Corrosion Performance of (TiAlZrTaNb)Nx High-Entropy Nitrides Thin Films Deposited on 304 Stainless Steel via HiPIMS
Abstract
1. Introduction
2. Materials and Methods
- Acetic acid: 3% (v/v) CH3COOH solution in distilled water, simulating foods with a pH = 2.5;
- Citric acid: C6H8O7 solution at 5 g/L in distilled water, simulating foods with a pH = 2.18;
- Tap water: simulating aqueous, alcoholic, and fatty foods: pH 6.8 ± 0.5; Conductivity 75 μS∙cm−1.
3. Results and Discussion
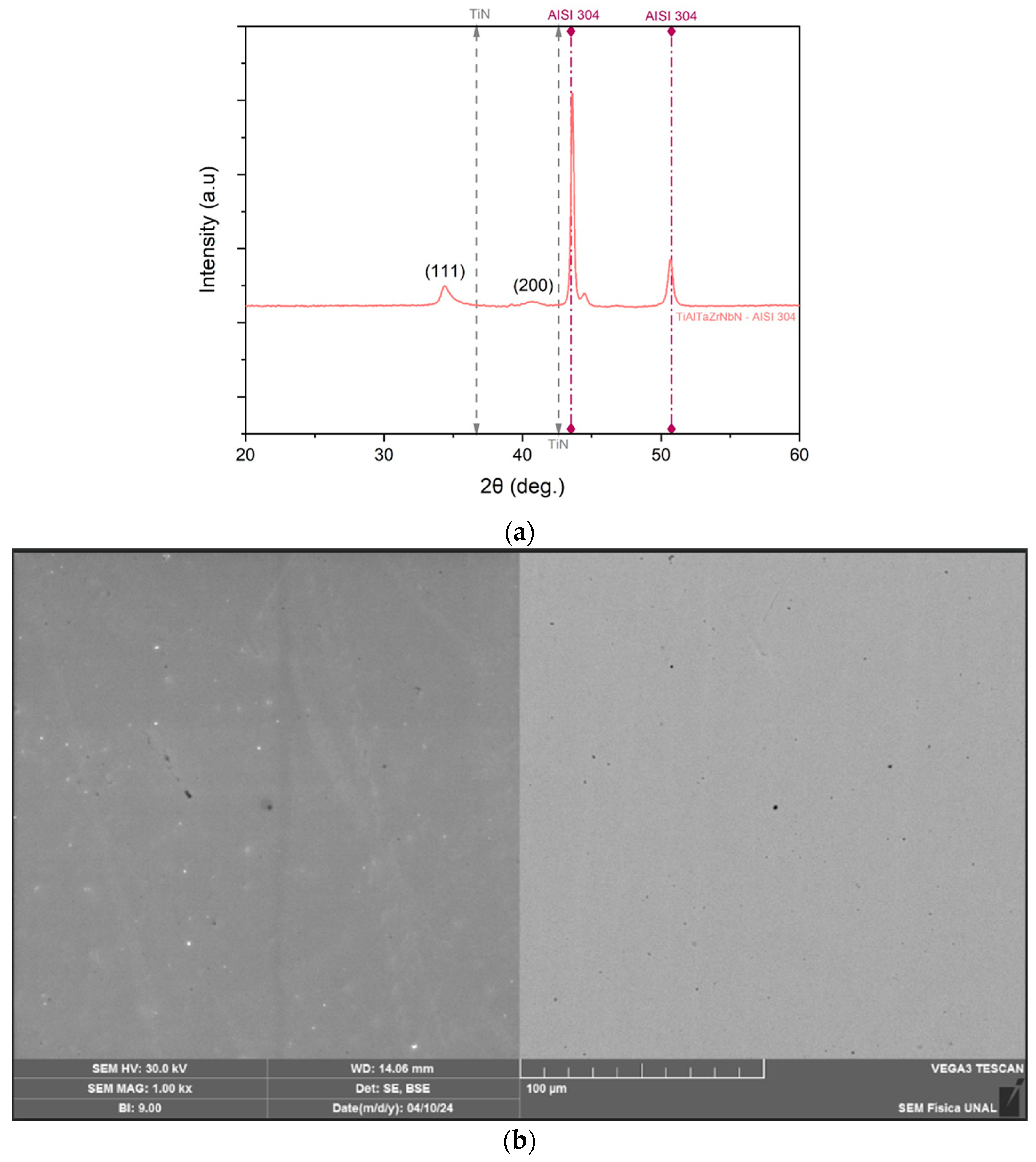
3.1. Elemental Composition
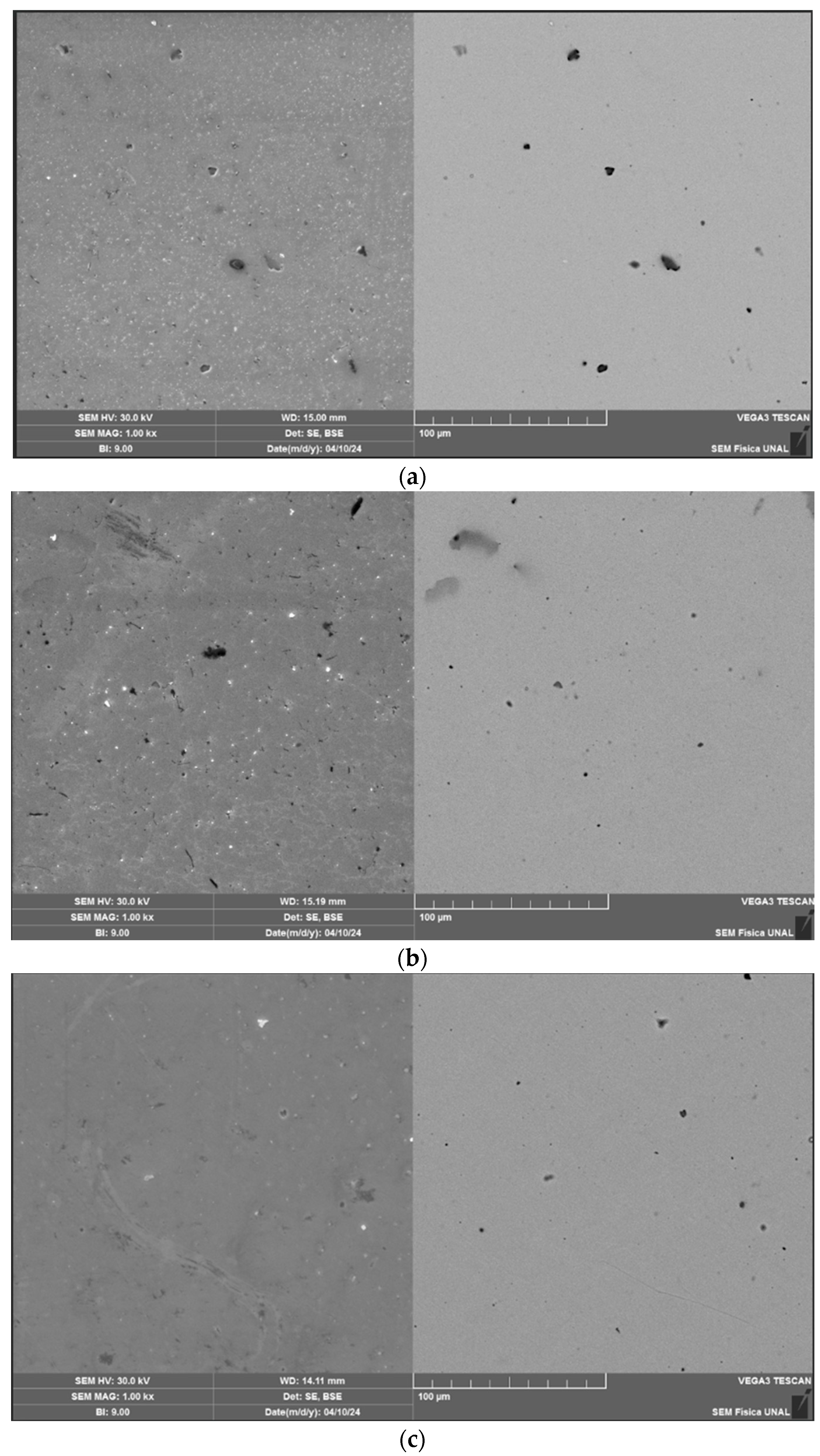
3.2. Microstructure and Morphology
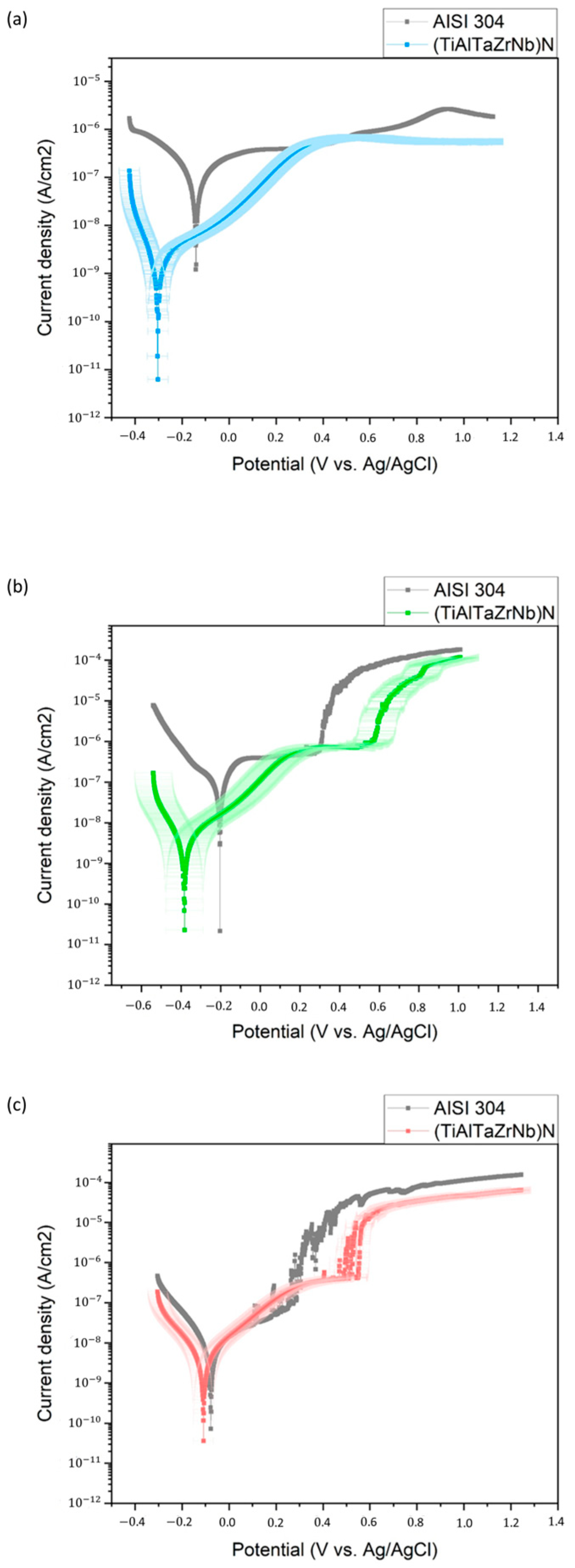
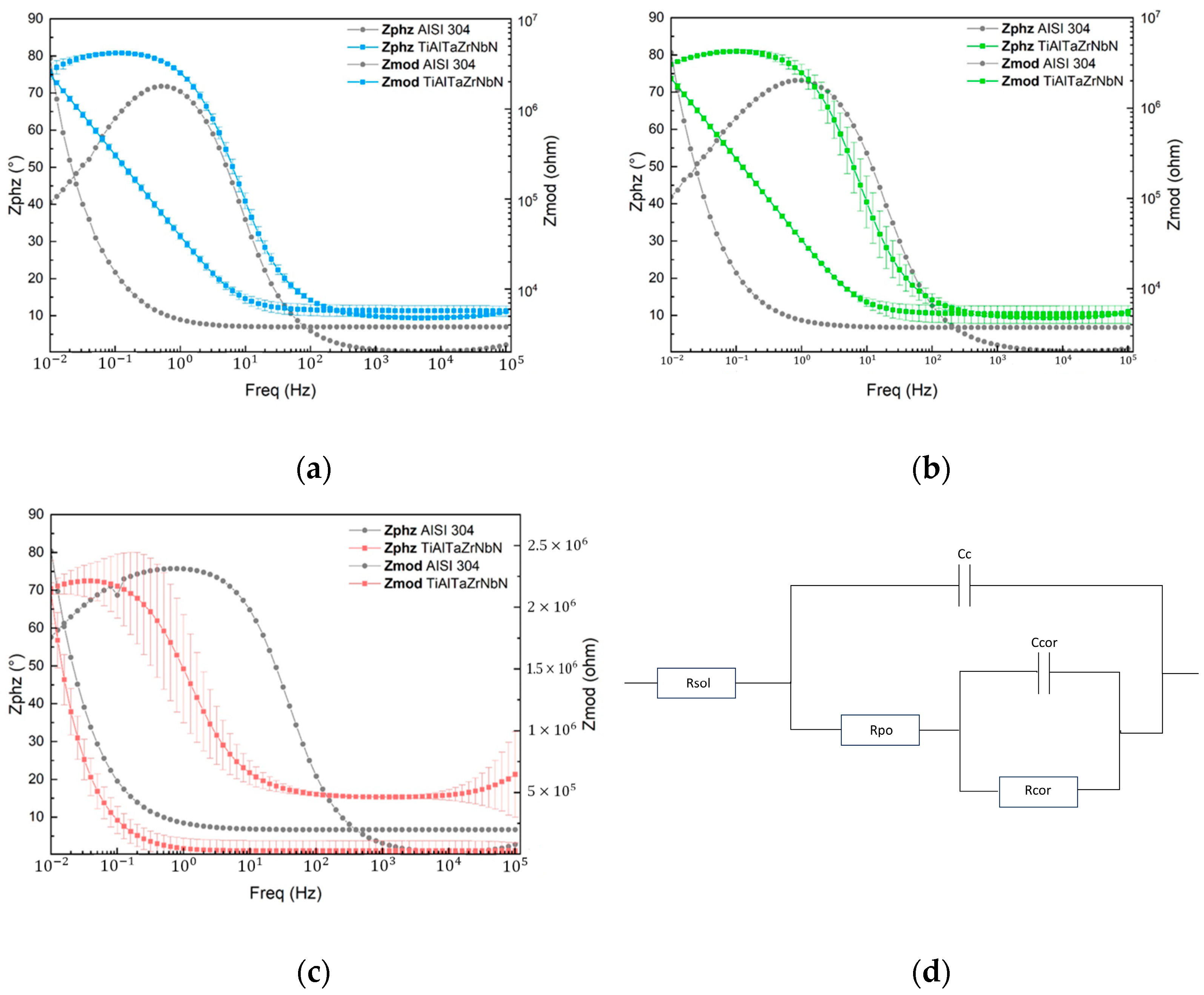
3.3. Corrosion Performance
3.4. Corrosion Mechanisms
3.4.1. Acetic Acid
3.4.2. Citric Acid
3.4.3. Tap Water
3.4.4. Proposed Mechanism of Corrosion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HEA | High-Entropy Alloy |
| HiPIMS | High-Power Impulse Magnetron Sputtering |
| PVD | Physical Vapor Deposition |
References
- Zhou, H.; Jiang, L.; Jia, L.; Zhu, S.; Wang, L.; Wu, A.; Zhang, X. Interstitial boron-doped FeCoNiCr high entropy alloys with excellent electromagnetic-wave absorption and resistance to harsh environments. J. Alloys Compd. 2023, 959, 170579. [Google Scholar] [CrossRef]
- Braic, V.; Vladescu, A.; Balaceanu, M.; Luculescu, C.R.; Braic, M. Nanostructured multi-element (TiZrNbHfTa) N and (TiZrNbHfTa) C hard coatings. Surf. Coat. Technol. 2012, 211, 117–121. [Google Scholar] [CrossRef]
- Bachani, S.K.; Wang, C.-J.; Lou, B.-S.; Chang, L.-C.; Lee, J.-W. Fabrication of TiZrNbTaFeN high-entropy alloys coatings by HiPIMS: Effect of nitrogen flow rate on the microstructural development, mechanical and tribological performance, electrical properties and corrosion characteristics. J. Alloys Compd. 2021, 873, 159605. [Google Scholar] [CrossRef]
- APogrebnjak, D.; Beresnev, V.M.; Smyrnova, K.V.; Kravchenko, Y.O.; Zukowski, P.V.; Bondarenko, G.G. The influence of nitrogen pressure on the fabrication of the two-phase superhard nanocomposite (TiZrNbAlYCr) N coatings. Mater. Lett. 2018, 211, 316–318. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar]
- Pshyk, A.V.; Vasylenko, A.; Bakhit, B.; Hultman, L.; Schweizer, P.; Edwards, T.E.J.; Michler, J.; Greczynski, G. High-entropy transition metal nitride thin films alloyed with Al: Microstructure, phase composition and mechanical properties. Mater. Des. 2022, 219, 110798. [Google Scholar]
- Yuan, M.; Gao, X.; Gu, X.; Dong, C.; Wang, S.; Wen, M.; Zhang, K. Simultaneous enhancement of hardness and wear and corrosion resistance of high-entropy transition-metal nitride. J. Am. Ceram. Soc. 2023, 106, 1356–1368. [Google Scholar]
- Lou, B.-S.; Lin, Y.-C.; Lee, J.-W. Mechanical Properties and Corrosion Resistance of AlCrNbSiTiN High Entropy Alloy Nitride Coatings. Coatings 2023, 13, 1724. [Google Scholar] [CrossRef]
- Arshad, M.; Amer, M.; Hayat, Q.; Janik, V.; Zhang, X.; Moradi, M.; Bai, M. High-Entropy Coatings (HEC) for High-Temperature Applications: Materials, Processing, and Properties. Coatings 2022, 12, 691. [Google Scholar] [CrossRef]
- Shu, R.; Paschalidou, E.-M.; Rao, S.G.; Bakhit, B.; Boyd, R.; Moro, M.V.; Primetzhofer, D.; Greczynski, G.; Nyholm, L.; le Febvrier, A.; et al. Effect of nitrogen content on microstructure and corrosion resistance of sputter-deposited multicomponent (TiNbZrTa) Nx films. Surf. Coat. Technol. 2020, 404, 126485. [Google Scholar] [CrossRef]
- Tian, C.; Cai, H.; Xue, Y. Effect of Working Pressure on Tribological Properties of Ce-Ti/MoS2 Coatings Using Magnetron Sputter. Coatings 2022, 12, 1576. [Google Scholar] [CrossRef]
- Ehiasarian, A. High-power impulse magnetron sputtering and its applications. Pure Appl. Chem. 2010, 82, 1247–1258. [Google Scholar] [CrossRef]
- Lundin, D. The HiPIMS Process. 2010. Available online: https://urn.kb.se/resolve?urn=urn:nbn:se:liu:diva-56748 (accessed on 24 June 2024).
- Dias, N.F.L.; Meijer, A.L.; Biermann, D.; Tillmann, W. Structure and mechanical properties of TiAlTaN thin films deposited by dcMS, HiPIMS, and hybrid dcMS/HiPIMS. Surf. Coat. Technol. 2024, 487, 130987. [Google Scholar] [CrossRef]
- Samuelsson, M.; Lundin, D.; Jensen, J.; Raadu, M.A.; Gudmundsson, J.T.; Helmersson, U. On the film density using high power impulse magnetron sputtering. Surf. Coat. Technol. 2010, 205, 591–596. [Google Scholar]
- Mazinanian, N.; Wallinder, I.O.; Hedberg, Y. Comparison of the influence of citric acid and acetic acid as simulant for acidic food on the release of alloy constituents from stainless steel AISI 201. J. Food Eng. 2015, 145, 51–63. [Google Scholar] [CrossRef]
- Sputtering Yields. Available online: https://www.angstromsciences.com/sputtering-yields (accessed on 26 June 2024).
- Ren, B.; Yan, S.Q.; Zhao, R.F.; Liu, Z.X. Structure and properties of (AlCrMoNiTi) Nx and (AlCrMoZrTi) Nx films by reactive RF sputtering. Surf. Coat. Technol. 2013, 235, 764–772. [Google Scholar] [CrossRef]
- Yeh, J.-W. Alloy Design Strategies and Future Trends in High-Entropy Alloys. JOM 2013, 65, 1759–1771. [Google Scholar] [CrossRef]
- Huaizhi, Q.; Minglong, G.; Dongdong, Z.; Wenda, S.; Fengfang, L.; Jing, B.; Qiuzhi, G.; Xiang, Z. Effect of heat treatment time on the microstructure and properties of FeCoNiCuTi high-entropy alloy. J. Mater. Res. Technol. 2023, 24, 4510–4516. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Yeh, J.-W. High-Entropy Alloys: A Critical Review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Karimzadeh, M.; Malekan, M.; Mirzadeh, H.; Li, L.; Saini, N. Effects of titanium addition on the microstructure and mechanical properties of quaternary CoCrFeNi high entropy alloy. Mater. Sci. Eng. A 2022, 856, 143971. [Google Scholar] [CrossRef]
- Miletić, A.; Panjan, P.; Škorić, B.; Čekada, M.; Dražič, G.; Kovač, J. Microstructure and mechanical properties of nanostructured Ti–Al–Si–N coatings deposited by magnetron sputtering. Surf. Coat. Technol. 2014, 241, 105–111. [Google Scholar] [CrossRef]
- Kaciulis, S.; Mezzi, A.; Montesperelli, G.; Lamastra, F.; Rapone, M.; Casadei, F.; Valente, T.; Gusmano, G. Multi-technique study of corrosion resistant CrN/Cr/CrN and CrN: C coatings. Surf. Coat. Technol. 2006, 201, 313–319. [Google Scholar]
- Cheng, K.-H.; Lai, C.-H.; Lin, S.-J.; Yeh, J.-W. Structural and mechanical properties of multi-element (AlCrMoTaTiZr)Nx coatings by reactive magnetron sputtering. Thin Solid Film. 2011, 519, 3185–3190. [Google Scholar] [CrossRef]
- Mendez, A.; Monclus, M.; Santiago, J.; Fernandez-Martinez, I.; Rojas, T.; Garcia-Molleja, J.; Avella, M.; Dams, N.; Panizo-Laiz, M.; Molina-Aldareguia, J. Effect of Al content on the hardness and thermal stability study of AlTiN and AlTiBN coatings deposited by HiPIMS. Surf. Coat. Technol. 2021, 422, 127513. [Google Scholar] [CrossRef]
- Wu, X.; Xu, X.; Jiang, Y.; Chen, C.; Han, H.; Zhao, L.; Xu, J.; Yu, L. Nitriding high entropy alloy films: Opportunities and challenges. Surf. Coat. Technol. 2024, 476, 130157. [Google Scholar]
- Kumar, A.; Malik, G.; Chandra, R.; Mulik, R.S. Sputter-grown hierarchical nitride (TiN & h-BN) coatings on BN nanoplates reinforced Al7079 alloy with improved corrosion resistance. Surf. Coat. Technol. 2022, 432, 128061. [Google Scholar] [CrossRef]
- Wetzel, A.; von der Au, M.; Dietrich, P.M.; Radnik, J.; Ozcan, O.; Witt, J. The comparison of the corrosion behavior of the CrCoNi medium entropy alloy and CrMnFeCoNi high entropy alloy. Appl. Surf. Sci. 2022, 601, 154171. [Google Scholar] [CrossRef]
- Ahmed, M.K.; Shahid, M.; Khan, Z.A.; Ammar, A.U.; Saboor, A.; Khalid, A.; Hayat, A.; Saeed, A.; Koohgilani, M. Electrochemical comparison of SAN/PANI/FLG and ZnO/GO coated cast iron subject to corrosive environments. Materials 2018, 11, 2239. [Google Scholar]
- Berríos, J.C. ▷ Propiedades dieléctricas de los materiales aislantes (fórmula y constante) | TELCOM® 2024. TELCOM: Aprenda ingeniería eléctrica y electrónica (gratis). Available online: https://telcomplus.org/propiedades-dielectricas-del-aislamiento/ (accessed on 20 July 2024).
- Song, G.L.; Liu, M. Corrosion and electrochemical evaluation of an Al–Si–Cu aluminum alloy in ethanol solutions. Corrosion Science 2013, 72, 73–81. [Google Scholar]
- González-Hernández, A.; Morales-Cepeda, A.B.; Flores, M.; Caicedo, J.C.; Aperador, W.; Amaya, C. Electrochemical Properties of TiWN/TiWC Multilayer Coatings Deposited by RF-Magnetron Sputtering on AISI 1060. Coatings 2021, 11, 797. [Google Scholar] [CrossRef]
- Zuñiga-Diaz, K.; Arrieta-Gonzalez, C.D.; Porcayo-Calderon, J.; Gonzalez-Rodriguez, J.G.; Casales-Diaz, M.; Martinez-Gomez, L. Electrochemical Behavior of Austenitic Stainless Steels Exposed to Acetic Acid Solution. Int. J. Electrochem. Sci. 2020, 15, 1242–1263. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, H.; Xu, Y.; Li, H.; Liu, J.; Fan, Y.; Wang, S. Corrosion Behavior of Aluminum in Dilute Acetic Acid Solution Simulating Cooling Water in HVDC Transmission. Int. J. Electrochem. Sci. 2022, 17, 220324. [Google Scholar] [CrossRef]
- Singh, S.K.; Mukherjee, A.K. Kinetics of Mild Steel Corrosion in Aqueous Acetic Acid Solutions. J. Mater. Sci. Technol. 2010, 26, 264–269. [Google Scholar] [CrossRef]
- Moreno Amado, M. Resistencia a la corrosión y al desgaste de recubrimientos nanoestructurados de Zirconia (ZrO2)—Plata (Ag) y/o Alúmina (Al2O3) obtenidos con técnica de “Sputtering” reactivo con magnetrón desbalanceado. June 2019. Available online: https://repositorio.unal.edu.co/handle/unal/78428 (accessed on 25 July 2024).
- Shahidi, M.; Gholamhosseinzadeh, M.R. Electrochemical evaluation of AA6061 aluminum alloy corrosion in citric acid solution without and with chloride ions. J. Electroanal. Chem. 2015, 757, 8–17. [Google Scholar] [CrossRef]
- Li, X.; Deng, S. Ce(SO4)2 as an efficient corrosion inhibitor for cold rolled steel in citric acid solution. J. Taiwan Inst. Chem. Eng. 2021, 122, 273–283. [Google Scholar] [CrossRef]
- Mazinanian, N.; Herting, G.; Odnevall, I.; Hedberg, Y. Metal Release and Corrosion Resistance of Different Stainless Steel Grades in Simulated Food Contact. Corrosion 2016, 72, 775–790. [Google Scholar] [CrossRef]
- Mazinanian, N.; Wallinder, I.O.; Hedberg, Y.S. Influence of citric acid on the metal release of stainless steels. Corros. Sci. Technol. 2015, 14, 166–171. [Google Scholar]
- Mazinanian, N.; Hedberg, Y.S. Metal Release Mechanisms for Passive Stainless Steel in Citric Acid at Weakly Acidic pH. J. Electrochem. Soc. 2016, 163, C686. [Google Scholar] [CrossRef]
- Vasyliev, G.S. The influence of flow rate on corrosion of mild steel in hot tap water. Corros. Sci. 2015, 98, 33–39. [Google Scholar]
- Grips, V.K.W.; Selvi, V.E.; Barshilia, H.C.; Rajam, K.S. Effect of electroless nickel interlayer on the electrochemical behavior of single layer CrN, TiN, TiAlN coatings and nanolayered TiAlN/CrN multilayer coatings prepared by reactive dc magnetron sputtering. Electrochim. Acta 2006, 51, 3461–3468. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, F.; Chen, Q.; Zhang, W.; Zhu, C.; Deng, J.; Zhong, Y.; Liao, J.; Yang, Y.; Liu, N.; et al. Effect of Au-ions irradiation on mechanical and LBE corrosion properties of amorphous AlCrFeMoTi HEA coating: Enhanced or deteriorated? Corros. Sci. 2021, 192, 109862. [Google Scholar] [CrossRef]
- Zhang, Z.; Han, E.-H.; Xiang, C. Effect of helium ion irradiation on short-time corrosion behavior of two novel high-entropy alloys in simulated PWR primary water. Corros. Sci. 2021, 191, 109742. [Google Scholar] [CrossRef]
- Influence of Citric Acid on the Metal Release of Stainless Steels-Corrosion Science and Technology|Korea Science. Available online: https://koreascience.or.kr/article/JAKO201528551642095.page (accessed on 11 August 2024).
- Lodhi, M.J.K.; Deen, K.M.; Haider, W. Corrosion behavior of additively manufactured 316L stainless steel in acidic media. Materialia 2018, 2, 111–121. [Google Scholar] [CrossRef]


| Parameters | Values |
|---|---|
| Deposition time [min] | 30 |
| Frecuency [Hz] | 500 |
| Pulse width [μs] | 60 |
| N2 flow rate [m3/s] | 33.3 × 10−9 |
| Ar Flow rate [m3/s] | 233 × 10−9 |
| Working pressure [10−3 mbar] | 5.07 ± 0.15 |
| Substrate temperature [°C] | 250.43 ± 0.98 |
| Discharge Voltage Udc [V] | 628 |
| Discharge current Idc [mA] | 302.86 ± 3.44 |
| Peak Power Pavg [W] | 190.57 ± 2.57 |
| Peak Current Ipk [A] | 13 ± 0.58 |
| Nb | Ti | Zr | Al | Ta | N | |
|---|---|---|---|---|---|---|
| Element concentration %at. | 11.87 ± 0.46 | 12.08 ± 0.12 | 9.70 ± 0.27 | 8.89 ± 0.19 | 8.24 ± 0.24 | 49.24 ± 0.48 |
| HEA | ∆Sconf | Reference |
|---|---|---|
| (TiNbZrTa)N | 1.54 R | [22] |
| (TiZrNbTaFe)N | 1.67 R | [2] |
| (TiZrNbAlYCr)N | 1.89 R | [3] |
| Electrolyte | Sample | Icorr (nA/cm2) | Ecorr (mV) | Corrosion Rate (mpy) | Pitting Potential (mV) |
|---|---|---|---|---|---|
| Acetic acid | AISI 304 | 283.0 | −141.0 | 658.9 × 10−3 | - |
| (TiAlTaZrNb)N | 6.050 | −59.20 | 14.1 × 10−3 | - | |
| Citric acid | AISI 304 | 472.0 | −205.0 | 1100 × 10−3 | 0.3 |
| (TiAlTaZrNb)N | 5.47 | −97.40 | 12.7 × 10−3 | 0.58 | |
| Tap water | AISI 304 | 13.50 | −77.6 | 31.4 × 10−3 | 0.3 |
| (TiAlTaZrNb)N | 7.55 | −94.80 | 17.6 × 10−3 | 0.5 |
| Electrolyte | Sample | Pa (µm) | Psk (µm) | Pv (µm) |
|---|---|---|---|---|
| Acetic acid | Before | 0.049 ± 0.02 | 0.425 ± 0.15 | 0.079 ± 0.02 |
| After | 0.027 ± 0.001 | −0.403 ± 0.12 | 0.102 ± 0.03 | |
| Citric acid | Before | 0.028 ± 0.003 | 0.296 ± 0,26 | 0.065 ± 0.02 |
| After | 0.024 ± 0.019 | −0.193 ± 0,37 | 0.056 ± 0.09 | |
| Tap water | Before | 0.032 ± 0.002 | −1.287 ± 0,92 | 0.244 ± 0.09 |
| After | 0.030 ± 0.006 | −0.20 ± 0,42 | 0.101 ± 0.05 |
| Electrolyte—Sample | Time (min) | Rsol (Ω) | Rpo (Ω) | Cc (S × sa) | m | Rcor (Ω) | Ccor (S × sa) | n | Rp (Ω) |
|---|---|---|---|---|---|---|---|---|---|
| Acetic acid AISI 304 | 0 | 5.5 × 103 | 7.29 × 105 | 5.46 × 10−6 | 0.89 | 8.29 × 105 | 8.30 × 10−6 | 0.90 | 1.56 × 106 |
| 168 | 5.6 × 103 | 2.35 × 107 | 3.80 × 10−6 | 0.91 | 7.18 × 103 | 1.57 × 10−4 | 0.56 | 2.35 × 107 | |
| Acetic acid TiAlTaZ-rNbN | 0 | 4.6 × 103 | 2.52 × 107 | 5.58 × 10−6 | 0.91 | 2.78 × 104 | 1.15 × 10−5 | 0.58 | 2.52 × 107 |
| 168 | 5.4 × 103 | 5.59 × 107 | 3.60 × 10−6 | 0.92 | 8.40 × 104 | 1.33 × 10−5 | 0.58 | 5.60 × 107 | |
| Citric acid AISI 304 | 0 | 1.1 × 103 | 7.30 × 105 | 12.6 × 10−6 | 0.87 | 3.65 × 108 | 1.44 × 10−7 | 0.62 | 3.66 × 105 |
| 168 | 4.4 × 103 | 5.15 × 107 | 7.41 × 10−6 | 0.92 | 7.47 × 103 | 2.55 × 10−6 | 0.56 | 5.15 × 107 | |
| Citric Acid TiAlTaZ-rNbN | 0 | 3.6 × 103 | 1.76 × 107 | 5.18 × 10−6 | 0.91 | 2.67 × 107 | 2.40 × 10−2 | 0.53 | 4.43 × 107 |
| 168 | 4.5 × 103 | 5.48 × 107 | 3.97 × 10−6 | 0.92 | 1.07 × 107 | 2.62 × 10−2 | 0.53 | 6.55 × 107 | |
| Tap water AISI 304 | 0 | 2.8 × 103 | 2.99 × 105 | 2.52 × 10−6 | 0.90 | 1.64 × 107 | 5.45 × 10−7 | 0.58 | 1.67 × 107 |
| 168 | 13.5 × 103 | 4.06 × 106 | 1.93 × 10−6 | 0.90 | 1.40 × 108 | 2.39 × 10−7 | 0.64 | 1.44 × 108 | |
| Tap water TiAlTaZrNbN | 0 | 28.1 × 103 | 5.85 × 106 | 5.58 × 10−6 | 0.90 | 1.28 × 107 | 1.84 × 10−9 | 0.65 | 1.87 × 107 |
| 168 | 36.1 × 103 | 1.22 × 106 | 3.93 × 10−6 | 0.92 | 7.03 × 107 | 3.43 × 10−8 | 0.59 | 7.16 × 107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castañeda, M.-C.; Piamba, O.; Olaya, J. Corrosion Performance of (TiAlZrTaNb)Nx High-Entropy Nitrides Thin Films Deposited on 304 Stainless Steel via HiPIMS. Metals 2025, 15, 988. https://doi.org/10.3390/met15090988
Castañeda M-C, Piamba O, Olaya J. Corrosion Performance of (TiAlZrTaNb)Nx High-Entropy Nitrides Thin Films Deposited on 304 Stainless Steel via HiPIMS. Metals. 2025; 15(9):988. https://doi.org/10.3390/met15090988
Chicago/Turabian StyleCastañeda, Maria-Camila, Oscar Piamba, and Jhon Olaya. 2025. "Corrosion Performance of (TiAlZrTaNb)Nx High-Entropy Nitrides Thin Films Deposited on 304 Stainless Steel via HiPIMS" Metals 15, no. 9: 988. https://doi.org/10.3390/met15090988
APA StyleCastañeda, M.-C., Piamba, O., & Olaya, J. (2025). Corrosion Performance of (TiAlZrTaNb)Nx High-Entropy Nitrides Thin Films Deposited on 304 Stainless Steel via HiPIMS. Metals, 15(9), 988. https://doi.org/10.3390/met15090988






