High-Purity Tungsten Oxide Production from Low-Grade Scheelite Concentrates at Pilot Plant Scale
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Characterization
2.2. Laboratory Scale Methods
2.2.1. Laboratory Scale Leaching
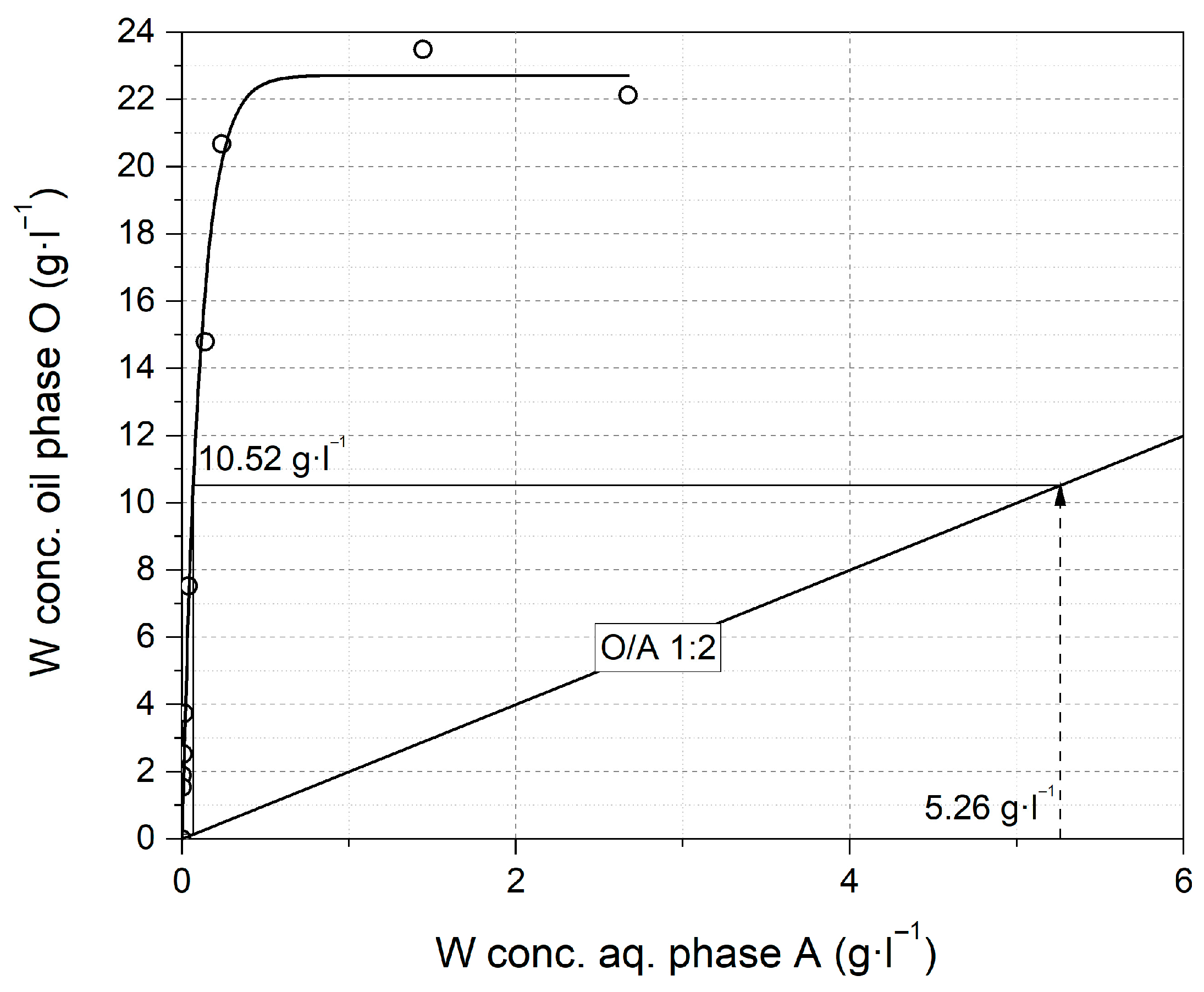
2.2.2. Laboratory Scale Solvent Extraction
2.3. Pilot Plant Equipment and Operation
2.3.1. Pilot Plant Pre-Treatment
2.3.2. Pilot Plant Leaching
2.3.3. Pilot Plant Solvent Extraction
2.3.4. Crystallization and Calcination
3. Results
3.1. Laboratory Scale Results
3.2. Pilot Plant Results
3.2.1. Flow-Sheet
3.2.2. Pilot Plant Conditions
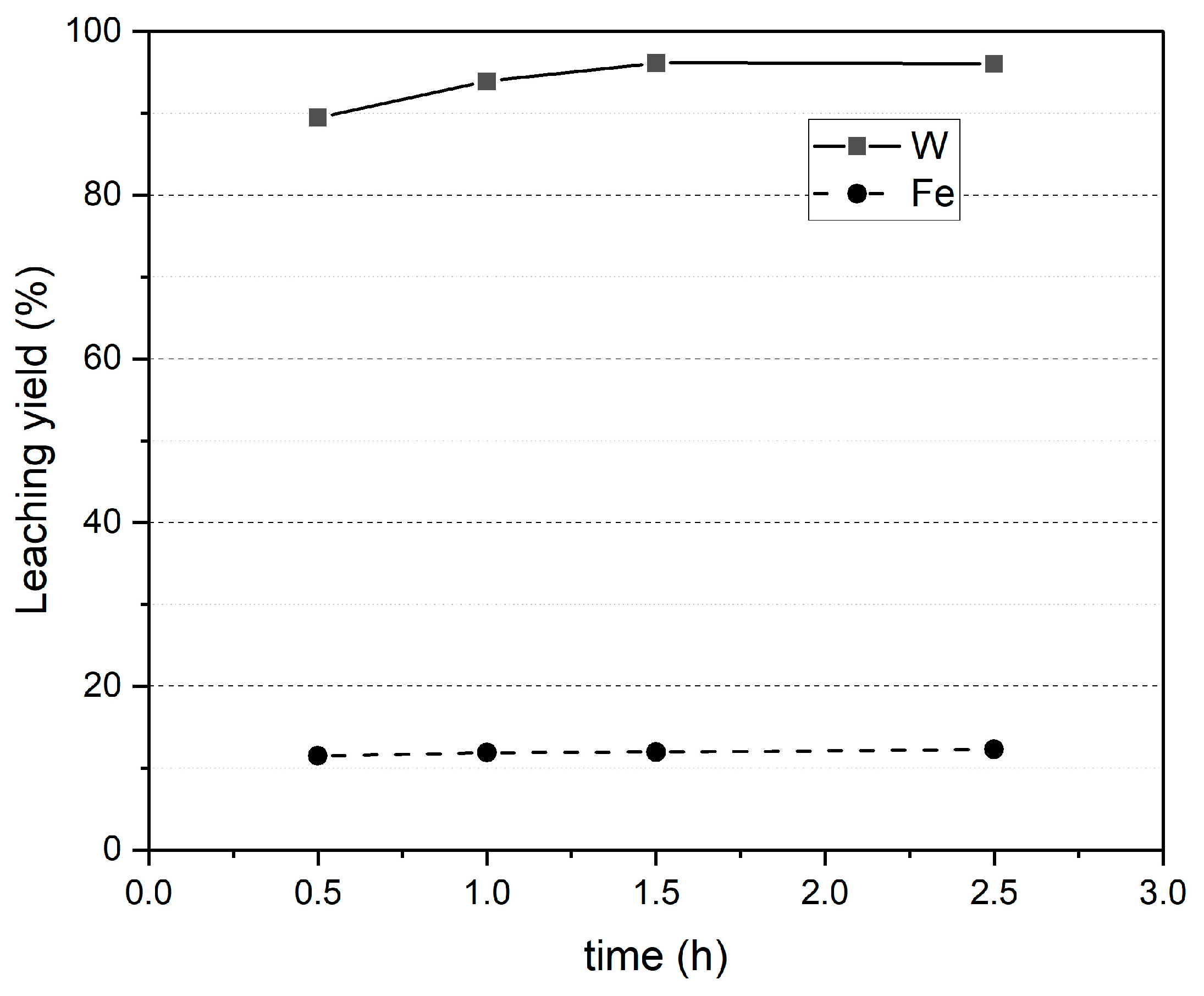
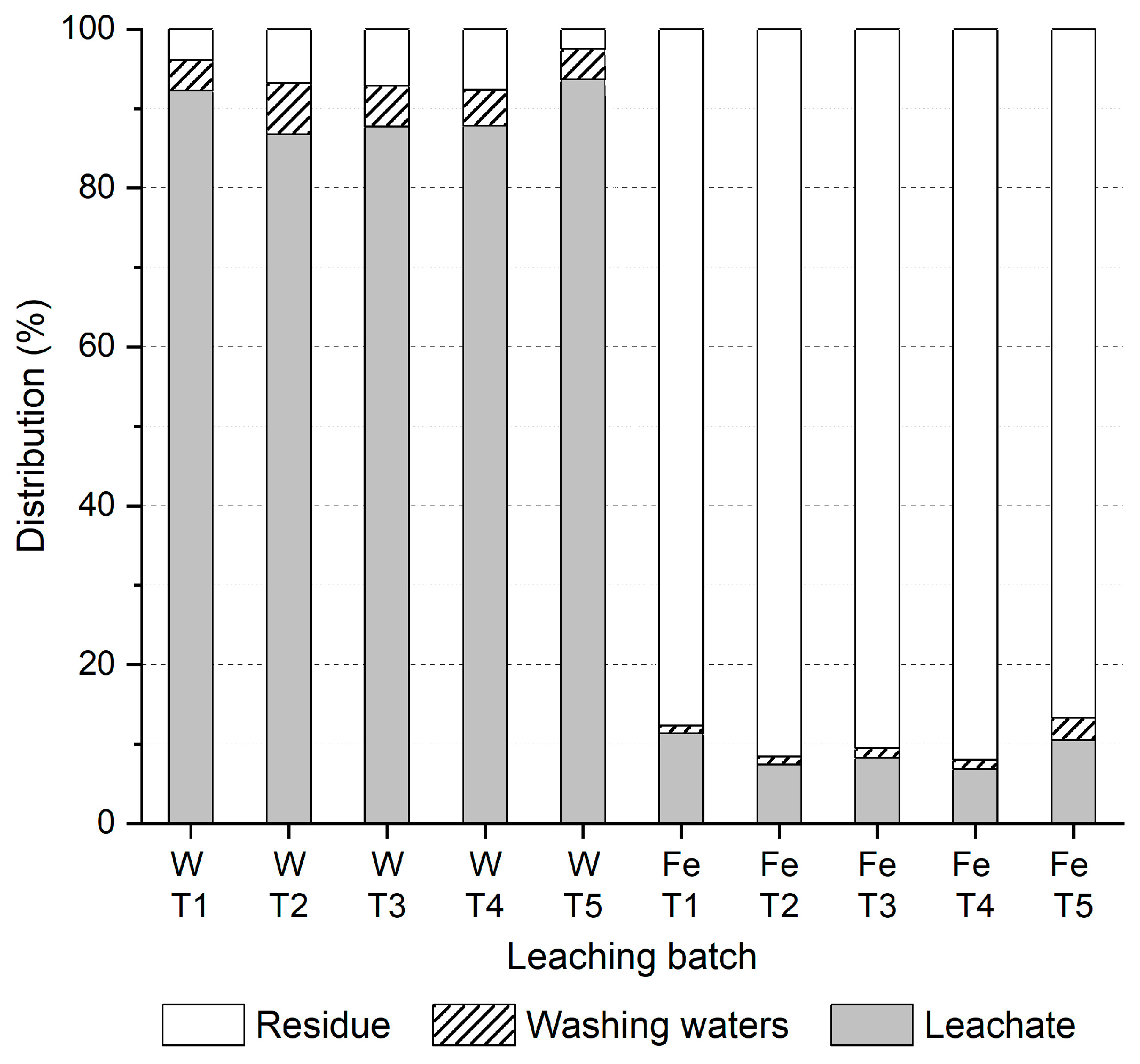
3.2.3. Leaching Results
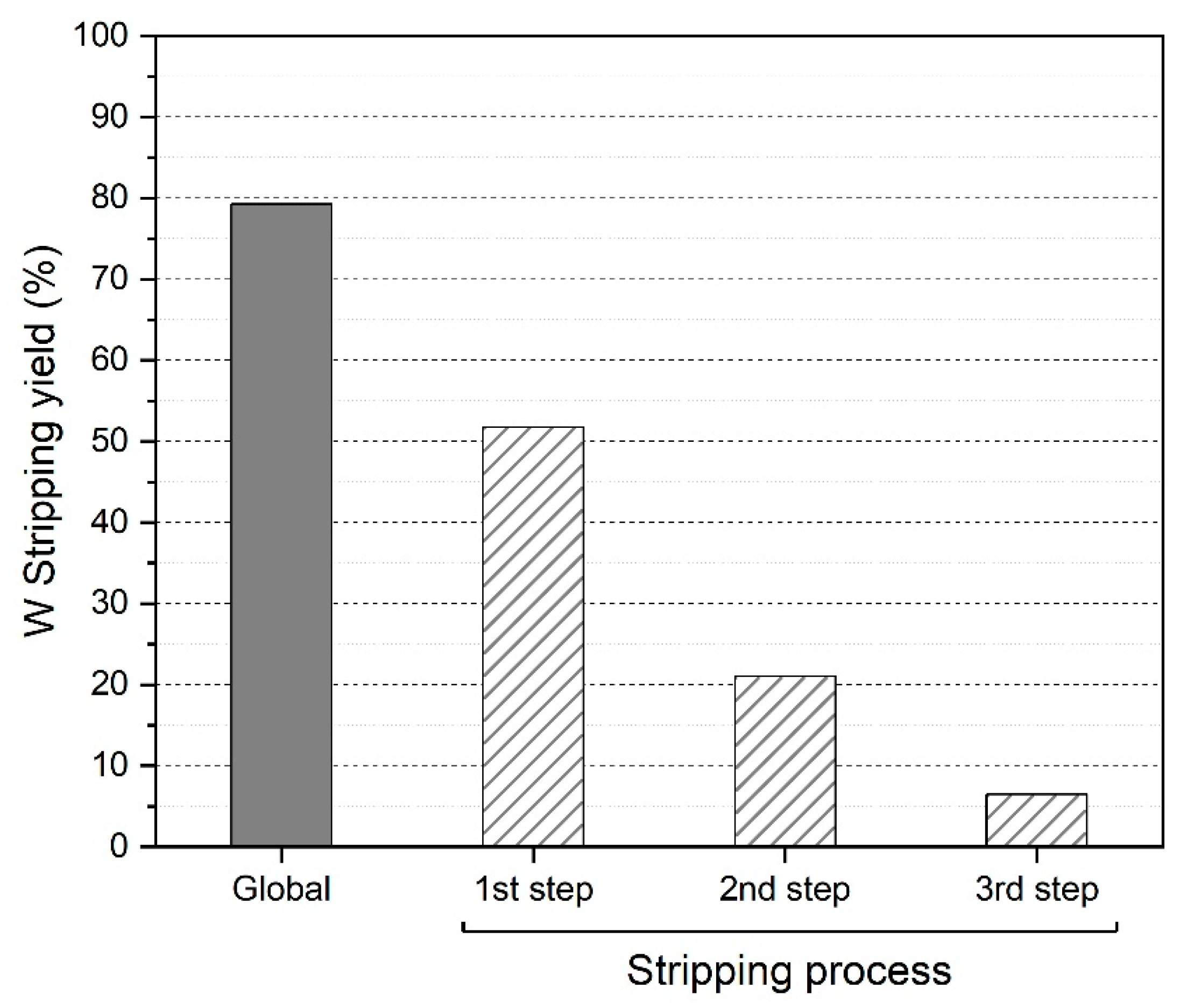
3.2.4. Solvent Extraction Results
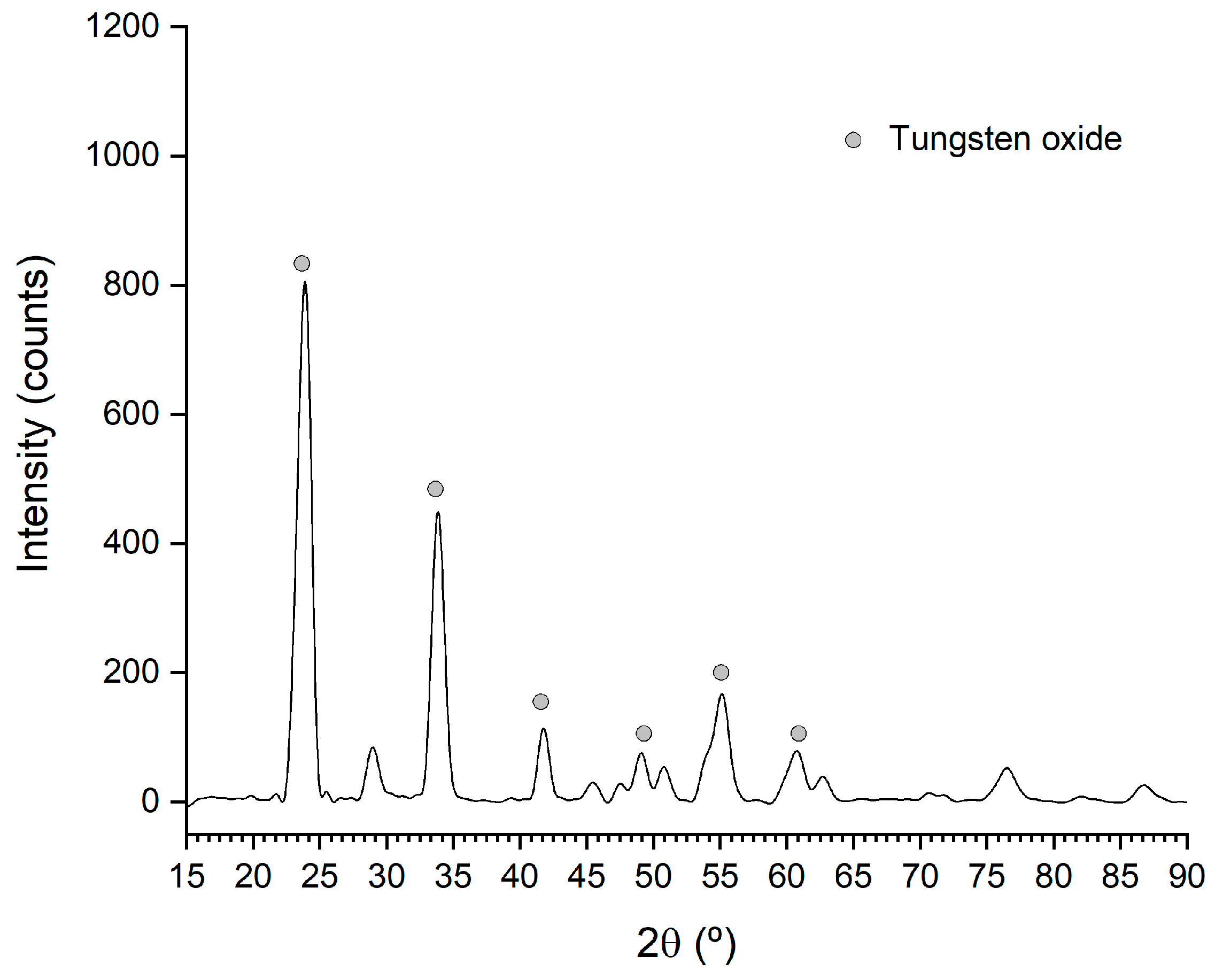
3.2.5. Crystallization and Calcination Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Is Tungsten a Good Conductor of Electricity? Available online: https://domadia.net/blog/is-tungsten-a-good-conductor-of-electricity/ (accessed on 22 November 2024).
- Tungsten (W)—The Different Properties and Applications. Available online: https://www.azom.com/article.aspx?ArticleID=9119 (accessed on 22 November 2024).
- China Dominates Tungsten Mine Production|China Is the Top Supplier of Tungsten While US Produces Zero—MINING.COM. Available online: https://www.mining.com/web/tungsten-miner-says-clients-in-shock-as-china-chokes-supply/china-dominates-tungsten-mine-production-china-is-the-top-supplier-of-tungsten-while-us-produces-zero/ (accessed on 11 March 2025).
- Michaud, D. Extraction of Tungsten from Scheelite Ore—911Metallurgist. Available online: https://www.911metallurgist.com/blog/extraction-tungsten-scheelite-ore/#Tungsten-TablingCircuit (accessed on 22 November 2024).
- Ahn, H.H.; Lee, M.-S. Hydrometallurgical Processes for the Recovery of Tungsten from Ores and Secondary Resources. Resour. Recycl. 2018, 27, 3–10. [Google Scholar] [CrossRef]
- Orefice, M.; Nguyen, V.T.; Raiguel, S.; Jones, P.T.; Binnemans, K. Solvometallurgical Process for the Recovery of Tungsten from Scheelite. Ind. Eng. Chem. Res. 2022, 61, 754–764. [Google Scholar] [CrossRef]
- Li, J.; Ma, Z.; Liu, X.; Chen, X.; Zhao, Z. Sustainable and Efficient Recovery of Tungsten from Wolframite in a Sulfuric Acid and Phosphoric Acid Mixed System. ACS Sustain. Chem. Eng. 2020, 8, 13583–13592. [Google Scholar] [CrossRef]
- Yang, J.H.; He, L.H.; Liu, X.H.; Ding, W.T.; Song, Y.F.; Zhao, Z.W. Comparative Kinetic Analysis of Conventional and Ultrasound-Assisted Leaching of Scheelite by Sodium Carbonate. Trans. Nonferrous Met. Soc. China Engl. Ed. 2018, 28, 775–782. [Google Scholar] [CrossRef]
- Bhosale, S.N.; Mookherjee, S.; Pardeshi, R.M. Current Practices in Tungsten Extraction and Recovery. High. Temp. Mater. Process. 1990, 9, 147–162. [Google Scholar] [CrossRef]
- Yang, W.; Wang, W.; Wu, X.; Yang, K.; Li, Q.; He, J. Co-Extraction of Tungsten and Molybdenum from Refractory Scheelite—Powellite Blend Concentrates by Roasting with Na2 CO3 and SiO2 and Leaching with Water. Can. Metall. Q. 2018, 57, 447–454. [Google Scholar] [CrossRef]
- Sreenivas, T.; Srinivas, K.; Natarajan, R.; Padmanabhan, N. An Integrated Process for the Recovery of Tungsten and Tin from a Combined Wolframite-Scheelite-Cassiterite Concentrate. Miner. Process. Extr. Metall. Rev. 2010, 25, 193–203. [Google Scholar] [CrossRef]
- Spooren, J.; Wouters, W.; Michielsen, B.; Seftel, E.M.; Koelewijn, S.-F. Microwave-Assisted Alkaline Fusion Followed by Water-Leaching for the Selective Extraction of the Refractory Metals Tungsten, Niobium and Tantalum from Low-Grade Ores and Tailings. Miner. Eng. 2024, 217, 108963. [Google Scholar] [CrossRef]
- Kalpakli, A.O.; Ilhan, S.; Kahruman, C.; Yusufoglu, I. Dissolution Behavior of Calcium Tungstate in Oxalic Acid Solutions. Hydrometallurgy 2012, 121–124, 7–15. [Google Scholar] [CrossRef]
- Yurramendi, L.; Nieto, J.; Siriwardana, A. Recovery of Tungsten from Downstream Mineral Processing Fractions by Deep Eutectic Solvents. Mater. Proc. 2021, 5, 96. [Google Scholar] [CrossRef]
- Nieto, J.; Yurramendi, L.; Aldana, J.L.; del Río, C.; Siriwardana, A. A Process Based on Ionic Liquids for Tungsten Valorization from Low Grade Scheelite Concentrates. In Proceedings of the ISEC 2022 Gothenburg Sweden 2023; Zenodo: Geneve, Switzerland, 2023. [Google Scholar]
- UNE-EN 13656:2020; Soil, Treated Biowaste, Sludge and Waste—Digestion with a Hydrochloric (HCl), Nitric (HNO3) and Tetrafluoroboric (HBF4) or Hydrofluoric (HF) Acid Mixture for Subsequent Determination of Elements. AENOR: Madrid, Spain, 2020.
- Deferm, C.; Onghena, B.; Nguyen, V.T.; Banerjee, D.; Fransaer, J.; Binnemans, K. Non-Aqueous Solvent Extraction of Indium from an Ethylene Glycol Feed Solution by the Ionic Liquid Cyphos IL 101: Speciation Study and Continuous Counter-Current Process in Mixer-Settlers. RSC Adv. 2020, 10, 24595–24612. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Devi, N. Separation and Recovery of Gallium(III) Ions from Aqueous Phase by Liquid–Liquid Extraction Using a Novel Extractant, Cyphos IL 101. Turk. J. Chem. 2017, 41, 892–903. [Google Scholar] [CrossRef]
- Zhu, Z.; Tulpatowicz, K.; Pranolo, Y.; Cheng, C.Y. Solvent Extraction of Molybdenum and Vanadium from Sulphate Solutions with Cyphos IL 101. Hydrometallurgy 2015, 154, 72–77. [Google Scholar] [CrossRef]
- Kumari, A.; Sinha, M.K.; Sahu, S.K.; Pandey, B.D. Solvent Extraction and Separation of Trivalent Lanthanides Using Cyphos IL 104, a Novel Phosphonium Ionic Liquid as Extractant. Solvent Extr. Ion. Exch. 2016, 34, 469–484. [Google Scholar] [CrossRef]
- Lloyd, P.J.D. Principles of Industrial Solvent Extraction. In Solvent Extraction Principles and Practice; Rydberg, J., Cox, M., Musikas, C., Choppin, G.R., Eds.; Marcel Dekker: New York, NY, USA, 2004; pp. 347–353. [Google Scholar]
- Fernández-Seara, J.; Sieres, J.; Rodríguez, C.; Vázquez, M. Ammonia-Water Absorption in Vertical Tubular Absorbers. Int. J. Therm. Sci. 2005, 44, 277–288. [Google Scholar] [CrossRef]
- Hadlocon, L.J.; Zhao, L. Production of Ammonium Sulfate Fertilizer Using Acid Spray Wet Scrubbers. In Proceedings of the 18th World Congress of CIGR, Beijing, China, 16–19 September 2014; pp. 41–51. [Google Scholar]




| W | Si | Al | Fe | K | Ca | Na | |
|---|---|---|---|---|---|---|---|
| Conc. (%) | 2.6 | 31.5 | 4.6 | 3.5 | 4.0 | 1.1 | 1.8 |
| Stage | Conditions |
|---|---|
| Leaching | T 60 °C time 1 h L/S ratio 5 |
| IL Extraction Stripping Regeneration | IL Extraction Organic phase:
Stripping NH3 1M (first mixer settler) NH3 0.5M (2nd and 3rd mixer settlers) O/A 1:1 Regeneration HCl 1M O/A 1:1 |
| Crystallization | T 105 °C |
| Calcination | T 650 °C time 1 h |
| W | Fe | |||
|---|---|---|---|---|
| Yield (%) | Conc. (g·L−1) | Yield (%) | Conc. (g·L−1) | |
| Extraction | 97.6 | 9.5 | 5.0 | 0.03 |
| Stripping | 98.3 | 3.2 | 14.2 | 0.01 |
| Regeneration | −0.2 | 0.02 | - | - |
| Overall | 95.7 | - | 0.3 | - |
| WO3 | SiO2 | P2O5 | TiO2 | Al2O3 | CaO | Fe2O3 | Rest |
|---|---|---|---|---|---|---|---|
| 99.30 | 0.34 | 0.16 | 0.07 | 0.04 | 0.02 | 0.01 | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nieto, J.; Yurramendi, L.; Antoñanzas, J.; Aldana, J.L. High-Purity Tungsten Oxide Production from Low-Grade Scheelite Concentrates at Pilot Plant Scale. Metals 2025, 15, 1051. https://doi.org/10.3390/met15091051
Nieto J, Yurramendi L, Antoñanzas J, Aldana JL. High-Purity Tungsten Oxide Production from Low-Grade Scheelite Concentrates at Pilot Plant Scale. Metals. 2025; 15(9):1051. https://doi.org/10.3390/met15091051
Chicago/Turabian StyleNieto, Javier, Lourdes Yurramendi, Javier Antoñanzas, and Jose Luis Aldana. 2025. "High-Purity Tungsten Oxide Production from Low-Grade Scheelite Concentrates at Pilot Plant Scale" Metals 15, no. 9: 1051. https://doi.org/10.3390/met15091051
APA StyleNieto, J., Yurramendi, L., Antoñanzas, J., & Aldana, J. L. (2025). High-Purity Tungsten Oxide Production from Low-Grade Scheelite Concentrates at Pilot Plant Scale. Metals, 15(9), 1051. https://doi.org/10.3390/met15091051






