Numerical Simulation on the Influence of Oxygen Content and Coke Size on the Performance of Fuel Layered-Distribution Sintering Process
Abstract
1. Introduction
2. Mathematical Model
2.1. Gas Phase Conservation Equations
2.2. Solid-Phase Conservation Equations
2.3. Chemical Reactions
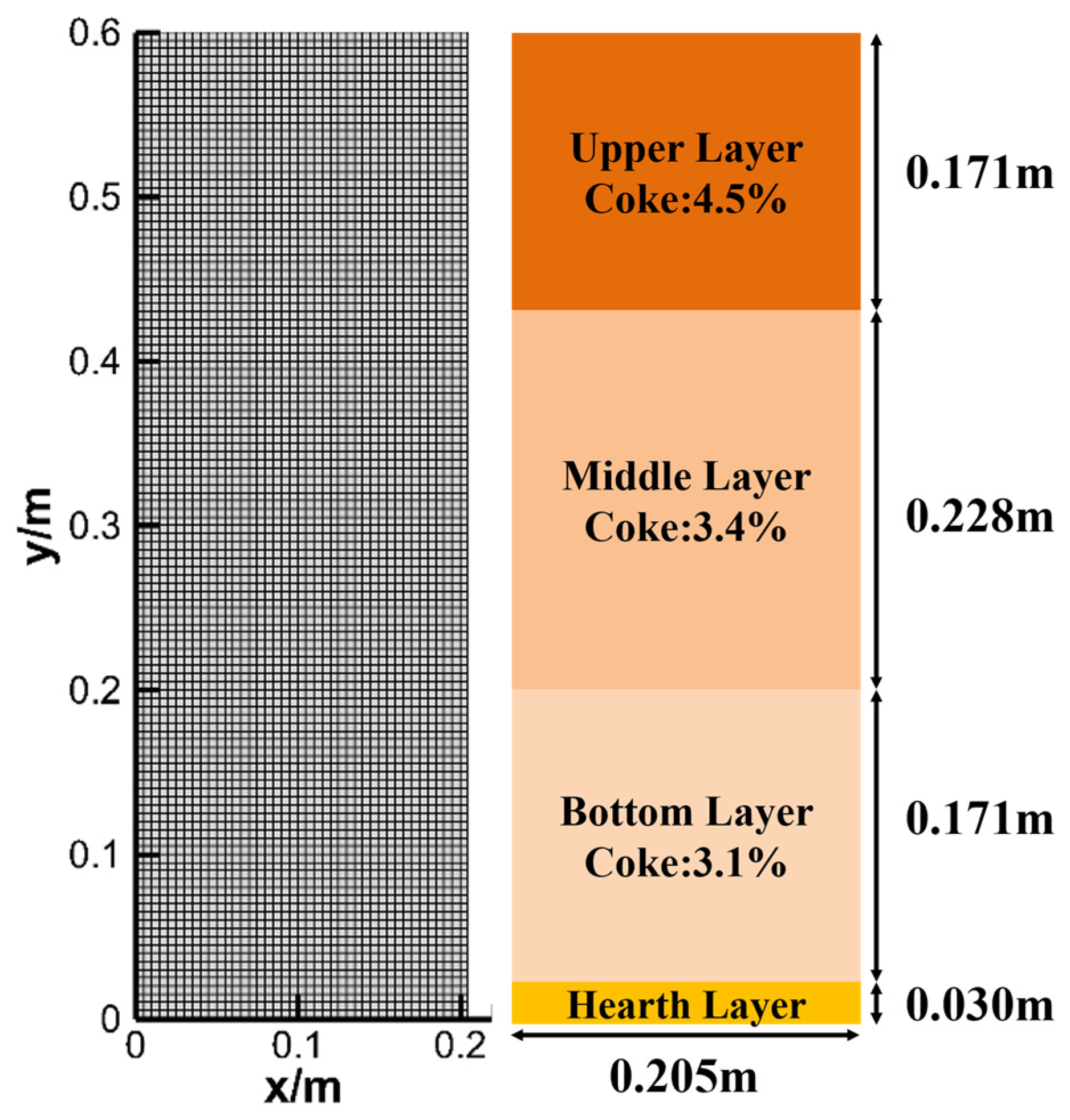
3. Model Construction and Simulation Conditions
3.1. Model Construction
3.2. Model Validation
3.3. Sintering Performance Indicators
4. Results and Discussion
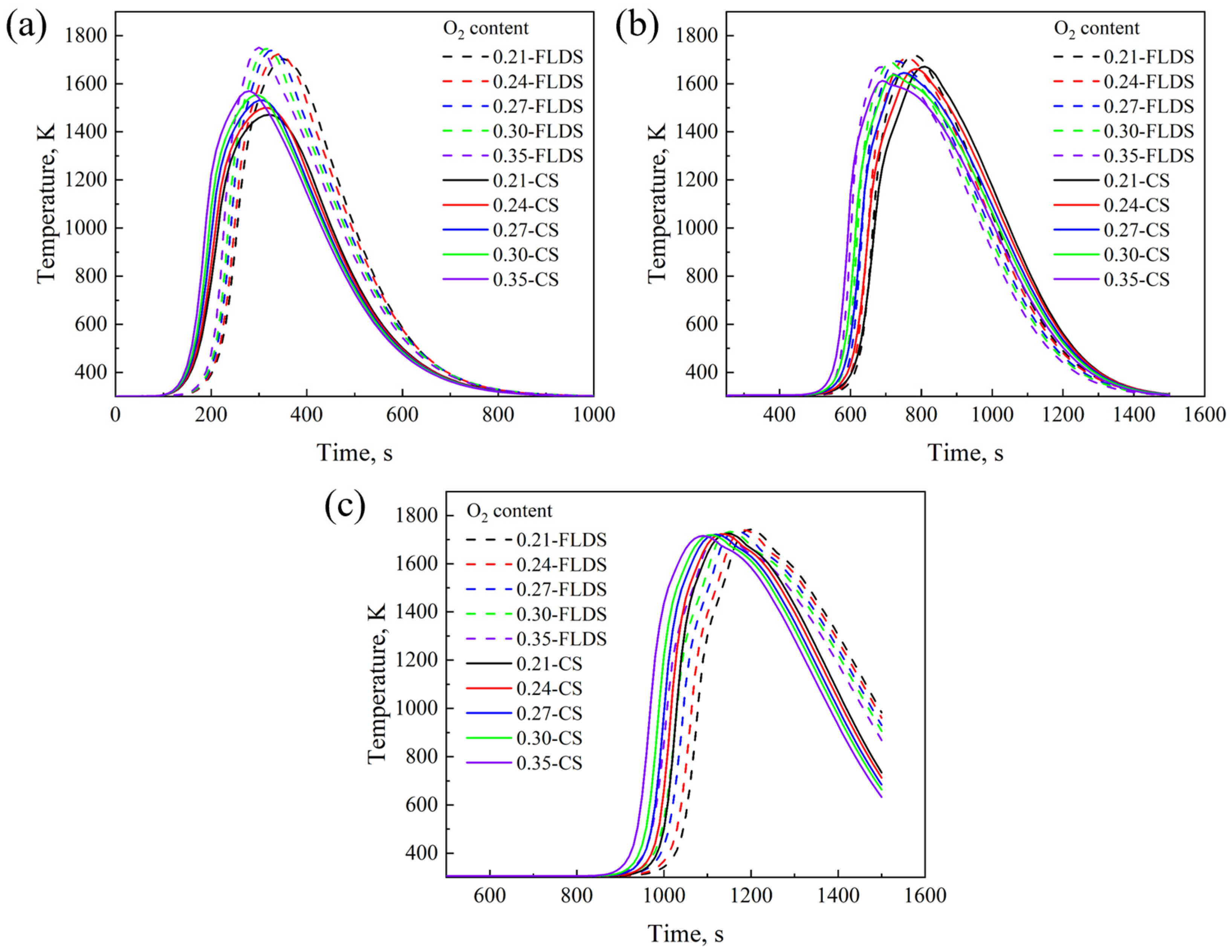
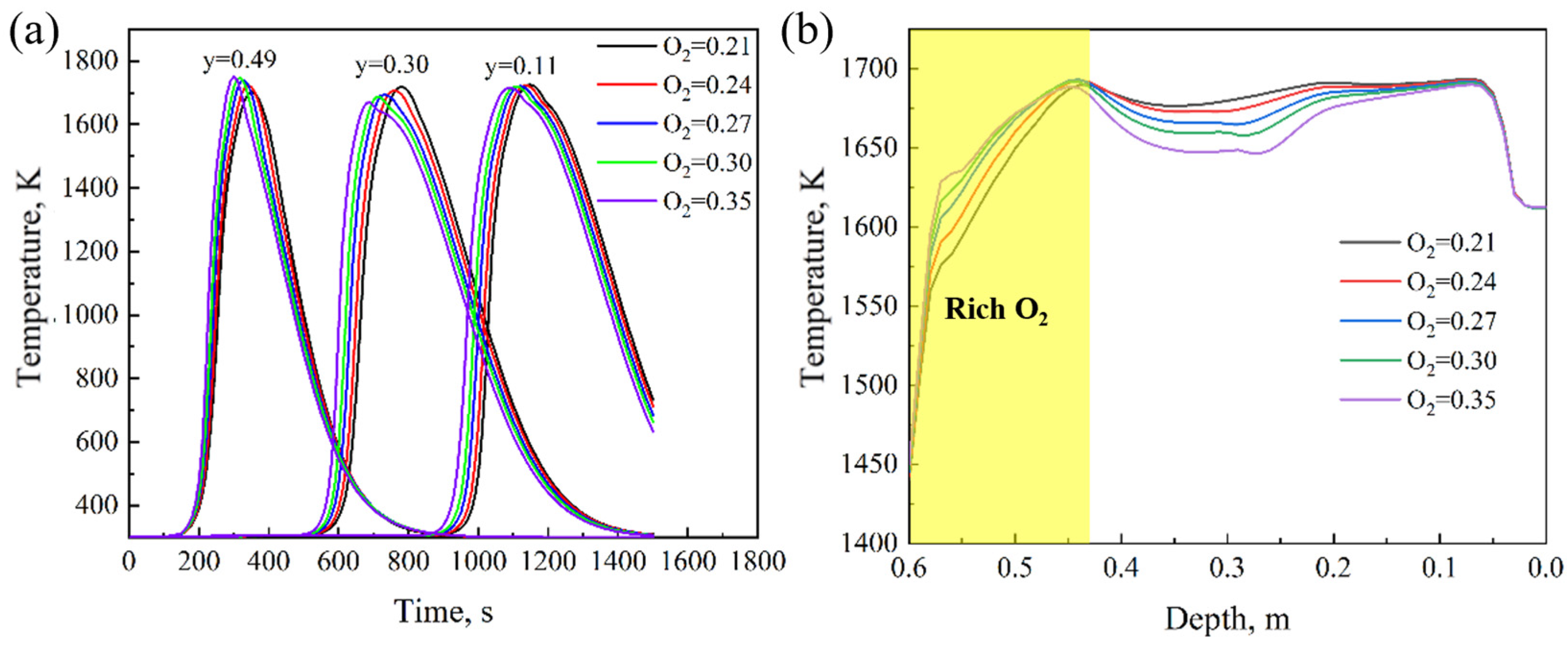
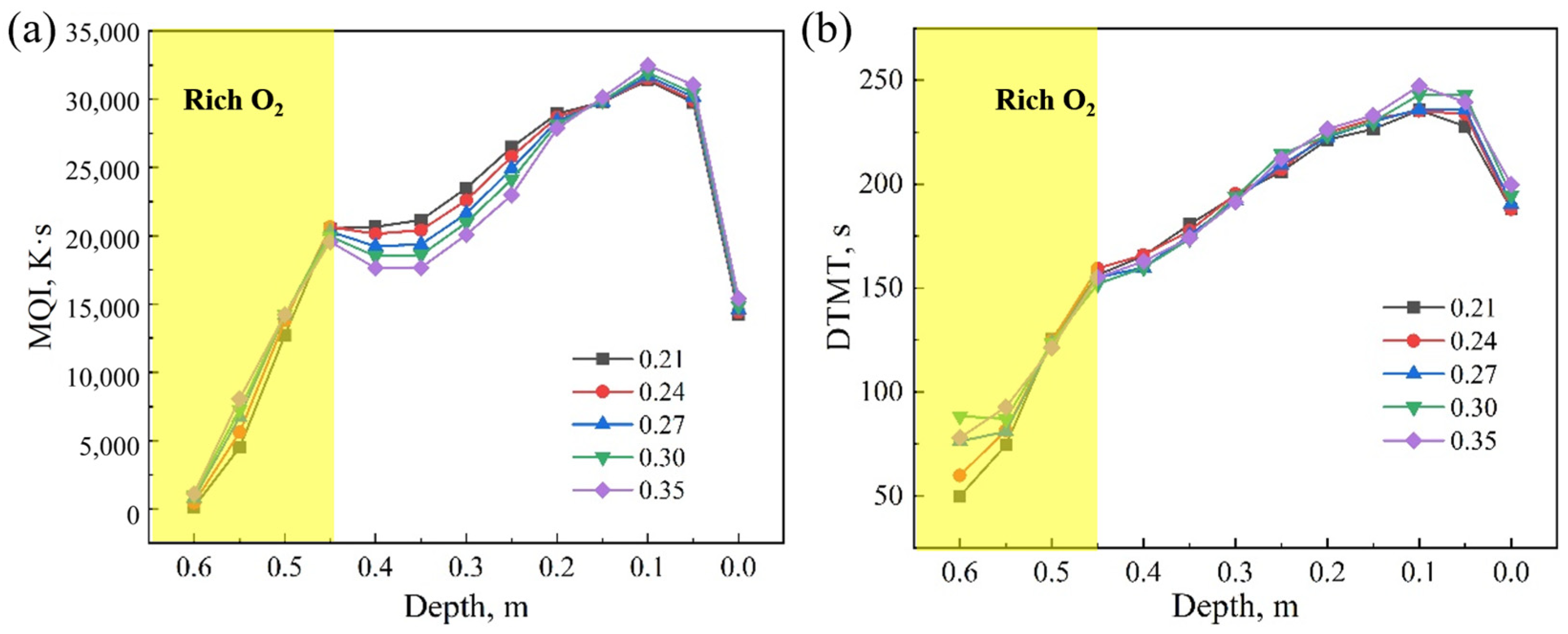
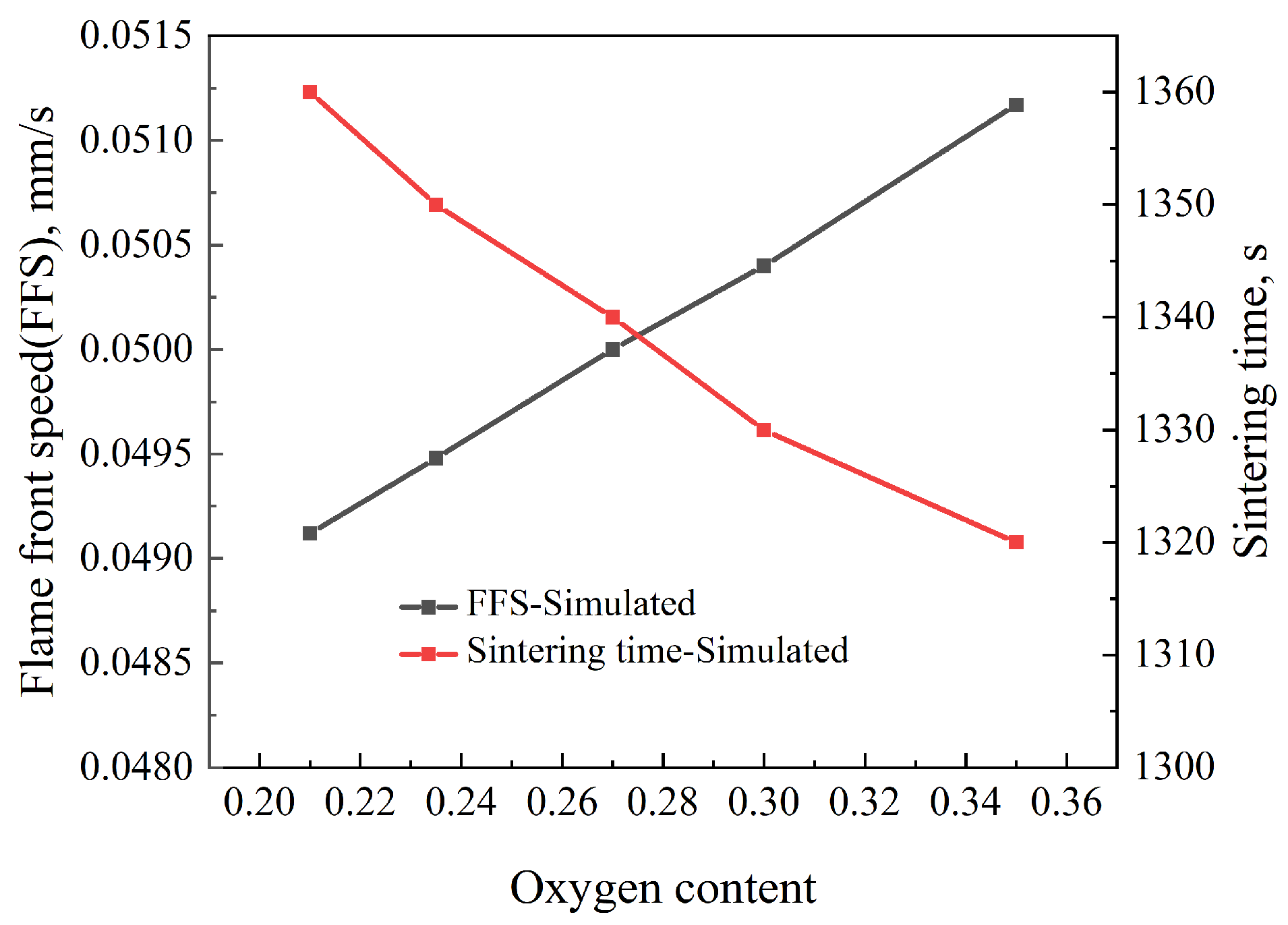
4.1. Effect of Oxygen Content
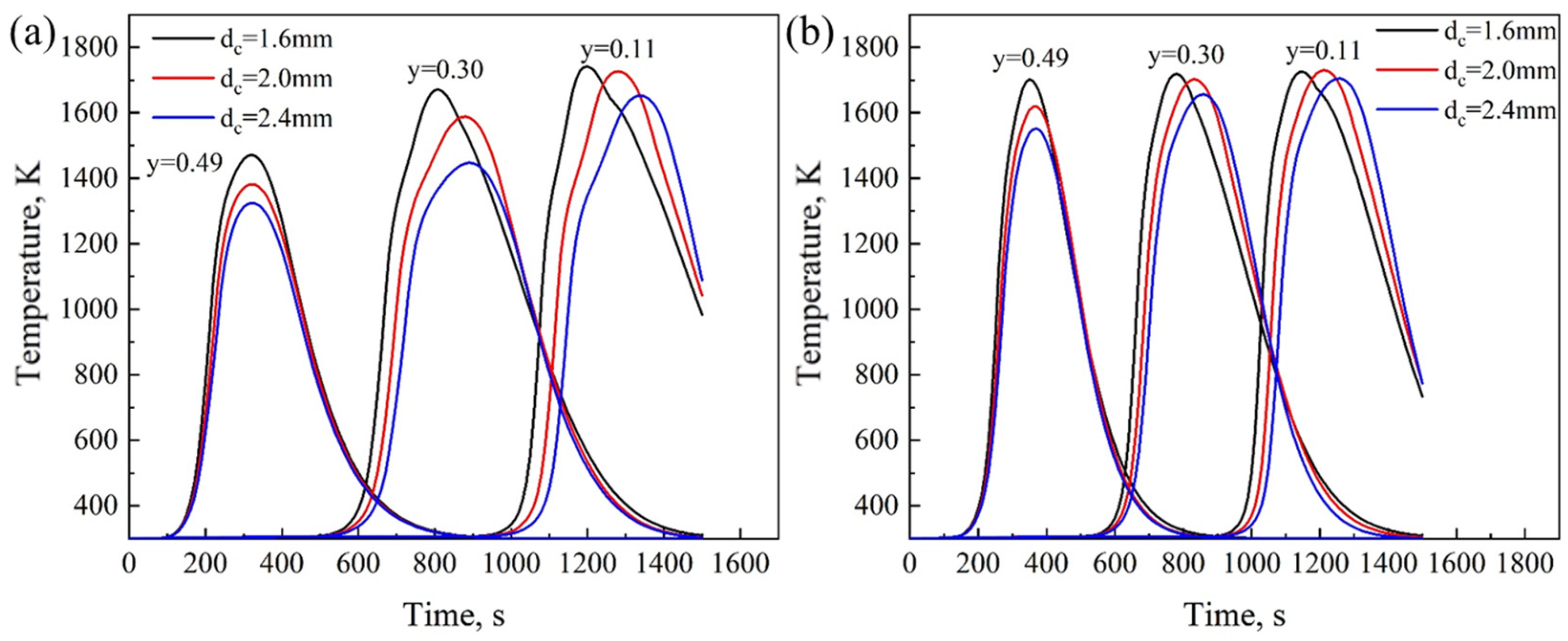
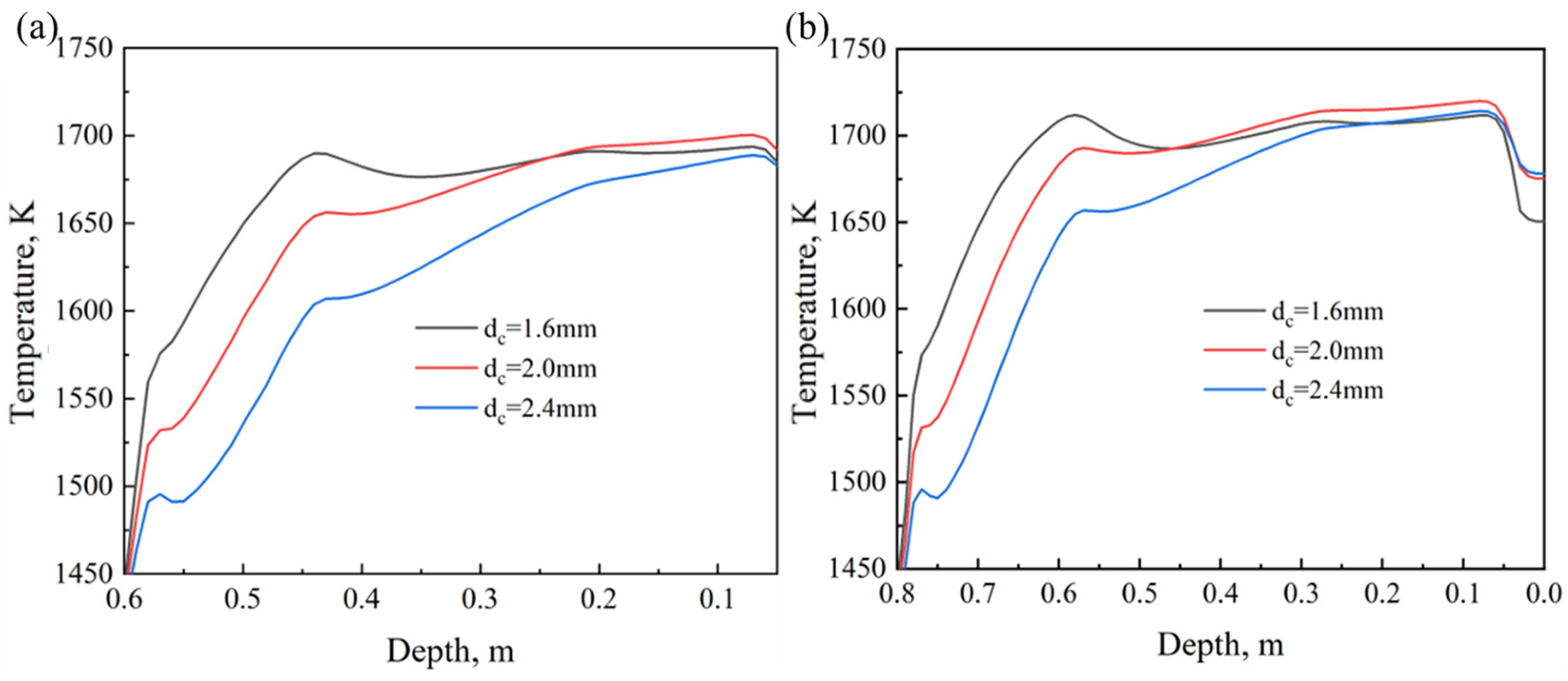
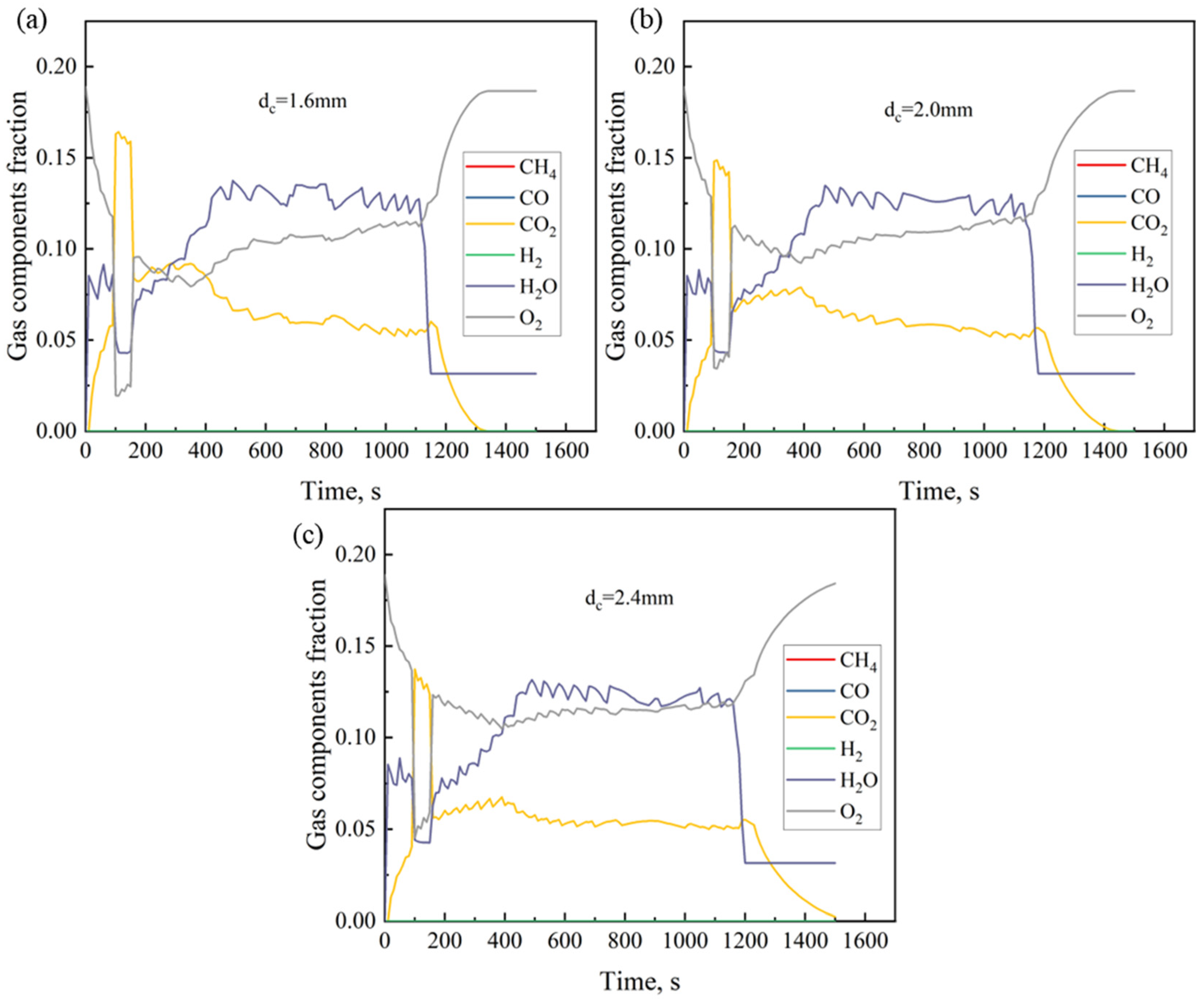
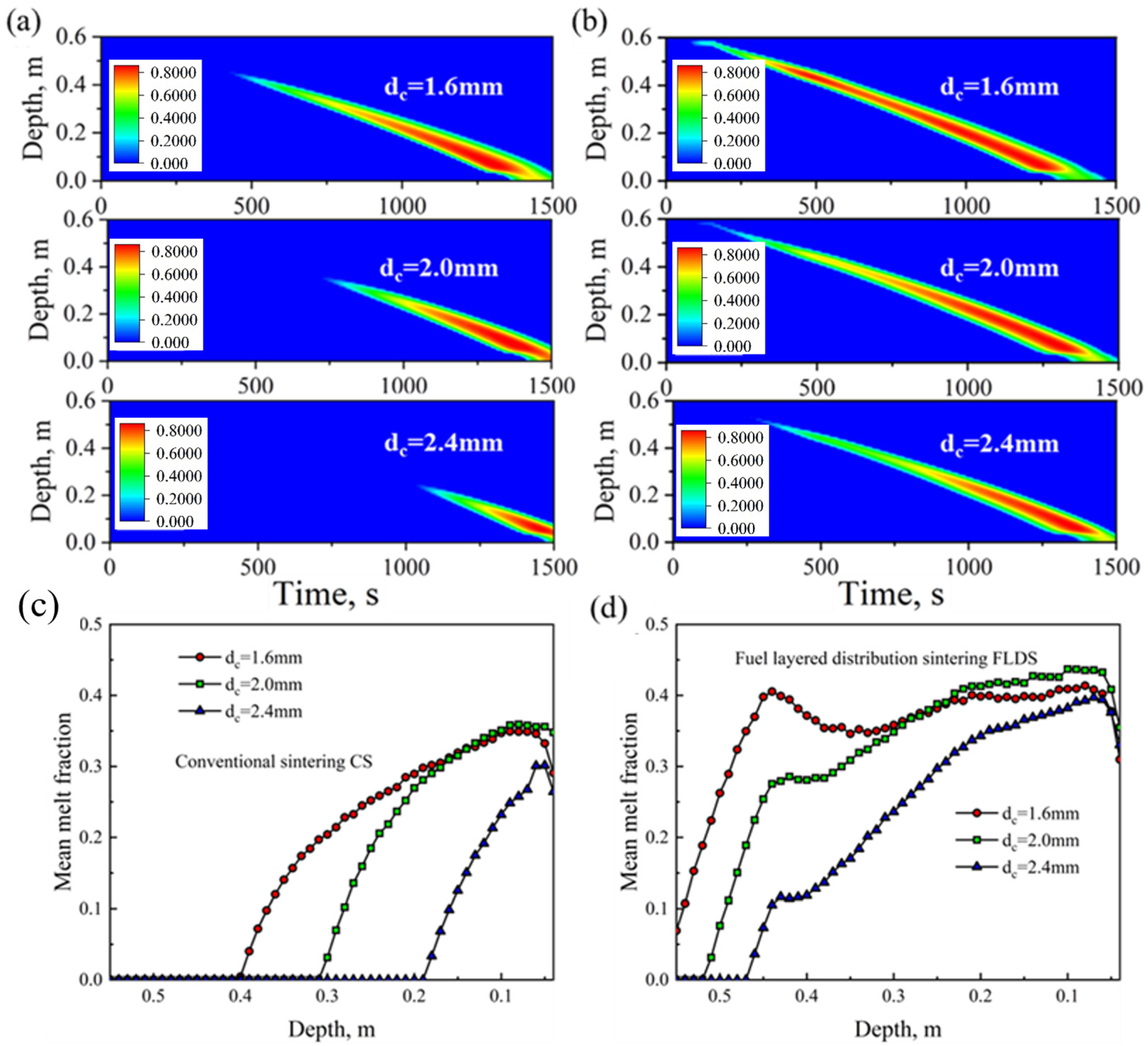
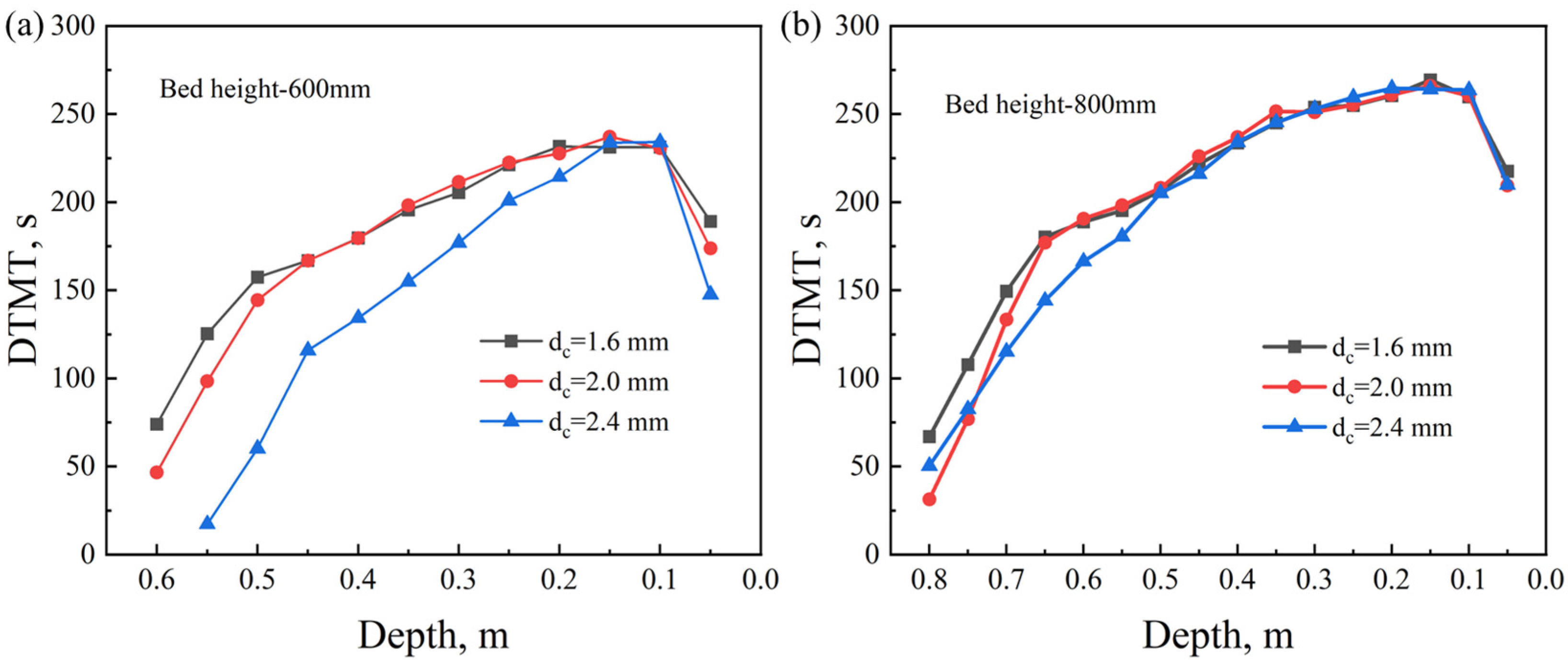
4.2. Effect of Coke Size
5. Conclusions
- (1)
- With the increase in oxygen concentration, the FFS increases and the sintering time decreases. At the same time, the maximum temperature in the bed layer and the MQI both improve. During the oxygen-enriched period, as the flame front advances downward, the DTMT increases with the amount of oxygen enrichment, ultimately reaching its maximum at the bottom of the bed. The results indicate that for a 600 mm sintering bed layer, an oxygen enrichment time of 6 min and an oxygen concentration of 27% can balance sintered ore quality, sintering time, and flame front speed, ensuring the yield of sintered ore.
- (2)
- As the coke size increases, the heating rate during the sintering process slows down, the FFS decreases, and the sintering time increases. Smaller coke particles (dc = 1.6 mm) perform better in the upper part of the sintering bed. However, in the middle and lower parts of the sintering bed, slightly larger coke particles (dc = 2.0 mm) can provide a longer DTMT and higher melt quality. Under thick bed-layer conditions, larger coke particles perform better at the bottom of the sintering bed. Based on the 600 mm sintering bed case, smaller coke particles with dc = 2.0 mm represent the balance point between flame front speed and sintering time, enabling the sintering process to be accelerated to the greatest extent while ensuring the quality of the sintered ore.
- (3)
- Under different oxygen concentrations and coke sizes, The FLDS technology can provide more sufficient heat during the initial stage of sintering, promoting the generation of more melt in the upper part of the sintering bed compared to CS. Meanwhile, the FLDS technology can provide a more uniform melt mass, which helps improve the quality of the sintered ore. Currently, sintering technologies for thick bed layers of 800 mm, 900 mm, and 1000 mm have already been applied in actual production. Identifying suitable operating conditions for thick bed layers can help enterprises optimize production. Similar conclusions can be drawn for 800 mm thick bed layers compared to that of 600 mm. As the bed-layer thickness increases, using appropriately sized coke particles at different heights within the sintering bed can improve the quality of the sintered ore.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yamaoka, H.; Kawaguchi, T. Development of a 3-D sinter process mathematical simulation model. ISIJ Int. 2005, 45, 522–531. [Google Scholar] [CrossRef]
- Eggersdorfer, M.L.; Kadau, D.; Herrmann, H.J.; Pratsinis, S.E. Multiparticle sintering dynamics: From fractal-like aggregates to compact structures. Langmuir 2011, 27, 6358–6367. [Google Scholar] [CrossRef]
- Cho, K.; Biswas, P. A geometrical sintering model (GSM) to predict surface area change. J. Aerosol Sci. 2006, 37, 1378–1387. [Google Scholar] [CrossRef]
- Yu, J.Y.; Xu, R.S.; Zhang, J.L.; Zheng, A.Y. A review on reduction technology of air pollutant in current China’s iron and steel industry. J. Cleaner Prod. 2023, 414, 137659. [Google Scholar] [CrossRef]
- Zhang, X.H.; Feng, P.; Xu, J.R.; Feng, L.B.; Qing, S. Numerical research on combining flue gas recirculation sintering and fuel layered distribution sintering in the iron ore sintering process. Energy 2020, 192, 116660. [Google Scholar] [CrossRef]
- Gao, Q.J.; Bao, L.; Zhu, P.X.; Jiang, X.; Zheng, H.Y.; Shen, F.M. Mathematical simulation of iron ore fines sintering process with solid fuel segregation distribution and corresponding heat pattern study. Metals 2022, 12, 2126. [Google Scholar] [CrossRef]
- Dai, F.L.; Fan, X.H.; Huang, X.X.; Chen, X.L.; Gan, M.; Ji, Z.Y.; Sun, Z.Q. Fuel-appropriate distribution of the material layer based on numerical model of sintering with particle swarm optimization algorithm. Minerals 2023, 13, 511. [Google Scholar] [CrossRef]
- Shrestha, S.; Xu, J.; Yu, A.B.; Zhou, Z.Y. Numerical simulation of fuel layered distribution iron ore sintering technology. Ironmak. Steelmak. 2022, 49, 83–100. [Google Scholar] [CrossRef]
- Menapace, C.; Cipolloni, G.; Hebda, M.; Ischia, G. Spark plasma sintering behaviour of copper powders having different particle sizes and oxygen contents. Powder Technol. 2016, 291, 170–177. [Google Scholar] [CrossRef]
- Kurinskiy, P.; Kim, J.H.; Nakamichi, M. Effect of plasma sintering on densification and oxygen content of beryllium powder. Nucl. Mater. Energy 2018, 17, 206–210. [Google Scholar] [CrossRef]
- Feng, D.; Ren, Q.X.; Ru, H.Q.; Wang, W.; Jiang, Y.; Ren, S.Y.; Zhang, C.P. Effect of oxygen content on the sintering behaviour and mechanical properties of SiC ceramics. Ceram. Int. 2019, 45, 23984–23992. [Google Scholar] [CrossRef]
- Cheng, P.C.; Chang, K.C.; Shiau, J.S.; Liu, S.H.; Hsieh, K.C.; Lin, S.K. Impact of low-grade iron ore on sintering reactions: Rapid heating experiments and thermodynamic modeling. J. Taiwan Inst. Chem. Eng. 2024, 165, 105817. [Google Scholar] [CrossRef]
- Rajak, D.K.; Ballal, N.B.; Viswanathan, N.N.; Singhai, M. Effect of oxygen enrichment on top layer sinter properties. ISIJ Int. 2021, 61, 79–85. [Google Scholar] [CrossRef]
- Peng, S.H.; Liu, H.; Sun, Z.Z.; Li, C.W.; Qin, Y.L.; Liu, W.Q.; Wang, G. Effect of oxygen enrichment on sintering behavior of high proportion vanadium–titanium magnesite concentrates. J. Iron Steel Res. Int. 2023, 30, 2122–2132. [Google Scholar] [CrossRef]
- Takehara, K.; Higuchi, T.; Yamamoto, T. Effect of oxygen enrichment on melting behavior in sintering process. ISIJ Int. 2024, 64, 521–529. [Google Scholar] [CrossRef]
- Gan, M.; Zheng, H.X.; Fan, X.H.; Ji, Z.Y.; Li, Q.; Wu, M.; Wang, Y.F.; Wang, X.L.; Wu, Y.F.; Chen, X.L.; et al. Influence of oxygen-rich hot air composite gas medium on sintering performance and function mechanism. J. Iron Steel Res. Int. 2024, 31, 1071–1081. [Google Scholar] [CrossRef]
- Ni, W.J.; Li, H.F.; Zhang, Y.Y.; Zou, Z.S. Effects of fuel type and operation parameters on combustion and NOx emission of the iron ore sintering process. Energies 2019, 12, 213. [Google Scholar] [CrossRef]
- Pahlevaninezhad, M.; Emami, M.D.; Panjepour, M. The effects of kinetic parameters on combustion characteristics in a sintering bed. Energy 2014, 73, 160–176. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, Z.; Cheng, M.; Zhou, M.X.; Liu, R.P. Influence of coke combustion on NOx emission during iron ore sintering. Energy Fuels 2015, 29, 974–984. [Google Scholar] [CrossRef]
- Xiong, L.; Peng, Z.W.; Gu, F.Q.; Ye, L.; Wang, L.C.; Rao, M.J.; Zhang, Y.B.; Li, G.H.; Jiang, T. Combustion behavior of granulated coke breeze in iron ore sintering. Powder Technol. 2018, 340, 131–138. [Google Scholar] [CrossRef]
- Zhao, J.P.; Loo, C.E.; Dukino, R.D. Modelling fuel combustion in iron ore sintering. Combust. Flame 2015, 162, 1019–1034. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, J.M.; Li, M. Prediction of sinter yield and strength in iron ore sintering process by numerical simulation. Appl. Therm. Eng. 2018, 131, 70–79. [Google Scholar] [CrossRef]
- Park, J.; Lee, S.J.; Park, J.Y. Review of computational fluid dynamics modeling of iron sintering process. J. Mech. Sci. Technol. 2022, 36, 4501–4508. [Google Scholar] [CrossRef]
- Liu, Z.J.; Li, Z.; Wang, Y.Z.; Zhang, J.L.; Wang, J.B.; Niu, L.L.; Li, S.D.; Feng, B. Numerical simulation of heat and mass transfer behavior during iron ore sintering: A review. Metals 2023, 13, 1277. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, J.M.; Li, M.; Li, Y. Modeling and simulation of iron ore sintering process with consideration of granule growth. ISIJ Int. 2018, 58, 17–24. [Google Scholar] [CrossRef]
- Zhou, H.; Zhou, M.X.; Cheng, M.; Guo, W.S.; Cen, K.F. Experimental study and X-ray microtomography based CFD simulation for the characterization of pressure drop in sinter bed. Appl. Therm. Eng. 2017, 112, 811–819. [Google Scholar] [CrossRef]
- Cheng, Z.L.; Fu, P.; Guo, Z.G.; Yang, J.; Wang, Q.W. CFD prediction of heat/mass transfer in multi-layer sintering process assisted with gaseous fuel injection. Int. Commun. Heat Mass Transf. 2021, 128, 105654. [Google Scholar] [CrossRef]
- Wang, G.; Wen, Z.; Lou, G.F.; Dou, R.F.; Li, X.W.; Liu, X.W.Y.; Su, F. Mathematical modeling and combustion characteristic evaluation of a flue gas recirculation iron ore sintering process. Heat Mass Transf. 2016, 97, 964–974. [Google Scholar] [CrossRef]
- Castro, J.A.D.; Sazaki, Y.; Yagi, J.I. Three dimensional mathematical model of the iron ore sintering process based on multiphase theory. Mater. Res. 2012, 15, 848–858. [Google Scholar] [CrossRef]
- Hobbs, M.L.; Radulovic, P.T.; Smoot, L.D. Combustion and gasification of coals in fixed-beds. Prog. Energy Combust. Sci. 1993, 19, 505–586. [Google Scholar] [CrossRef]
- Patisson, F.; Bellot, J.P.; Ablitzer, D. Study of moisture transfer during the strand sintering process. Metall. Trans. B 1990, 21, 37–47. [Google Scholar] [CrossRef]
- Rangarao, G.C.P.; Chetana, U.V.; Veerraju, P. Mathematical model for computer simulation of moisture transfer in multiple package systems. LWT 1995, 28, 38–42. [Google Scholar] [CrossRef]
- Yi, M.; Wang, W.X.; Xue, M.; Gong, Q.H.; Xu, B.X. Modeling and simulation of sintering process across scales. Arch. Comput. Methods Eng. 2023, 30, 3325–3358. [Google Scholar] [CrossRef]
- Ramos, M.V.; Kasai, E.; Kano, J.; Nakamura, T. Numerical simulation model of the iron ore sintering process directly describing the agglomeration phenomenon of granules in the packed bed. ISIJ Int. 2000, 40, 448–454. [Google Scholar] [CrossRef]
- Pahlevaninezhad, M.; Emami, M.D.; Panjepour, M. Identifying major zones of an iron ore sintering bed. Appl. Math. Model. 2016, 40, 8475–8492. [Google Scholar] [CrossRef]
- Huang, X.X.; Fan, X.H.; Chen, X.L.; Zhao, X.Z.; Gan, M. Optimisation model of fuel distribution in materials bed of iron ore sintering process. Ironmak. Steelmak. 2018, 46, 649–655. [Google Scholar] [CrossRef]
- Yang, W.; Ryu, C.; Choi, S.; Choi, E.; Lee, D.; Huh, W. Modeling of combustion and heat transfer in an iron ore sintering bed with considerations of multiple solid phases. ISIJ Int. 2004, 44, 492–499. [Google Scholar] [CrossRef]
- Cheng, Z.L.; Yang, J.; Zhou, L.; Liu, Y.; Wang, Q.W. Sinter strength evaluation using process parameters under different conditions in iron ore sintering process. Appl. Therm. Eng. 2016, 105, 894–904. [Google Scholar] [CrossRef]
- González, D.F.; Ruiz-Bustinza, I.; Mochón, J.; González-Gasca, C.; Verdeja, L.F. Iron ore sintering: Quality indices. Miner. Process. Extr. Metall. Rev. 2017, 38, 254–264. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, Z.H.; Cheng, M.; Liu, R.P.; Cen, K.F. Effect of flame-front speed on the pisolite-ore sintering process. Appl. Therm. Eng. 2015, 75, 307–314. [Google Scholar] [CrossRef]
- Than, V.T.; Wang, C.C.; Ngo, T.T.; Lin, C.H. Thermal behavior of polytetrafluoroethylene in the sintering process. Therm. Sci. Eng. Prog. 2022, 30, 101247. [Google Scholar] [CrossRef]
- Chen, Y.A.; Tan, J.L.; Sun, J.X.; Guo, H.S.; Bai, J.M.; Zhou, P.; Zhang, D.Y.; Liu, G. Effect of sintering temperature on the microstructures and mechanical properties of ZrO2 ceramics fabricated by additive manufacturing. Ceram. Int. 2024, 50, 11392–11399. [Google Scholar] [CrossRef]
- Wang, J.K.; Meng, H.X.; Zhou, H. Effect of biochar substitution on iron ore sintering characteristics based on optimization of fuel distribution through the bed. Fuel Process. Technol. 2023, 247, 107817. [Google Scholar] [CrossRef]
- Cheng, Z.L.; Tan, Z.T.; Guo, Z.G.; Yang, J.; Wang, Q.W. Recent progress in sustainable and energy-efficient technologies for sinter production in the iron and steel industry. Renew. Sustain. Energy Rev. 2020, 131, 110034. [Google Scholar] [CrossRef]
- Ji, M.; Xu, J.Y.; Yu, D.D.; Chen, M.; Mansori, M.E. Influence of sintering temperatures on material properties and corresponding milling machinability of zirconia ceramics. J. Manuf. Process. 2021, 68, 646–656. [Google Scholar] [CrossRef]
- Dai, F.L.; Huang, X.X.; Fan, X.H.; Chen, X.L.; Gan, M.; Ji, Z.Y.; Sun, Z.Q.; Zhao, G.G. Mathematical modeling and distribution characteristics evaluation of fuel particles in iron ore sintering process. Adv. Powder Technol. 2022, 33, 103688. [Google Scholar] [CrossRef]
- Ni, W.J.; Jiang, L.L.; Zhu, X.F.; Yi, X.; Li, H.F.; Shao, L.; Meng, F.C.; Zou, Z.S. Numerical simulation of iron ore sintering process with coke oven gas injection and oxygen enrichment. Int. J. Chem. React. Eng. 2022, 20, 1035–1051. [Google Scholar] [CrossRef]








| Expressions of Reaction | Reaction Rate |
|---|---|
| Coke combustion [18,30] | |
| Drying and condensation [31,32,33] | |
| Decomposition of limestone [22,34] | |
| Iron oxide reduction and oxidation | |
| Melting and solidification [35] | , |
| Expressions of Reaction | Reaction Rate |
|---|---|
| CO + 0.5O2 → CO2 | |
| H2 + 0.5O2 → H2O | |
| CO + H2O → H2 + CO2 | |
| CO2 → CO + 0.5O2 | |
| CH4 + 1.5O2 → CO + 2H2O | |
| H2 + CO2 → CO + H2O |
| Parameters | Value | Parameters | Value |
|---|---|---|---|
| Coke diameter | 0.0008 m | Inlet Air Velocity | 0.45 m/s |
| Limestone diameter | 0.0008 m | Mass fraction of Coke | 3.6% |
| Iron ore diameter | 0.0016 m | Mass fraction of Limestone | 13% |
| Coke apparent density | 1200 kg/m3 | Mass fraction of Iron ore | 83.4% |
| Limestone apparent density | 1600 kg/m3 | Outlet pressure during ignition | 10,000 Pa |
| Iron ore apparent density | 2000 kg/m3 | Outlet Pressure | 15,000 Pa |
| Initial porosity | 0.43 | Grid size | 0.0025 m × 0.0025 m |
| Sintering bed height | 0.6 m | Ignition temperature | 1400 K |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Yang, X.; Tian, Z.; Zhou, Z.; Wang, Y.; Zhang, Q. Numerical Simulation on the Influence of Oxygen Content and Coke Size on the Performance of Fuel Layered-Distribution Sintering Process. Metals 2025, 15, 953. https://doi.org/10.3390/met15090953
Xu J, Yang X, Tian Z, Zhou Z, Wang Y, Zhang Q. Numerical Simulation on the Influence of Oxygen Content and Coke Size on the Performance of Fuel Layered-Distribution Sintering Process. Metals. 2025; 15(9):953. https://doi.org/10.3390/met15090953
Chicago/Turabian StyleXu, Jin, Xiaobo Yang, Ziyue Tian, Zongyan Zhou, Yuelei Wang, and Qibin Zhang. 2025. "Numerical Simulation on the Influence of Oxygen Content and Coke Size on the Performance of Fuel Layered-Distribution Sintering Process" Metals 15, no. 9: 953. https://doi.org/10.3390/met15090953
APA StyleXu, J., Yang, X., Tian, Z., Zhou, Z., Wang, Y., & Zhang, Q. (2025). Numerical Simulation on the Influence of Oxygen Content and Coke Size on the Performance of Fuel Layered-Distribution Sintering Process. Metals, 15(9), 953. https://doi.org/10.3390/met15090953







