Abstract
With the gradual depletion of high-quality iron ore resources, global steel enterprises have shifted their focus to low-grade, high-impurity iron ores. Using low-grade iron ore to produce pellets for blast furnaces is crucial for companies to control production costs and diversify raw material sources. However, producing qualified pellets from limonite and other low-grade iron ores remains highly challenging. This study investigates the mechanism by which basicity affects the consolidation and reduction behavior of high-Al2O3 limonite pellets from a thermodynamic perspective. As the binary basicity of the pellets increased from 0.01 under natural conditions to 1.2, the compressive strength of the roasted pellets increased from 1100 N/P to 5200 N/P. The enhancement in basicity led to an increase in the amount of low-melting-point calcium ferrite in the binding phase, which increased the liquid phase in the pellets, thereby strengthening the consolidation. CaO infiltrated into large-sized iron particles and reacted with Al and Si elements, segregating the contiguous large-sized iron particles and encapsulating them with liquid-phase calcium ferrite. Calcium oxide reacts with the Al and Si elements in large hematite particles, segmenting them and forming liquid calcium ferrite that encapsulates the particles. Additionally, this study used thermodynamic analysis to characterize the influence of CaO on aluminum elements in high-aluminum iron ore pellets. Adding CaO boosted the liquid phase’s ability to incorporate aluminum, lessening the inhibition by high-melting-point aluminum elements of hematite recrystallization. During the reduction process, pellets with high basicity exhibited superior reduction performance.
1. Introduction
Pellet production has low energy consumption and less pollutant emissions, making it a high-quality raw material for blast furnace ironmaking [1]. The gradual depletion of high-grade iron ore compels steel enterprises to seek value in low-grade, high-impurity ores [2]. High-aluminum iron ores, a low-grade iron ore resource, are widely distributed in China, Australia, India, Brazil, and other regions [3]. The total iron content in the ore ranges from 40% to 60%, and the content of aluminum oxide is generally around 2%, which also makes it difficult to use directly as a raw material for ironmaking [4,5,6]. Consequently, the development and utilization of high aluminum iron ore pellets to provide suitable burden materials for blast furnaces have become a focal point of research [7].
High-aluminum iron ores are characterized by their high aluminum content, which poses specific difficulties during the smelting process [8,9]. When the content of Al2O3 is high, the fluidity of the slag becomes poor, causing trouble for blast furnace operation. During the oxidation consolidation process, the high melting point of Al2O3 particles in the ore hinders the oxidation of magnetite and the recrystallization of hematite, resulting in product strength that does not meet production requirements. In acid pellets, Al2O3 alters the compressive strength of roasted pellets by affecting the recrystallization of hematite [10,11,12]. A lower Al2O3 content could replace iron in iron olivine, promoting the recrystallization of hematite [13,14]. However, when the content of Al2O3 increases, its high melting point and low reactivity have a negative impact on compressive strength [15,16]. In basic pellets, Al2O3 primarily affects the performance of pellets by influencing the binding phase calcium ferrite [17,18,19]. Hariswijaya studied the impact of alumina on the development of calcium ferrite during roasting and established a relationship between initial melting temperature and aluminum content [20]. Hessien investigated the relationship between the stability of calcium aluminate and the content of alumina in roasting, concluding that calcium aluminates could only stably exist when the content of Al2O3 exceeded 2.5% [21]. Tian studied the impact of different types of aluminous ores on the crystallization of hematite and found that kaolinite had better consolidation properties compared to other aluminous ores [22]. Zhang’s research indicated that the inhibitory effect of MgO on pellet reduction swelling decreased with increasing aluminum content [23].
The addition of alkaline fluxes such as limestone and dolomite to pellets significantly enhanced their physical and metallurgical properties. During the oxidation process, basicity influenced the sintering strength by altering the composition, formation temperature, and quantity of the liquid phase during sintering [24]. Adding CaO, on one hand, formed low-melting-point calcium ferrite bonding phases with Fe, and on the other hand, reacted with gangue to form low-melting-point liquid phases, which tightly bound solid particles through surface tension [25,26]. The initial product at the Ca-Fe-Si diffusion interface was Ca2Fe2O5, which gradually transformed into CaFe2O4 as more Fe2O3 was added to the reaction [27]. Park et al.’s research indicated that at low basicity, the main component of the calcium-containing bonding phase was calcium ferrite silicate, and as basicity increased, the bonding phase gradually transitioned to calcium ferrite [28]. Additionally, as basicity increased, the decomposition of calcium carbonate also led to an increase in the porosity of the roasted pellets [29]. These changes in micro-components and structure contribute to improvement of pellet quality [30]. During the reduction stage, the impact of basicity on the metallurgical properties of pellets is complex. It is generally believed that at low basicity, the reduction swelling rate of pellets increases, and when basicity is raised to 1.0, the reduction swelling rate of hematite pellets decreases [31]. Studies have also shown that the addition of CaO can cause the expansion of particles in the inner and outer layers during reduction to be out of sync, providing better resistance to reduction stress [32]. An increase in basicity has a positive effect on the reducibility of pellets. Calcium ferrite has superior reducibility compared to other bonding phases, and the increased porosity generated during the oxidation stage also promotes the reduction of pellets [33,34,35,36]. Previous studies have largely focused on hematite pellets with high aluminum content and the impact of Al on pellet performance under high basicity conditions. These studies typically regulate aluminum content by adding pure Al2O3 reagents, which does not accurately reflect the occurrence state of Al in minerals. At present, the solidification mechanism of high-alumina iron ore pellets is not very clear, especially high-alumina limonite pellets.
This study selected high-aluminum blended ore from actual production processes as the research subject, and prepared high-aluminum pellets with varying basicity by adding limestone to serve as a raw material for blast furnace smelting. The study investigated the impact of basicity on the compressive strength and metallurgical properties of high-aluminum iron ore pellets. The phase compositions and microscopic structures were analyzed to revealed the solidification and reduction mechanism of high-aluminum iron ore pellets.
2. Materials and Methods
2.1. Raw Materials
In this study, the high-aluminum iron ore used was Australian iron ore (Aquila Inc., Brisbane Australia) (AIO), which was mixed with Mauritanian fine ore (SNIM Inc., Zoueratt, Mauritania) (MAF) in a ratio of 68:32, following the on-site production proportion. The chemical compositions and particle size distributions of the two types of iron ores, blended ore, bentonite, and limestone are presented in Table 1 and Table 2. The alumina content in the blended iron ore was 2.54%, classifying it as a high-aluminum limonite mineral, with high total iron (TFe) and silica (SiO2) content. The blended iron ore had a high loss on ignition (LOI) of 7.62%. The particle size screening results indicate that 66.66% of the iron ore passed through a sieve with a diameter of 45 µm, while the contents of bentonite and limestone smaller than 45 µm were 69.04% and 76.52%, respectively. The two main raw materials AIO and MAF (accounting for more than 95% of the mixture) were analyzed using X-ray diffraction (PANalytical Inc., Almelo, the Netherlands), and the results are shown in Figure 1. The iron-bearing phases of AIO were hematite and goethite, with gangue primarily present in the form of quartz. The main phases of MAF were hematite and a small amount of magnetite, with gangue being quartz. MIRA-IIII Scanning electron microscopy (TESCAN Inc., Brno, Czech Republic) was used to analyze the particle morphology, which is shown in Figure 2. The crystalline water contained in AIO caused the formation of cementing materials between particles, making it impossible to observe clear particle boundaries. The particles of MAF were smooth and had clear boundaries.

Table 1.
Chemical composition of the raw materials (wt%).

Table 2.
Size classification of raw materials.
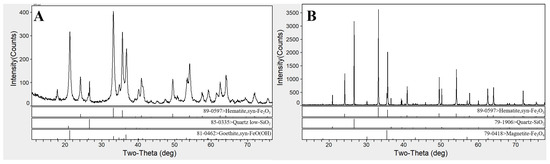
Figure 1.
X-ray diffraction analysis pattern of iron ore AIO (A) and MAF (B).
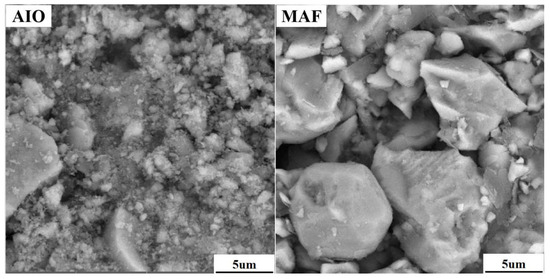
Figure 2.
SEM maps of iron ore AIO and MAF.
2.2. Methods
The experimental procedure is depicted in Figure 3. The blended ore, bentonite, and limestone were mixed together with the addition of water. The mixture was placed into a disk pelletizer with a diameter of 1000 mm and a rotation speed of 24 r/min, where manual intermittent spraying of water was used to obtain green pellets. During the production of green pellets, the pelletizing time was controlled at 14 min, the moisture content of the blended material was maintained at 7%, and 1.0% bentonite was added as a binder for the pellets. All basicities referred to in this study are binary basicity (B2), which is the CaO/SiO2 ratio of the pellets. In this study, the basicity of the pellets was varied within the range of 0.01 to 1.2 by adding limestone. The green pellets were sieved to select particles of 10–16 mm and placed in a constant temperature oven at 105 °C for drying for 5 h. The oxidation roasting process was carried out in a tube resistance furnace. In the preheating stage, the dried pellets were preheated at 1070 °C or 1150 °C for 5 min, followed by the determination of compressive strength. Pellets preheated at 1070 °C for 5 min were selected for roasting, which was carried out at 1250 °C for 10 min. Subsequently, the compressive strength was tested and characterized.
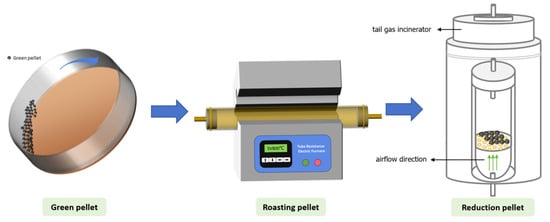
Figure 3.
Schematic diagram for pellet preparation.
The compressive strength test was conducted on an automatic pressure testing machine in accordance with the ISO 4700 standard [37]. Ten green pellets were selected and naturally dropped from a height of 0.5 m. The average number of drops required for the pellets to fracture was recorded as the drop strength. The bursting temperature of the pellets was determined in a tube furnace with dimensions of Φ650 mm × 1000 mm. Hot air with a flow rate of 18 L/min was blown through 50 green pellets and held for 5 min. Subsequently, the number of burst pellets was observed. The temperature at which 4% of the pellets burst was defined as the bursting temperature. The reduction swelling index was determined in accordance with GB/T13240-1991 [38]. This standard requires the oxidized pellets to be reduced at 900 °C with 30 vol.% CO and 70 vol.% N2 gas at a flow rate of 15 L/min for 1 h. Under these conditions, the percentage of pellet volume expansion was measured as the reduction swelling index (RSI). The reduction disintegration index and reduction degree were determined in accordance with the GB/T 13242-2017 standard [39]. The pellets were reduced under gas conditions of 500 °C, 20 vol.% CO, 20 vol.% CO2, and 60 vol.% N2 at a flow rate of 15 L/min for 1 h before being placed in a drum. The percentage of mass passing through the sieve was measured as the reduction disintegration index of the pellets.
2.3. Analysis
The chemical composition of two iron ore powders, bentonite, and limestone were analyzed using chemical analysis methods. To investigate the effect of different basicities on the micro-characteristics of pellets, X-ray diffraction (XRD) was used to analyze the main phase composition of the sintered pellets, with a scan range (2θ) of 5° to 80°, a step size of 0.02°, and a scan speed of 2°/min. FactSage 8.1 software was used to simulate the phase equilibrium of the roasted pellets, theoretically predicting the transformation of various phases and the formation temperature of the liquid phase. A Leica DM RXP microscope and scanning electron microscope (SEM), equipped with an energy-dispersive spectrometer (EDS) for point and surface scanning, were used to study the microstructure and crystallization of the pellets.
3. Results and Discussion
3.1. Effect of Basicity on Green Pellet Properties
Figure 4 displays the addition amount of limestone in pellets with different basicities and their green pellet performance. The moisture content of green pellets was controlled at an optimal level of around 8%. With little difference in moisture content, the compressive strength of alkaline pellets with added limestone was higher than that of natural pellets. With increasing basicity, the drop strength of pellets first increased and then decreased, while the bursting temperature gradually decreased. When the basicity was increased to 1.2, the bursting temperature of the pellets was only 350 °C. Overall, the effect of basicity on green pellets of high-aluminum ores was positive, and the drop strength and compressive strength of pellets at various basicities can meet the needs of iron and steel enterprises (Compressive strength ≥ 10 N/P; Drop numbers ≥ 3 times/0.5 m).
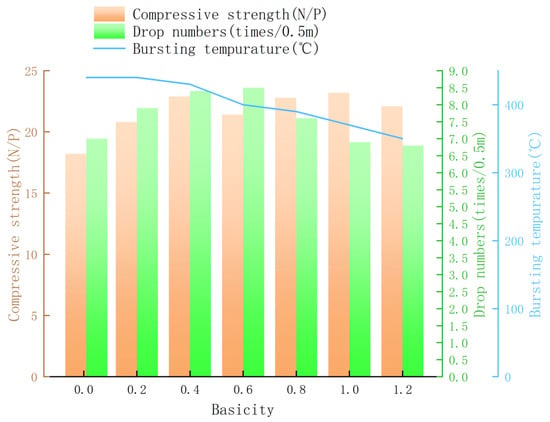
Figure 4.
Effect of basicity on green pellet properties.
3.2. Impact of Basicity on the Consolidation Characteristics of Oxidized Pellets
3.2.1. Compressive Strength of Oxidized Pellets
Figure 5 illustrates the impact of basicity on the compressive strength of preheated and roasting pellets. It can be seen that the strength of preheated pellets was relatively low, and the strength of preheated pellets decreased after the addition of limestone. Although increasing the temperature can improve the strength of preheated pellets, under a preheating time of 5 min, the preheating temperatures of 1070 °C and 1150 °C both resulted in preheated pellet strengths of less than 400 N/P. The influence of basicity on the compressive strength of high-aluminum pellets was studied with a preheating time of 5 min at 1070 °C and a roasting time of 15 min at 1250 °C. The strength of roasted pellets significantly increased with the enhancement of basicity. By merely increasing the basicity to 0.2, the compressive strength of roasted pellets could reach 2283 N/P. When the basicity reached 1.2, the compressive strength of roasted pellets could reach 5791 N/P.
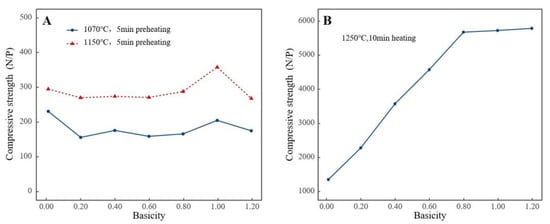
Figure 5.
Effect of basicity on compressive strength of pellets ((A). Preheating stage; (B). Roasting stage).
3.2.2. Analysis of the Consolidation Mechanism of Oxidized Pellets
Changes in the microstructure of the pellet during oxidation roasting are significant factors in the development of compressive strength. To further explore the mechanism by which basicity affected the compressive strength of pellets, scanning electron microscopy–energy dispersive spectroscopy (SEM-EDS) was used to observe sintered pellets with R = 0.01 and R = 1.0, with results shown in Figure 6. At basicity 0.01, large-sized iron ore particles could be observed in high-aluminum iron ore pellets. Some well-developed iron particles had complete morphology, while most iron ore particles formed irregular clusters interspersed with slender, irregularly shaped pores. In the pellets with R = 1.0, large iron ore particles were distinctly observed to be segmented by a light gray phase. In pellets with R = 1.0, the iron particles were smaller in size, with no obvious cracks or pores inside the particles.
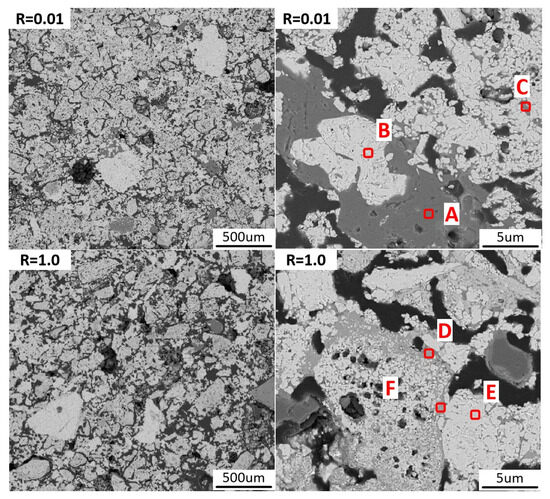
Figure 6.
SEM-EDS maps of oxidized pellets with basicity 0.01 and 1.0.
Table 3 presents the elemental composition of the points depicted in Figure 6. Combined with SEM-EDS analysis, it is known that under basicity 0.01 conditions, the consolidation of high-aluminum iron ore pellets mainly relied on the recrystallization of hematite. During the roasting process, the formed aluminosilicate phase filled the gaps between hematite particles, playing a role in auxiliary consolidation. In pellets with a basicity of 1.0, high-aluminum iron ore particles were relatively small, and the light gray phase was distributed in a reticular pattern between larger iron ore particles. The presence of iron elements in the light gray phase was clearly observable. The light gray phase showed obvious iron elements, and its composition indicates it was a calcium ferrite phase. In iron ore particles at basicity 0.01, significant Al and Si elements could be observed, but these elements were not observed in iron ore particles at a basicity of R = 1.0. This indicates that the addition of CaO would erode those poorly crystallized iron particles, reacting with Al and Si to form slag phases. Ultimately, this led to iron particles in pellets with R = 1.0 being smaller and more complete.

Table 3.
EDS analysis results (at%) for the spots in roasted pellets in Figure 6.
Figure 7 displays the phase diagram of the Al2O3-SiO2-CaO-Fe2O3 system, where the ratio of Fe2O3 to the total (Al2O3-SiO2-CaO-Fe2O3) is 0.3339, and the oxygen partial pressure is 0.21 atm. It can be seen from Figure 7a that at 1250 °C, the liquid phase region of the slag system was very small. To the right of the liquid phase region, there was a large melilite phase area, indicating that the slag system had low solubility for melilite when the aluminum content in the system increased. At lower basicities, the first liquid phase formed at the roasting temperature of 1250 °C was a calcium iron silicate. As the reaction continued, Al from the gangue dissolved into the liquid phase, increasing the aluminum content in the liquid phase. Once more Al2O3 was produced in the liquid phase, solid phase melilite precipitated from the slag. When the basicity increased, the content of CaO in the system increased, and the calcium iron aluminum silicate in the liquid phase enhanced the liquid phase’s capacity to accommodate Al, resulting in an increase in the amount of liquid phase in the system.
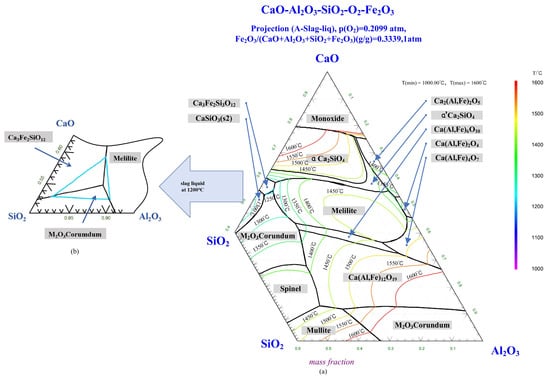
Figure 7.
(a) Phase diagram of Al2O3-SiO2-CaO-15 (wt%) Fe3O4 and (b) Projection of 1200 °C liquid phase.
The phase composition during the roasting process at 1250 °C under different basicities was calculated using FactSage 8.1 software, with the results shown in Figure 8. With the increase in basicity, the overall amount of liquid phase produced by high-aluminum pellets during the sintering stage showed an increasing trend. At a basicity of 0.6–0.8, melilite began to form, leading to a slight decrease in the amount of liquid phase. As basicity further increased, more melilite was dissolved in the liquid phase, which continued to increase the amount of liquid phase while reducing the content of melilite. An increase in basicity also led to an elevation in the iron content of the liquid phase. This indicates that CaO reacted with iron ore particles during the roasting process, breaking the crystal structure of the iron ore and promoting the recrystallization of iron particles.
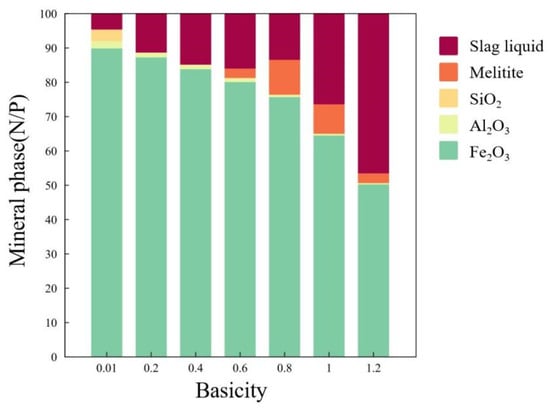
Figure 8.
Phase composition of high-aluminum pellets with different basicities at 1250 °C.
Overall, an increase in basicity has a positive impact on the oxidation of high-aluminum iron ore pellets. During the oxidation process, the strength of high-aluminum pellets at basicity 0.01 is mainly provided by solid-phase consolidation. An increase in basicity leads to an increase in the volume of the liquid phase. Liquid phases with higher basicity have greater capacity to accommodate aluminum, mitigating the inhibitory effect of less reactive Al2O3 particles on the crystallization of hematite. The added CaO reacts with Al and Si elements in the iron ore particles, segmenting the larger particles and encapsulating them with a calcium ferrite phase. Higher basicity enhances the formation of calcium ferrite, which has excellent binding properties, thereby increasing the agglomeration strength of the pellets.
3.3. Impact of Basicity on the Metallurgical Performance of High-Aluminum Pellets
3.3.1. Reduction Disintegration and Reduction Swelling Characteristics
Basicity also affects the metallurgical performance of high-aluminum pellets. Figure 9 and Figure 10 respectively present the reduction disintegration index and swelling performance of pellets with different basicities. High-aluminum iron ore pellets inherently possess an excellent reduction disintegration index, with RDI + 3.15 content above 97.5% at basicity 0.01 levels. The enhancement of their reduction disintegration performance by basicity was not significant. At R = 1.2, the reduction disintegration performance of the pellets deteriorated. At lower basicities, basicity had a negative impact on the reduction swelling of high-aluminum pellets. As basicity increased, the reduction swelling index of the pellets increased, reaching a peak at R = 0.6, after which the reduction swelling index gradually decreased.
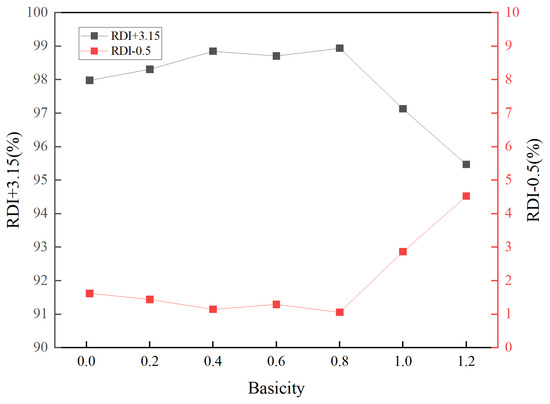
Figure 9.
Effect of basicity on the RDI of oxidized pellets.
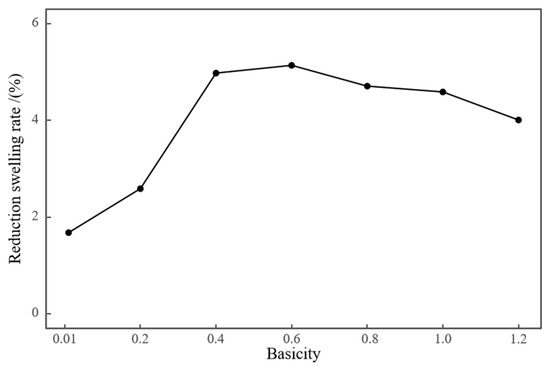
Figure 10.
Effect of basicity on swelling rate.
3.3.2. Microstructural Analysis of Reduced Pellets
Figure 11 shows the microstructures of pellets with different basicities under the optical microscope. Significant differences in porosity were observed among pellets with different basicities. This is consistent with the liquid phase mass shown in Figure 9. An appropriate amount of liquid phase helps reduce pellet porosity, but excessive liquid phase can impede the contact between iron ore particles, thereby increasing porosity. These porosity changes clarify the variation rules of different pellets’ reduction expansion and powdering rates. At natural and low basicity levels, pellets were not fully reduced, retaining the excellent mechanical properties of oxidized pellets. Consequently, both the reduction swelling index and reduction degradation index were low. As the basicity increased to R = 0.8, the porosity of the pellets decreased, and the reduced pellet structure became denser. This enhanced resistance to impact and friction during the reduction degradation test. However, the denser structure left no room for expansion during reduction, leading to an increase in the reduction expansion rate. Increasing the basicity to 1.0 or 1.2 increased the amount of liquid phase, increasing pellet porosity. This reduced the pellets’ resistance to impact and friction, increasing the reduction degradation index. However, the increasing porosity accommodated the volume increase of iron ore particles during reduction, decreasing the reduction swelling index. It is worth noting that in this study, the research on porosity is currently only at the stage of qualitative observation through microstructural images. No quantitative determination of porosity was conducted in this study (for example, using the mercury intrusion method). The impact of porosity on pellet consolidation strength and metallurgical properties will be demonstrated in subsequent research.

Figure 11.
Microstructures of different basicity pellets under optical microscope.
SEM-EDS was applied to the analysis of reduced pellets with basicities of 1.0 and 0.01, with results shown in Table 4 and Figure 12. Reduced pellets were mainly composed of metallic iron phases and slag phases. The bright white parts are the metallic iron phases after reduction, the grayish-white parts are the partially reduced iron-oxygen wüstite, and the dark gray parts are mostly slag phases. In pellets with basicity 0.01, most of the Fe elements were only reduced to the Fe-O wüstite stage. A significant amount of oxygen was still present in the small areas of bright white metallic iron. Despite this, the presence of Fe-O wüstite could still be observed in large metallic particles and metal particles encapsulated by silicate phases in pellets with a basicity of 1.0. In pellets with a basicity of 1.0, the number of bright white metallic iron areas increased, and their area enlarged. The content of oxygen elements within these areas was also lower. This also indicates that an increase in basicity promoted the reduction of high-aluminum pellets. Ca elements were hardly observed in the metallic iron phase but generally enriched in the aluminosilicate phase. This is due to the participation of CaO in the reaction with gangue minerals during the oxidation process, forming aluminosilicate complex oxides, disrupting the original mineral phases, and thereby playing a role in increasing the degree of reduction.

Table 4.
EDS analysis results (at%) for the spots in roasted pellets in Figure 12.
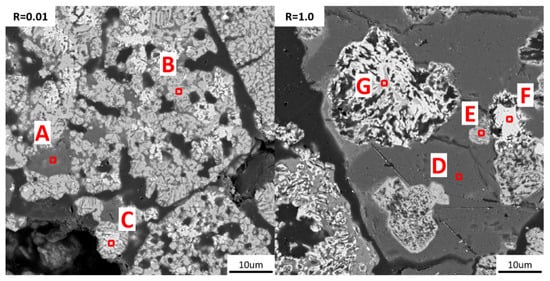
Figure 12.
SEM maps of reduced pellets with basicity 0.01 and 1.0, with areas marked for EDS analysis.
3.3.3. Reduction of High-Alumina Iron Ore Pellets
Reduction atmosphere and temperature are important factors affecting the reduction degree of pellet ore. Using pellets with R = 0.01 and R = 1.0 as raw materials, this study investigated the impact of basicity on the reduction degree of pellets under different reduction atmospheres and temperatures, with results shown in Figure 13.
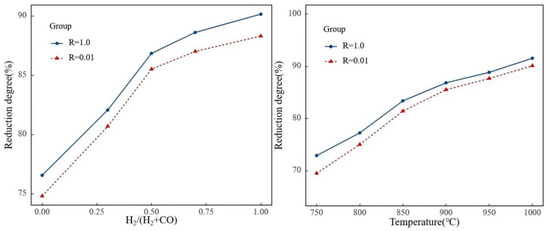
Figure 13.
Degree of reduction at different temperatures and gas compositions.
Under different reduction atmospheres and temperatures, the reduction degree of alkaline pellets was higher than that of R = 0.01 pellets. This means that the promotional effect of basicity on the reduction of high-alumina pellets discussed in the previous section still applied under a broader range of atmosphere conditions and thermal regimes. Increasing the proportion of H2 in the reduction atmosphere and raising the reduction temperature both increased the reduction degree of high-alumina ore pellets. When the proportion of H2 increased, the increase in the reduction degree of R = 1.0 pellets was greater than that of R = 0.01 pellets. Within the reduction temperature range of 800–1000 °C, the increase in the reduction degree of R = 0.01 pellets was higher than that of R = 1.0 pellets.
3.4. Industrial Applicability
The production of high-Al2O3 limonite pellets leads to high energy consumption and emissions due to the special properties of the high-alumina limonite raw material. Its roasting requires a higher temperature to promote the recrystallization and consolidation of hematite, increasing fuel consumption. Meanwhile, the high moisture content of limonite intensifies energy consumption during the drying stage. In terms of emissions, high-temperature calcination intensifies the emission of waste gases such as CO2, SOx, and NOx. To evaluate the scalability of high-basicity recipes, we must consider economic and operational factors. While high-basicity limonite pellets enhance consolidation, challenges such as flux cost and slag handling arise. However, recent industrial practices demonstrate viable solutions. Some companies have successfully integrated waste slags as flux carriers, reducing raw material costs while improving pellet performance. Finally, these strategies mitigate economic and environmental concerns, suggesting that high-basicity recipes are technically feasible at scale. Future work could explore low-cost composite fluxes derived from industrial waste.
4. Conclusions
In this study, the addition of limestone was employed as a means to regulate basicity. The study investigated the impact of basicity on the oxidation consolidation and reduction processes of high-aluminum pellets and discussed the underlying principles. The conclusions drawn from this study are as follows:
High-aluminum green pellets possess excellent performance. Increasing basicity improves the compressive strength of high-aluminum green pellets and reduces the bursting temperature.
Basicity has a positive effect on the strength of both preheated and sintered high-aluminum pellets. As basicity increases, the compressive strength of high-aluminum pellets gradually increases. The main sources of compressive strength in high-basicity pellets are twofold. First, increasing the basicity enhances the liquid phase’s ability to accommodate Al elements, alleviating the inhibitory effect of unreactive Al elements on solid-phase agglomeration. Second, higher basicity introduces more calcium ferrite binding phases with excellent binding properties, thereby increasing agglomeration strength.
Basicity has a complex impact on the metallurgical properties of high-aluminum pellets. At high basicity levels, the excessive liquid phase generated during the oxidation process increases the porosity of the pellets. This leads to an increase in the reduction degradation index and a decrease in the reduction swelling index. The impact of basicity on the reduction rate of pellets is positive. This is partly due to the excellent reduction properties of calcium ferrite formed at high basicity, and partly that the increase in porosity facilitates the diffusion of reducing gases within the pellets. Additionally, large iron particles are divided into smaller iron ore particles by calcium oxide and encapsulated with calcium ferrite, which is also a reason for the improved reducibility of high-basicity pellets.
Author Contributions
Conceptualization, L.Y.; Methodology, Y.Z.; Software, F.C.; Validation, L.Y.; Formal analysis, Y.Z. and X.X.; Investigation, Y.X.; Resources, Y.X.; Data curation, X.X.; Writing—original draft, Y.Z.; Writing—review and editing, Y.G. and S.W.; Visualization, F.C.; Supervision, Y.G. and S.W.; Funding acquisition, Y.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lv, W.; Sun, Z.; Su, Z. Life cycle energy consumption and greenhouse gas emissions of iron pelletizing process in China, a case study. J. Clean. Prod. 2019, 233, 1314–1321. [Google Scholar] [CrossRef]
- Shaik, S.; Chen, Z.; Sahoo, P.P.; Borra, C.R. Kinetics of solid-state reduction of chromite overburden. Int. J. Miner. Metall. Mater. 2023, 30, 2347–2355. [Google Scholar] [CrossRef]
- Singh, S.; Sahoo, H.; Rath, S.S.; Sahu, A.K.; Das, B. Recovery of iron minerals from Indian iron ore slimes using colloidal magnetic coating. Powder Technol. 2015, 269, 38–45. [Google Scholar] [CrossRef]
- Kim, S.W.; Jeon, J.W.; Suh, I.K.; Jung, S.M. Improvement of sintering characteristics by selective granulation of high Al iron ores and ultrafine iron ores. Ironmak. Steelmak. 2016, 43, 500–507. [Google Scholar] [CrossRef]
- Long, F. The Effection on Ironmaking Raw Materials and Metallurgical Process by High Content of Al2O3 Iron Ore. Ph.D. Thesis, Wuhan University of Technology, Wuhan, China, 2006; p. 1. [Google Scholar]
- Umadevi, T.; Deodar, A.V.; Mahapatra, P.C.; Prabhu, M.; Ranjan, M. Influence of Alumina on Iron Ore Sinter Properties and Productivity in the Conventional and Selective Granulation Sintering Process. Steel Res. Int. 2009, 80, 686–692. [Google Scholar]
- Hou, J.; Zhang, J.; Yan, Z.; Yang, X.; Wu, H.; Huang, X.; Bai, C.; Zhang, S. Effect of increasing the proportion of high-alumina iron ore on structures and properties of sinter produced in iron ore blending sintering process. Ironmak. Steelmak. 2024, 1, 12. [Google Scholar] [CrossRef]
- Kumar, R.; Sagar, R.K.; Sah, R.; Kumar, V.; Srinidhi, R.; Balachandran, G. Utilization of High-Alumina Iron Ore in Pellet Making Through Process Optimization. Trans. Indian Inst. Met. 2024, 77, 3293–3302. [Google Scholar] [CrossRef]
- Zhang, J.-L.; Wang, Z.-Y.; Xing, X.-D.; Liu, Z.-J. Effect of aluminum oxide on the compressive strength of pellets. Int. J. Miner. Metall. Mater. 2014, 21, 339–344. [Google Scholar] [CrossRef]
- Yi, Z.; Liu, Q.; Shao, H. Effect of MgO on Highly Basic Sinters with High Al2O3. Min. Metall. Explor. 2021, 38, 2175–2183. [Google Scholar] [CrossRef]
- Li, X.-B.; Wang, H.-Y.; Zhou, Q.-S.; Qi, T.-G.; Liu, G.-H.; Peng, Z.-H.; Wang, Y.-L. Reaction behavior of kaolinite with ferric oxide during reduction roasting. Trans. Nonferr. Met. Soc. China 2019, 29, 186–193. [Google Scholar] [CrossRef]
- Shen, F.-M.; Gao, Q.-J.; Jiang, X.; Wei, G.; Zheng, H.-Y. Effect of magnesia on the compressive strength of pellets. Int. J. Miner. Metall. Mater. 2014, 21, 431–437. [Google Scholar] [CrossRef]
- Dong, J.-J.; Wang, G.; Gong, Y.-G.; Xue, Q.-G.; Wang, J.-S. Effect of high alumina iron ore of gibbsite type on sintering performance. Ironmak. Steelmak. 2016, 42, 34–40. [Google Scholar] [CrossRef]
- Pal, J.; Ghorai, S.; Nandi, B.; Chakraborty, T.; Das, G.; Venugopalan, T. Effect of pyroxenite and olivine minerals as source of MgO in hematite pellet on improvement of metallurgical properties. J. Cent. South Univ. 2015, 22, 3302–3310. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Chen, B.-X.; Hou., J.; Chen, M.; Bai, C.-G.; Hu, M.-L. Hydrogen affection on softening and melting behaviours of high alumina sinter in gas-injection blast furnace. Ironmak. Steelmak. 2024; published. [Google Scholar] [CrossRef]
- Xue, Y.; Pan, J.; Zhu, D.; Guo, Z.; Yang, C.; Lu, L.; Tian, H. Improving High-Alumina Iron Ores Processing via the Investigation of the Influence of Alumina Concentration and Type on High-Temperature Characteristics. Minerals 2020, 10, 802. [Google Scholar] [CrossRef]
- Guo, Y.; Xie, J.; Gao, J.; Xu, H.; Qie, J. Study on the Production and Metallurgical Properties of Fluxed Pellets with High Hematite Content. Metallurgist 2017, 61, 638–645. [Google Scholar] [CrossRef]
- Ranjan, P.; Pal, J. Salt solution treatment to prevent the low temperature reduction degradation of haematite pellet. Ironmak. Steelmak. 2016, 43, 688–696. [Google Scholar] [CrossRef]
- Dhiraj, M.-K.; Chandra, S.-M.-S.; Srinivas, D.; Saurabh, K.; Pradeep, C.; Mithilesh, K.-J.; Uttam, S. Development and application of a novel fluxing compound for improving sintering performance of high alumina iron ore. Ironmak. Steelmak. 2021, 48, 1261–1276. [Google Scholar] [CrossRef]
- Hariswijaya, D.; Da Rocha, L.T.; Chiwandika, E.K.; Jung, S.-M. Effect of Alumina on Calcium Ferrites Development in the Goethite Ore Sinters. J. Sustain. Metall. 2022, 8, 257–273. [Google Scholar] [CrossRef]
- Hessien, M.M.; Kashiwaya, Y.; Ishii, K.; Nasr, M.I.; El-Geassy, A.A. Sintering and heating reduction processes of alumina containing iron ore samples. Ironmak. Steelmak. 2008, 35, 191–204. [Google Scholar] [CrossRef]
- Tian, H.; Zhu, D.; Pan, J.; Yang, C.; Huang, W.; Chu, M. Effect mechanism of aluminum occurrence and content on the induration characteristics of iron ore pellets. Int. J. Miner. Metall. Mater. 2023, 30, 2334–2346. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Chen, R.; Sun, Y.; Zhu, Y.-D.; Li, X.-L.; Li, L.-L.; Zou, Z.-S. Non-isothermal reduction characteristics of roasted high alumina iron ore pellets. Can. Metall. Q. 2017, 56, 148–155. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, D.; Pan, J.; Guo, Z.; Yang, C. Effect of basicity on the structure characteristics of chromium-nickel bearing iron ore pellets. Powder Technol. 2019, 342, 409–417. [Google Scholar] [CrossRef]
- Shi, Y.; Zhu, D.; Pan, J.; Guo, Z.; Lu, S.; Xu, M. Improving hydrogen-rich gas-based shaft furnace direct reduction of fired hematite pellets by modifying basicity. Powder Technol. 2022, 408, 117782. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, K.; Chen, F.; Wang, S.; Zheng, F.; Yang, L.; Liu, Y. Effect of basicity on the reduction swelling behavior and mechanism of limestone fluxed iron ore pellets. Powder Technol. 2021, 393, 291–300. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Li, Q.; Zou, Z.S. Reduction properties of high alumina iron ore cold bonded pellet with CO-H2 mixtures. Ironmak. Steelmak. 2014, 41, 561–567. [Google Scholar] [CrossRef]
- Park, H.; Sahajwalla, V. Influence of CaO-SiO2-Al2O3 Ternary Oxide System on the Reduction Behavior of Carbon Composite Pellet: Part I. Reaction Kinetics. Metall. Mater. Trans. B-Process Metall. Mater. Process. Sci. 2013, 44, 1379–1389. [Google Scholar] [CrossRef]
- Kotta, A.B.; Narsimhachary, D.; Karak, S.K.; Kumar, M. Studies on the Mechanical and Physical Properties of Hematite Iron Ore Pellets Prepared Under Different Conditions. Trans. Indian Inst. Met. 2020, 73, 2561–2575. [Google Scholar] [CrossRef]
- Zeng, J.; Wang, J.; Wang, R.; Zhang, N.; Li, Y.; Xue, Y.; Lv, X. Promoting the Utilization of High-Alumina Iron Ores During Sintering by Pre-preparing a Low-Melting-Point Flux. J. Sustain. Metall. 2024, 10, 2205–2215. [Google Scholar] [CrossRef]
- Umadevi, T.; Sah, R. Effect of olivine as MgO-Bearing Flux on Low And High-Alumina iron ore pellets. J. Min. Metall. Sect. B-Metall. 2023, 59, 455–464. [Google Scholar] [CrossRef]
- Li, G.; Jiang, T.; Liu, M.; Zhou, T.; Fan, X.; Qiu, G. Beneficiation of High-Aluminium-Content Hematite Ore by Soda Ash Roasting. Miner. Process. Extr. Metall. Rev. 2010, 31, 150–164. [Google Scholar] [CrossRef]
- Hu, X.; Zheng, H.; Guo, Y.; Jiang, X.; Gao, Q.; Shen, F. Determination of Al2O3 Activity by Reference Slag Method in CaO-SiO2-Al2O3-MgO Melts for Blast Furnace Slag with High Al2O3 at 1873 K. Steel Res. Int. 2020, 91, 1900285. [Google Scholar] [CrossRef]
- Cavaliere, P.; Perrone, A.; Dijon, L.; Laska, A.; Koszelow, D. Direct reduction of pellets through hydrogen: Experimental and model behaviour. Int. J. Hydrogen Energy 2024, 49, 1444–1460. [Google Scholar] [CrossRef]
- Sadeghi, B.; Najafizadeh, M.; Cavaliere, P.; Shabani, A.; Aminaei, M. Effect of composition and processing conditions on the direct reduction of iron oxide pellets. Powder Technol. 2024, 444, 120061. [Google Scholar] [CrossRef]
- Sarkar, A.; Chavan, V.; Pai, N.N.; Prakash, A.; Hazra, B.; Raut, P.; Sunilkumar, D.; Sivananda, C.; Kundu, S.; Nag, S.; et al. Reduction of Iron Ore Pellets: A Microstructural Perspective? Metall. Mater. Trans. A 2024, 55, 537–549. [Google Scholar] [CrossRef]
- ISO 4700:2007; Iron Ore Pellets for Blast Furnace and Direct Reduction Feedstocks—Determination of the Crushing Strength. ISO: Geneva, Switzerland, 2007.
- GB/T 13240-1991; Determination of Particle Size Distribution of Iron Ore. Standard Press of China: Beijing, China, 1991.
- GB/T 13242-2017; Determination of Tumbler Index of Sinter and Pellets. Standard Press of China: Beijing, China, 2017.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).