Study on the Corrosion Behavior and Mechanism of Supersonic Flame Sprayed C276 Coating in Simulated Seawater Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Coating Deposition
2.2. Coating Morphology Characterization
2.3. Electrochemical Testing
2.4. Weightlessness Testing
3. Results
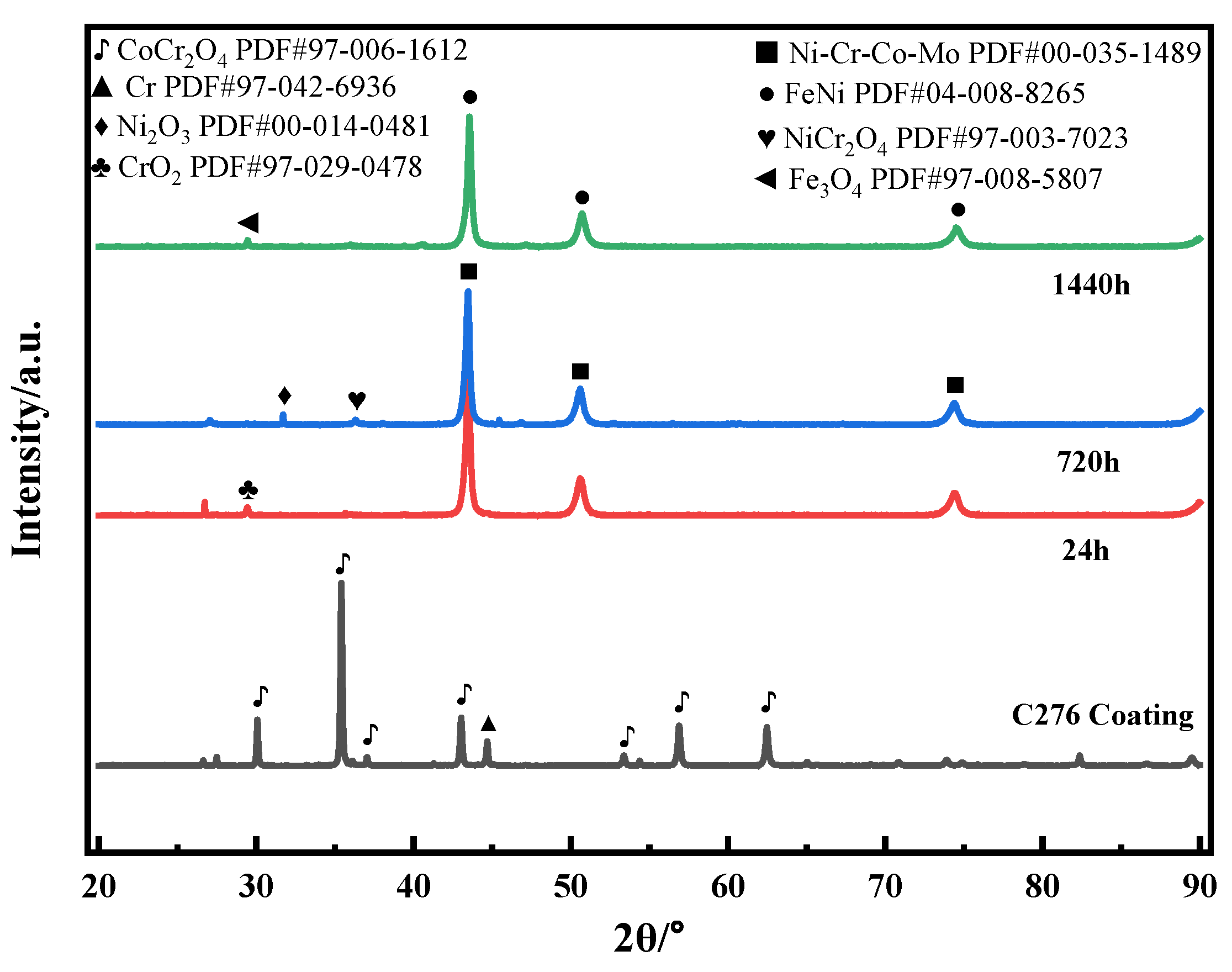
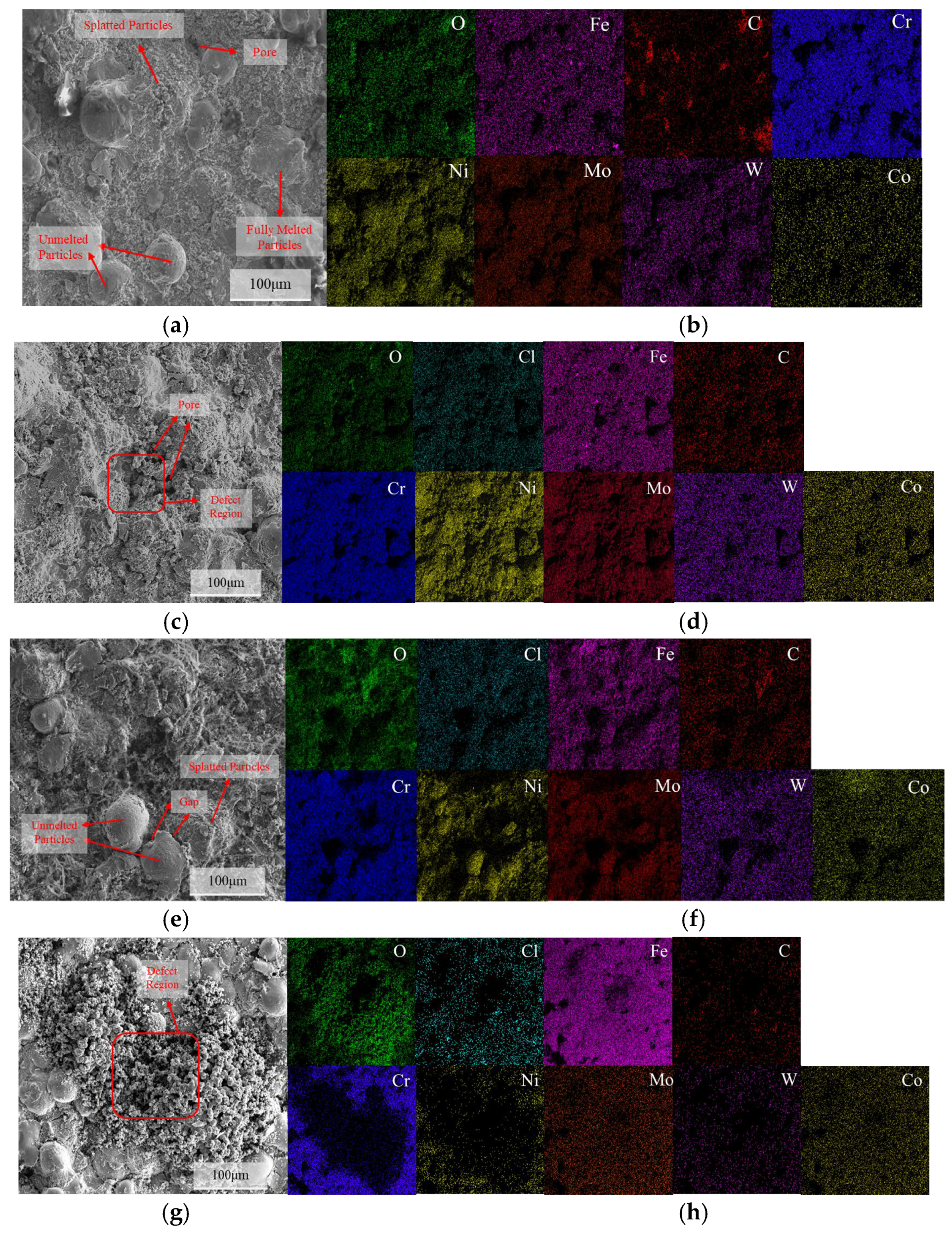
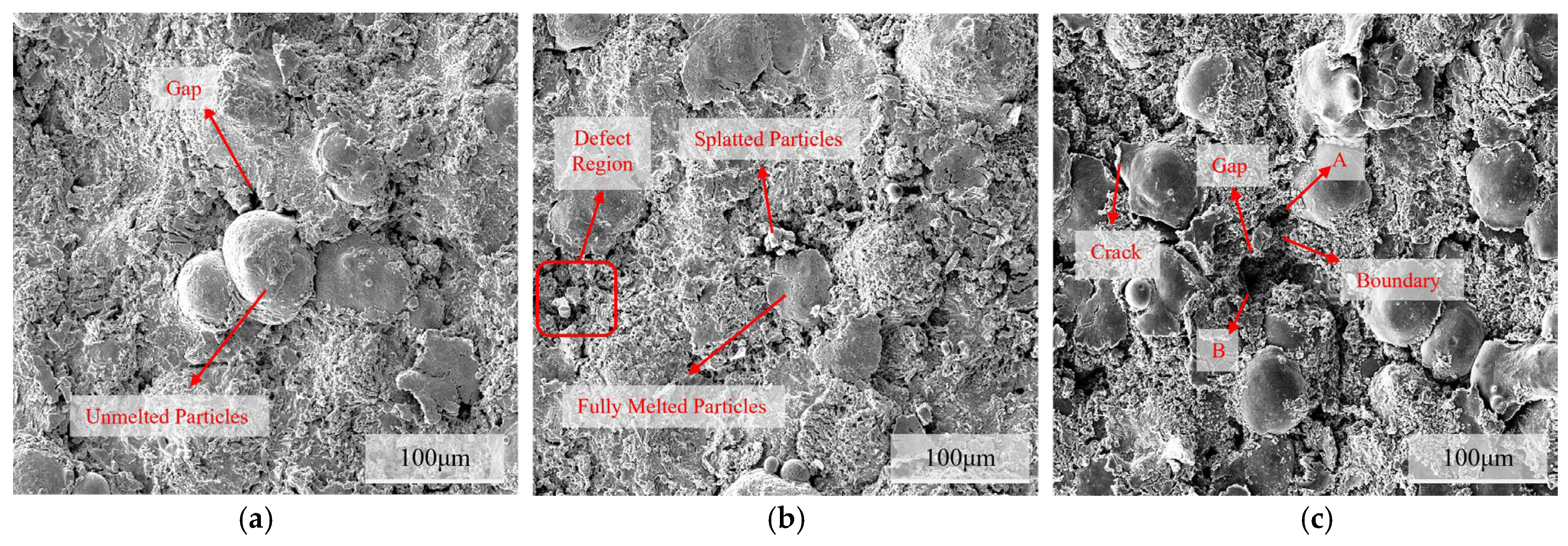
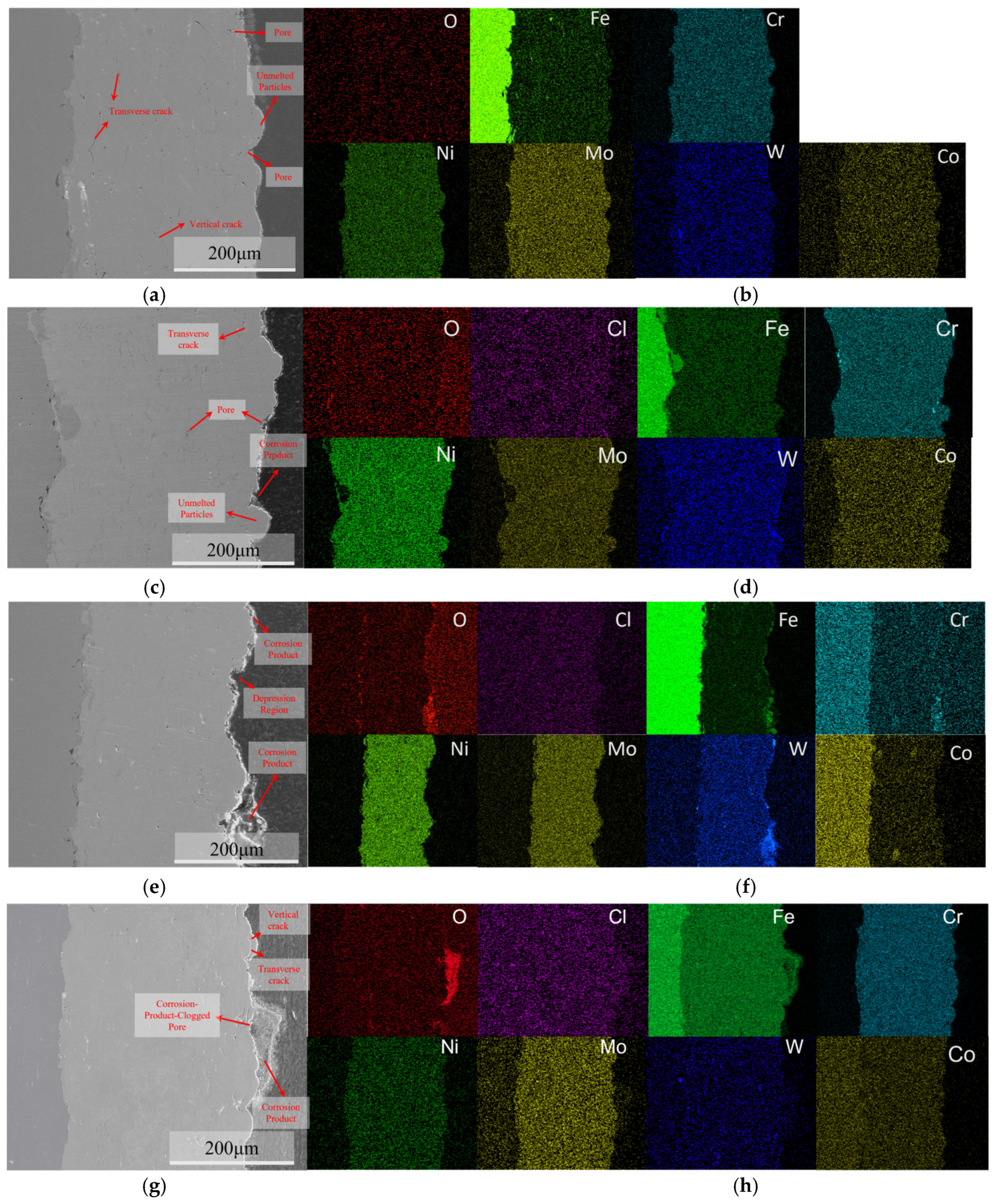
3.1. Microstructural Characterization of C276 Coating
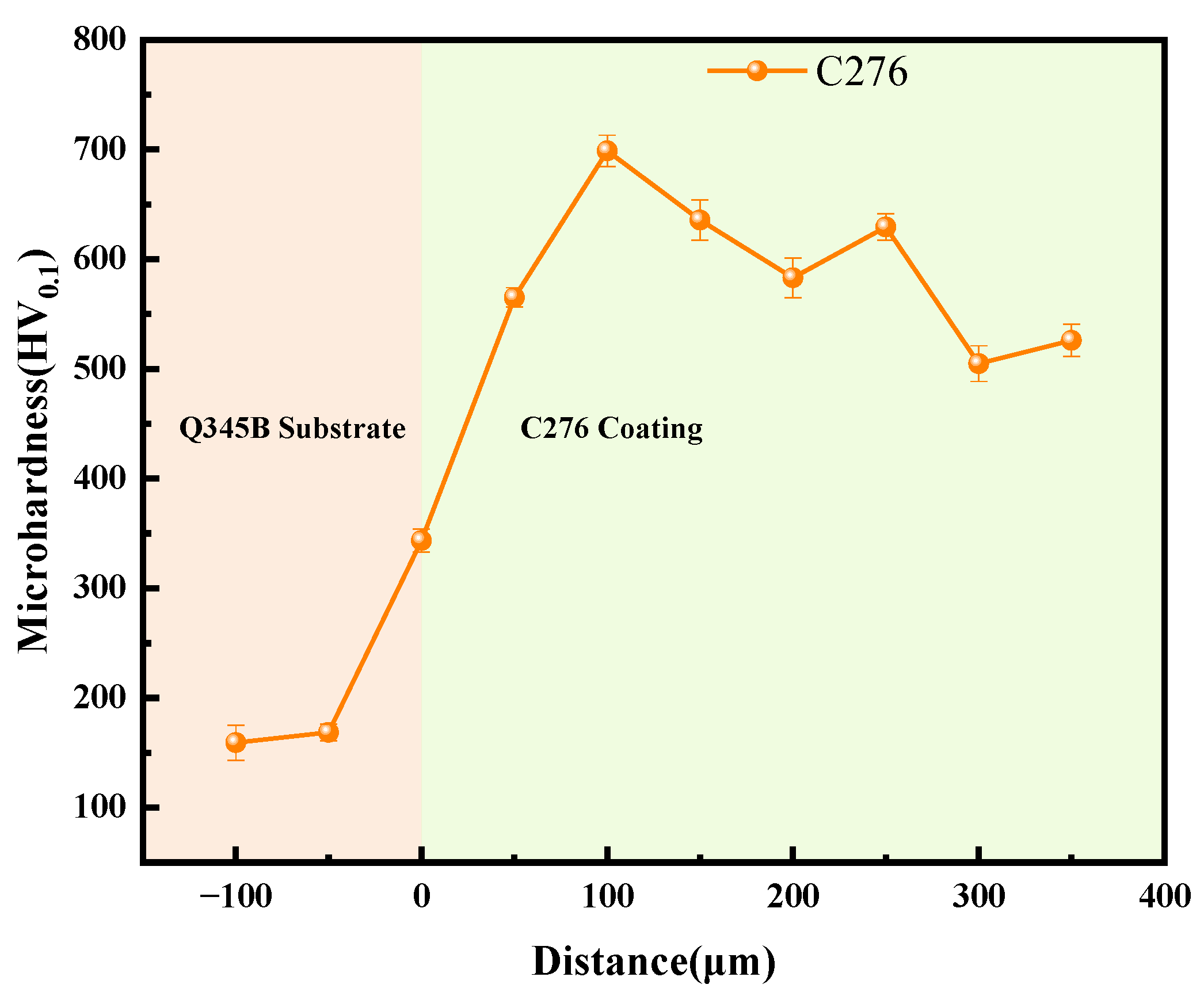
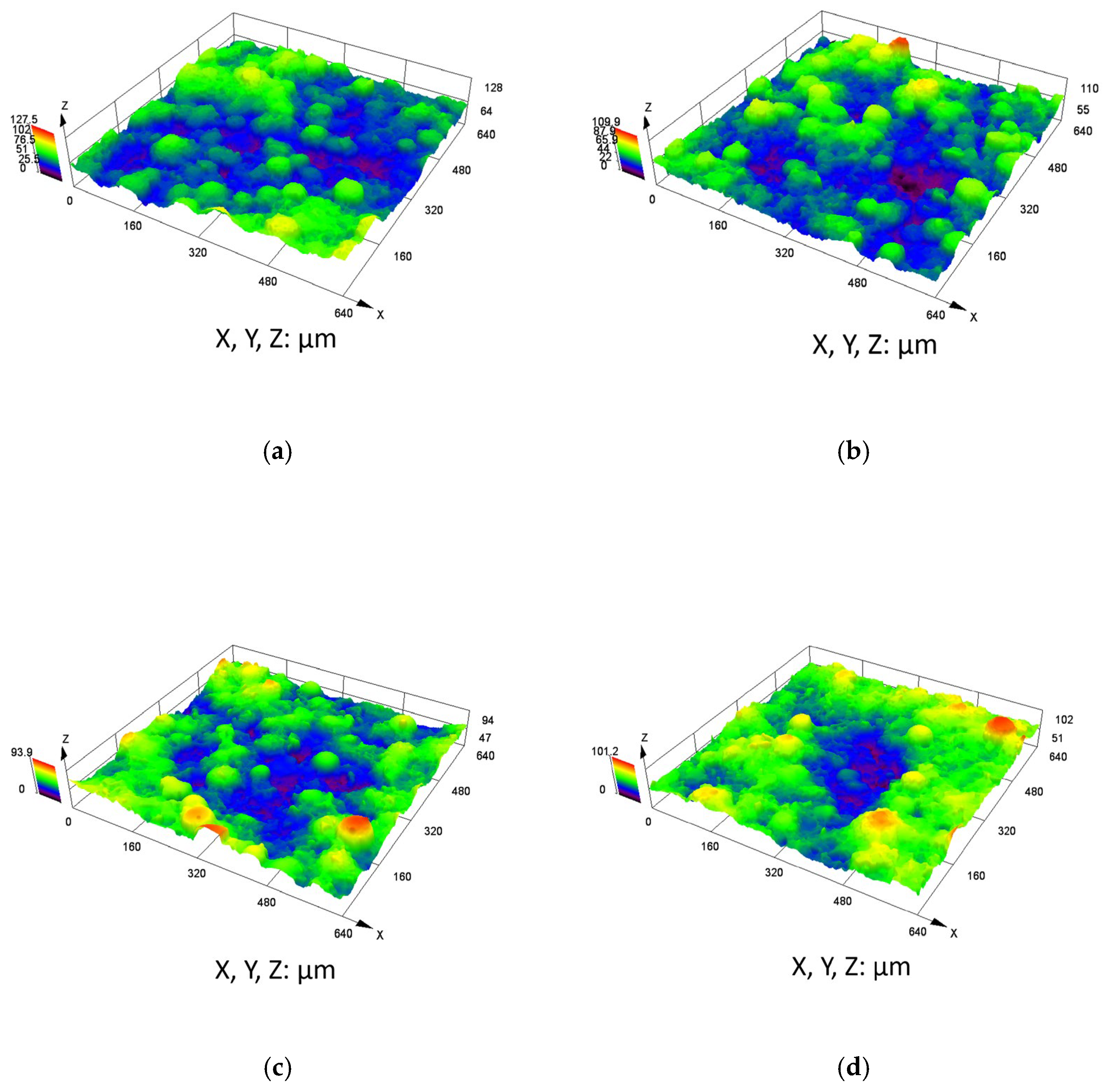
3.2. Physical Properties of the Coating
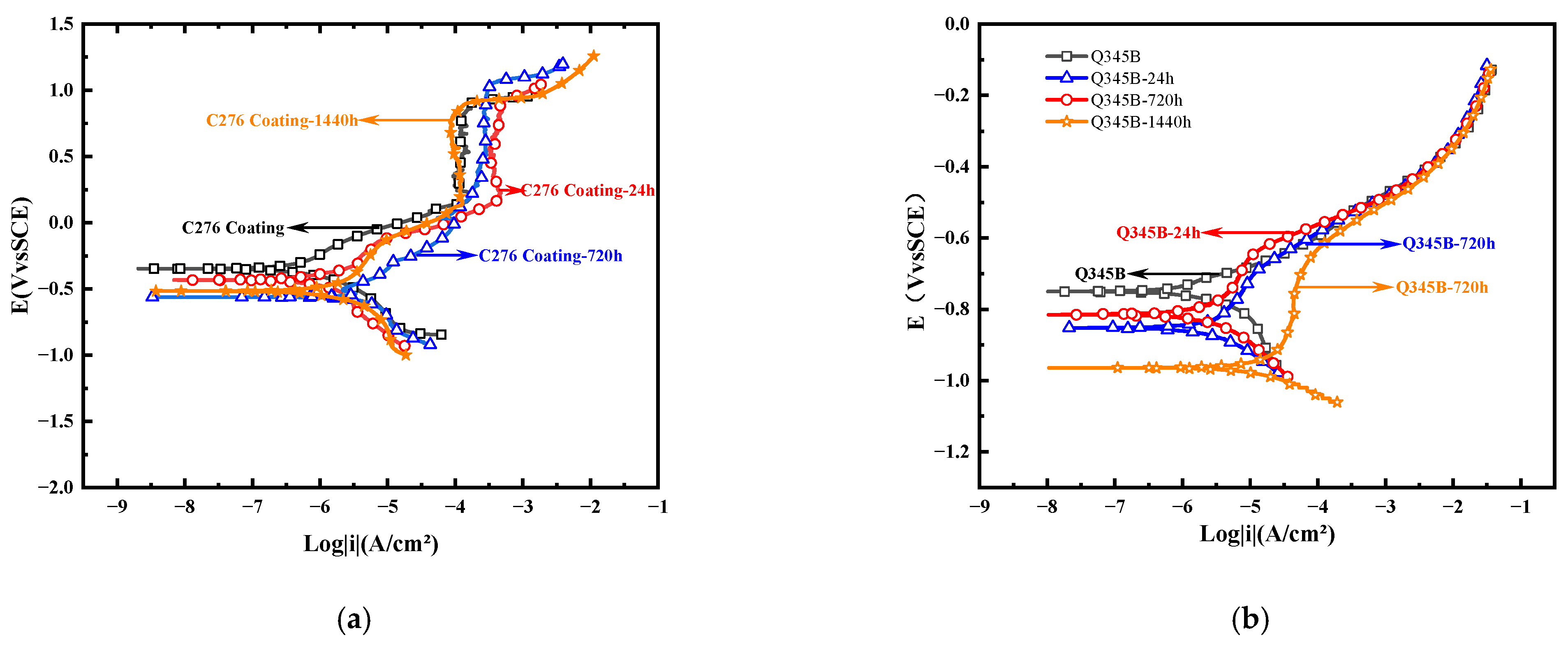
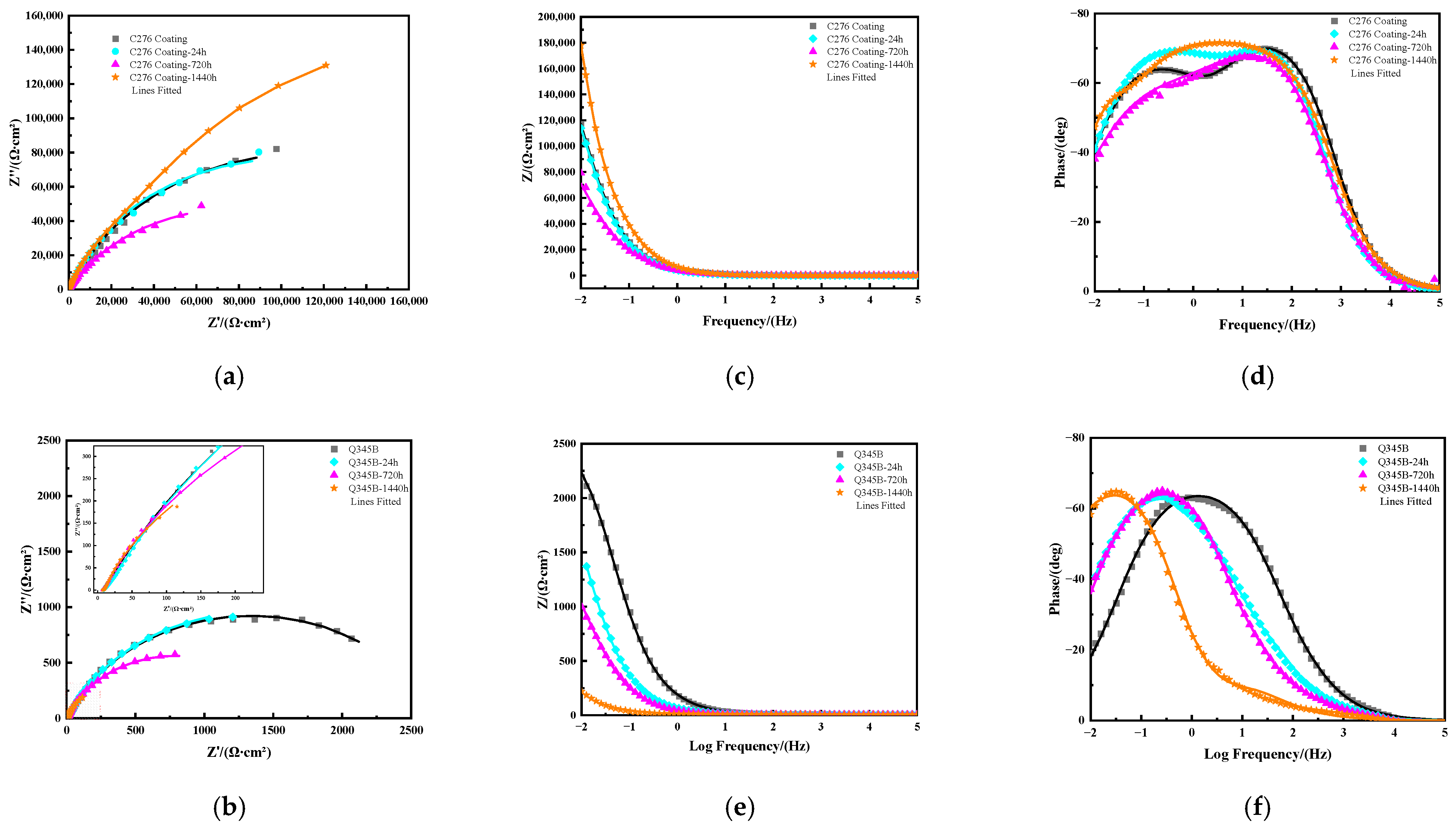
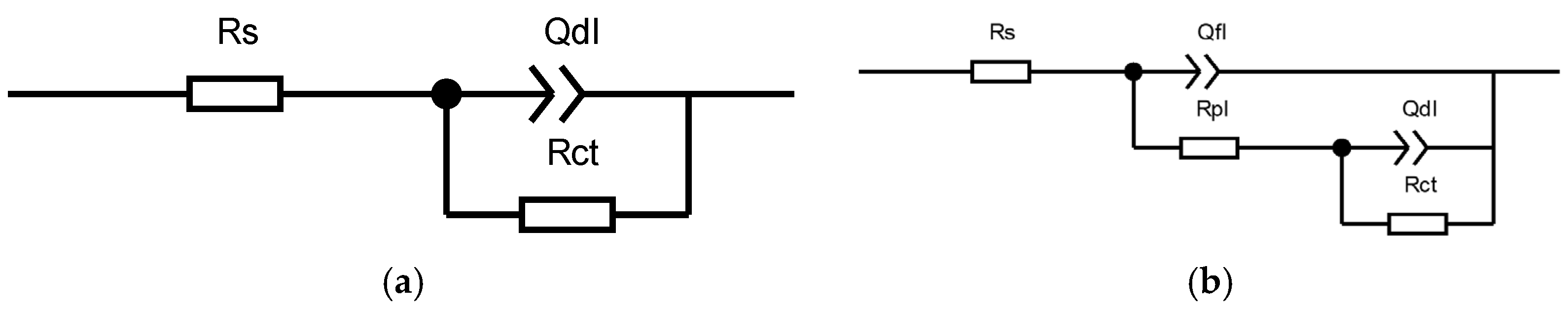
3.3. Electrochemical Testing Results
3.4. The Immersion Test Results
4. Discussion
4.1. Coating Electrochemical Corrosion Mechanism
4.2. The Influence of Pores and Corrosion Products on the Corrosion Behavior of Coatings
5. Conclusions
- (1)
- Using HVOF technology, high-performance coatings with excellent corrosion resistance (compared to Q345B) and good economic efficiency can be successfully prepared on a Q345B steel substrate. The resulting C276 coating exhibited low porosity, few microcracks, and high microhardness values.
- (2)
- The corrosion rate of the coating initially increased, and then decreased during immersion. The corrosion resistance of the coating decreased after 24 h of immersion compared to that in the initial state, with the corrosion rate reaching a maximum at 720 h. However, when the immersion time was extended to 1440 h, the corrosion rate decreased, demonstrating optimal comprehensive corrosion resistance among the three immersion periods.
- (3)
- In the initial stage of immersion, the coating experienced pitting initiation, resulting in a decrease in corrosion resistance compared to that before immersion. As the soaking time increased, the corrosion products gradually accumulated at the defect sites to form a dense oxide layer, effectively blocking the corrosive medium from further penetrating the coating through the pores. Simultaneously, during the later stage of immersion, FeNi phases and dense Fe3O4 formed on the surface of the C276 coating, enhancing the stability of the surface passive film and thus exhibiting excellent long-term corrosion resistance.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, B.; Pan, Y.; Yang, J.; Guo, J.; Zhao, B.; Liu, X.; Liu, Z.; Li, X. Microstructure evolution and SSCC behavior of strain-strengthened 304 SS pre-strained at room temperature and cryogenic temperature. Corros. Sci. 2023, 210, 110855. [Google Scholar] [CrossRef]
- Sun, B.; Wang, X.; Li, Y.; Huang, X.; Pan, Y.; Yan, L.; Zhang, Y.; Sun, M.; Lu, L.; Liu, Z.; et al. Investigating the environmental factors on corrosion and stress corrosion cracking behavior of Ti-6Al-3Nb-2Zr-1Mo alloy in simulated seawater. Corros. Sci. 2026, 259, 113487. [Google Scholar] [CrossRef]
- Little, B.J.; Blackwood, D.J.; Hinks, J.; Lauro, F.M.; Marsili, E.; Okamoto, A.; Rice, S.A.; Wade, S.A.; Flemming, H.C. Microbially influenced corrosion—Any progress? Corros. Sci. 2020, 170, 108641. [Google Scholar] [CrossRef]
- Shokri, A.; Sanavi Fard, M. Corrosion in seawater desalination industry: A critical analysis of impacts and mitigation strategies. Chemosphere 2022, 307, 135640. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; Wang, H.; Li, B.; Hu, Q.; Shao, T.; Yang, R.; Wang, B.; Wan, Q.; Li, Z.; et al. Experimental Study on Neutral Salt Spray Accelerated Corrosion of Metal Protective Coatings for Power-Transmission and Transformation Equipment. Coatings 2023, 13, 480. [Google Scholar] [CrossRef]
- Guipont, V.; Fauvarque, J.P.; Beauvais, S.; Jeandin, M.; Le Guyader, H.; Lepresle, H.; Grolleau, A.M. Ceramic Coating of Alloy 625 using Controlled Atmosphere Plasma Spraying for Sea Water Corrosion Protection. Int. Therm. Spray Conf. 2003, 83638, 255–261. [Google Scholar] [CrossRef]
- Wielage, B.; Hofmann, U.; Steinhauser, S.; Zimmermann, G. Improving wear and corrosion resistance of thermal sprayed coatings. Surf. Eng. 1998, 14, 136–138. [Google Scholar] [CrossRef]
- Qin, Y.; Wu, Y.; Zhang, J.; Hong, S.; Guo, W.; Chen, L.; Liu, H. Optimization of the HOVF Spray Parameters by Taguchi Method for High Corrosion-Resistant Fe-Based Coatings. J. Mater. Eng. Perform. 2015, 24, 2637–2644. [Google Scholar] [CrossRef]
- Kawakita, J.; Kuroda, S.; Fukushima, T.; Kodama, T. Corrosion Resistance of HastelloyC Coatings Formed by an Improved HVOF Thermal Spraying Process. Mater. Trans. 2003, 44, 253–258. [Google Scholar] [CrossRef]
- GB/T 11373-2008; Thermal Spraying—Metallic and Non-Metallic Coatings—General Technical Requirements. Standards Press of China: Beijing, China, 2008.
- GB/T 16545-2015; Corrosion of Metals and Alloys—Removal of Corrosion Products from Corrosion Test Specimens. Standards Press of China: Beijing, China, 2015.
- Palanisamy, K.; Gangolu, S.; Mangalam Antony, J. Effects of HVOF spray parameters on porosity and hardness of 316L SS coated Mg AZ80 alloy. Surf. Coat. Technol. 2022, 448, 128898. [Google Scholar] [CrossRef]
- Sadeghimeresht, E.; Markocsan, N.; Nylén, P. A Comparative Study of Corrosion Resistance for HVAF-Sprayed Fe- and Co-Based Coatings. Coatings 2016, 6, 16. [Google Scholar] [CrossRef]
- Sadeghi, E.; Joshi, S. Chlorine-induced high-temperature corrosion and erosion-corrosion of HVAF and HVOF-sprayed amorphous Fe-based coatings. Surf. Coat. Technol. 2019, 371, 20–35. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, E.; An, Y.; Hou, G.; Zhao, X.; Zhou, H. The interaction mechanism of cavitation erosion and corrosion on HVOF sprayed NiCrWMoCuCBFe coating in artificial seawater. Appl. Surf. Sci. 2020, 525, 146499. [Google Scholar] [CrossRef]
- GB/Z 45463-2025; Determination of Porosity of Thermal Spray Coatings. Standards Press of China: Beijing, China, 2025.
- Sharma, R.K.; Kumar, S.R. Comparative investigation of high-velocity oxy fuel and plasma coating process on mechanical and wear properties of iron alloy-based coating materials. Proc. Inst. Mech. Eng. Part E J. Process Mech. Eng. 2025. [Google Scholar] [CrossRef]
- Liu, C.; Mao, Z.; Xu, X. Research on the coating parameters and properties of Cr3C2-NiCr coating sprayed by HVOF. J. Phys. Conf. Ser. 2025, 2961, 12001. [Google Scholar] [CrossRef]
- Wang, Q.; Kainuma, S.; Yang, H.; Kim, A.; Nishitani, T. Durability and corrosion resistance of defective Zn- and Al-based thermal spray coatings on carbon steel plates in high-chloride environments. Results Eng. 2025, 28, 108206. [Google Scholar] [CrossRef]
- Singh, J.K.; Lee, H.-S. Enhanced corrosion resistance properties of 15Al-85Zn coating by post-treatment with sodium hexa-meta phosphate in saline solution. Corros. Sci. 2024, 226, 111684. [Google Scholar] [CrossRef]
- Khalid, A.; Abbas, M.; Zhang, Y.; Hyland, M.; Munroe, P.R. Microstructural study of Ni and Ni-20Cr particles plasma sprayed on stainless steel substrate at 300 °C. Appl. Surf. Sci. 2022, 592, 153320. [Google Scholar] [CrossRef]
- Gao, R.; Huang, Y.; Zhou, X.; Ma, G.; Jin, G.; Li, T.; Wang, H.; Liu, M. Material system and tribological mechanism of plasma sprayed wear resistant coatings: Overview. Surf. Coat. Technol. 2024, 483, 130758. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, F.; Zhou, Y.; Cao, Y.; Zhang, Q.; Wang, C.; Li, Y.; Qing, Y. Enhanced thermal radiation blocking of plasma spraying thermal barrier coatings with a bimodal splat thickness distribution. J. Eur. Ceram. Soc. 2025, 45, 117068. [Google Scholar] [CrossRef]
- Yang, K.; Jiang, Z.; Chen, C.; Zhang, S.; Liu, X. Investigation on the microstructure, tribological performance and corrosion resistance of Ni–Mo coatings deposited by HVOF and APS methods. Vacuum 2022, 200, 111023. [Google Scholar] [CrossRef]
- Li, J.; Cui, J.; Yang, J.; Li, Y.; Qiu, H.; Yang, J. Reinforcement of graphene and its derivatives on the anticorrosive properties of waterborne polyurethane coatings. Compos. Sci. Technol. 2016, 129, 30–37. [Google Scholar] [CrossRef]
- Nayak, S.K.; Kumar, A.; Laha, T. Developing an Economical Wear and Corrosion Resistant Fe-Based Metallic Glass Composite Coating by Plasma and HVOF Spraying. J. Therm. Spray Technol. 2021, 31, 1317–1329. [Google Scholar] [CrossRef]
- Dhinakarraj, C.K.; Perumal, G.; Senthilkumar, N.; Deepanraj, B. Exploring the morphologies and corrosion performances of AZ31 alloy composites reinforced with silicon nitride. Results Surf. Interfaces 2024, 14, 100180. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, X.; Xiao, P. Effect of surface curvature on oxidation of a MCrAlY coating. Corros. Sci. 2020, 163, 108256. [Google Scholar] [CrossRef]
- Verma, R.; Kaushal, G.; Bala, N. Comparative Assessment on the Behavior of HVOF Sprayed Ni-Based Alloy Coatings on SA213-T22 Boiler Tube Steel in Actual Biomass Fired Boiler Environment. J. Therm. Spray Technol. 2023, 32, 918–935. [Google Scholar] [CrossRef]
- Shi, J.; Wu, M.; Ming, J. Degradation effect of carbonation on electrochemical behavior of 2304 duplex stainless steel in simulated concrete pore solutions. Corros. Sci. 2020, 177, 109006. [Google Scholar] [CrossRef]
- Kiourtsidis, G.E.; Skolianos, S.M. Pitting corrosion of artificially aged T6 AA2024/SiCp composites in 3.5wt.% NaCl aqueous solution. Corros. Sci. 2007, 49, 2711–2725. [Google Scholar] [CrossRef]
- Sun, B.; Huang, X.; Pan, Y.; Yan, T.; Zhang, Y.; Sun, M.; Liu, Z.; Fan, L.; Li, X. A comparative study on the passive film and SCC behavior of Ti-6Al-3Nb-2Zr-1Mo alloy at various test temperatures in simulated seawater. Corros. Sci. 2024, 233, 112066. [Google Scholar] [CrossRef]
- Sun, B.; Wang, Q.; Pan, Y.; Liu, Z.; Du, C.; Li, X. Understanding the non-steady electrochemical mechanism on SCC of 304 SS under applied polarization potentials. Corros. Sci. 2024, 227, 111686. [Google Scholar] [CrossRef]
- Wang, Z.; Seyeux, A.; Zanna, S.; Maurice, V.; Marcus, P. Chloride-induced alterations of the passive film on 316L stainless steel and blocking effect of pre-passivation. arXiv 2019. [Google Scholar] [CrossRef]
- Cui, Y.-W.; Chen, L.-Y.; Chu, Y.-H.; Zhang, L.; Li, R.; Lu, S.; Wang, L.; Zhang, L.-C. Metastable pitting corrosion behavior and characteristics of passive film of laser powder bed fusion produced Ti–6Al–4V in NaCl solutions with different concentrations. Corros. Sci. 2023, 215, 111017. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, L.; Zhao, H.; Yuan, C.; Yan, J.; Li, Z.; Xin, L.; Li, C.; Wu, F.; Ye, S.; et al. Anticorrosion behavior and enhanced wear resistance of Ni–P–GO–Y composite coating on the surface of NdFeB permanent magnet. J. Mater. Sci. 2024, 59, 11510–11532. [Google Scholar] [CrossRef]
- Wang, X.; Mercier, D.; Zanna, S.; Seyeux, A.; Perriere, L.; Laurent-Brocq, M.; Guillot, I.; Maurice, V.; Marcus, P. Effects of Chloride Ions on Passive Oxide Films Formed on Cr-Fe-Co-Ni(-Mo) Multi-Principal Element Alloy Surfaces. J. Electrochem. Soc. 2023, 170, 41506. [Google Scholar] [CrossRef]
- Zamani, P.; Valefi, Z. Characterization and Early-Stage Oxidation Behavior of CoNiCrAlY/Nano-Al2O3 Composite Coatings Using Satellited Powders Deposited by HVOF and LPPS Processes. J. Therm. Spray Technol. 2023, 32, 2525–2538. [Google Scholar] [CrossRef]
- Patel, P.; Munagala, V.N.V.; Sharifi, N.; Roy, A.; Alidokht, S.A.; Harfouche, M.; Makowiec, M.; Stoyanov, P.; Chromik, R.R.; Moreau, C. Influence of HVOF spraying parameters on microstructure and mechanical properties of FeCrMnCoNi high-entropy coatings (HECs). J. Mater. Sci. 2024, 59, 4293–4323. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Q.; Chen, H.; Zhao, Y.; Huang, Y. Experimental study on erosion-corrosion of carbon steel in flowing NaCl solution of different pH. J. Mater. Res. Technol. 2022, 20, 4432–4451. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, H.; Hu, W.; Wang, Y.; Wu, X.; Zhang, Z.; Ren, Z.; Zhai, X.; Zeng, X.; Tang, M. Research on the Corrosion Behavior of Mo/C276 Coating Deposited by HVOF Method in Deep-Sea Cold Seep Environments. Coatings 2025, 15, 194. [Google Scholar] [CrossRef]
- Wang, K.; Hong, S.; Wei, Z.; Hu, N.; Cheng, J.; Wu, Y. Long-term corrosion behavior of HVOF sprayed Cr3C2–NiCr coatings in sulfide-containing 3.5 wt.% NaCl solution. J. Mater. Res. Technol. 2021, 15, 3122–3132. [Google Scholar] [CrossRef]
- Aghili, S.E.; Shamanian, M.; Amini Najafabadi, R.; Ashrafi, H.; Marzbanrad, E.; Mahmoodkhani, Y.; Toyserkani, E. Evaluation of Short-Term Oxidation Mechanism of Laser Cladded Ni–Cr–C Coating on Titanium Aluminide Substrate. High Temp. Corros. Mater. 2023, 99, 311–329. [Google Scholar] [CrossRef]
- Jiang, Z.; Ding, Y.; Chen, Z.; Zuo, B. Quantitative characterization of the interfacial failure of metallic coatings on epoxy substrates in salty atmospheres. Polym. J. 2025, 57, 1015–1023. [Google Scholar] [CrossRef]
- Sheng, M.Q.; Xu, J.F.; Wan, K.; Lv, C.K. Preparation and Corrosion Resistance Performance of Fe-Ni Alloy Coating on Surface of Mild Steel. Cailiao Yanjiu Xuebao/Chin. J. Mater. Res. 2013, 27, 183–188. [Google Scholar]
- Ni, J.; Ma, H.; Wei, W.; An, X.; Yu, M.; Hu, J. Novel Effect of Post-Oxidation on the Comprehensive Performance of Plasma Nitriding Layer. Coatings 2024, 14, 86. [Google Scholar] [CrossRef]
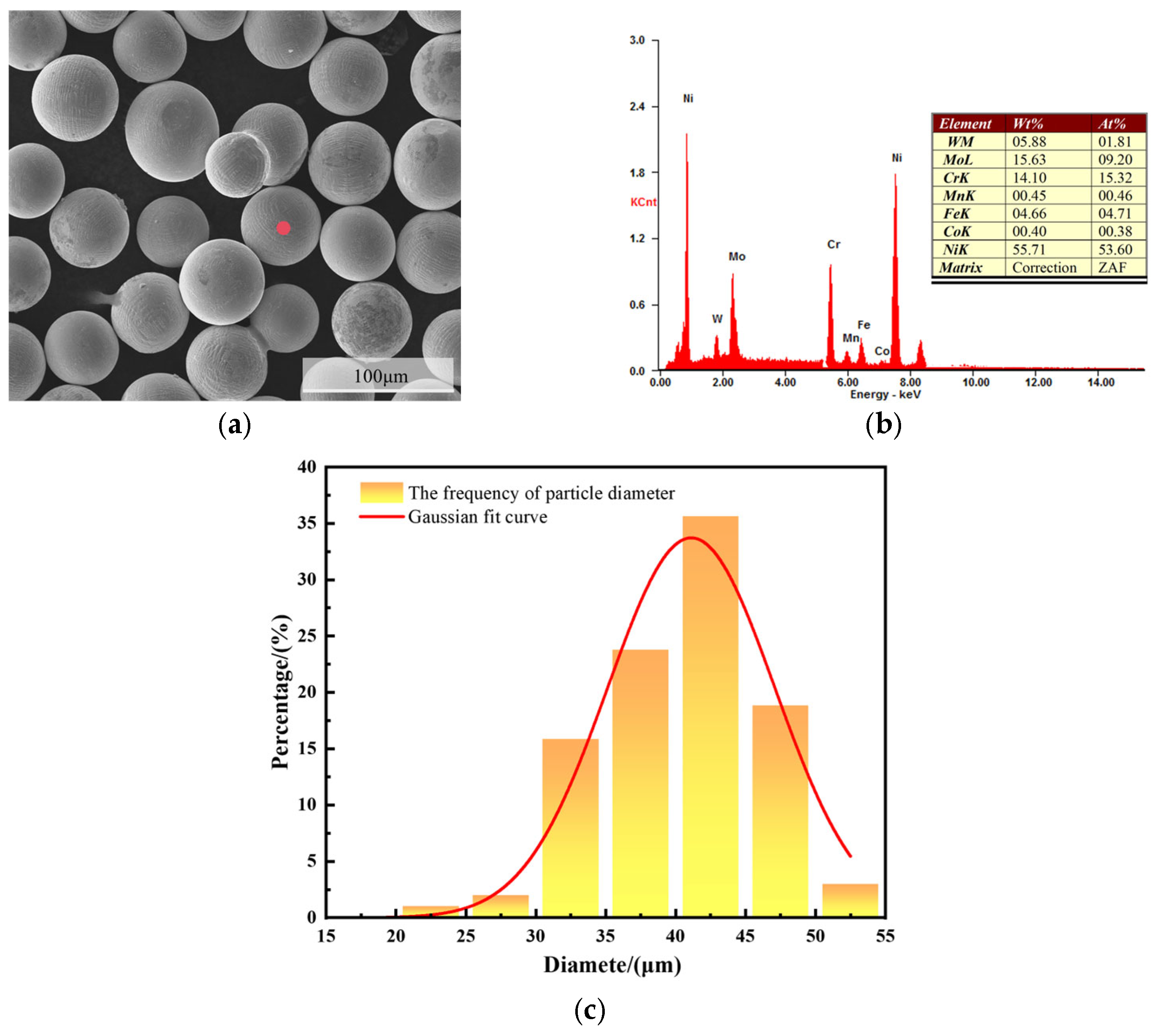
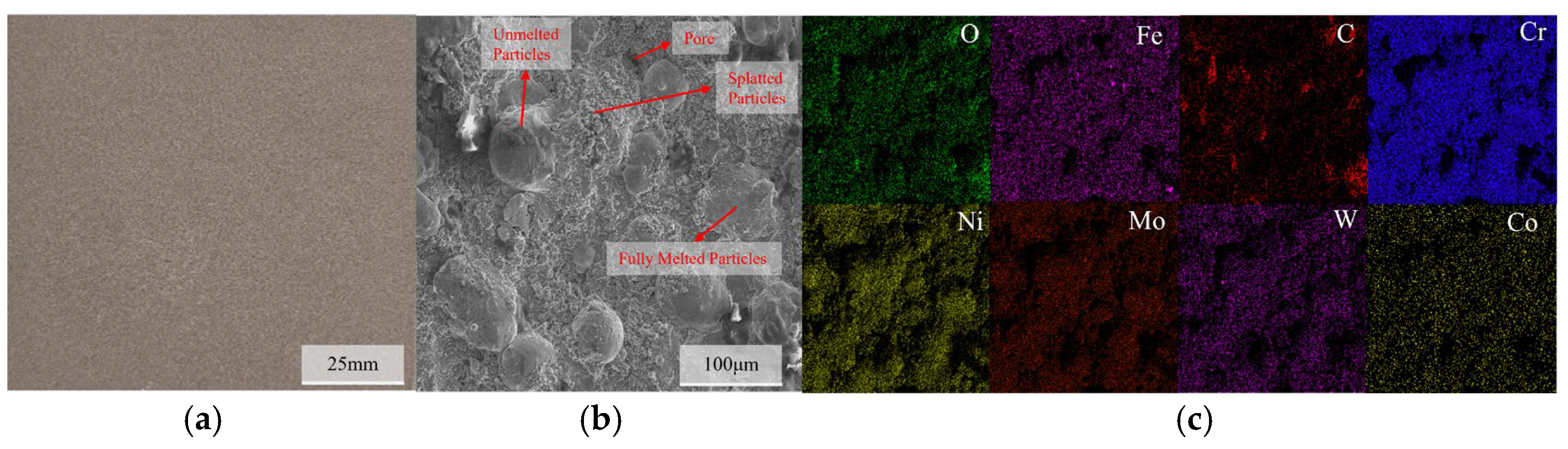
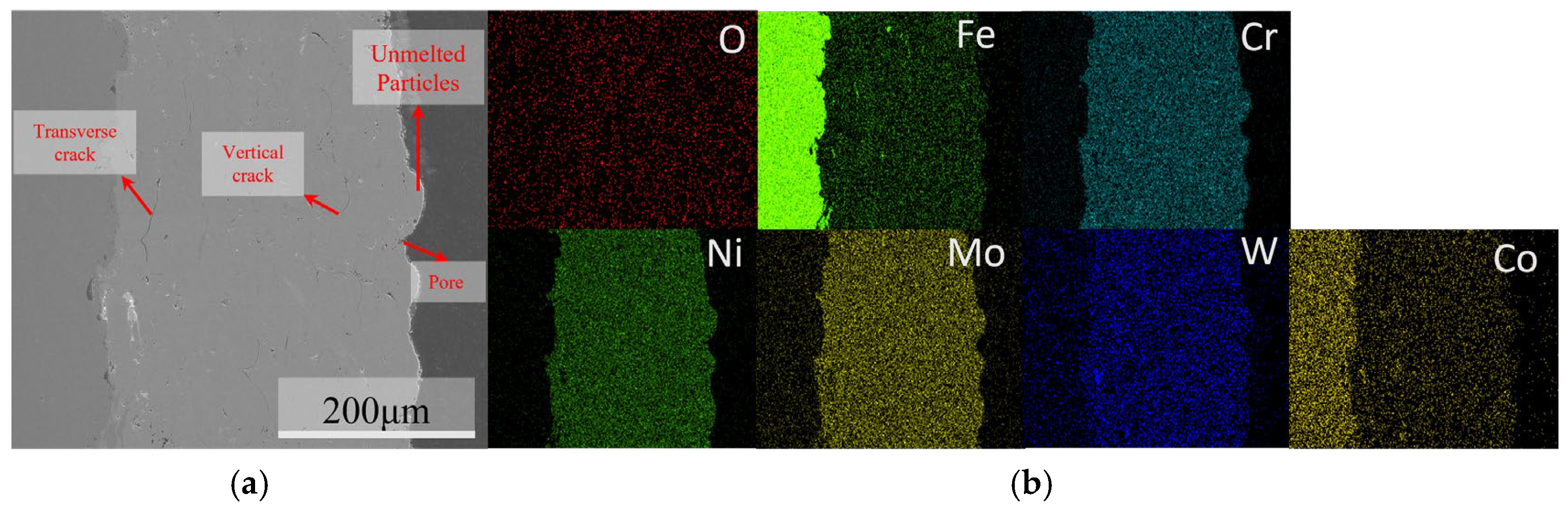
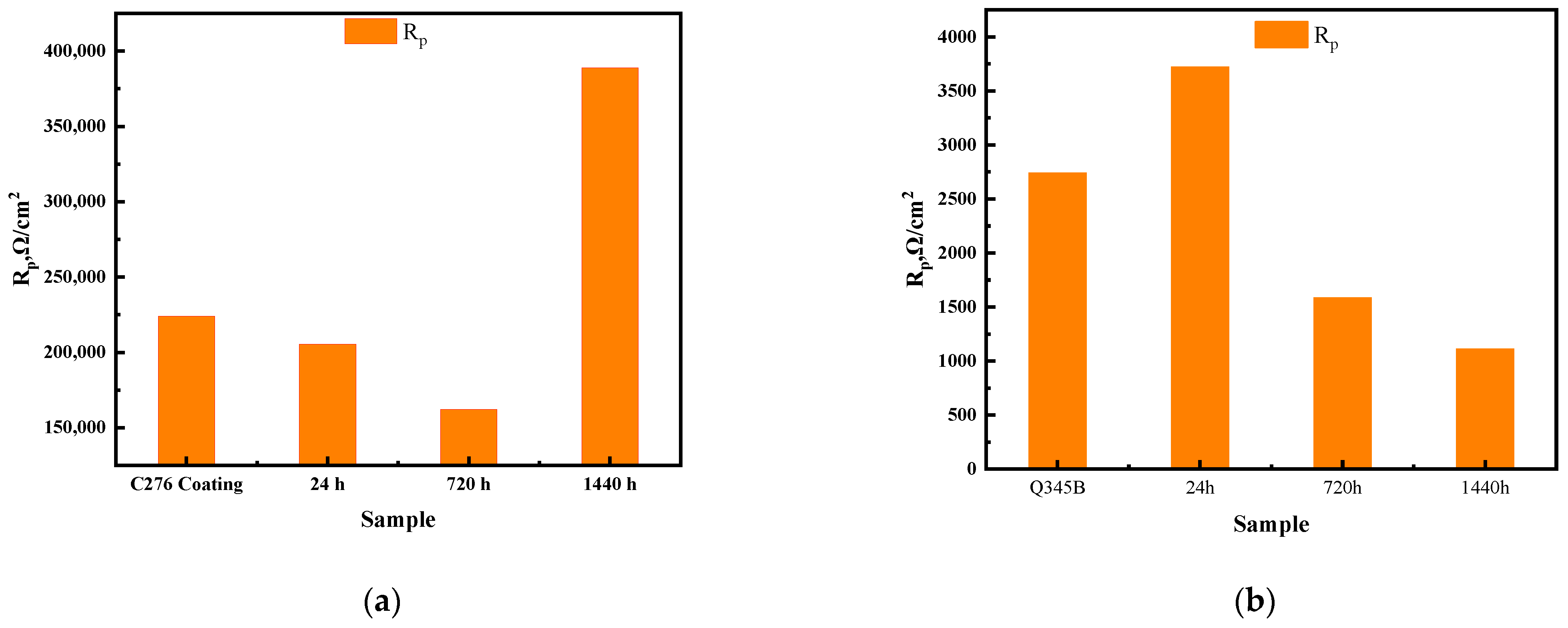
| Steel Element | C | Mn | Si | P | S | Ni | Cr | Cu | Fe |
|---|---|---|---|---|---|---|---|---|---|
| wt.% | 0.20 | 0.28 | 0.14 | 0.02 | 0.01 | 0.02 | 0.07 | 0.02 | Bal |
| Alloying Element | Cr | Mo | W | Fe | Co | Mn | Ni |
|---|---|---|---|---|---|---|---|
| wt.% | 14.5–16.5 | 15–17 | 3–4.5 | 4–7 | ≤2.5 | ≤1.0 | Bal |
| Parameter | Values |
|---|---|
| Kerosene flow rate (L/h) | 24.6 |
| Oxygen flow rate (L/min) | 850 |
| Powder disk rotational speed (rpm) | 4 |
| Carrier gas flow rate (L/min) | 12 |
| Combustion chamber pressure (MPa) | 8 |
| Spraying distance (mm) | 330 |
| Sample | Ecorr (V) | icorr (A/cm2) | βa (mv/dec) | βc (mv/dec) |
|---|---|---|---|---|
| C276 Coating-0 h | −0.35 | 2.85 × 10−7 | −107.28 | 149.09 |
| C276 Coating-24 h | −0.43 | 5.70 × 10−7 | 213.19 | 392.04 |
| C276 Coating-720 h | −0.62 | 3.72 × 10−6 | 332.96 | 318.02 |
| C276 Coating-1440 h | −0.51 | 1.81 × 10−6 | 520.66 | 302.17 |
| Q345B-0 h | −0.75 | 1.84 × 10−6 | 92.62 | 162.06 |
| Q345B-24 h | −0.85 | 5.76 × 10−6 | 429.23 | 178.42 |
| Q345B-720 h | −0.78 | 6.90 × 10−6 | 139.73 | 158.47 |
| Q345B-1440 h | −0.96 | 2.68 × 10−5 | 709.13 | 107.44 |
| Index | Rs/Ω∙cm2 | Qf1 × 10−4/Ω−1cm−2sn | npl | Rpl/Ω∙cm2 | Qdl × 10−4/Ω−1cm−2sn | ndl | Rct/Ω∙ cm2 | χ2 |
|---|---|---|---|---|---|---|---|---|
| C276 | 26.68 | 0.27 | 0.86 | 5.94 × 103 | 0.29 | 0.75 | 2.18 × 105 | 1.85 × 10−3 |
| 24 h | 27.29 | 0.38 | 0.86 | 4.41 × 103 | 0.21 | 0.76 | 2.01 × 105 | 1.53 × 10−3 |
| 720 h | 31.23 | 0.43 | 0.83 | 7.25 × 103 | 0.34 | 0.59 | 1.55 × 105 | 1.59 × 10−3 |
| 1440 h | 33.44 | 0.33 | 0.81 | 1.78 × 105 | 0.46 | 1 | 2.11 × 105 | 2.05 × 10−3 |
| Q345B | 7.84 | / | / | / | 0.13 | 0.76 | 2.73 × 103 | 0.24 × 10−3 |
| 24 h | 9.09 | / | / | / | 37.1 | 0.73 | 3.71 × 103 | 4.25 × 10−3 |
| 720 h | 7.24 | 33.23 | 0.76 | 8.22 | 21.92 | 0.85 | 1.57 × 103 | 2.45 × 10−4 |
| 1440 h | 7.3 | 132.3 | 0.72 | 4.49 | 285.9 | 0.87 | 1.10 × 103 | 4.34 × 10−4 |
| Sample (Exposed Area 10 mm × 10 mm) | C276-24 h | C276-720 h | C276-1440 h | Q345B-24 h | Q345B-720 h | Q345B-1440 h |
|---|---|---|---|---|---|---|
| Mass (g) | 0.0006 | 0.0241 | 0.0361 | 0.0016 | 0.0531 | 0.1127 |
| 0.0008 | 0.0272 | 0.0396 | 0.0018 | 0.0543 | 0.1011 | |
| 0.0006 | 0.0259 | 0.0377 | 0.0017 | 0.0535 | 0.1151 | |
| Vc (mm/year) | 0.274 | 0.352 | 0.259 | 0.790 | 0.831 | 0.850 |
| Sample | Ra (μm) | Rp (μm) | Rc (μm) | Rt (μm) |
|---|---|---|---|---|
| C276-0 h | 2.31 | 4.70 | 6.75 | 16.73 |
| C276-24 h | 2.52 | 5.18 | 6.85 | 21.67 |
| C276-720 h | 2.71 | 5.66 | 7.08 | 21.86 |
| C276-1440 h | 2.36 | 4.69 | 5.69 | 17.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Yan, L.; Liang, P.; Chen, Z.; Ma, H.; Li, Z.; Du, C.; Liu, Z. Study on the Corrosion Behavior and Mechanism of Supersonic Flame Sprayed C276 Coating in Simulated Seawater Environment. Metals 2026, 16, 172. https://doi.org/10.3390/met16020172
Yan L, Liang P, Chen Z, Ma H, Li Z, Du C, Liu Z. Study on the Corrosion Behavior and Mechanism of Supersonic Flame Sprayed C276 Coating in Simulated Seawater Environment. Metals. 2026; 16(2):172. https://doi.org/10.3390/met16020172
Chicago/Turabian StyleYan, Long, Ping Liang, Zengyao Chen, Hongchi Ma, Zhong Li, Cuiwei Du, and Zhiyong Liu. 2026. "Study on the Corrosion Behavior and Mechanism of Supersonic Flame Sprayed C276 Coating in Simulated Seawater Environment" Metals 16, no. 2: 172. https://doi.org/10.3390/met16020172
APA StyleYan, L., Liang, P., Chen, Z., Ma, H., Li, Z., Du, C., & Liu, Z. (2026). Study on the Corrosion Behavior and Mechanism of Supersonic Flame Sprayed C276 Coating in Simulated Seawater Environment. Metals, 16(2), 172. https://doi.org/10.3390/met16020172








