CrossRef]
- Akdim, O.; Demirci, U.B.; Muller, D.; Miele, P. Cobalt (II) salts, performing materials for generating hydrogen from sodium borohydride. Int. J. Hydrog. Energy 2009, 34, 2631–2637. [Google Scholar] [CrossRef]
- Wu, C.; Bai, Y.; Wu, F.; Yi, B.L.; Zhang, H.M. Highly active cobalt-based catalysts in situ prepared from CoX2 (X = Cl−, NO3−) and used for promoting hydrogen generation from NaBH4 solution. Int. J. Hydrog. Energy 2010, 35, 2675–2679. [Google Scholar] [CrossRef]
- Kuriyama, N.; Sakai, T.; Miyamura, H.; Uehara, I.; Ishikawa, H.; Iwasaki, T. Electrochemical impedance and deterioration behavior of metal hydride electrodes. J. Alloy. Compd. 1993, 202, 183–197. [Google Scholar] [CrossRef]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, S.; Huang, J.; Chu, H.; Zou, Y.; Xiang, C.; Yan, E.; Xu, F.; Sun, L. The Co-B Amorphous Alloy: A High Capacity Anode Material for an Alkaline Rechargeable Battery. Metals 2016, 6, 269. https://doi.org/10.3390/met6110269
Qiu S, Huang J, Chu H, Zou Y, Xiang C, Yan E, Xu F, Sun L. The Co-B Amorphous Alloy: A High Capacity Anode Material for an Alkaline Rechargeable Battery. Metals. 2016; 6(11):269. https://doi.org/10.3390/met6110269
Chicago/Turabian StyleQiu, Shujun, Jianling Huang, Hailiang Chu, Yongjin Zou, Cuili Xiang, Erhu Yan, Fen Xu, and Lixian Sun. 2016. "The Co-B Amorphous Alloy: A High Capacity Anode Material for an Alkaline Rechargeable Battery" Metals 6, no. 11: 269. https://doi.org/10.3390/met6110269
APA StyleQiu, S., Huang, J., Chu, H., Zou, Y., Xiang, C., Yan, E., Xu, F., & Sun, L. (2016). The Co-B Amorphous Alloy: A High Capacity Anode Material for an Alkaline Rechargeable Battery. Metals, 6(11), 269. https://doi.org/10.3390/met6110269