Evolution of Microstructure and the Influence of Carbides on Hardness Properties in Martensitic Stainless Steel 90Cr18MoV During Heat Treatment
Abstract
1. Introduction
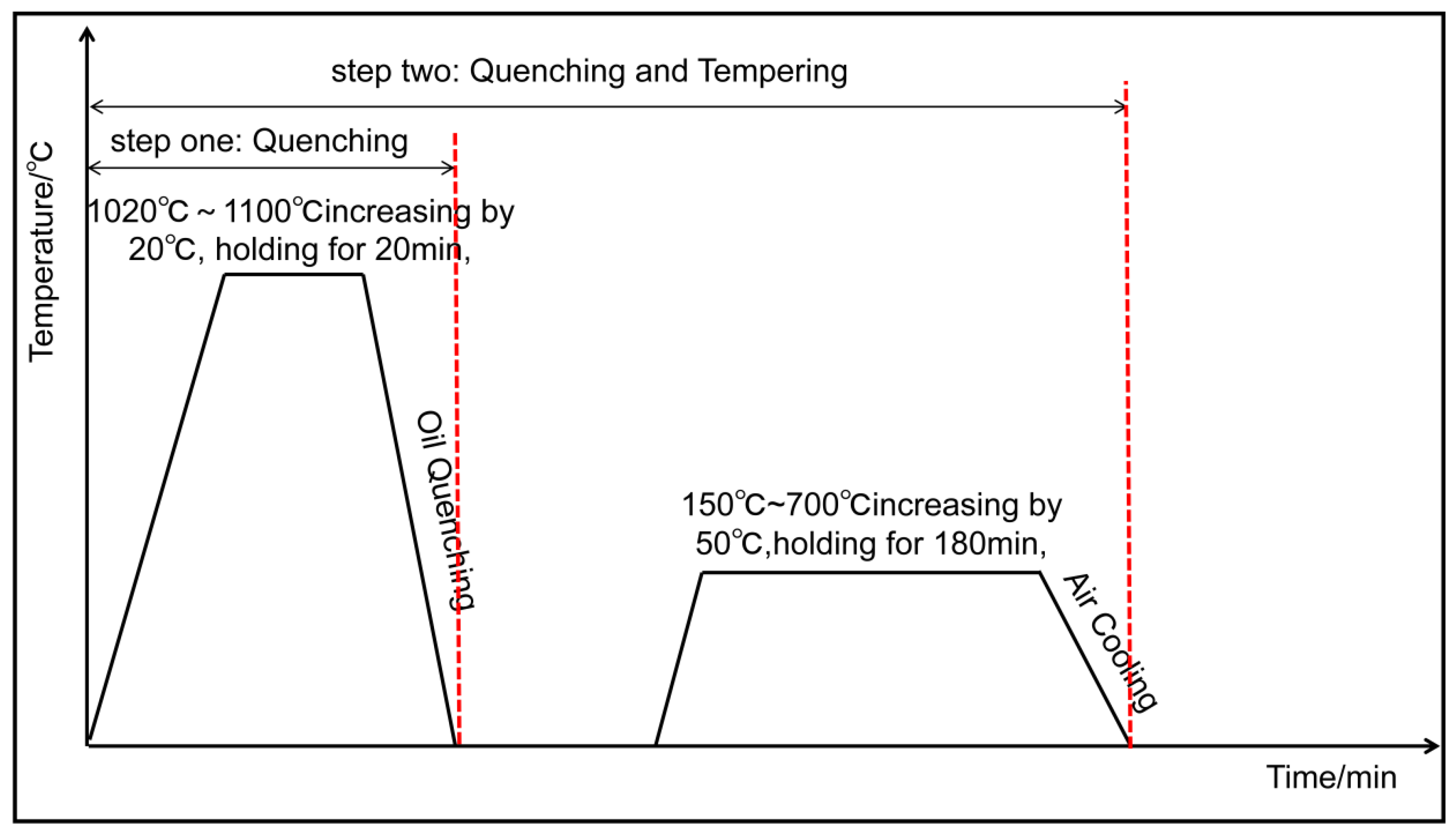
2. Materials and Methods
2.1. Materials
2.2. Characterization
3. Results
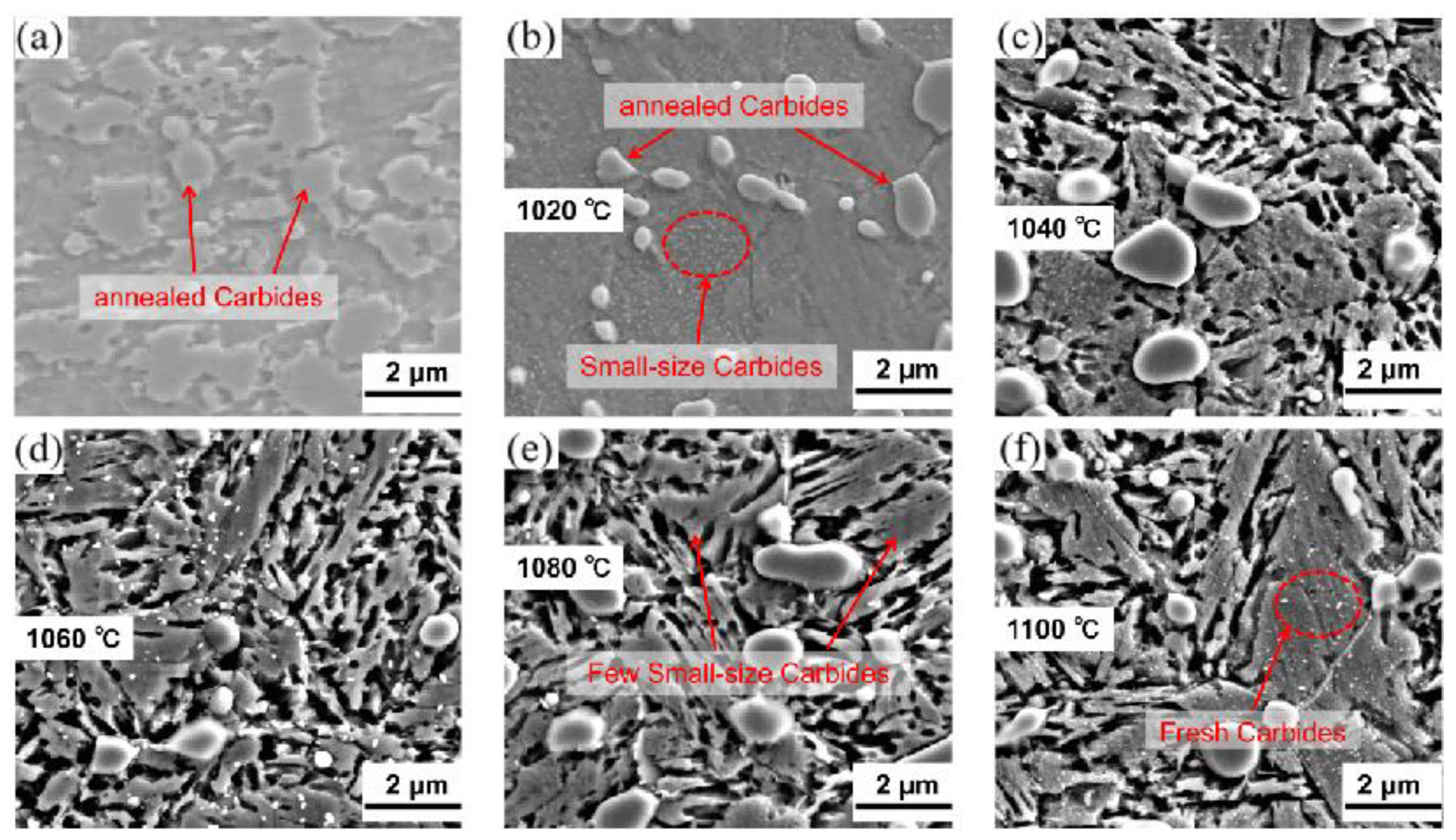
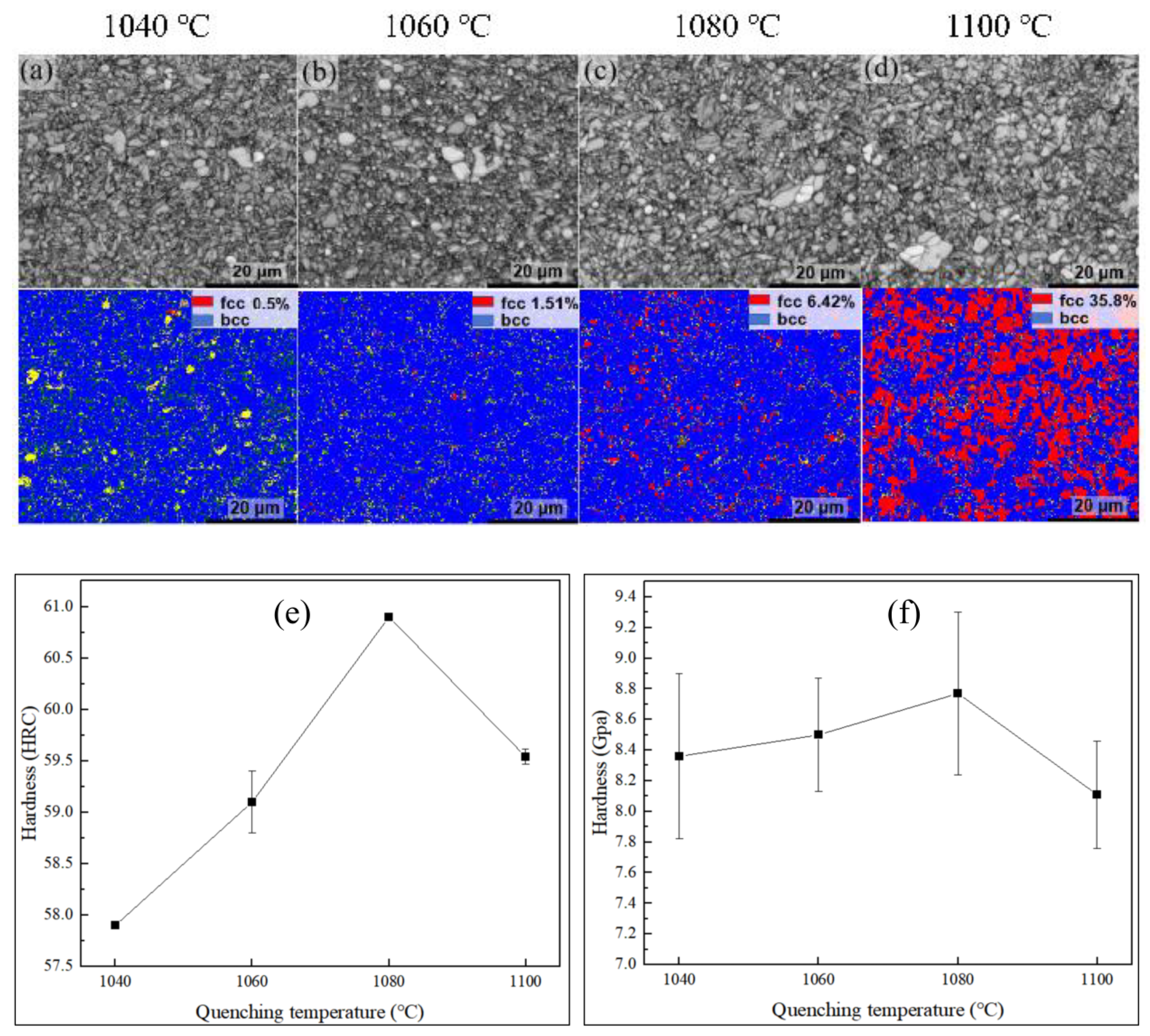
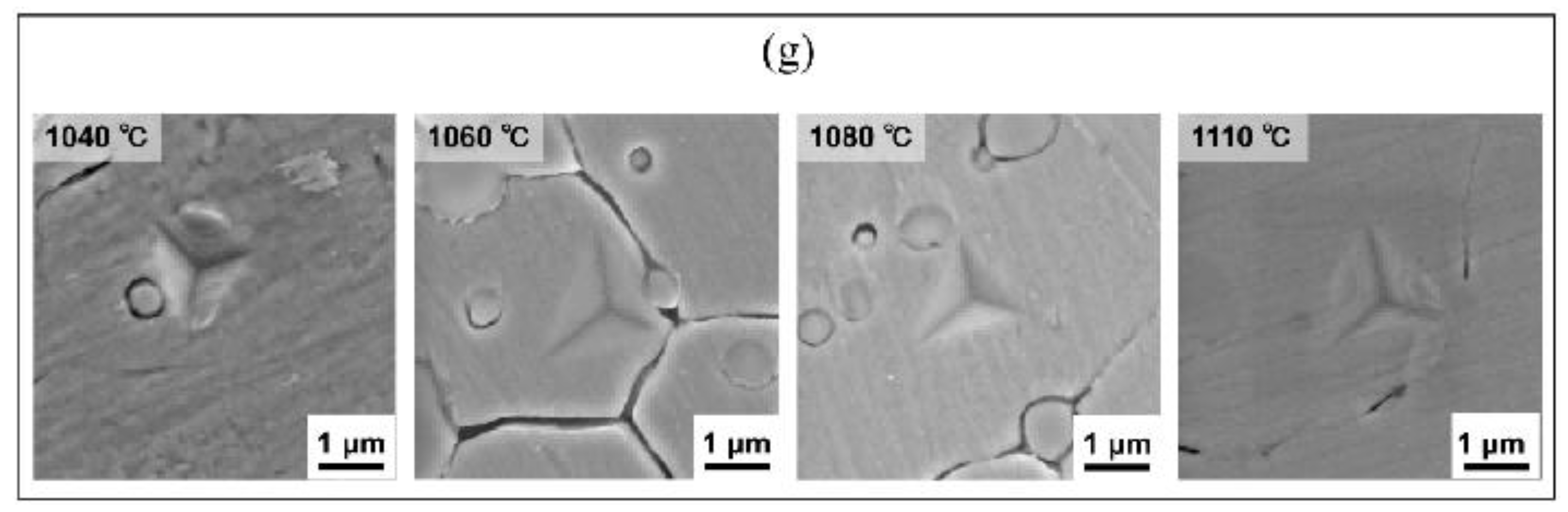
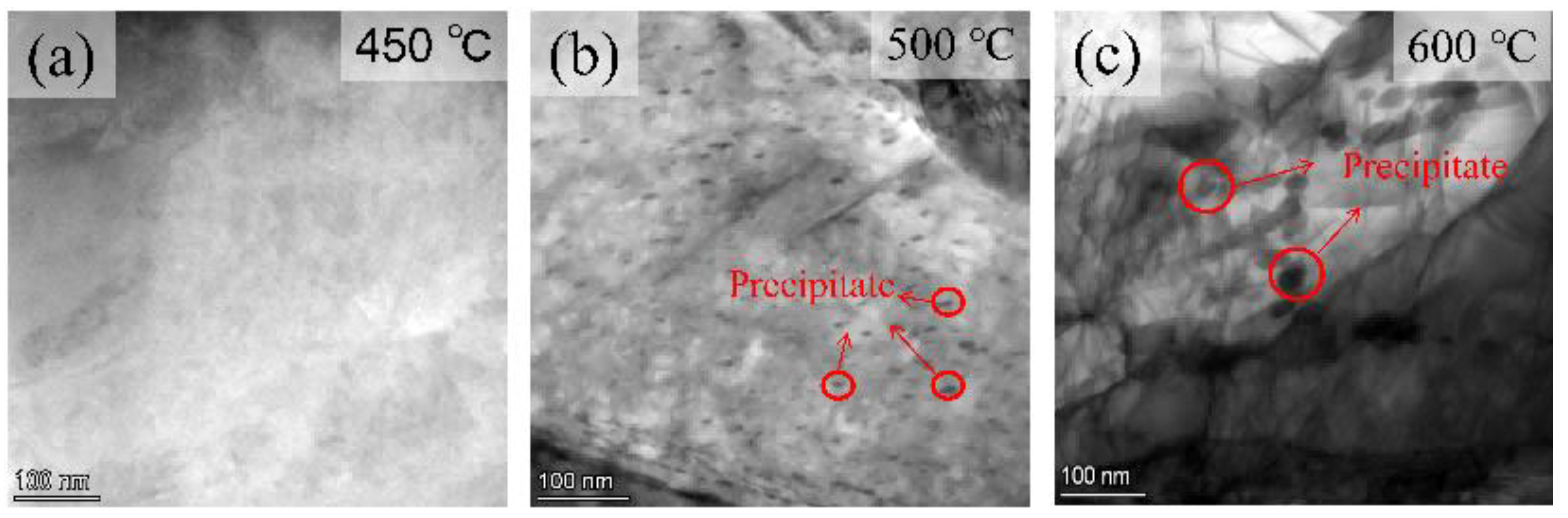
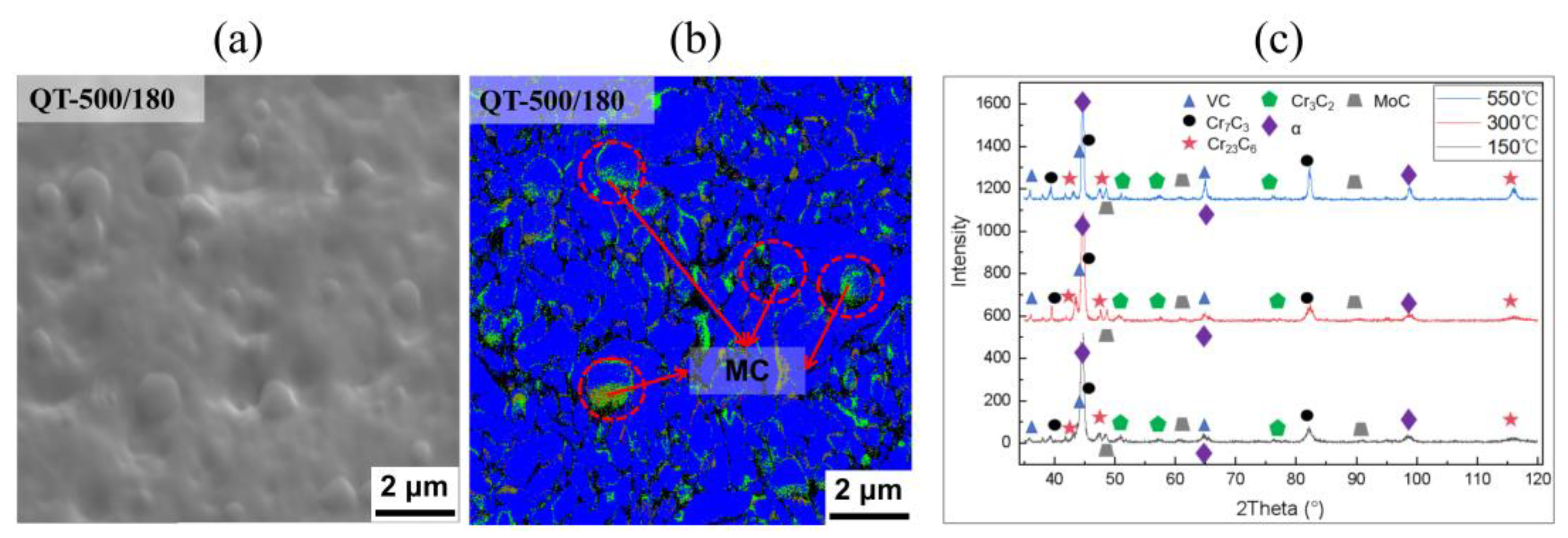
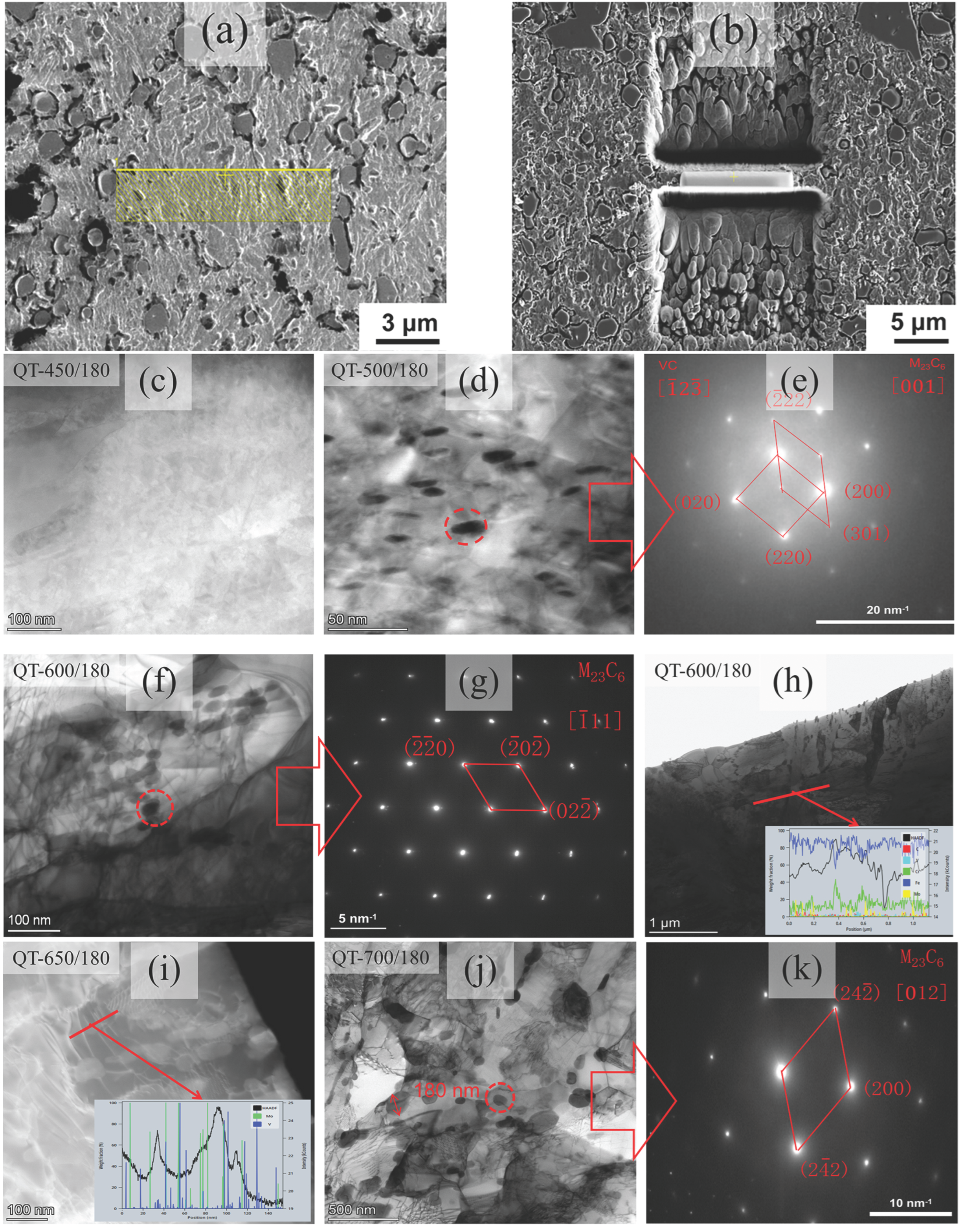
3.1. Quenching Treatment
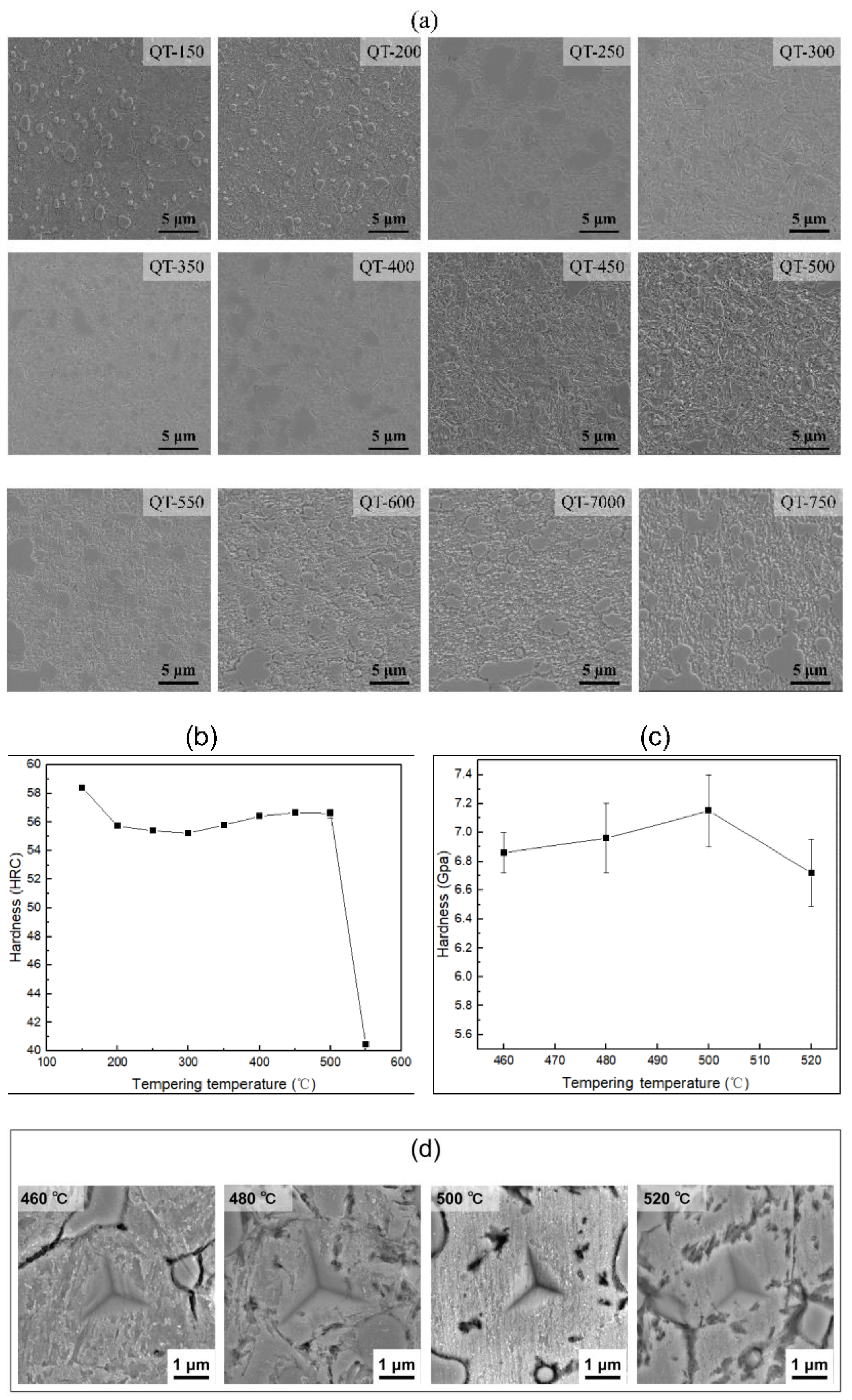
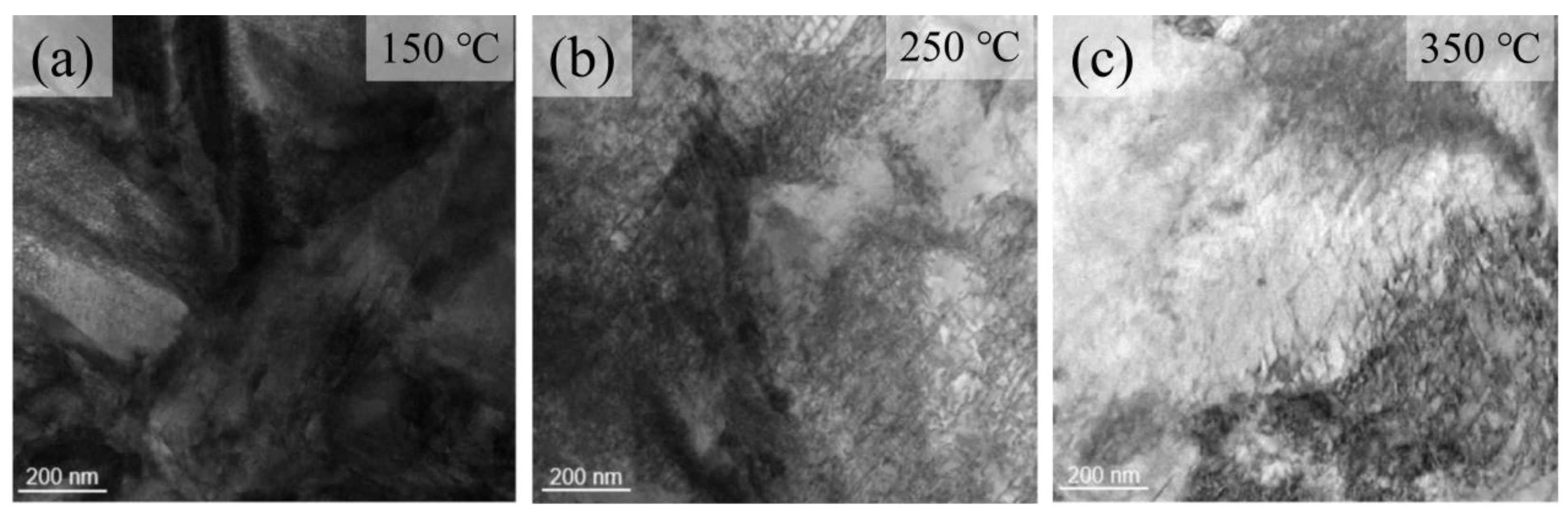
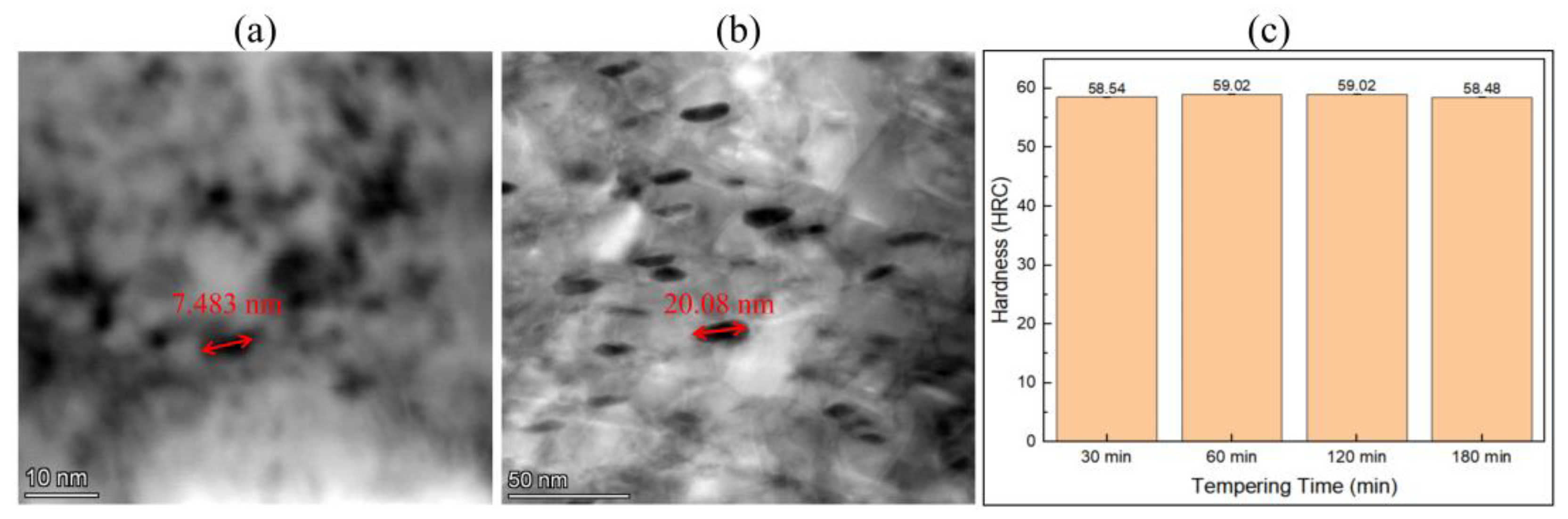
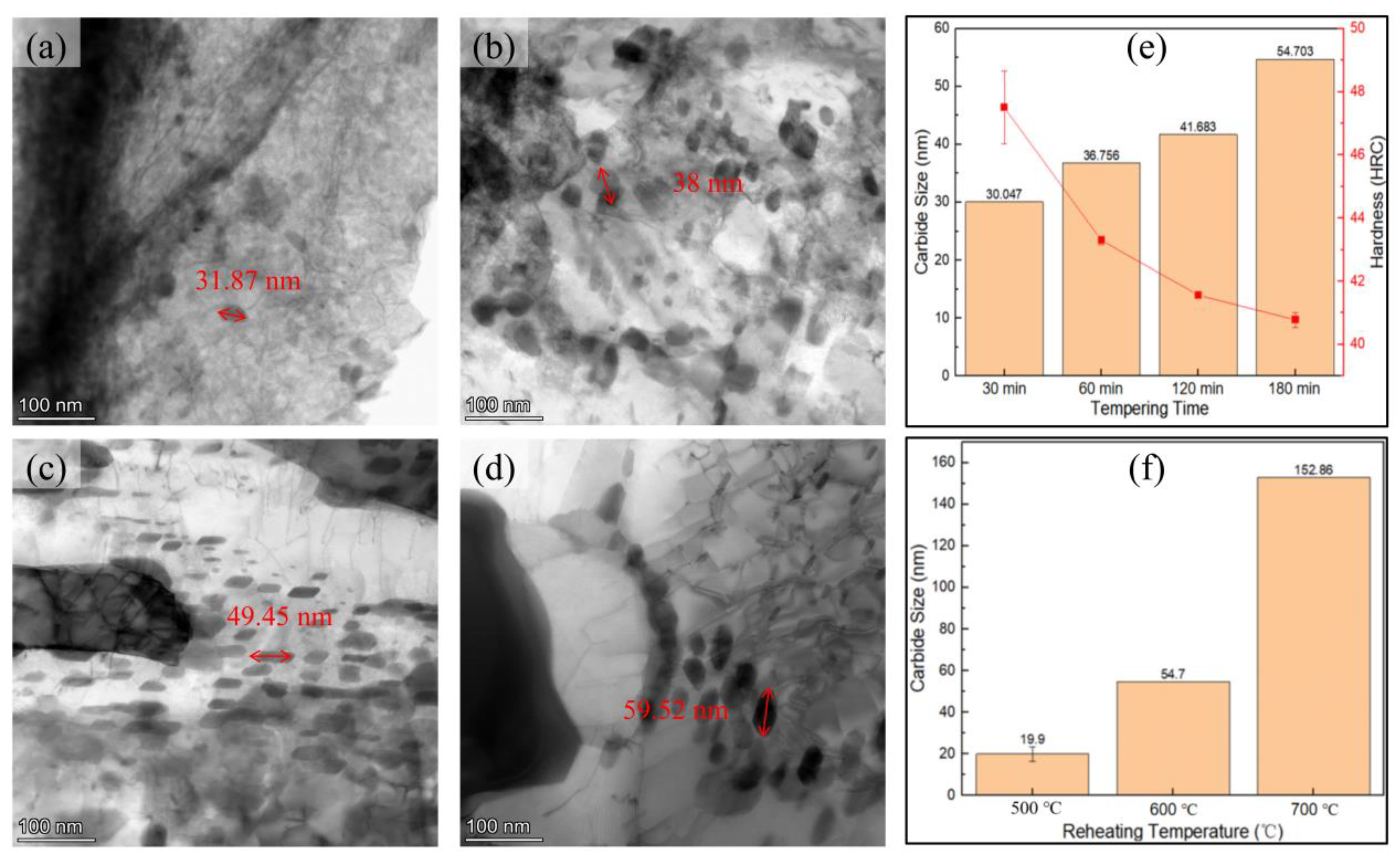
3.2. Tempering Treatment
4. Discussion
4.1. Phase Diagram Calculation
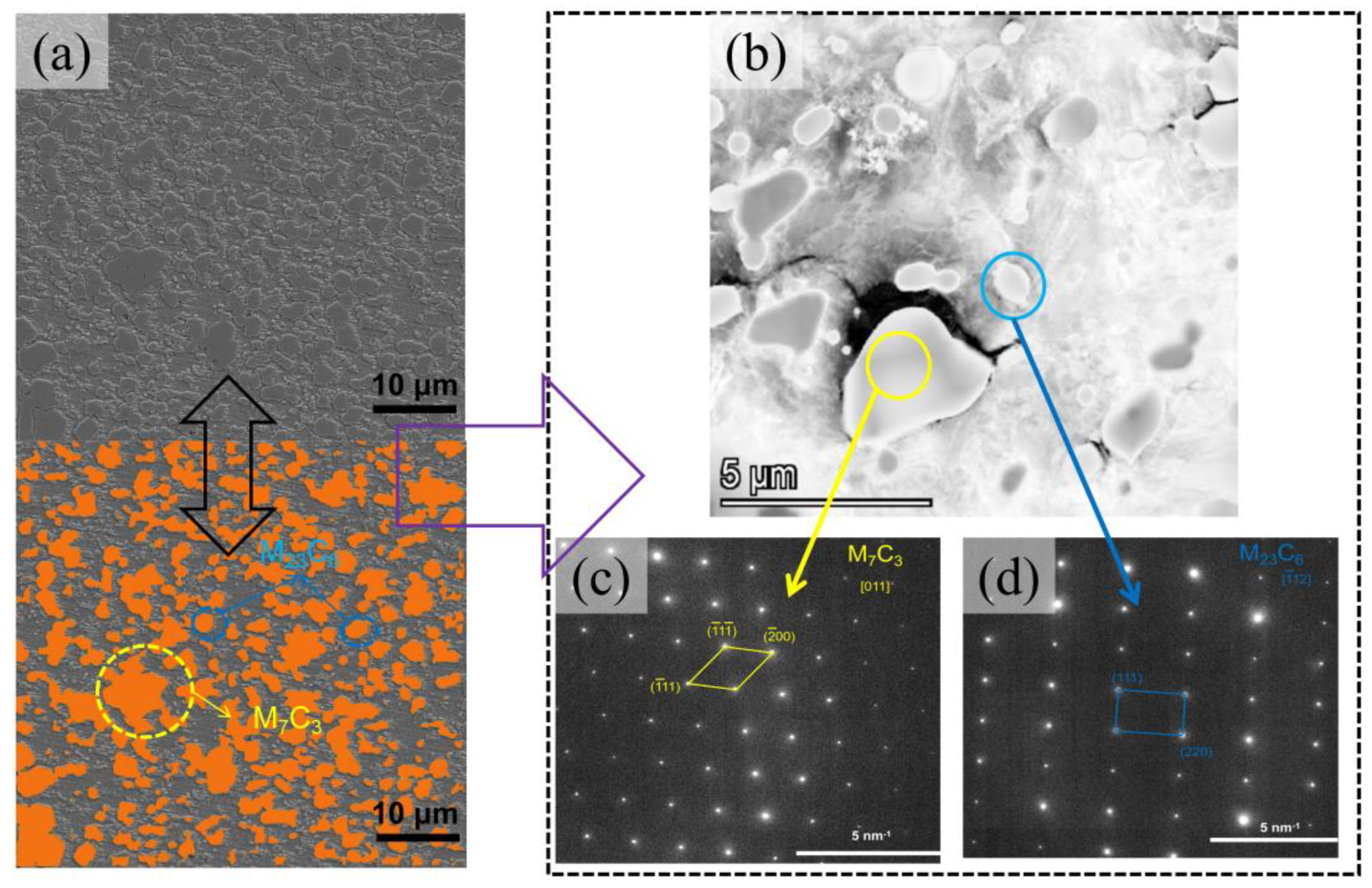
4.2. Microstructural Characterization
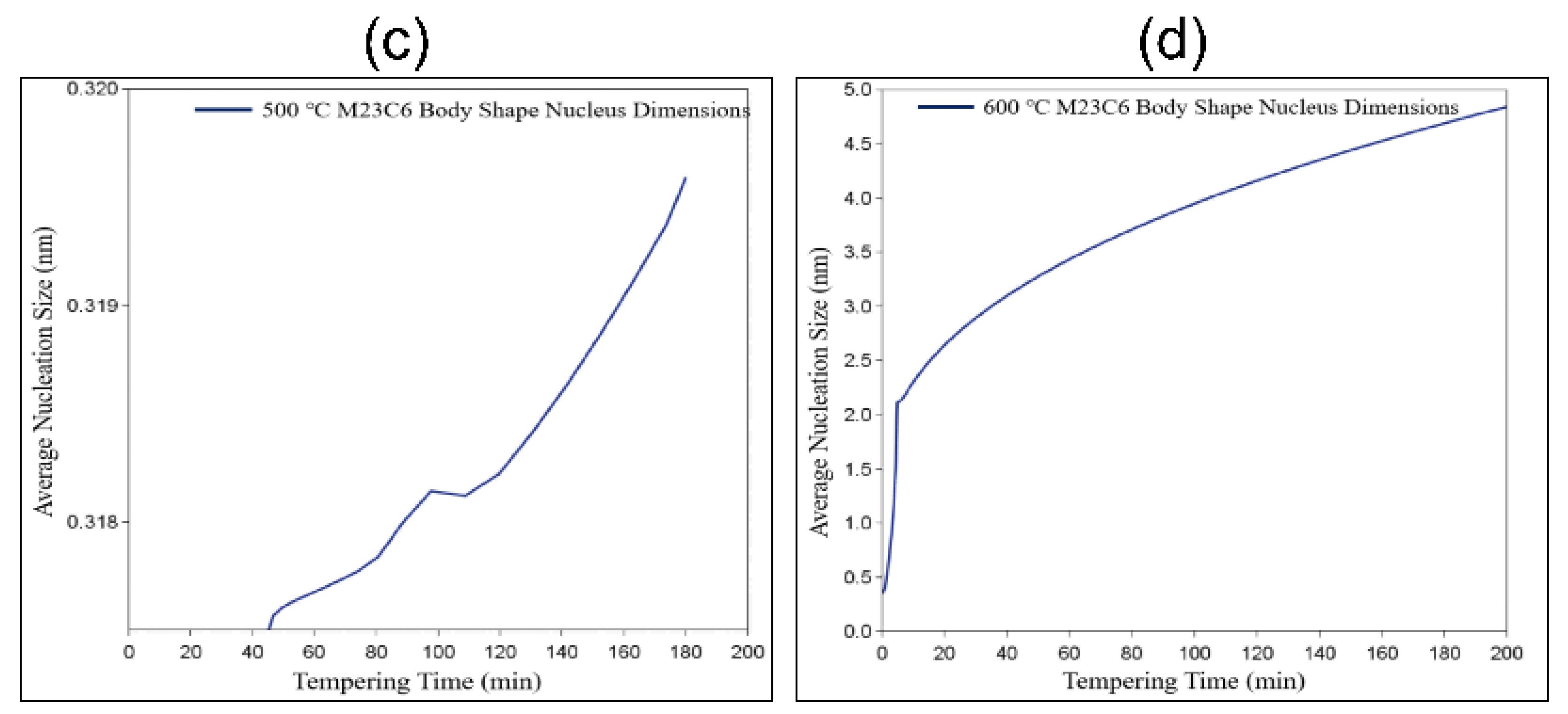
4.3. Refinement of Precipitate Size Statistics
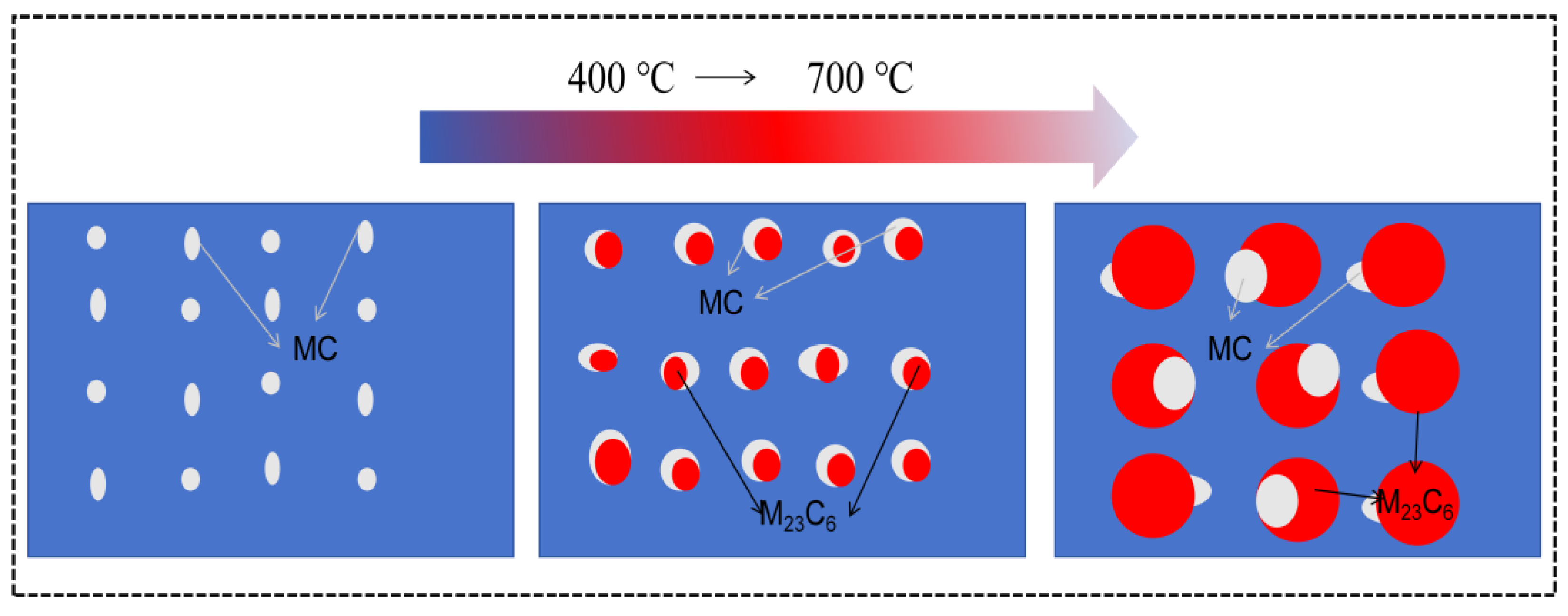
4.4. The Formation Mechanism of M23C6
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, X.L.; Huang, A.R.; Shang, C.J.; Xie, Z.J. Characterization of the cladding layer by laser cladding of 9Cr18Mo powder on 3Cr14 martensitic stainless steel and the impact of martensite obtained through post heat treatment on hardness. Mater. Today Commun. 2022, 32, 104057. [Google Scholar] [CrossRef]
- Mul, D.O.; Bushueva, E.G.; Lazurenko, D.V.; Lozhkina, E.A.; Domarov, E.V. Structure and tribological properties of “carbon steel-VC containing coating” compositions formed by non-vacuum electron-beam surfacing of vanadium-containing powder mixtures. Surf. Coat. Technol. 2023, 474, 130107. [Google Scholar] [CrossRef]
- Chen, T.; Hao, L.; Liu, T.; Zhong, Y.; Wang, Z.; Liu, C.; Cheng, X.; Li, X. Insights into the role of the Cr and rare element in improving the corrosion resistance of HRB400 rebars in simulated SO2-polluted marine environment. J. Build. Eng. 2024, 97, 110807. [Google Scholar] [CrossRef]
- Liu, L.; Lu, K.; Jing, Z.; Zhang, Z.; Xue, L.; Yuan, J.; Li, X.; Zhang, B.; Liang, X.; Cheng, J. Simultaneously improving strength and corrosion resistance of CoCrNi-based multi-principal element alloys via alloying with Mo content. J. Alloys Compd. 2025, 1010, 177119. [Google Scholar] [CrossRef]
- Hao, Y.; Xu, R.; Bi, H.; Zhang, Z.; Chen, Z.; Li, M.; Chen, B. The corrosion properties of ferritic stainless steel with varying Cr and Mo contents in the early stages of a simulated proton exchange membrane fuel cell environment investigated using experimental and joint calculation method HKD. Corros. Sci. 2024, 239, 112389. [Google Scholar] [CrossRef]
- Liu, Z.B.; Ling, J.-X.; Yang, Z.Y. Effect and application of Mo in martensitic precipitation hardening stainless steel. Contin. Cast. 2015, 34, 44–48. [Google Scholar]
- Krauss, G. Martensite in steel: Strength and structure. Mater. Sci. Eng. A 1999, 273–275, 40–57. [Google Scholar]
- Halfa, H.; Mattar, T.; Eissa, M. Precipitation behavior of modified as cast high nitrogen super hard high-speed tool steel. Steel Res. 2012, 83, 1071–1078. [Google Scholar] [CrossRef]
- Yue, X.; Chen, D.; Krishnan, A.; Lazar, I.; Niu, Y.; Golias, E.; Wiemann, C.; Gloskovskii, A.; Schlueter, C.; Jeromin, A.; et al. Unveiling nano-scale chemical inhomogeneity in surface oxide films formed on V- and N-containing martensite stainless steel by synchrotron X-ray photoelectron emission spectroscopy/microscopy and microscopic X-ray absorption spectroscopy. J. Mater. Sci. Technol. 2025, 205, 191–203. [Google Scholar] [CrossRef]
- Wieczerzak, K.; Bala, P.; Dziurka, R.; Tokarski, T.; Cios, G.; Koziel, T.; Gondek, L. The effect of temperature on the evolution of eutectic carbides and M7C3→M23C6 carbides reaction in the rapidly solidified Fe-Cr-C alloy. J. Alloys Compd. 2017, 698, 673–684. [Google Scholar] [CrossRef]
- Kong, F.; Zhao, W.; Li, H.; He, N. Effect of grain size and cobalt content on machining performance during milling tungsten carbide with PCD tool. Int. J. Refract. Met. Hard Mater. 2024, 123, 106780. [Google Scholar] [CrossRef]
- Kondrat’ev, S.Y.; Kraposhin, V.S.; Anastasiadi, G.P.; Talis, A.L. Experimental observation and crystallographic description of M7C3 carbide transformation in Fe-Cr-Ni-C HP type alloy. Acta Mater. 2015, 100, 275–281. [Google Scholar] [CrossRef]
- Wang, K.; Li, D. Formation of core (M7C3)-shell (M23C6) structured carbides in white cast irons: A thermo-kinetic analysis. Comput. Mater. Sci. 2018, 154, 111–121. [Google Scholar]
- Wiengmoon, A.; Chairuangsri, T.; Brown, A.; Brydson, R.; Edmonds, D.V.; Pearce, J.T.H. Microstructural and crystallographical study of carbides in 30wt.%Cr cast irons. Acta Mater. 2005, 53, 4143–4154. [Google Scholar]
- Wang, M.; Flahaut, D.; Zhang, Z.; Jones, I.P.; Chiu, Y.L. Primary carbide transformation in a high performance micro-alloy at 1000 °C. J. Alloys Compd. 2019, 781, 751–760. [Google Scholar]
- Yu, W.T. Study on Control Technology of Carbides in High-Carbon Martensitic Stainless Steel 8Cr13MoV Used as Knives and Shears. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2017. [Google Scholar]
- Zuo, J.Z.; He, X.; Zhao, Y.; Zhou, Y.; Chen, T.J. Influence of hot deformation process on network carbide of 100Cr6 bearing steel wire. Spec. Steel 2022, 43, 60–65. [Google Scholar] [CrossRef]
- Qian, S.; Teng, H.; Zhao, H.; Hu, P.; Man, T.; Dong, H. Microstructural evolution of secondary carbides during spheroidized annealing and quenching and tempering in 60Cr16MoMA martensitic stainless steel. J. Mater. Res. Technol. 2024, 28, 3207–3216. [Google Scholar] [CrossRef]
- Lu, S.Y.; Yao, K.F.; Chen, Y.B.; Wang, M.H.; Ge, X.Y. Influence of heat treatment on the microstructure and corrosion resistance of 13 Wt Pct Cr-type martensitic stainless steel. Metall. Mater. Trans. A 2015, 46, 6090–6102. [Google Scholar] [CrossRef]
- Muchiri, P.W.; Mwalukuku, V.M.; Korir, K.K.; Amolo, G.O.; Makau, N.W. Hardness characterization parameters of niobium carbide and niobium nitride: A first principles study. Mater. Chem. Phys. 2019, 229, 489–494. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.; Jiang, B.; Zhou, L.; Liu, Y. Effect of large-size M23C6-type carbides on the low-temperature toughness of martensitic heat-resistant steels. J. Alloys Compd. 2016, 685, 248–257. [Google Scholar] [CrossRef]
- Bonagania, S.K.; Bathulab, V.; Kain, V. Influence of tempering treatment on microstructure and pitting corrosion of 13 wt.% Cr martensitic stainless steel. Corros. Sci. 2018, 131, 340–354. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, H.; Sisson, R.D., Jr. The effect of carbon content on the c/a ratio of as-quenched martensite in Fe-C alloys. Mater. Sci. Eng. A 2017, 700, 592–597. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, D.X.; Xu, R.C.; Sun, Z.H.; Wu, S.S.; Zhang, X.T.; Chen, T. The Genetic Influence of Carbon Segregation in GCr15 Cast Billets on Microstructure and Friction-Wear Properties. Steel 2025, 1–14. [Google Scholar] [CrossRef]
- Luo, Q.; Chen, H.; Chen, W.; Wang, C.; Xu, W.; Li, Q. Thermodynamic prediction of martensitic transformation temperature in Fe-Ni-C system. Scr. Mater. 2020, 187, 413–417. [Google Scholar] [CrossRef]
- Navarrete-Cuadrado, J.; Soria-Biurrun, T.; Lozada-Cabezas, L.; Isasti, N.; Ibarreta-López, F.; Martínez-Pampliega, R.; Sánchez-Moreno, J.M. Effect of carbon content and cooling rate on the microstructure and hardness of TiC-Fe-Cr-Mo cermets. Int. J. Refract. Met. Hard Mater. 2024, 119, 106552. [Google Scholar] [CrossRef]
- Idayan, A.; Gnanavelbabu, A.; Rajkumar, K. Influence of deep cryogenic treatment on the mechanical properties of AISI 440C bearing steel. Procedia Eng. 2014, 97, 1683–1691. [Google Scholar]
- Xu, Z.; Xia, Z.; Xiong, Y.; Lu, J.; Guo, Z. Multiple morphologies and twin structure generated by a definite growth behavior of grain boundary M23C6 in tempered Fe–15Mn–3Al-0.7C steel. J. Mater. Res. Technol. 2024, 28, 774–781. [Google Scholar]
- Zhang, J.; Yu, L.; Liu, C.; Ding, R.; Liu, Y. Synergistic Strengthening Mechanisms and Deformation Heat Treatment Applications of High Chromium Martensitic Heat-Resistant Steel. Acta Metall. Sin. 2024, 60, 713–730. [Google Scholar]
- Wang, X.; Xu, L.; Jiao, L.; Li, W.; Mei, J.; Zhao, Y.; Qiao, L. Inhibition of the intergranular brittleness of HR3C heat-resistant steel by strain-aging induced nano-M23C6 dispersion precipitation. J. Mater. Sci. Technol. 2025, 213, 288–299. [Google Scholar]
- Wang, X.; Li, C.; Zhang, Z.; Zhao, X.; Tong, D.; Yan, G.; Han, L.; Gu, J. Tailoring the microstructure and mechanical properties of H13 steel by controlling the pre-precipitation of VC carbides from austenite. J. Mater. Res. Technol. 2024, 33, 9614–9621. [Google Scholar]
- Wang, Q.; Kong, D.; Li, X.; Zhou, S.; Zhang, Z. Additive manufacturing Cr-Mo-Si-V steel: Systematic parameter assessments, precipitation behavior of in-situ VC-M23C6 and strengthening mechanisms. Mater. Sci. Eng. A 2025, 919, 147504. [Google Scholar] [CrossRef]
- Jiao, Y.; Dan, W.J.; Zhang, W.G. The strain-induced martensitic phase transformation of Fe–C alloys considering C addition: A molecular dynamics study. J. Mater. Res. 2020, 35, 1803–1816. [Google Scholar] [CrossRef]

| C | Si | Mn | P | S | Cr | Ni | Mo | V |
|---|---|---|---|---|---|---|---|---|
| 0.903 | 0.512 | 0.420 | 0.026 | 0.004 | 17.19 | 0.329 | 1.069 | 0.161 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, S.; Wang, R.; Wang, X.; Jiang, F.; Shang, C.; Wu, X. Evolution of Microstructure and the Influence of Carbides on Hardness Properties in Martensitic Stainless Steel 90Cr18MoV During Heat Treatment. Metals 2025, 15, 999. https://doi.org/10.3390/met15090999
Yuan S, Wang R, Wang X, Jiang F, Shang C, Wu X. Evolution of Microstructure and the Influence of Carbides on Hardness Properties in Martensitic Stainless Steel 90Cr18MoV During Heat Treatment. Metals. 2025; 15(9):999. https://doi.org/10.3390/met15090999
Chicago/Turabian StyleYuan, Shengfu, Ruizhi Wang, Xuelin Wang, Fajian Jiang, Chengjia Shang, and Xinghua Wu. 2025. "Evolution of Microstructure and the Influence of Carbides on Hardness Properties in Martensitic Stainless Steel 90Cr18MoV During Heat Treatment" Metals 15, no. 9: 999. https://doi.org/10.3390/met15090999
APA StyleYuan, S., Wang, R., Wang, X., Jiang, F., Shang, C., & Wu, X. (2025). Evolution of Microstructure and the Influence of Carbides on Hardness Properties in Martensitic Stainless Steel 90Cr18MoV During Heat Treatment. Metals, 15(9), 999. https://doi.org/10.3390/met15090999








