Development Status of Production Purification and Casting and Rolling Technology of Electrical Aluminum Rod
Abstract
1. Introduction
2. Aluminum Alloy Cable Market Size and Classification
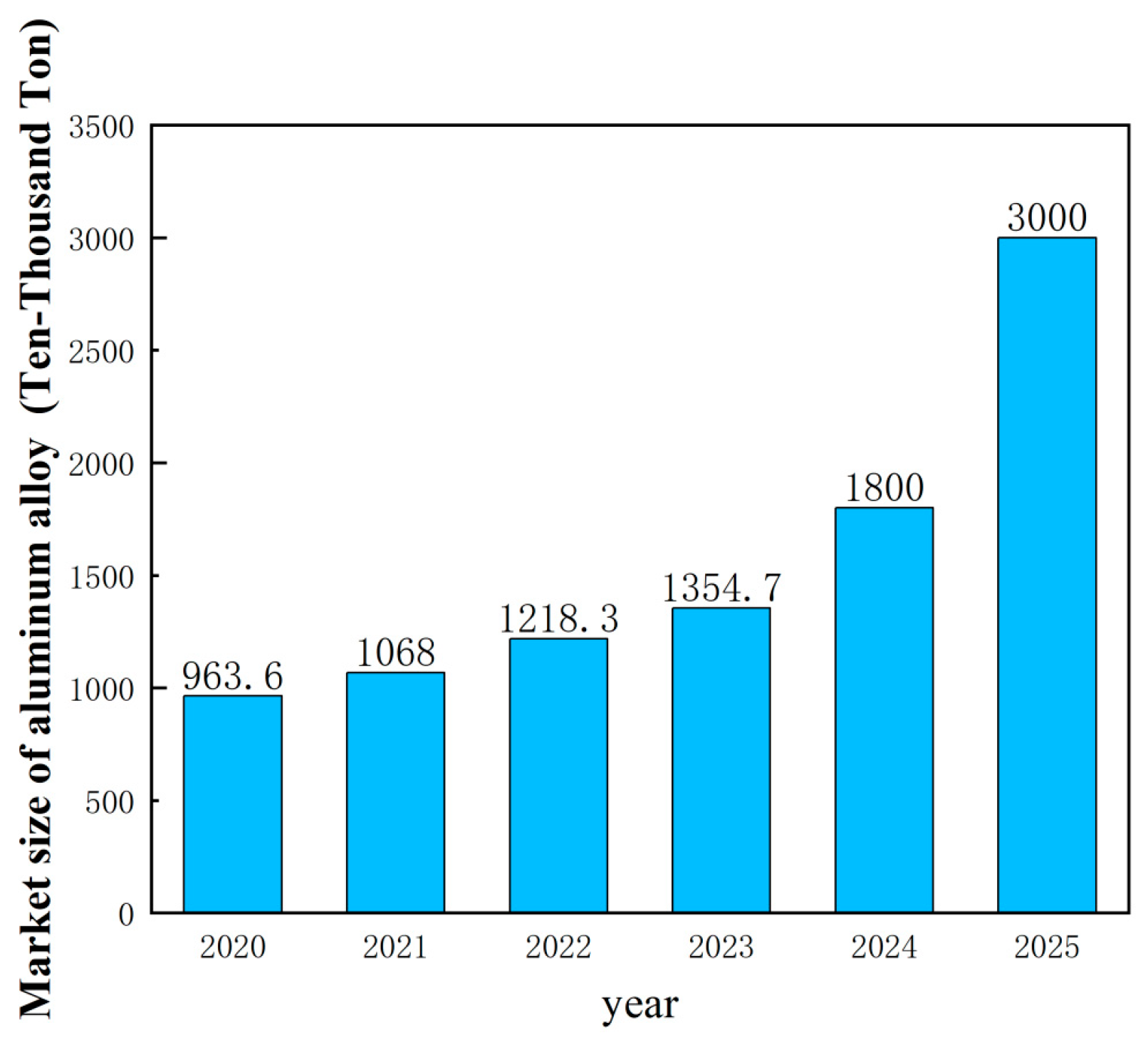
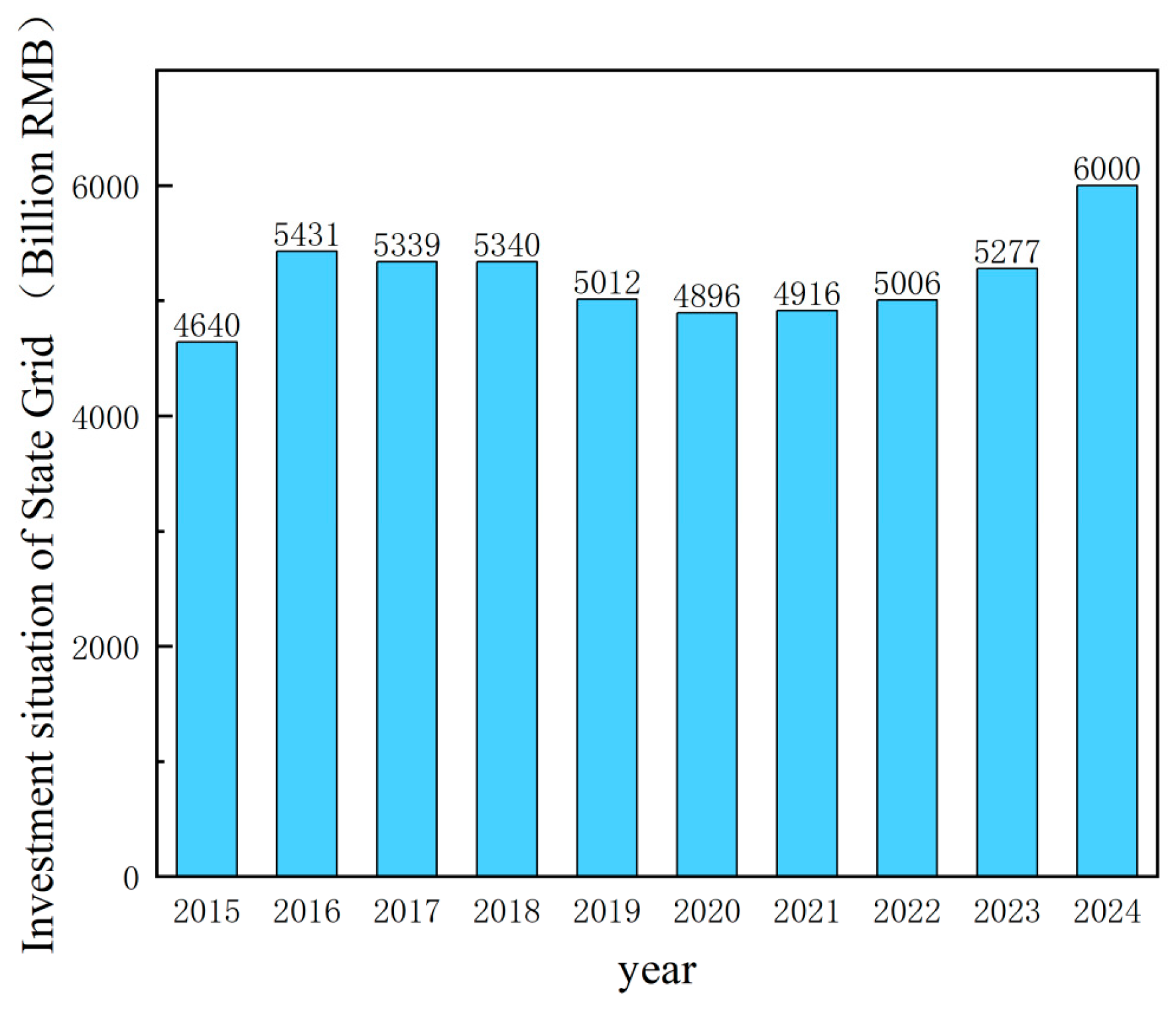
2.1. Aluminum Alloy Cable Market Size
2.2. Classification and Performance Requirements of Round Aluminum Rods for Electricians
3. Development of the Purification Process
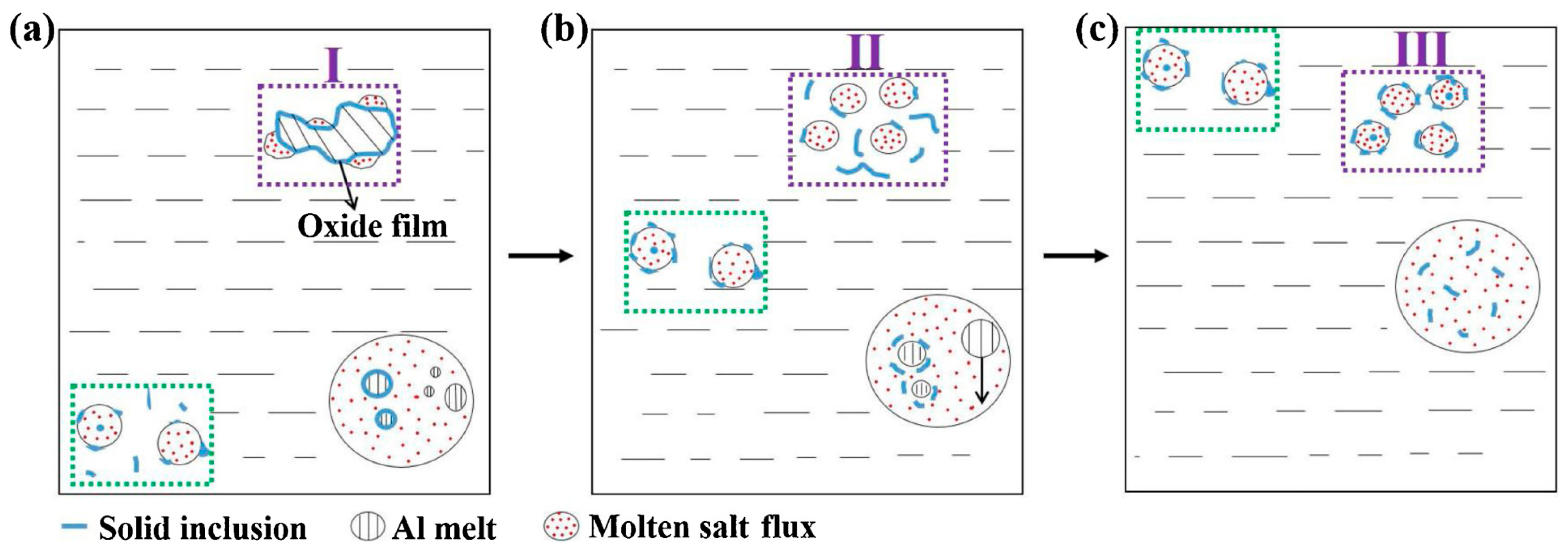
3.1. Fluxing Method
3.1.1. Refining Agent
3.1.2. Intermediate Alloy
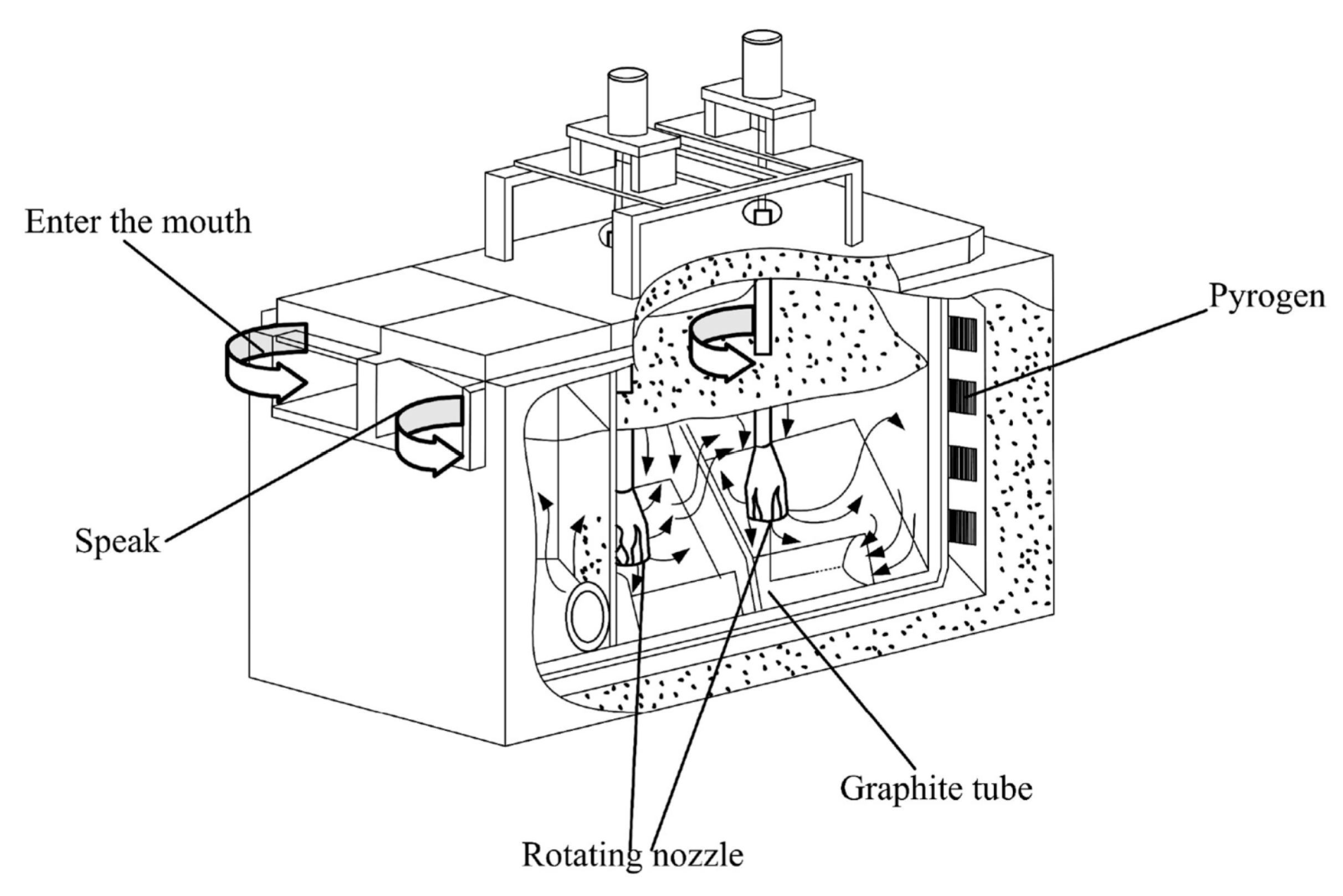
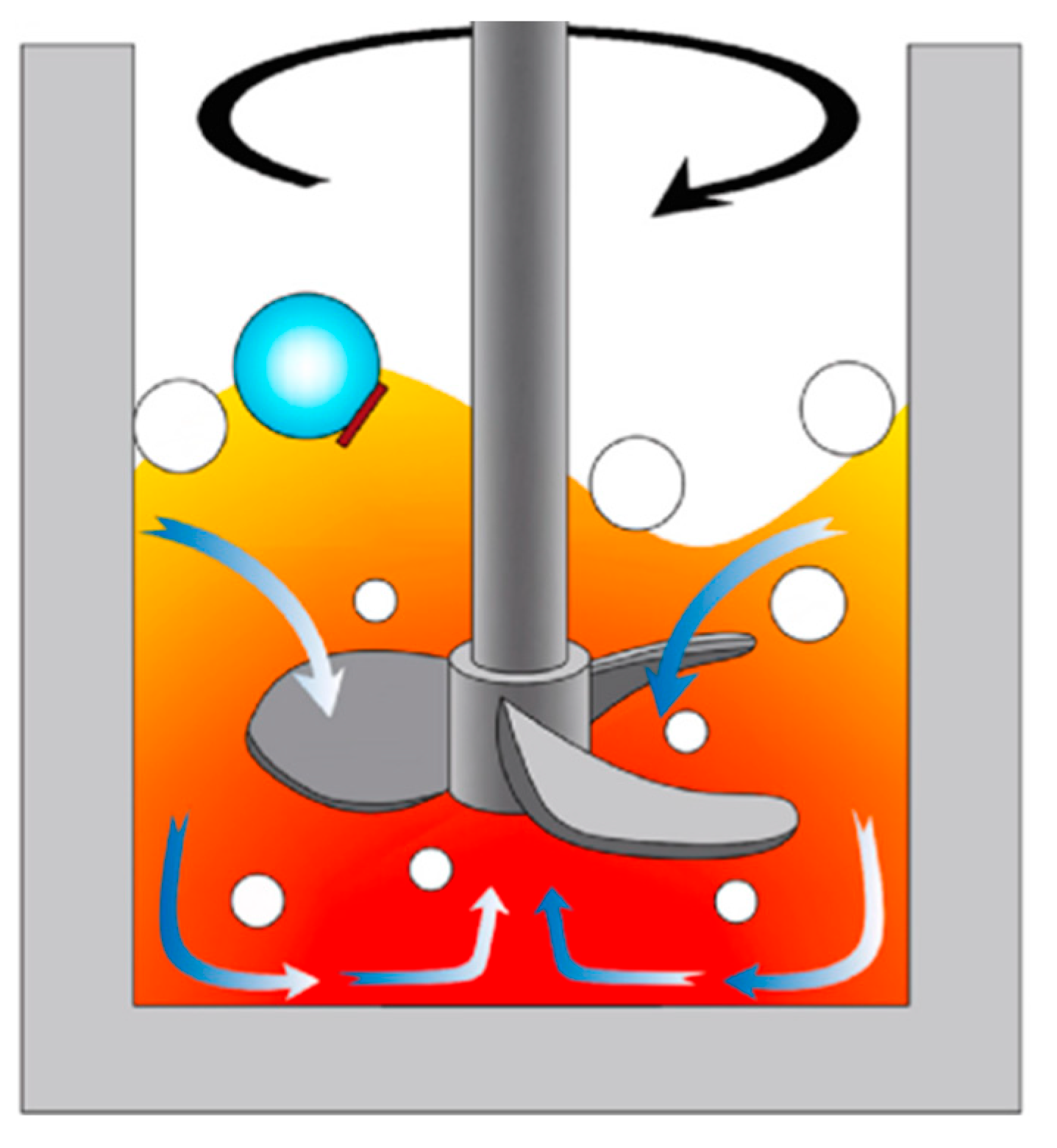
3.2. Gas Purification Method
3.3. Filtration Method
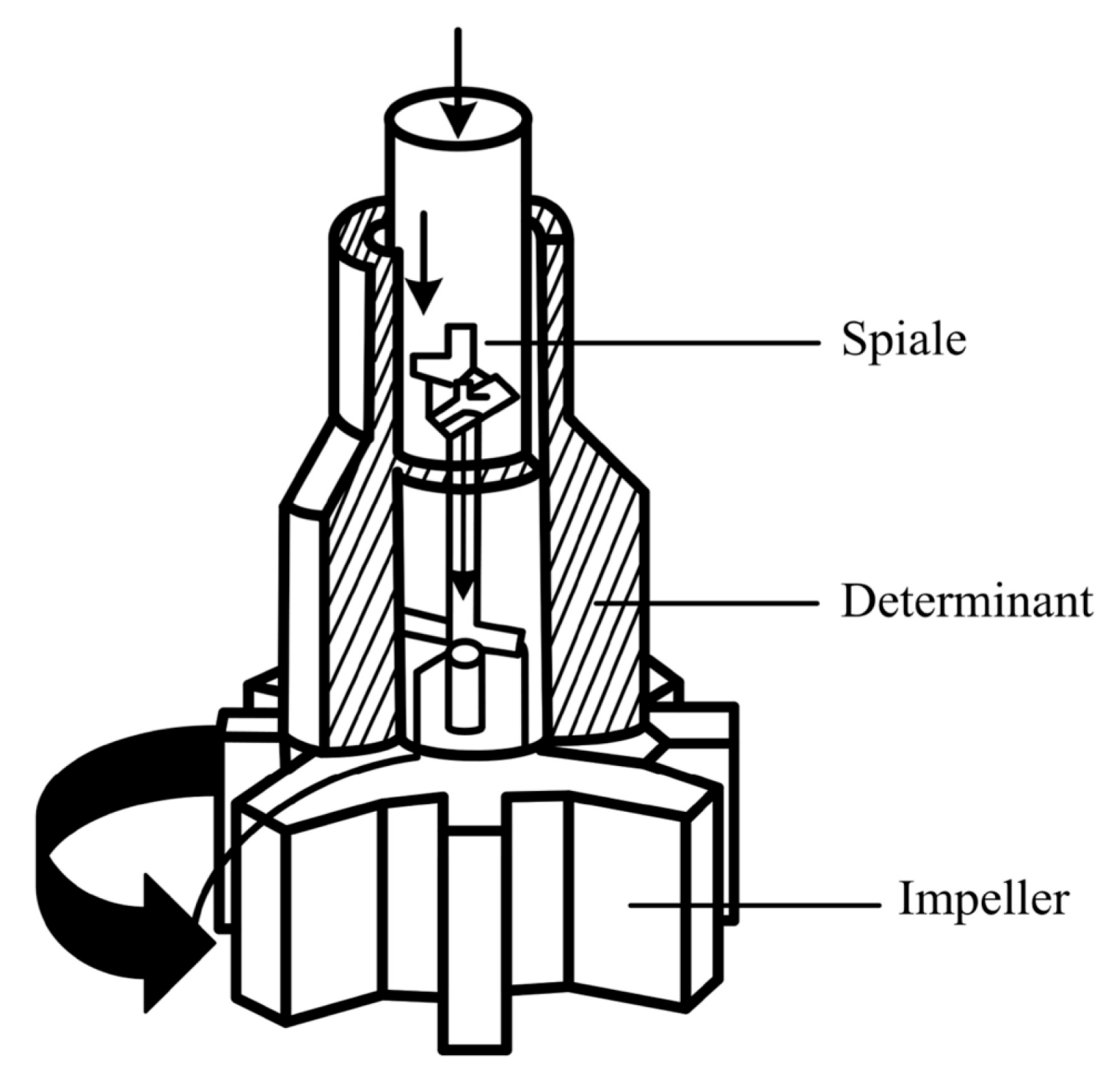
3.4. Rotary Injection Method
3.5. Other Auxiliary Methods
4. Cast-Rolling Process
4.1. Continuous Casting and Rolling Process
4.2. Two-Roll Cast-Rolling Process
4.3. Direct Water-Cooled Semi-Continuous Casting and Extrusion Process
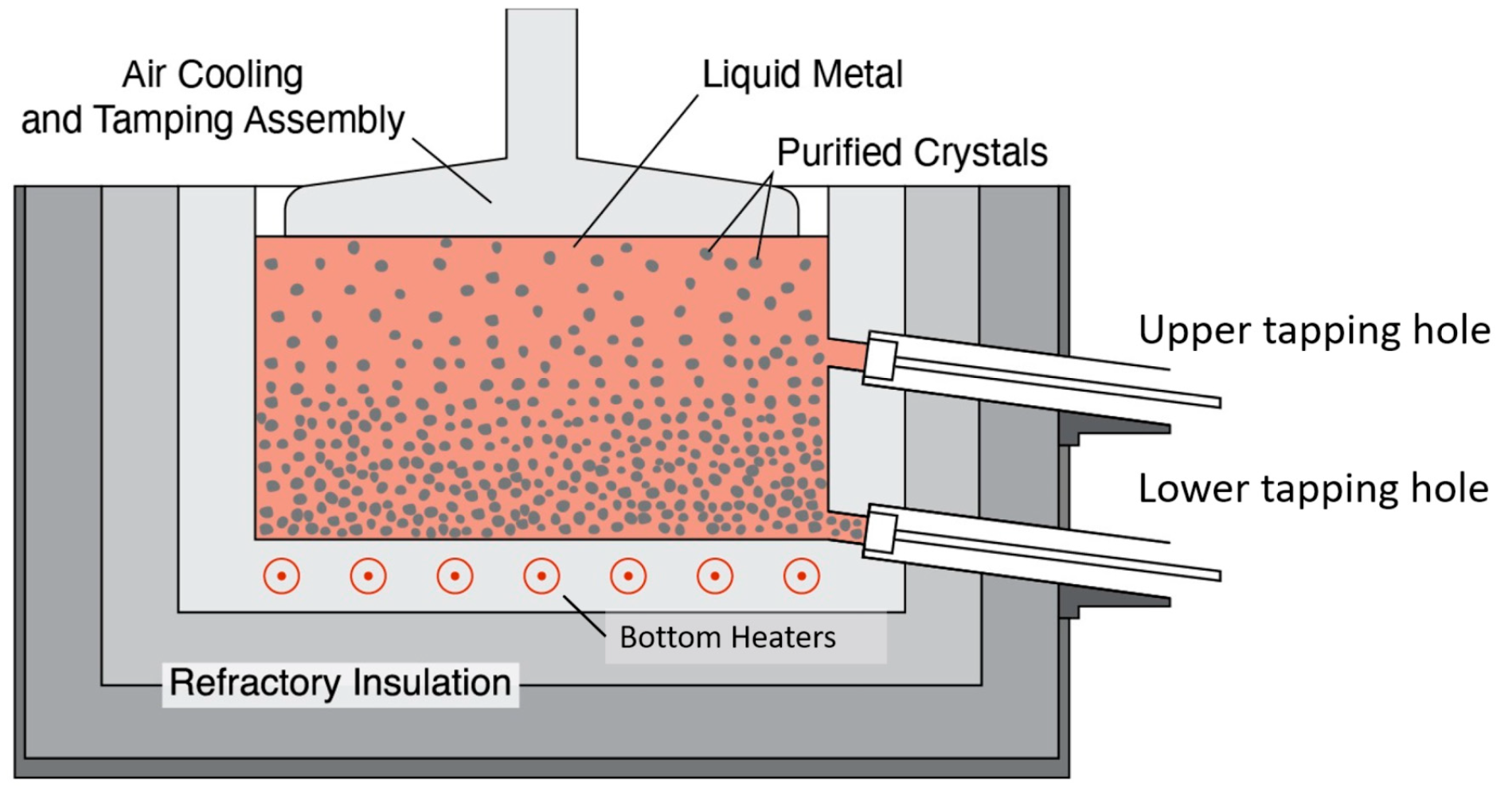
4.3.1. Direct Water-Cooled Semi-Continuous Casting
4.3.2. Extrusion Process
4.4. Continuous Casting and Extrusion Process
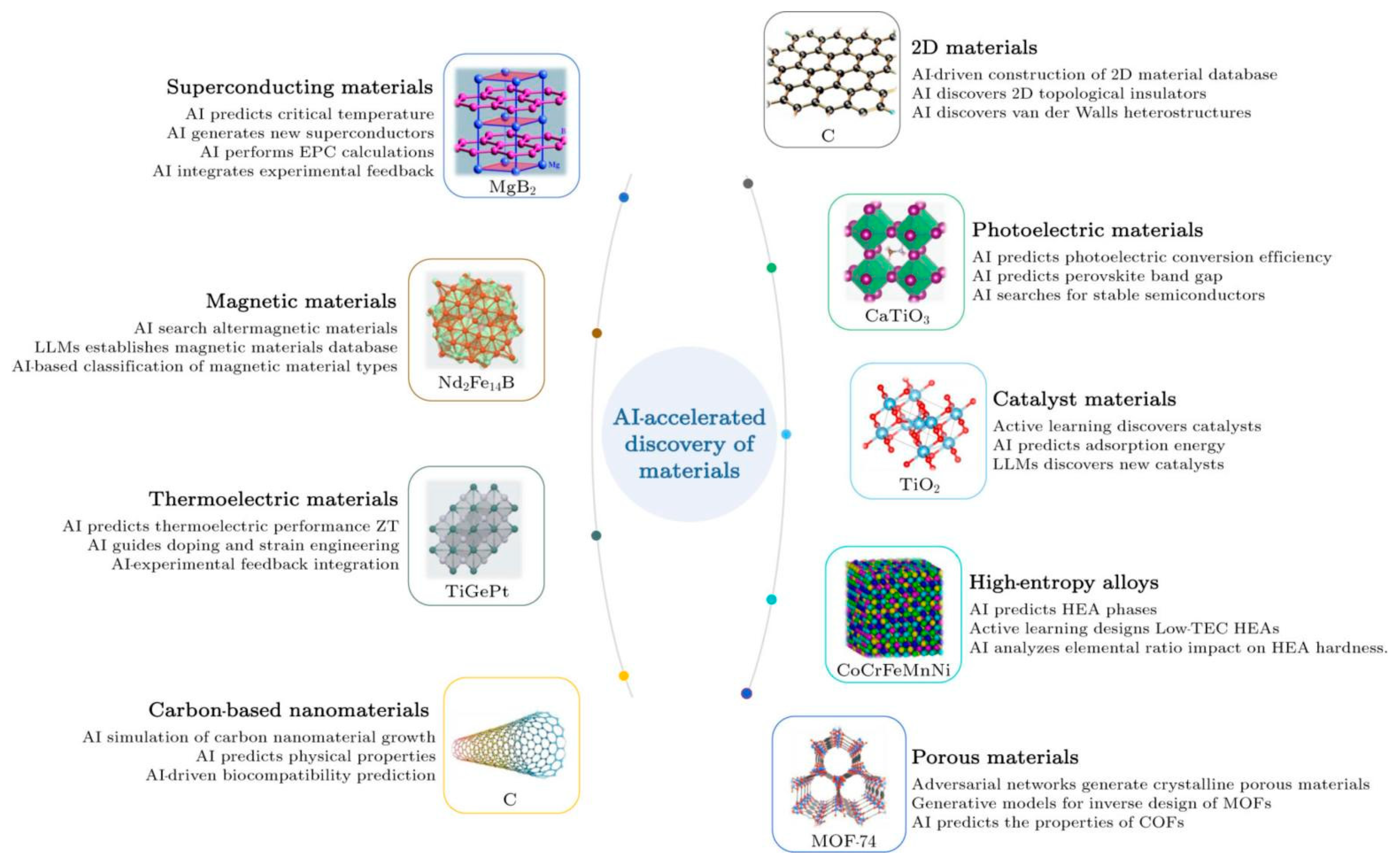
5. The Prospects of AI-Driven Process Innovation
5.1. AI Innovations in Purification Processes
5.2. AI Applications in Casting-Rolling Processes
5.3. AI Applications in ERAR Product Inspection
5.4. Summary and Prospects
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ERAR | Electrician Round Aluminum Rod |
| CRP | Cast-Rolling Process |
| IACS | International Annealed Copper Standard |
| UHV | Ultra-High Voltage |
| SNIF | Spinning Nozzle Inert Flotation |
| ALPUR | Aluminum Purification |
| FILD | Fumeless In-Line Degassing |
| RDU | Refining and Degassing Unit |
| GBF | Gas Bubbling Flotation |
| MINT | Melt In-Line Treatment System |
| Alcoa469 | Melt Purification by ALCOA469 Method |
| Heprojet | Helium Projection |
| LARSTM | Liquid Aluminum Refining System |
| SPD | Severe Plastic Deformation |
| ESCP | Equal Channel Angle Extrusion |
| DC | Direct Water-Cooled Semicontinuous |
| ML | Machine Learning |
| DL | Deep Learning |
References
- Shen, W.; Hu, A.; Liu, S.; Hu, H. Al-Mn alloys for electrical applications: A review. J. Alloys Metall. Syst. 2023, 2, 2949–9178. [Google Scholar] [CrossRef]
- Zhang, A.; Li, Y. Thermal conductivity of aluminum alloys—A review. Materials 2023, 16, 2972. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Peng, J. A review on aluminum alloy conductors influenced by alloying elements and thermomechanical treatments: Microstructure and properties. J. Mater. Res. 2023, 38, 1488–1509. [Google Scholar] [CrossRef]
- Sims, Z.C.; Rios, O.R.; Weiss, D.; Turchi, P.E.A.; Perron, A.; Lee, J.R.I.; Li, T.T.; Hammons, J.A.; Hansen, M.B.; Willey, T.M.; et al. High performance aluminum–cerium alloys for high-temperature applications. Mater. Horiz. 2017, 4, 1070–1078. [Google Scholar] [CrossRef]
- Haridas, R.S.; Agrawal, P.; Thapliyal, S.; Agrawal, P.; Dhal, A.; Shukla, S.; Zhou, L.; Sohn, Y.; Mishra, R.S. Synergy of tensile strength and high cycle fatigue properties in a novel additively manufactured Al-Ni-Ti-Zr alloy with a heterogeneous microstructure. Addit. Manuf. 2023, 62, 103380. [Google Scholar] [CrossRef]
- Czerwinski, F. Aluminum alloys for electrical engineering: A review. J. Mater. Sci. 2024, 59, 14847–14892. [Google Scholar] [CrossRef]
- Korotkova, N.O.; Belov, N.A.; Timofeev, V.N.; Motkov, M.M.; Cherkasov, S.O. Influence of heat treatment on the structure and properties of an Al–7% REM conductive aluminum alloy casted in an electromagnetic crystallizer. Phys. Met. Metallogr. 2020, 121, 173–179. [Google Scholar] [CrossRef]
- Olafsson, P.; Sandstrom, R.; Karlsson, Å. Comparison of experimental, calculated and observed values for electrical and thermal conductivity of aluminum alloys. J. Mater. Sci. 1997, 32, 4383–4390. [Google Scholar] [CrossRef]
- Yavaş, A.; Cilingir, C.; Turk, A.; Celik, E. Production and characterization of highly conductive aluminum metal for electric motor applications. J. Mater. Eng. Perform. 2025, 34, 1705–1716. [Google Scholar] [CrossRef]
- She, X.; Jiang, X.; Tan, X.; Guo, S. Status and prospect for aluminum industrial development in China. Chin. J. Nonferrous Met. 2020, 30, 709–718. [Google Scholar] [CrossRef]
- Levin, A.A.; Narykova, M.V.; Lihachev, A.I.; Kardashev, B.K.; Kadomtsev, A.G.; Brunkov, P.N.; Panfilov, A.G.; Prasolov, N.D.; Sultanov, M.M.; Kuryanov, V.N.; et al. Modification of the Structural, Microstructural, and Elastoplastic Properties of Aluminum Wires after Operation. Metals 2021, 11, 1955. [Google Scholar] [CrossRef]
- Levin, A.A.; Narykova, M.V.; Lihachev, A.I.; Kardashev, B.K.; Kadomtsev, A.G.; Panfilov, A.G.; Prasolov, N.D.; Sokolov, R.V.; Brunkov, P.N.; Sultanov, M.M.; et al. Structural, Microstructural, Elastic, and Microplastic Properties of Aluminum Wires (from AAAC (A50) Cables) after Fatigue Tests. Metals 2023, 13, 298. [Google Scholar] [CrossRef]
- Sebastian, M. Influence of Non-Uniform Temperature Distribution on Metallic Charge Length on Energy and Force Parameters During Groove-Rolling. J. Iron Steel Res. Int. 2012, 19, 17–24. [Google Scholar] [CrossRef]
- Nan, M.; Li, H.; Chen, S. Review of the effects of composition and processing on 8030 aluminum alloy cable conductors. J. Shanxi Univ. Technol. (Nat. Sci. Ed.) 2024, 40, 1–9. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, A. Application and market analysis of aluminum alloy cable. Rare Met. Cem. Carbide 2016, 44, 81–84. [Google Scholar] [CrossRef]
- Analysis of China’s Aluminum Alloy Industry Products Tend to Be High-End Under the Policy of Sufficient Supply Side Capacity. Available online: www.chinabaogao.com/market/202401/688881.html (accessed on 10 July 2025).
- Investment in Power Grid Construction of State Grid and China Southern Power Grid over the Years. Available online: https://bg.sgpjbg.com/hyshuju/2e56fe116d0325b4459a278b758387d5.html (accessed on 10 July 2025).
- SMM. Available online: https://price.smm.cn (accessed on 10 July 2025).
- GB/T 3954:2022; Aluminium and Aluminium Alloys Rod for Electrical Purpose. Nation Standardization Administration of the People’s Republic of China: Beijing, China, 2022.
- Li, J. Current situation and development of aluminum alloy refining technology. Low-Carbon World 2018, 6, 330–331. [Google Scholar] [CrossRef]
- Qiu, Y.; Chen, C.; Li, J.; Wang, L.; Lan, Y.; Zhao, F. Research status and trend of aluminum melt purification. China Metall. 2023, 33, 9–16. [Google Scholar] [CrossRef]
- Ma, L. Exploration of common impurity purification technology in aluminum alloy melting process. Shanxi Metall. 2024, 47, 93–94. [Google Scholar] [CrossRef]
- Wan, B.; Li, W.; Liu, F.; Lu, T.; Jin, S.; Wang, K.; Yi, A.; Tian, J.; Chen, W. Determination of fluoride component in the multifunctional refining flux used for recycling aluminum scrap. J. Mater. Res. Technol. 2020, 9, 3447–3459. [Google Scholar] [CrossRef]
- Cai, Y.; Song, D.; Yang, D.; Zhao, Y.; Ke, B.; Zhang, D.; Zhang, W. Effect of Na3AlF6 contents in refining flux on 3D characteristics of pore and mechanical properties of recycled Al-Mg-Si alloy. Mater. Today Commun. 2024, 39, 108797. [Google Scholar] [CrossRef]
- Kulikov, B.; Partyko, E.; Kosovich, A.; Yuryev, P.; Mansurov, Y.; Stepanenko, N.; Baykovskiy, Y.; Bozhko, D.; Durnopyanov, A.; Dombrovskiy, N.; et al. Analysis of composition, properties, and usage efficiency of different commercial salt fluxes for aluminum alloy refining. Metals 2025, 15, 448. [Google Scholar] [CrossRef]
- Shi, M.; Li, Y. Performance Improvement in Aluminum Alloy Treated by Salt Flux with Different Fluorides. J. Mater. Eng. Perform. 2023, 32, 3065–3072. [Google Scholar] [CrossRef]
- Chen, G.; Huang, S.; Fu, G.; Wu, J.; Chen, R.; Zheng, X. Optimization of impurity removal and purification flux components and their application in the production of A356 aluminum alloy. J. Phys. Conf. Ser. 2024, 2845, 012008. [Google Scholar] [CrossRef]
- Yuan, S.; Hao, F.; Zhou, F.; Wang, Z.; Jin, H.; Huang, R.; Gu, W. Current status and prospects of aluminum melt furnace refining process for electrical round aluminum rod. J. Netshape Form. Eng. 2024, 16, 71–78. [Google Scholar] [CrossRef]
- Zhao, P.; Jiang, X.; Zhao, Y.; Zhu, Y.; Mu, H.; Cui, T. Use of a new environment-friendly AJ401K smokeless particle refiner. Chongqing China 2021, 325–329. [Google Scholar] [CrossRef]
- Tkacheva, O.; Kataev, A.; Rudenko, A.; Dedyukhin, A.; Zaikov, Y. Novel molten salts media for production of functional materials. MATEC Web Conf. 2016, 67, 06044. [Google Scholar] [CrossRef]
- Dhaneswara, D.; Fatriansyah, J.F.; Firmansyah, M.R.; Prasetyo, Y. Removal of oxide inclusions in aluminum scrap casting process with sodium based fluxes. MATEC Web Conf. 2019, 269, 07002. [Google Scholar] [CrossRef]
- Chen, S.; Jin, Y.; Wei, H.; Li, Z. Study on the effect of rare earth elements La, Ce, Y and Sc doping on the mechanical properties of aluminum–lithium alloys. Appl. Surf. Sci. 2025, 680, 161334. [Google Scholar] [CrossRef]
- Zhang, G.; Lu, W.; Wu, X.; Wu, X.; Yang, B.; Tan, Y.; Xu, Z.; Tang, H.; Zeng, J.; Wang, J. A new strategy on designing fluxes for aluminum alloy melt refinement. Materials 2023, 16, 2322. [Google Scholar] [CrossRef]
- Yang, H.; Qian, Z.; Chen, H.; Zhao, X.; Han, G.; Du, W.; Nie, X.; Zhao, K.; Liu, G.; Sun, Q.; et al. A new insight into heterogeneous nucleation mechanism of Al by non-stoichiometric TiCx. Acta Mater. 2022, 233, 117977. [Google Scholar] [CrossRef]
- Chen, G.; Huang, S.; Fu, G.; Wu, J.; Chen, R. Study on the Effect of Al-Ti-B-RE refiner on the refining effect of A356 aluminum alloy after different melt treatments. J. Phys. Conf. Ser. 2024, 2845, 12039. [Google Scholar] [CrossRef]
- Ait, E.H.B.; Hamadellah, A.; Bouayad, A.; El, A.C. Review of grain refinement performance of aluminum cast alloys. Metall. Mater. Eng. 2023, 29, 1–15. [Google Scholar] [CrossRef]
- Cui, X.; Wu, Y.; Zhang, G.; Liu, Y.; Liu, X. Study on the improvement of electrical conductivity and mechanical properties of low alloying electrical aluminum alloys. Compos. Part B Eng. 2017, 110, 381–387. [Google Scholar] [CrossRef]
- Liu, G.; Ren, Y.; Ma, W.; Morita, K.; Lei, Y.; Zhan, S.; Lv, G.; Li, S.; Zeng, Y.; Li, R. Development process and future trends of chemical refining agents’ influence on grain refinement in aluminum alloys. J. Mater. Res. Technol. 2024, 29, 242–257. [Google Scholar] [CrossRef]
- Zeng, P.; Zhang, X.; Zeng, H.; Liu, Y. Influence of rare earth content on microstructure properties of 8030 aluminum alloy. Light Met. 2017, 2, 47–50. [Google Scholar] [CrossRef]
- Lin, G.; Li, L.; Guo, Z.; Jia, X.; Wang, X.; Yuan, Z.; Zhang, G.; Zhan, Y.; Shan, Q.; Li, Z. Influence of cerium and yttrium addition on strength and electrical conductivity of pure aluminum alloys. J. Rare Earths 2024, 42, 600–611. [Google Scholar] [CrossRef]
- Teng, D.; Zhang, G.; Zhang, S.; Li, J.; Guan, R. Response of mechanical properties of A356 alloy to continuous rheological extrusion Al-5Ti-0.6C-xCe alloy addition upon different Ce contents. J. Mater. Res. Technol. 2023, 27, 209–224. [Google Scholar] [CrossRef]
- Al-Alimi, S.; Yusuf, N.K.; Ghaleb, A.M.; Lajis, M.A.; Shamsudin, S.; Zhou, W.; Altharan, Y.M.; Abdulwahab, H.S.; Saif, Y.; Didane, D.H.; et al. Recycling aluminum for sustainable development: A review of different processing technologies in green manufacturing. Results Eng. 2024, 23, 102566. [Google Scholar] [CrossRef]
- Du, S.; Zhang, S.; Wang, J.; Lv, Z.; Xu, Z.; Liu, C.; Liu, J.; Liu, B. Sustainable recycling of aerospace-grade ultra-clean 7050 aluminum alloy melts through argon refining without secondary aluminum dross generation. J. Mater. Res. Technol. 2023, 27, 2102–2116. [Google Scholar] [CrossRef]
- Qin, Y.; Xu, G.; Li, Z.; Li, Y.; Long, M.; Wu, Q.; Li, J.; Wu, G.; Hu, W.; Chen, Z. Research and application of aluminum alloy refining Technology for automobile. Pop. Sci. Technol. 2023, 25, 58–61. [Google Scholar] [CrossRef]
- Wu, J.; Djavanroodi, F.; Gode, C.; Attarilar, S.; Ebrahimi, M. Melt refining and purification processes in Al alloys: A comprehensive study. Mater. Res. Express 2022, 9, 032001. [Google Scholar] [CrossRef]
- Hoppach, D.; Peuker, U.A. Increasing cleanliness of Al-melts by addition of ceramic fibers. In Multifunctional Ceramic Filter Systems for Metal Melt Filtration; Aneziris, C.G., Biermann, H., Eds.; Springer Series in Materials Science; Springer: Cham, Switzerland, 2024; Volume 337. [Google Scholar] [CrossRef]
- Wu, Y.; Yan, H.; Wang, J.; Zheng, J.; Na, X.; Wang, X. Research on Online Monitoring Technology and Filtration Process of Inclusions in Aluminum Melt. Sensors 2024, 24, 2757. [Google Scholar] [CrossRef]
- Schoß, J.P.; Baumann, B.; Keßler, A.; Szucki, M.; Wolf, G. Filtration efficiency in the recycling process of particle-reinforced aluminum alloys using different filter materials. Int. J. Met. 2023, 17, 1681–1696. [Google Scholar] [CrossRef]
- Liu, G.; Ren, Y.; Ma, W.; Morita, K.; Lei, Y.; Zhan, S.; Lv, G.; Li, S.; Wang, Z.; Li, R. Recent advances and future trend of aluminum alloy melt purification: A review. J. Mater. Res. Technol. 2024, 28, 2238–7854. [Google Scholar] [CrossRef]
- Curtolo, D.C.; Xiong, N.; Friedrich, S.; Friedrich, B. High- and ultra-high-purity aluminum, a review on technical production methodologies. Metals 2021, 11, 1407. [Google Scholar] [CrossRef]
- Zhou, W.; Li, J.; Bian, Y.; Han, X.; Guan, R. Exploration of deep purification of aluminum alloy in vacuum based on adsorption of oxides by hydrogen bubbles. Vacuum 2024, 229, 113594. [Google Scholar] [CrossRef]
- Jaime, R.F.; Puga, H.; Prokic, M.; Söderhjelm, C.; Apelian, D. Fundamentals of Ultrasonic Treatment of Aluminum Alloys. Int. J. Met. 2024, 18, 2783–2807. [Google Scholar] [CrossRef]
- Zhou, W.; Li, J.; Bian, Y.; Han, X.; Jiang, J.; Guan, R. The impact of frequency and power on the ultrasonic purification of aluminum alloy. Ultrason. Sonochem. 2024, 109, 107006. [Google Scholar] [CrossRef]
- Wan, H.; Xu, B.; Yang, B.; Zhao, J.; Dai, Y. The impurities distribution and purification efficiency of high-purity aluminum preparation by zone melting in vacuum. Vacuum 2020, 171, 108839. [Google Scholar] [CrossRef]
- Li, L.; Chen, Q.; Luo, F.; Wang, X.; Zhu, Q.; Zhao, Z. Structure of the moderate-strength aluminum alloy for overhead conductors during the rotary-wheel casting process. Light Alloy Fabr. Technol. 2015, 43, 28–31. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Bai, J.; Xue, H.; Tian, N.; Zhao, Z.; Qin, G. Research progress on microstructure tuning of heat-resistant cast aluminum alloys. J. Mater. Sci. 2024, 59, 10035–10057. [Google Scholar] [CrossRef]
- Liu, X.; Wang, C.; Li, X.; Zhu, B.; Li, R. Investigation of 7075 aluminum alloy TIG welding joint using 7075 aluminum alloy wire before and after heat treatment. Mater. Res. Express 2023, 10, 046512. [Google Scholar] [CrossRef]
- Jia, X.; Fan, Z.; Luo, Z.; Hu, G.; Zhang, H. Impact of Heat Treatment Parameters on the Plastic Properties of 6061 Aluminum Alloy. Materials 2025, 18, 1705. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Wang, L.; Nie, J.; Zhao, Y. Optimizing strength and electrical conductivity of 6201 aluminum alloy wire through rotary swaging and aging processes. J. Mater. Process. Technol. 2024, 331, 118497. [Google Scholar] [CrossRef]
- Md Ali, A.; Omar, M.Z.; Salleh, M.S.; Mohamed, I.F.; Hashim, H.; Wakhi Anuar, N.F.B. Effect of Heat Treatment on the Microstructures and Mechanical Properties of Thixoformed Graphene-reinforced Aluminium Alloy Composite. Int. J. Met. 2024, 18, 1695–1709. [Google Scholar] [CrossRef]
- Yu, G.; Wu, Z. Manufacturing technologies and quality controls of 8030 aluminum alloy rod. Electr. Wires Cables 2017, 2, 12–16+46. [Google Scholar] [CrossRef]
- Jia, T.; Xu, Z.; Tian, S.; Qian, A.; Chen, Y.; Chen, Z. Technological research status and development trend of horizontal direct casting of aluminum alloy. Non-Ferr. Met. Process. 2025, 54, 7–13. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, X. Research on casting properties and relative parameters database of aluminum alloy. Cast 2024, 73, 514–519. [Google Scholar] [CrossRef]
- EN 754-2; Aluminium and Aluminium Alloys—Cold Drawn Rod/Bar and Tube. European Committee for Standardization: Brussels, Belgium, 2016.
- EN 755-2; Aluminium and Aluminium Alloys—Extruded Rod/Bar, Tube and Profiles. European Committee for Standardization: Brussels, Belgium, 2016.
- Sidelnikov, S.; Galiev, R.; Bersenev, A.; Voroshilov, D. Application and research twin roll casting-extruding process for production longish deformed semi-finished products from aluminum alloys. Mater. Sci. Forum 2018, 918, 13–20. [Google Scholar] [CrossRef]
- Wang, J.; Fu, J.; Cong, F.; Ren, W.; Wu, Y.; Li, G.; Zhang, X.; Wang, F.; Li, Y.; Liu, C. Progress and development trend of aluminum alloy twin-roll casting process. Light Alloy Fabr. Technol. 2024, 52, 1–10. [Google Scholar] [CrossRef]
- Cho, H.-G.; Kim, Y.D.; Kim, M.-S. Effect of in situ Al roll coating on Strip Surface quality in traditional twin-roll casting of aluminum alloys. Metals 2025, 15, 377. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, X.; Chen, C.; Lan, S.; Wang, R.; Fan, Y.; Zhang, Y.; Dang, P. Microstructure and properties of Al-0.65Mg-0.58Si-0.17Fe alloy prepared by horizontal continuous casting. Min. Metall. Eng. 2024, 44, 174–178. [Google Scholar] [CrossRef]
- Guo, X.; Zhao, Z. The current research status of aluminum alloy welding wire production. Light Alloy Fabr. Technol. 2023, 51, 1–6. [Google Scholar] [CrossRef]
- Engler, O. Microchemistry evolution during industrial DC casting and subsequent homogenization of aluminum alloy AlMn0.5Mg0.5 (AA 3105). Mater. Today Commun. 2023, 36, 106724. [Google Scholar] [CrossRef]
- Duan, W.; Li, J.; Pan, Y.; Han, F.; Zhu, X.; Mao, H.; Zhou, G.; Lang, Y.; Wang, S.; Zhang, Z. Development of semi-continuous casting technology under external physical field. Spec. Cast. Nonferrous Alloys 2024, 44, 745–752. [Google Scholar] [CrossRef]
- Baserinia, A.R.; Ng, H.; Weckman, D.C.; Weiis, M.A.; Barker, S.; Gallerneault, M. A simple model of the mold boundary condition in direct-chill (DC) casting of aluminum alloys. Metall. Mater. Trans. B 2012, 43, 887–901. [Google Scholar] [CrossRef]
- Tokunaga, H.; Motoyama, Y.; Okane, T. Particle method simulation of direct-chill casting process including breakout. Int. J. Adv. Manuf. Technol. 2023, 129, 4407–4418. [Google Scholar] [CrossRef]
- Zhou, X.; Tian, Z.; Liu, Y. Rolling deformation and microstructure properties on 7050 aluminum alloy under different rolling deformation amounts. Forg. Stamp. Technol. 2024, 49, 80–87. [Google Scholar] [CrossRef]
- Jin, H.; Guan, R.; Tie, D. Mechanical and conductive performance of aged 6xxx aluminum alloy during rotary swaging. Crystals 2022, 12, 530. [Google Scholar] [CrossRef]
- Sun, W.; Zhu, Y.; Marceau, R.; Wang, L.; Zhang, Q.; Gao, X.; Hutchinson, C. Precipitation strengthening of aluminum alloys by room-temperature cyclic plasticity. Science 2019, 363, 972–975. [Google Scholar] [CrossRef]
- Zhao, D.; Qin, J.; Lü, S.; Li, J.; Guo, W.; Wu, S.; Zhang, Y. Mechanisms of extrusion-ratio dependent ultrafine-grain fabrication in AZ31 magnesium alloy by continuous squeeze casting-extrusion. J. Mater. Process. Technol. 2022, 310, 117755. [Google Scholar] [CrossRef]
- Afifi, M.A.; Wang, Y.C.; Pereira, P.H.R.; Wang, Y.; Li, S.; Huang, Y.; Langdon, T.G. Characterization of precipitates in an Al-Zn-Mg alloy processed by ECAP and subsequent annealing. Mater. Sci. Eng. A 2018, 712, 146–156. [Google Scholar] [CrossRef]
- Murashkin, M.; Medvedev, A.; Kazykhanov, V.; Krokhin, A.; Raab, G.; Enikeev, N.; Valiev, R.Z. Enhanced mechanical properties and electrical conductivity in ultrafine-grained Al 6101 alloy processed via ECAP-conform. Metals 2015, 5, 2148–2164. [Google Scholar] [CrossRef]
- Krbaťa, M.; Eckert, M.; Križan, D.; Barényi, I.; Mikušová, I. Hot Deformation Process Analysis and Modelling of X153CrMoV12 Steel. Metals 2019, 9, 1125. [Google Scholar] [CrossRef]
- Wu, S. Study on Microstructure and Properties of Al-Zr Alloy with Gas Assisted Continuous Casting. Master’s Thesis, Kunming University of Science and Technology, Kunming, China, 2024. [Google Scholar] [CrossRef]
- Chen, J.; Liu, C.; Guan, R.; Wen, F.; Zhou, Q.; Zhao, H. Improving the comprehensive mechanical property of the rheo-extruded Al-Fe alloy by severe rolling deformation. J. Mater. Res. Technol. 2020, 9, 1768–1779. [Google Scholar] [CrossRef]
- Li, J.; Gao, M.; Yang, L.; Shen, C.; Guan, R. Effect of cold drawing on the microstructure and property of precipitation strengthening Al–Mg–Si–Cu alloy prepared by continuous casting-extrusion. Mater. Sci. Eng. A 2025, 922, 147669. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, X.; Chen, C. Experimental Study on continuous casting and extrusion forming technology of high electrical conductivity round aluminum rods. J. Shenyang Univ. (Nat. Sci. Ed.) 2012, 24, 31–33. [Google Scholar] [CrossRef]
- Han, X.; Wang, X.; Xu, M.; Feng, Z.; Yao, B.; Guo, P.; Gao, Z.; Lu, Z. AI-driven inverse design of materials: Past, present, and future. Chin. Phys. Lett. 2025, 42, 27403. [Google Scholar] [CrossRef]
- Meng, S.; Shi, Z.; Peng, M.; LI, G.; Zheng, H.; Liu, L.; Zhang, L. Landslide displacement prediction with step-like curve based on convolutional neural network coupled with bi-directional gated recurrent unit optimized by attention mechanism. Eng. Appl. Artif. Intell. 2024, 133, 108078. [Google Scholar] [CrossRef]
- Yang, L.; Wang, H.; Leng, D.; Fang, S.; Yang, Y.; Du, Y. Machine learning applications in nanomaterials: Recent advances and future perspectives. Chem. Eng. J. 2024, 500, 156687. [Google Scholar] [CrossRef]
- Hao, M.; Yu, Q.; Wei, C.; Chen, Y.; Chai, L.; Ge, Y. Statistical determination of Johnson-Cook model parameters for porous materials by machine learning and particle swarm optimization algorithm. J. Mater. Eng. Perform. 2022, 31, 7176–7190. [Google Scholar] [CrossRef]
- Park, S.; Kayani, S.; Euh, K.; Seo, E.; Kim, H.; Park, S.; Yadav, B.; Park, S.; Sung, H.; Jung, I. High strength aluminum alloys design via explainable artificial intelligence. J. Alloys Compd. 2022, 903, 163828. [Google Scholar] [CrossRef]
- Houria, M.; Nadot, Y.; Fathallah, R.; Roy, M.; Maijer, D. Influence of casting defect and SDAS on the multiaxial fatigue behaviour of A356-T6 alloy including mean stress effect. Int. J. Fatigue 2015, 80, 90–102. [Google Scholar] [CrossRef]
- Yang, B.; Chen, S.; Song, H.; Zhang, S.; Chang, H.; Xu, S.; Zhu, Z.; Li, C. Effects of microstructure coarsening and casting pores on the tensile and fatigue properties of cast A356-T6 aluminum alloy: A comparative investigation. Mater. Eng. A 2022, 857, 144106. [Google Scholar] [CrossRef]
- Han, J.; Dong, G.; Li, S.; Zheng, J.; Wang, J.; Li, H.; Mikhail, D.; Bi, J. Uneven distribution of cooling rate, microstructure and mechanical properties for A356-T6 wheels fabricated by low pressure die casting. J. Manuf. Process. 2024, 127, 196–210. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Xue, C.; Wang, S.; Zhang, Y.; Li, Q.; Miao, Y.; Wang, J. Hydrogen microporosity evolution and dendrite growth during long solidification of Al-Cu-Li alloys: Modeling and experiment. J. Mater. Process. Technol. 2023, 321, 118135. [Google Scholar] [CrossRef]
- Zhang, A.; Guo, Z.; Jiang, B.; Du, J.; Wang, C.; Huang, G.; Zhang, D.; Liu, F.; Xiong, S.; Pan, F. Multiphase and multiphysics modeling of dendrite growth and gas porosity evolution during solidification. Acta Mater. 2021, 214, 117005. [Google Scholar] [CrossRef]
- Xue, C.; Mao, P.; Liu, C.; Guo, Y.; Qian, F.; Zhang, C.; Liu, K.; Zhang, M.; Tang, S.; Wang, J. Improving mechanical properties of wire arc additively manufactured AA2196 Al-Li alloy by controlling solidification defects. Addit. Manuf. 2021, 43, 102019. [Google Scholar] [CrossRef]
- Chen, R.; Xu, Q.; Liu, B. Cellular automaton simulation of three-dimensional dendrite growth in Al-7Si-Mg ternary aluminum alloys. Comput. Mater. Sci. 2015, 105, 90–100. [Google Scholar] [CrossRef]
- Liu, X.; Wang, B.; Li, Q.; Wang, J.; Xue, C.; Yang, X.; Tian, G.; Chang, X.; Liu, X. Quantifying the effects of grain refiners (AlTiB and Y) on microstructure and properties in W319 alloys. Mater. Today Commun. 2022, 33, 104671. [Google Scholar] [CrossRef]
- Hou, Q.; Wu, X.; Li, Z.; Feng, S.; Kong, D.; Wang, S.; Ma, X.; Miao, Y.; Qiao, H.; Li, X.; et al. Artificial intelligence enabled microstructure prediction in Al alloy castings. J. Mater. Sci. Technol. 2026, 241, 21–34. [Google Scholar] [CrossRef]
- Tiwari, T.; Jalalian, S.; Mendis, C.; Eskin, D. Classification of T6 Tempered 6XXX Series Aluminum Alloys Based on Machine Learning Principles. JOM 2023, 75, 4526–4537. [Google Scholar] [CrossRef]
- Chehri, H.; Chehri, A.; Kiss, L.; Zimmerman, A. Automatic Anode Rod Inspection in Aluminum Smelters using Deep-Learning Techniques: A Case Study. Procedia Comput. Sci. 2020, 176, 3536–3544. [Google Scholar] [CrossRef]
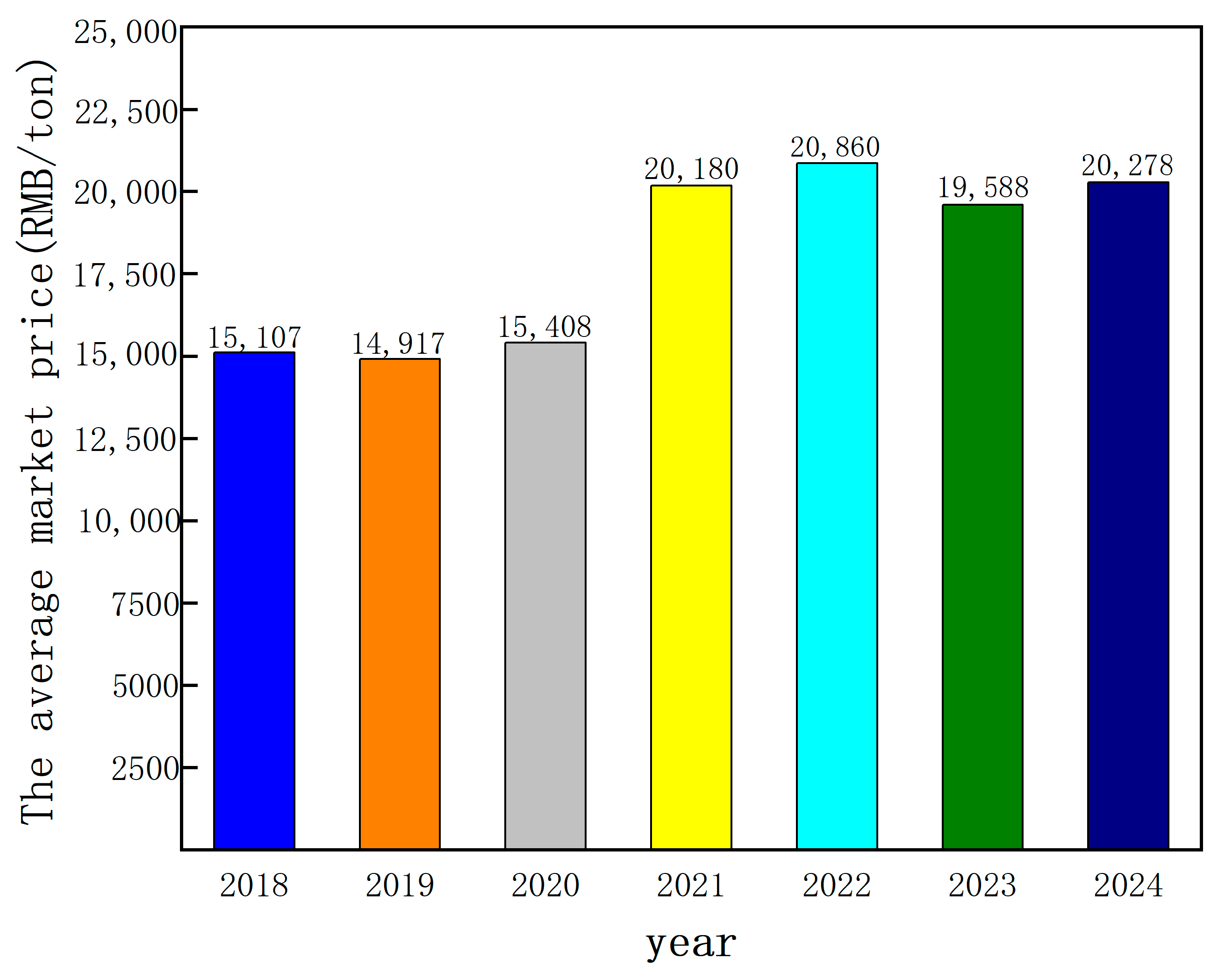
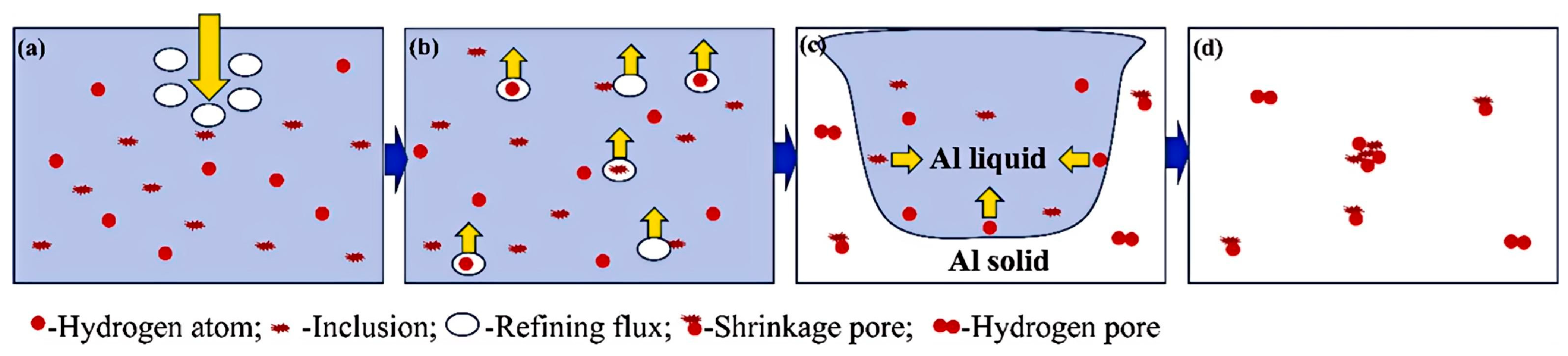

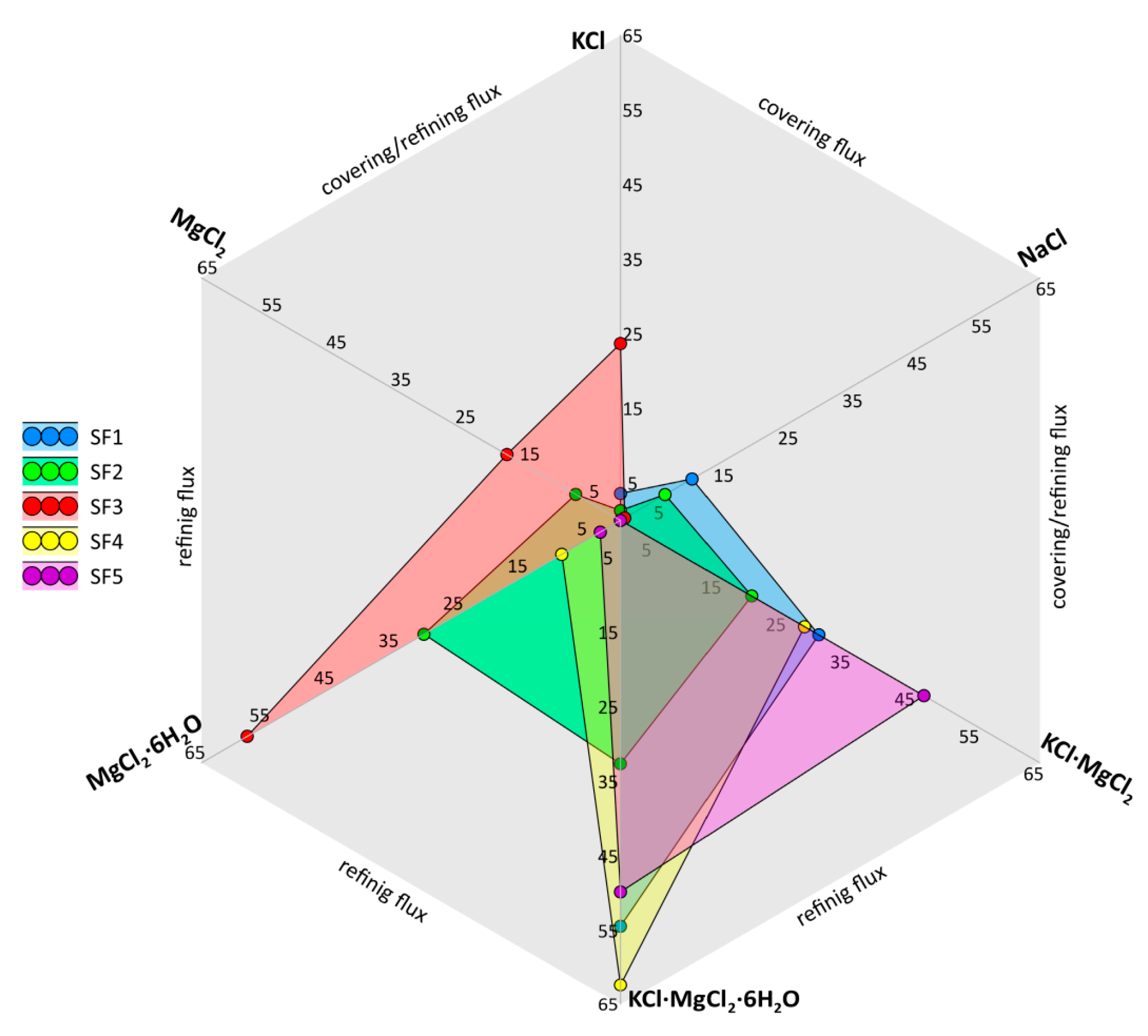


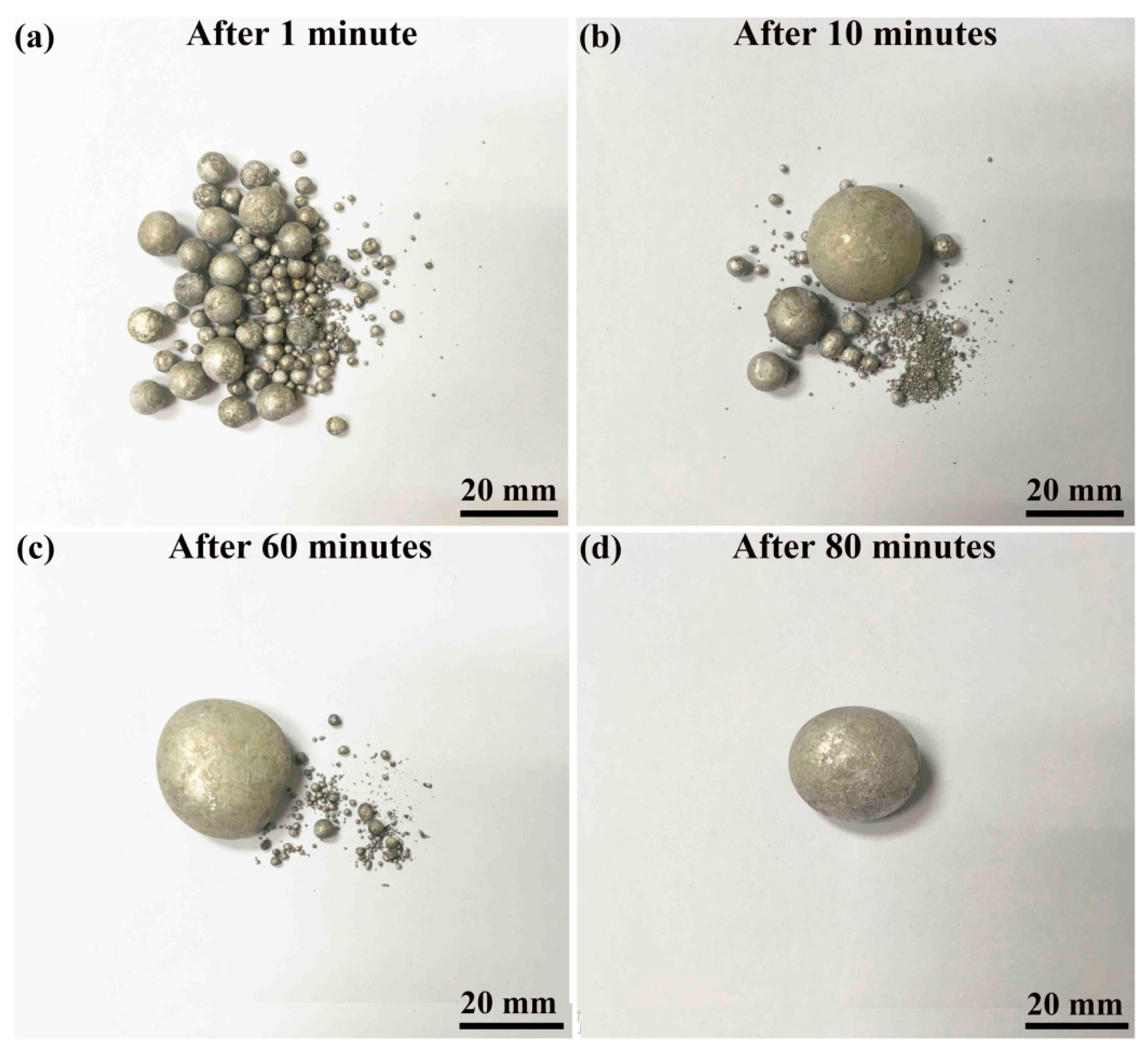
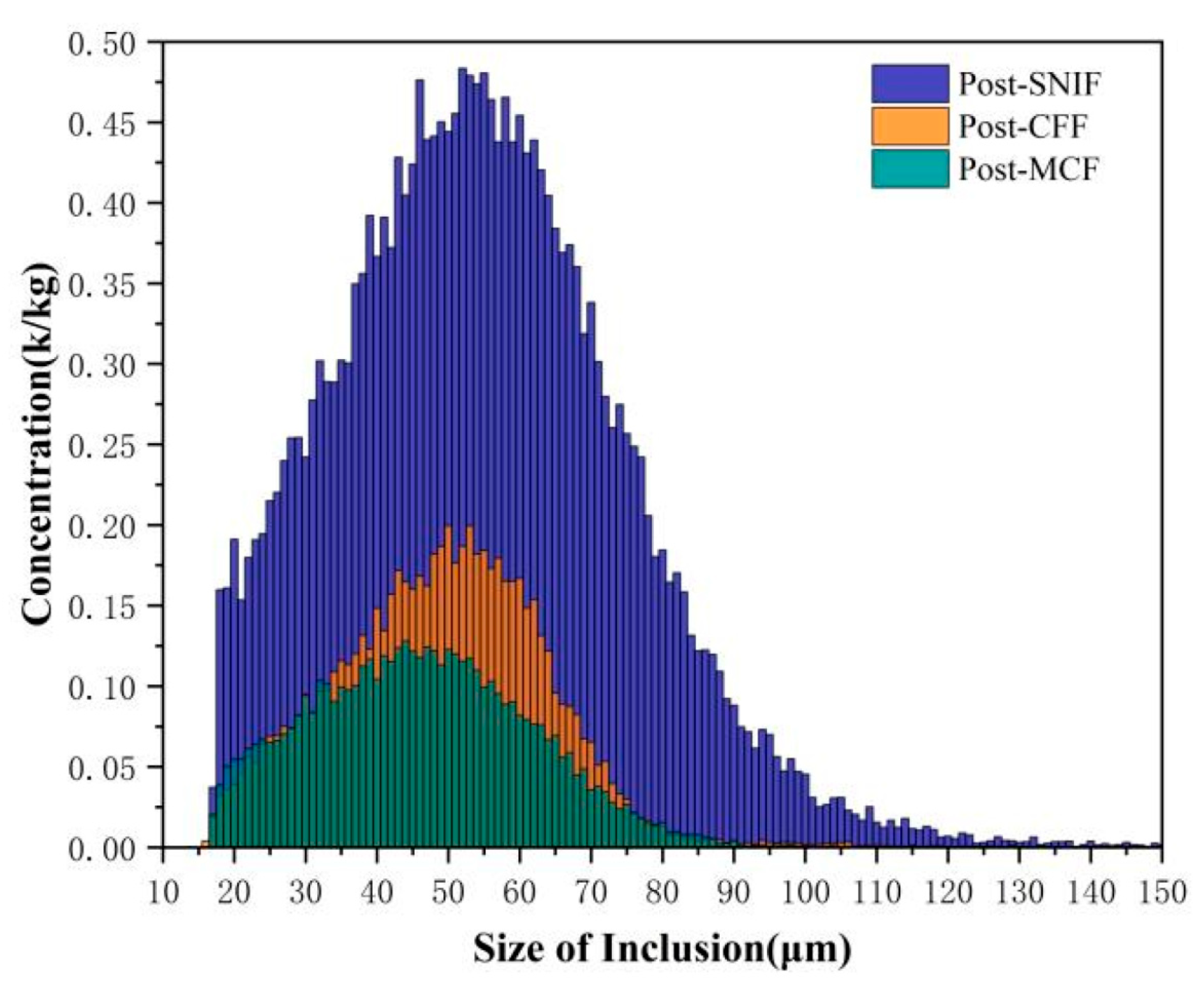

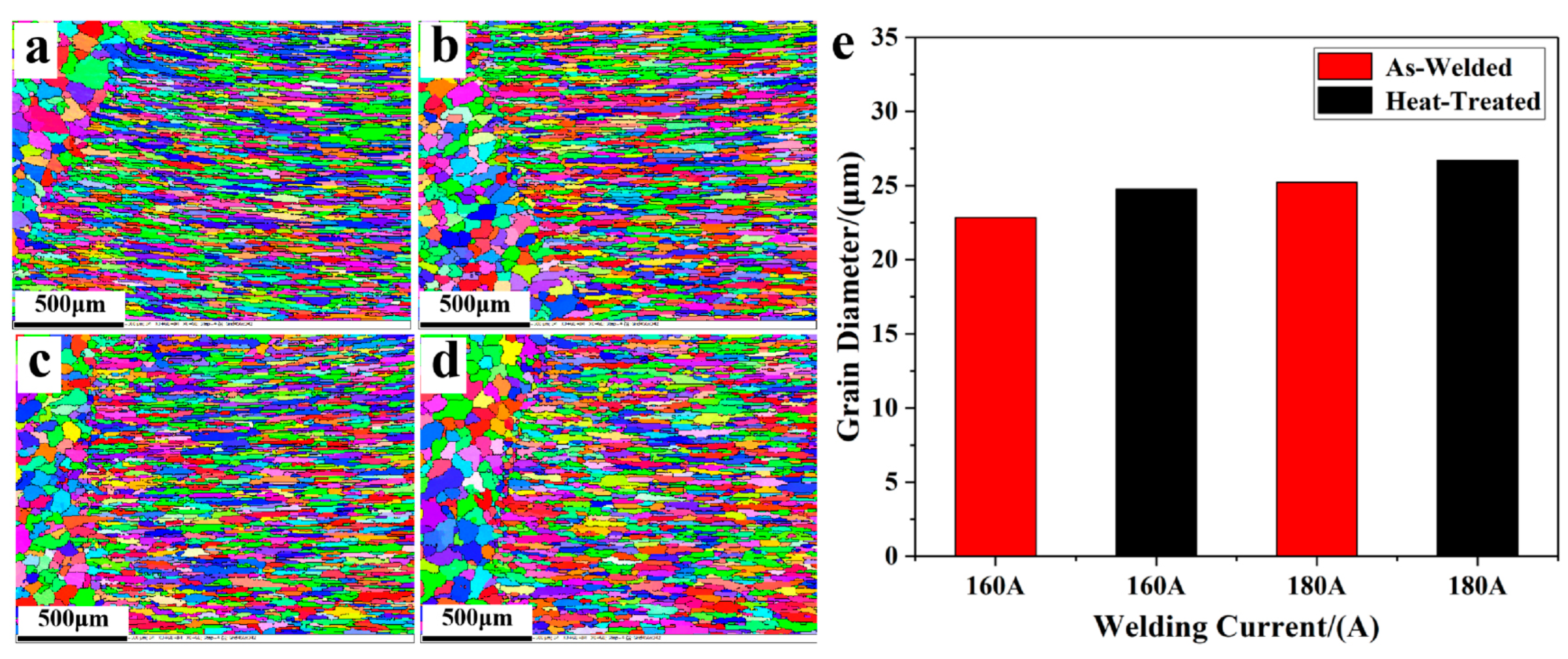
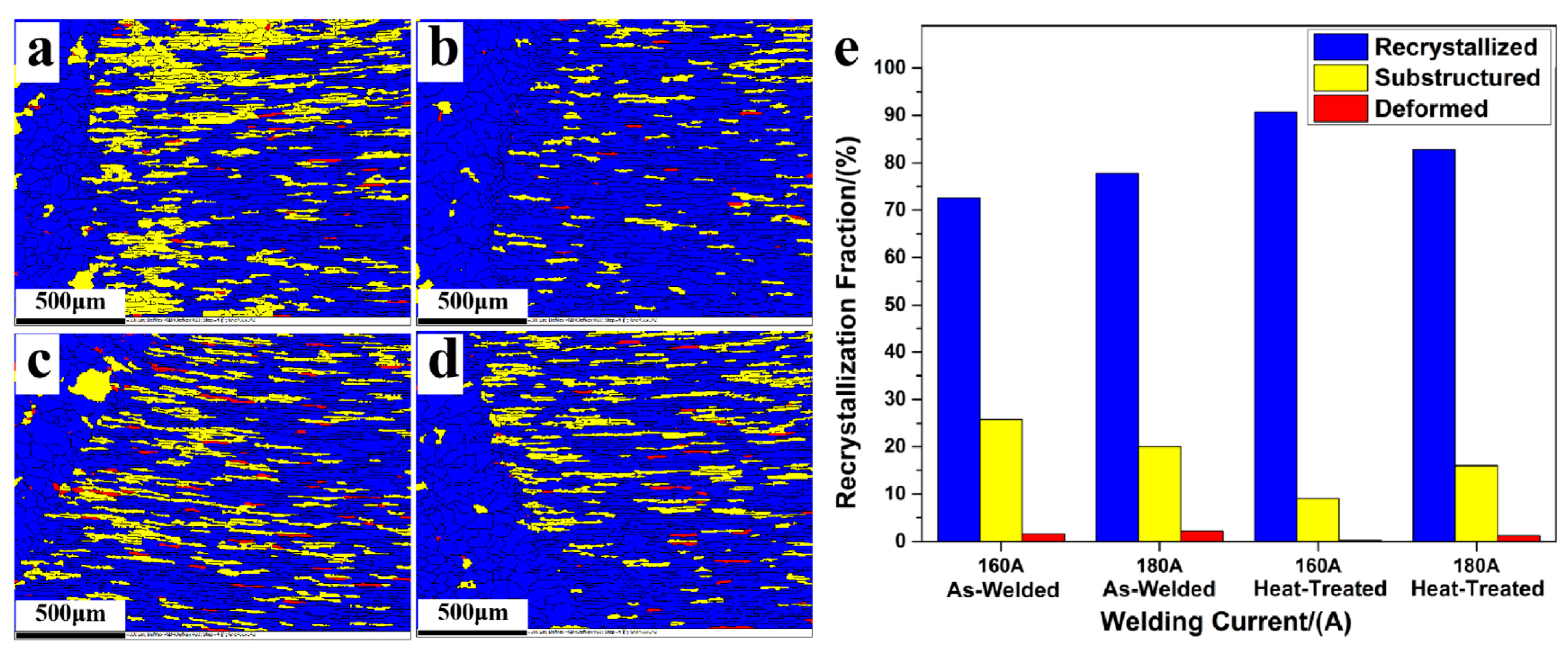

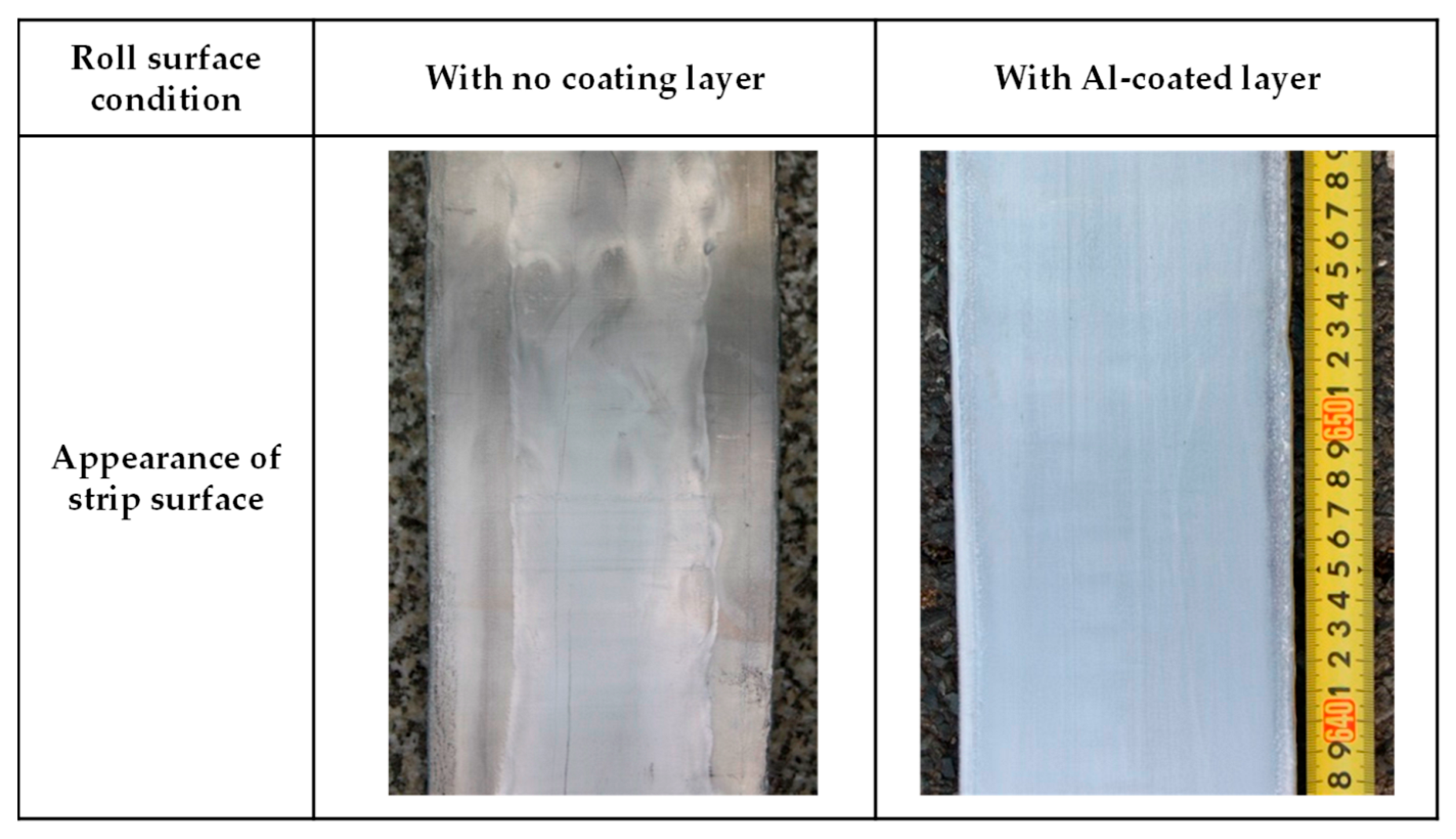
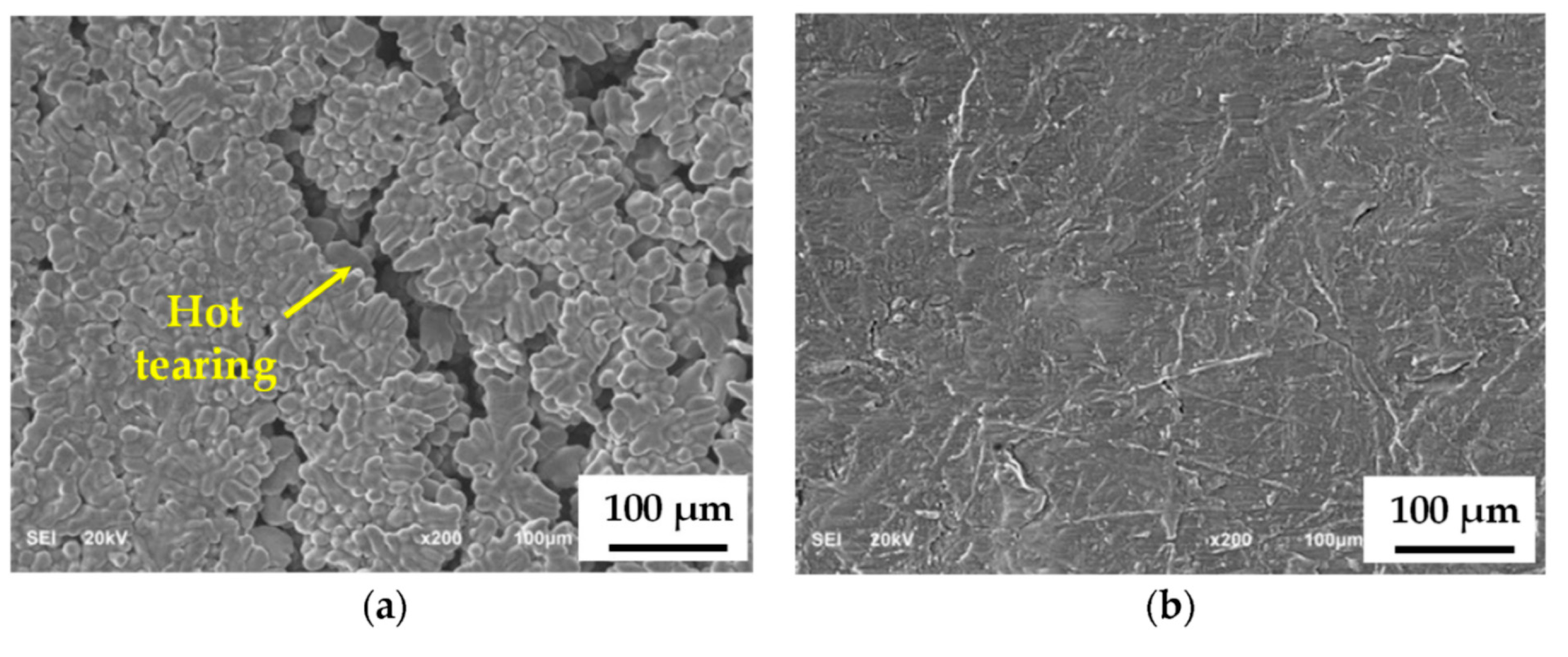
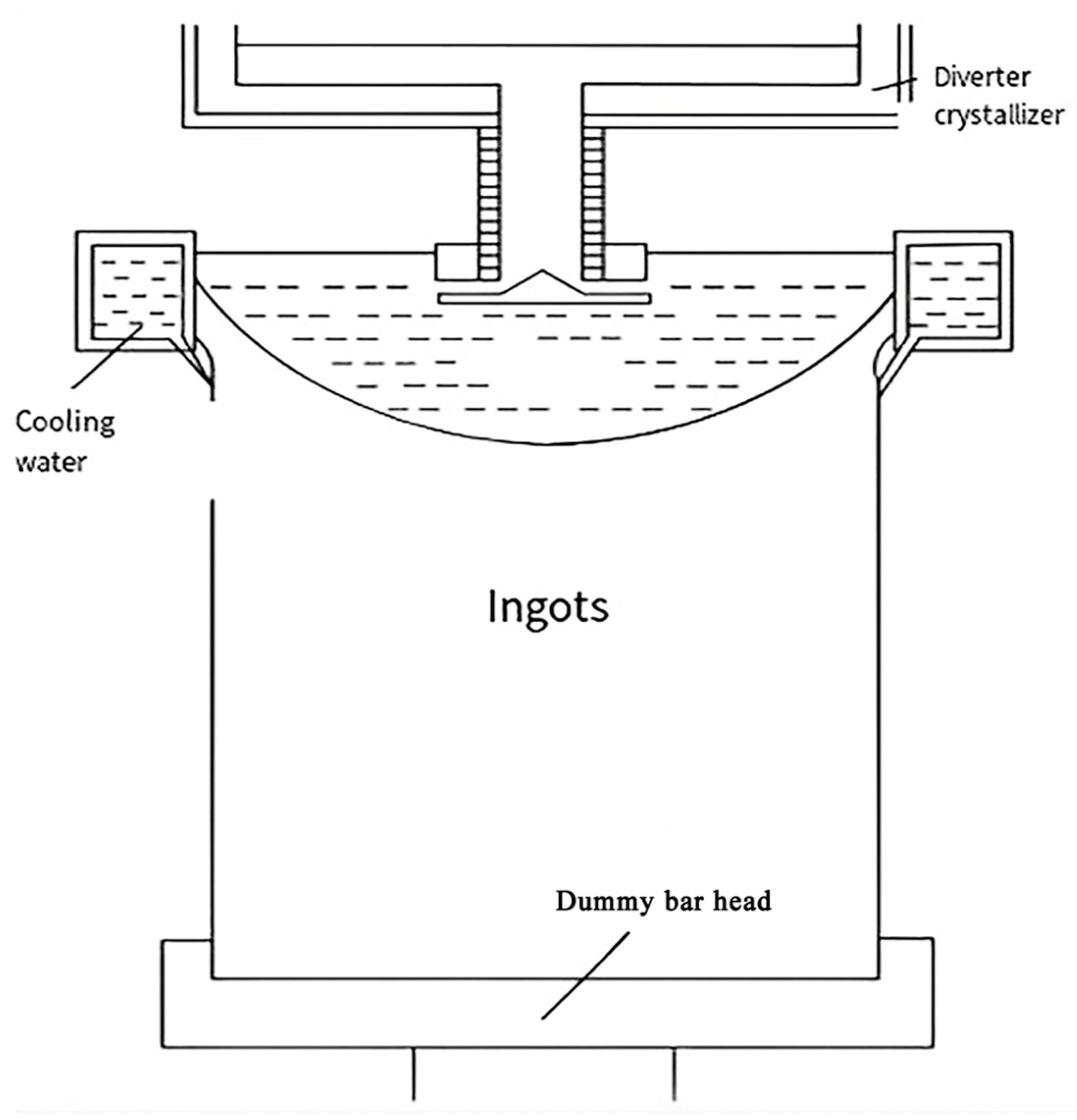
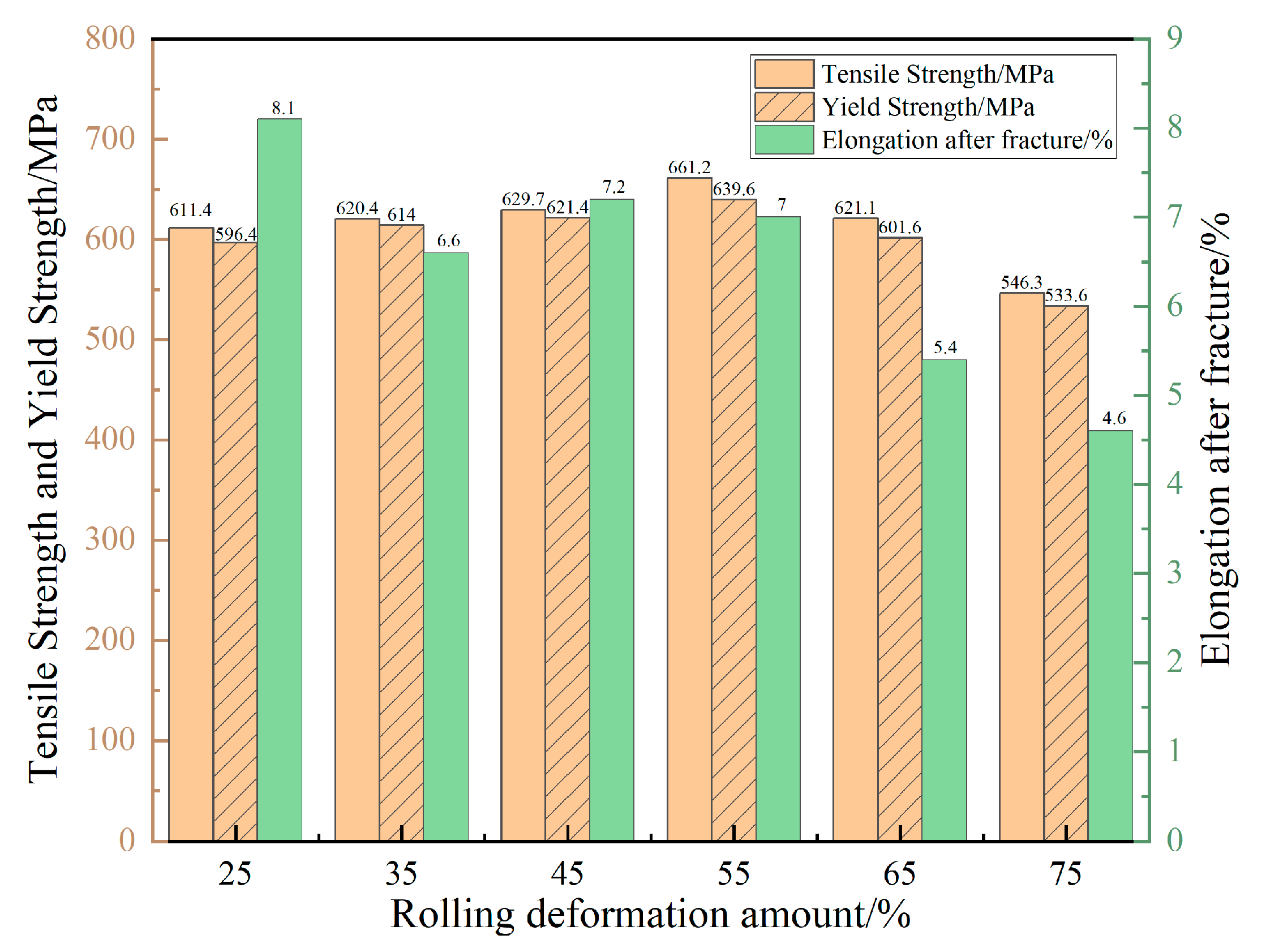
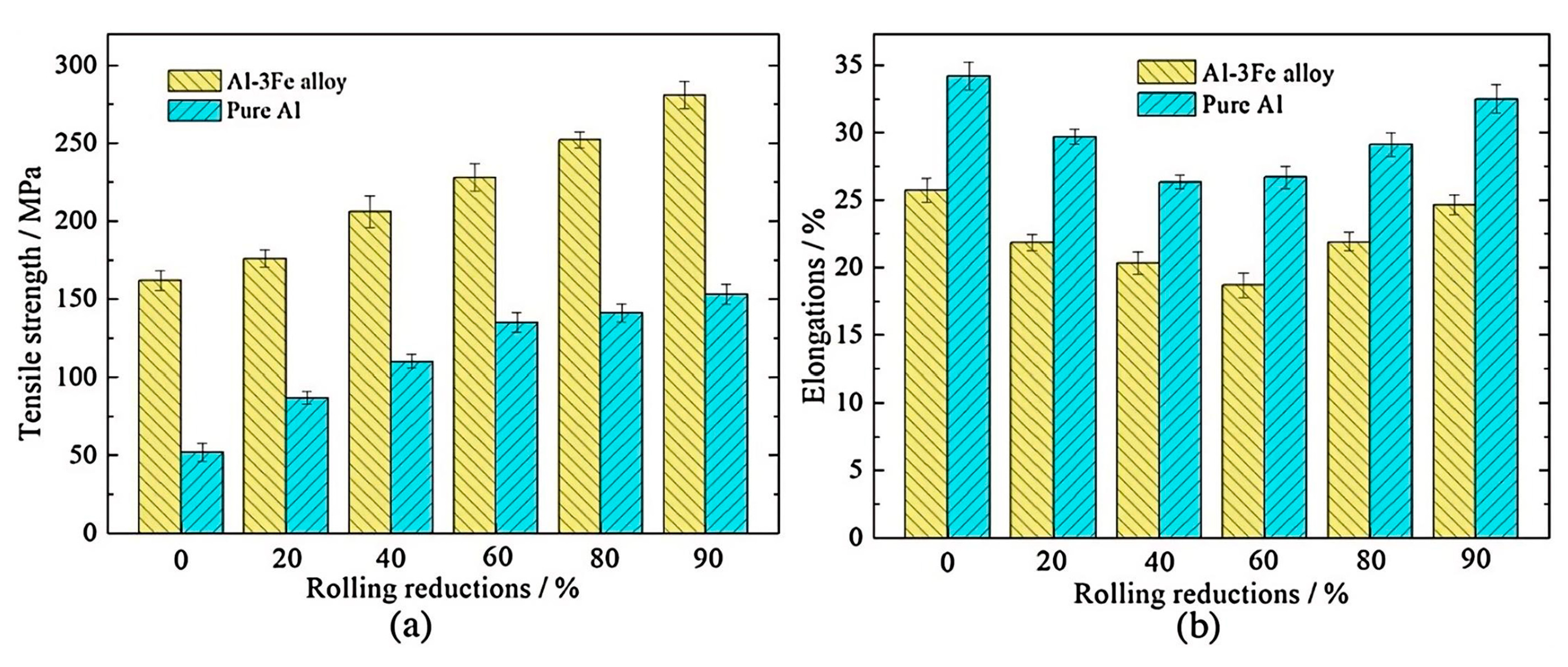

| Type of Electrical Conductor | Density /(g/mm3) | Melting Point /°C | Conductivity /(%IACS) | RTC/(°C−1) | TS/MPa | YS/MPa | E/% |
|---|---|---|---|---|---|---|---|
| 8030 | 2.7 | 660 | 61.8 | 4.03 | 113.8 | 53.9 | 30 |
| Cu | 8.89 | 1083 | 100 | 3.93 | 220–270 | 60–80 | 30–45 |
| Al | 2.7 | 660 | 62 | 4.03 | 70–110 | 20–30 | 23–25 |
| Brand Number | Status | T/MPa | E/% | Resistivity/nΩ·m |
|---|---|---|---|---|
| 1B90, 1B93, 1B95, 1B97 | O | 35~65 | 35 | 27.15 |
| H14 | 60~90 | 15 | 27.25 | |
| 1B85 | H14 | 105~130 | 5 | 27.95 |
| 1370 | O | 60~95 | 25 | 27.90 |
| H12 | 85~115 | 11 | 28.01 | |
| H13 | 105~135 | 8 | 28.03 | |
| H14 | 115~150 | 6 | 28.05 | |
| H16 | 130~160 | 5 | 28.08 | |
| 1A60, 1R50 | O | 60~90 | 25 | 27.55 |
| H12 | 80~110 | 13 | 27.85 | |
| H13 | 95~115 | 11 | 28.01 | |
| H14 | 110~130 | 8 | 28.01 | |
| H16 | 120~150 | 6 | 28.01 | |
| 1R60 | O | 95~130 | 8 | 28.03 |
| H15 | 100~135 | 7 | 28.34 | |
| 1350 | O | 60~95 | 25 | 27.90 |
| H12 | 85~115 | 12 | 28.03 | |
| H14 | 105~135 | 10 | 28.08 | |
| H16 | 120~150 | 8 | 28.12 | |
| 6101 | T4 | 150~200 | 10 | 34.50 |
| 6201 | T4 | 160~220 | 10 | 34.50 |
| 8A07 | H15 | 95~135 | 7 | 28.64 |
| H17 | 120~160 | 6 | 31.25 | |
| 8030 | H14 | 105~155 | 10 | 29.73 |
| 8E76 | O | 60~100 | 25 | 28.00 |
| H12 | 85~125 | 13 | 28.45 | |
| 8R76 | H13 | 95~120 | 11 | 28.78 |
| H14 | 105~140 | 8 | 28.89 | |
| H16 | 115~150 | 6 | 29.00 |
| Compare Dimensions | European Aluminum Conductor Grades | American Aluminum Conductor Grades |
|---|---|---|
| Main ingredients | Basically consistent | Basically consistent |
| Applicable market | European project | Worldwide |
| Testing key point | Dimensional accuracy of wire | Annealing Process |
| Impurity Type | Ingredients | Morphology | Density/(g/cm3) | Size/(μm) | Mass Fraction | Sources |
|---|---|---|---|---|---|---|
| Gas | H2 | Atom | 0.00002~0.00006 | Raw materials, melting environment, chemical reactions | ||
| Oxides | Al2O3 | Granules, membranes | 3.97 | Granules: 0.2~30 Membrane: 10–5000 | 0.0006~0.0016 | Oxidation reaction High-temperature melting Casting Refractory |
| MgO | Granules, membranes | 3.58 | Pellets: 0.1~5.0 Membrane: 10~5000 | |||
| MgAl2O4 | Granules, membranes | 3.60 | Pellets: 0.1~5.0 Membrane: 10~5000 | |||
| SiO2 | Pellets | 2.66 | 0.5~5.0 | |||
| Salt | Fluoride, chloride | Particle | 1.98–2.16 | 0.1~5.0 | <0.0001 | Chloride, cryolite electrolyte, etc. |
| Carbides | Al4C3 | Pellets | 2.36 | 0.5~25.0 | 0.0002~0.0012 | High-temperature reaction |
| SiC | Pellets | 3.22 | High-temperature reaction | |||
| Nitrides | AlN | Granules, membranes | 3.25 | 10~50 | 0.0003~0.0012 | High-temperature reaction |
| Borides | TiB2 | Particle | 4.50 | 1~30 | <0.0001 | Grain refiner |
| AlB2 | Pellets | 3.19 | 0.1~3.0 | 0.0001~0.0100 | Grain refiner used incorrectly | |
| Intermetallic compounds | Al (FeMnCr) Si | Granules | >4.00 | 1~50 | Melting process tool |
| Refining Agent Type | Hydrogen Removal Capacity/(mL/100 g Al) | Impurity Removal Capacity | Cost Estimation | Environmental Friendly | Basis |
|---|---|---|---|---|---|
| Traditional chlorinated salt refining agent | 0.15~0.20 | 40%~60% | Low | Large amounts of smoke and slag | NaCl, KCl, KF, KAlF4 |
| New type of compound refining agent | ≤0.12 | 70%~90% | Medium | The amount of flue gas and slag is significantly reduced | K2SO4, NaNO3, KF, K2CO3, RE |
| Nano-composite refining agent | ≤0.10 | >85% | High | Low slag output, high energy consumption | C2Cl6, Na2CO3, nano-Al2O3 |
| Sample | B Addition/% | |||||
|---|---|---|---|---|---|---|
| 0 | 0.03 | 0.06 | 0.09 | 0.12 | 0.24 | |
| Al-0.5Mg-0.35Si | 52.2 | 57.0 | 54.3 | 53.9 | 55.0 | 58.7 |
| Al-0.5Fe-0.2Si | 53.0 | 54.1 | 56.6 | 56.9 | 58.3 | 57.4 |
| Al-0.8Fe-0.2Cu | 56.2 | 57.0 | 58.0 | 58.3 | 59.8 | 58.7 |
| Alloy Composition/wt.% | Electrical Conductivity/%IACS | Ultimate Tensile Strength/MPa | Yield Strength/MPa | Elongation/% |
|---|---|---|---|---|
| Pure Al | 59.9–60.3 | 91.4 | 85.4 | 13.6 |
| Al-0.2Ce | 60.3–61.2 | 100.3 | 94.7 | 14.2 |
| Al-0.2Ce-0.1Y | 62.9–63.3 | 106.4 | 101.3 | 14.3 |
| Al-0.25Zr-0.03Y | ~57.2 | |||
| Al-0.2Y-0.05Sc | 60.8 | 144 ± 2 | 12.6 ± 0.2 | |
| Al-0.2Y-0.2Sc | 60.2 | 194 ± 1 | 12.2 ± 0.4 | |
| Al-0.2Y-0.2Sc-0.3Er | 59.7 | 207 | 14 | |
| Al-0.2Ce-0.2Sc-0.1Y | 61.77 ± 0.11 | 198 ± 2 | 8.5 ± 0.2 | |
| Al-0.2Y-0.2Sc-0.3Yb | 54.9 | 228 ± 2 | 11.5 ± 0.8 |
| Al | Si | Fe | Cu | Mn | Mg | Cr | Ni | Zn | Ti | V | B | Ga | Zr |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3N6 | 135 | 165 | 10 | 13 | 4 | 2 | 10 | 10 | 10 | 10 | 6 | 70 | 10 |
| 3N8 | 69 | 60 | 3 | 9 | 4 | 2 | 3 | 5 | 6 | 13 | 4 | 42 | 10 |
| 4N3 | 22 | 18 | 2 | 5 | 1 | 1 | 0 | 5 | 0 | 2 | 2 | 11 | 3 |
| 4N7 | 7 | 4 | 3 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 2 | 1 |
| 5N5 | 0.7 | 0.2 | 0.3 | 0.1 | 0.5 | 0.1 | 0.005 | 0.05 | 0.05 | 0.01 | 0.1 | 0.005 | 0.01 |
| Equipment Investment | Energy Consumption | Advantage | |
|---|---|---|---|
| continuous casting and rolling process | medium | medium | continuous production, high efficiency |
| two-roll cast-rolling process | low | low | short process |
| DEP | high | high | better mechanical properties |
| Continuous Casting and Extrusion Process | medium | medium | high flexibility |
| Element | Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | Other Elements | Al |
|---|---|---|---|---|---|---|---|---|---|---|
| 7075/T6 Base Metal | 0.08 | 0.20 | 1.41 | 0.06 | 2.55 | 0.20 | 5.60 | 0.02 | ≤0.05 | Bal. |
| 7075Wire | <0.01 | 0.03 | 2.18 | 0.16 | 1.96 | <0.01 | 5.80 | <0.01 | ≤0.05 | Bal. |
| Mg | Si | Mn | Fe | Cu | Cr | Zn | Ti | Al |
|---|---|---|---|---|---|---|---|---|
| 0.97 | 0.13 | 0.12 | 0.56 | 0.27 | 0.12 | 0.08 | 0.023 | Bal. |
| Strain Rate/(S−1) | Direction | Yield Strength/MPa | Tensile Strength/MPa | Elongation Rate/% |
|---|---|---|---|---|
| 0.05 | RD | 347 | 395 | 9.0 |
| ST | 306 | 355 | 7.9 | |
| LT | 321 | 378 | 9.8 | |
| 0.01 | RD | 346 | 395 | 10.9 |
| ST | 312 | 357 | 7.4 | |
| LT | 334 | 378 | 10.5 | |
| 0.001 | RD | 345 | 391 | 10.4 |
| ST | 345 | 355 | 7.4 | |
| LT | 310 | 367 | 8.2 |
| Sample | Yield Strength/MPa | Tensile Strength/MPa | Uniform Elongation/% | Elongation at Failure/% | Electrical Conductivity/%IACS |
|---|---|---|---|---|---|
| ST | 121 ± 11 | 210 ± 9 | 21 ± 3 | 32 ± 4 | 45.2 ± 0.2 |
| UA | 153 ± 10 | 235 ± 14 | 16 ± 2 | 29 ± 2 | 48.6 ± 0.6 |
| PA | 226 ± 17 | 274 ± 14 | 11 ± 1 | 19 ± 2 | 50.5 ± 0.2 |
| ST + RS | 328 ± 13 | 347 ± 16 | 2.0 ± 0.3 | 6.8 ± 0.5 | 43.9 ± 0.3 |
| UA + RS | 367 ± 10 | 371 ± 9 | 1.7 ± 0.1 | 6.5 ± 0.3 | 45.5 ± 0.4 |
| PA + RS | 384 ± 8 | 387 ± 14 | 0.7 ± 0.1 | 6.1 ± 0.2 | 50.3 ± 0.7 |
| ST + RS + RA160 | 341 ± 21 | 360 ± 16 | 5.1 ± 0.2 | 12.8 ± 0.3 | 47.0 ± 0.2 |
| UA + RS + RA160 | 350 ± 15 | 363 ± 14 | 4.2 ± 0.3 | 11.8 ± 0.4 | 50.6 ± 0.3 |
| PA + RS + RA160 | 348 ± 17 | 352 ± 13 | 4.0 ± 0.2 | 11.5 ± 0.3 | 51.7 ± 0.2 |
| ST + RS + RA180 | 319 ± 14 | 330 ± 10 | 4.2 ± 0.1 | 11.3 ± 0.3 | 49.8 ± 0.2 |
| UA + RS + RA180 | 313 ± 10 | 320 ± 9 | 3.5 ± 0.2 | 10.6 ± 0.5 | 51.5 ± 0.4 |
| PA + RS + RA180 | 308 ± 7 | 312 ± 10 | 2.3 ± 0.1 | 10.2 ± 0.4 | 53.0 ± 0.5 |
| ST + RS + RA200 | 258 ± 11 | 278 ± 8 | 2.5 ± 0.1 | 11.0 ± 0.2 | 52.8 ± 0.3 |
| UA + RS + RA200 | 269 ± 13 | 277 ± 11 | 2.8 ± 0.1 | 10.8 ± 0.6 | 52.9 ± 0.2 |
| PA + RS + RA200 | 268 ± 9 | 275 ± 8 | 3.0 ± 0.1 | 11.4 ± 0.4 | 53.5 ± 0.3 |
| Condition | Bar Length Selection Zone/mm | Ultimate Tensile Strength/MPa | Yield Strength/MPa | Elongation to Failure/% |
|---|---|---|---|---|
| hot-extruded | output | 285 | 165 | 22.2 |
| middle | 213 | 133 | 23.8 | |
| in the end | 210 | 126 | 25.3 | |
| heat-treated | output | 397 | 294 | 15.6 |
| 369 | 258 | 14.0 | ||
| middle | 386 | 326 | 15.6 | |
| 373 | 305 | 15.8 | ||
| In the end | 371 | 307 | 10.7 | |
| 378 | 315 | 12.4 | ||
| Requirements for EN 755-2 for extruded rods to 25 mm from alloy 6082 in condition T6 | ≥295 | ≥250 | ≥8 | |
| Condition | Ultimate Tensile Strength/MPa | Yield Strength/MPa | Elongation to Failure/% |
|---|---|---|---|
| hot-extruded | 290 | 268 | 12.0 |
| 284 | 260 | 12.3 | |
| 269 | 251 | 12.0 | |
| heat-treated | 331 | 268 | 16.0 |
| 327 | 264 | 18.5 | |
| 335 | 276 | 16.5 | |
| 343 | 287 | 19.5 | |
| 343 | 280 | 17.5 | |
| 331 | 276 | 18.7 | |
| Requirements for EN 755-2 for extruded rods to 25 mm from alloy 6082 in condition T6 | ≥310 | ≥255 | ≥10 |
| Indicators | Traditional Extruding Technology | TRCR Technology | |
|---|---|---|---|
| The yield/% | melting | 77 | 96 |
| extruding | 63 | 97 | |
| Electricity/(kW·h on 1 ton) | melting | 832 | 95 |
| extruding | 5303 | 3073 | |
| Cost price at variable costs/(RUR/ton) | 193,754 | 163,692 | |
| Reducing the cost price/(RUR/ton) | 30,062 | ||
| Indicators | Initial Peak Aging State | Rate of Area Reduction (19%) | Rate of Area Reduction (91%) |
|---|---|---|---|
| average grain size/μm | 184.47 | 81.22 | 8.80 |
| yield strength/MPa | 289.61 | 351.00 | 410.72 |
| Elongation/% | 9.44 | 6.71 | |
| Conductivity/%IACS | 52.85 | 52.78 | |
| dislocation density/m−2 | 4.4 × 1014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Jin, H.; Jiang, J. Development Status of Production Purification and Casting and Rolling Technology of Electrical Aluminum Rod. Metals 2025, 15, 981. https://doi.org/10.3390/met15090981
Liu X, Jin H, Jiang J. Development Status of Production Purification and Casting and Rolling Technology of Electrical Aluminum Rod. Metals. 2025; 15(9):981. https://doi.org/10.3390/met15090981
Chicago/Turabian StyleLiu, Xiaoyu, Huixin Jin, and Jiajun Jiang. 2025. "Development Status of Production Purification and Casting and Rolling Technology of Electrical Aluminum Rod" Metals 15, no. 9: 981. https://doi.org/10.3390/met15090981
APA StyleLiu, X., Jin, H., & Jiang, J. (2025). Development Status of Production Purification and Casting and Rolling Technology of Electrical Aluminum Rod. Metals, 15(9), 981. https://doi.org/10.3390/met15090981





