Quantifying Latent Heat in AlSi5Cu Alloys (with 1, 2, and 4% of Cu by Mass) via DSC, Thermal Analysis, and Commercial Software
Abstract
1. Introduction
2. Materials and Methods
Newtonian Method
3. Results
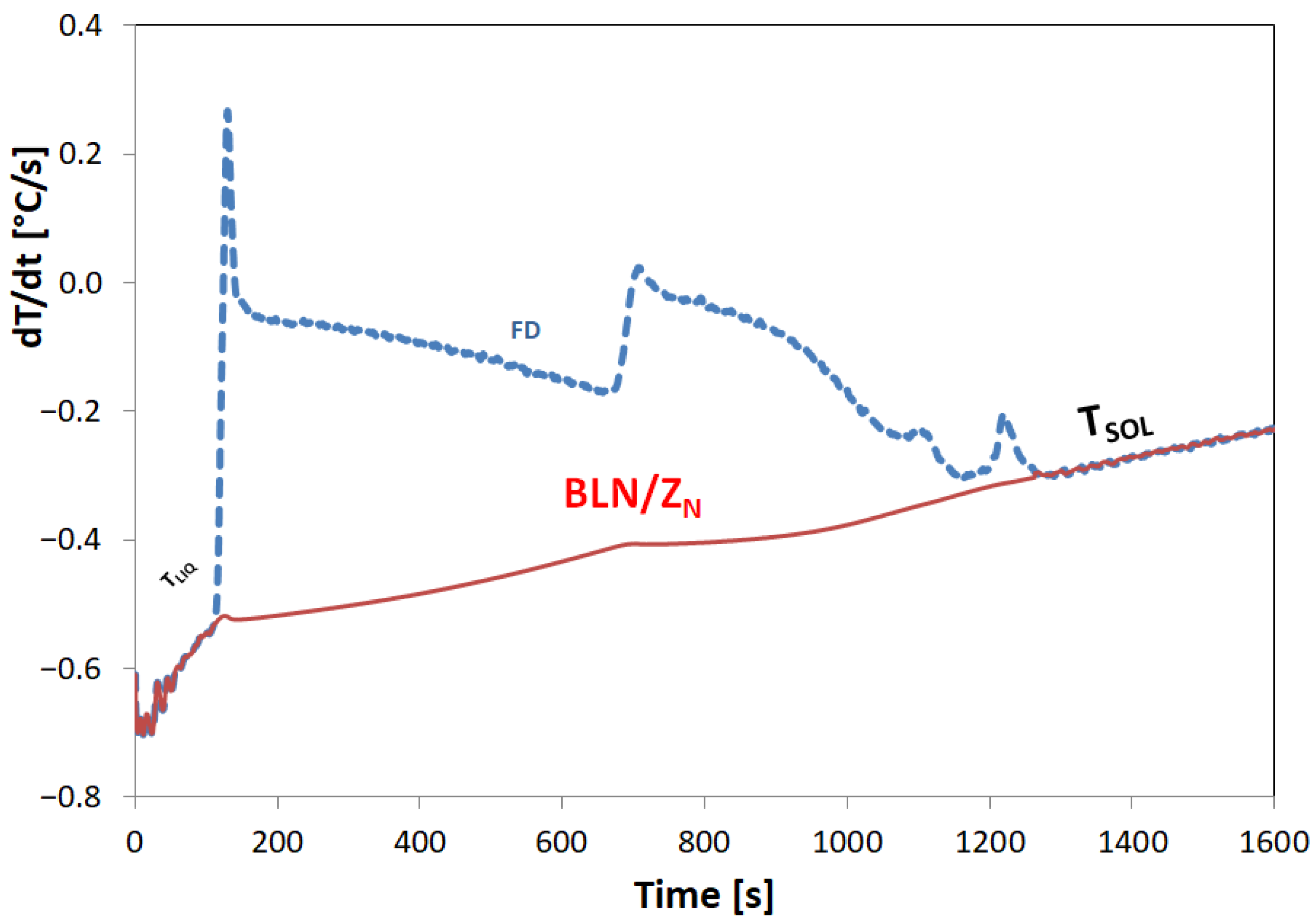
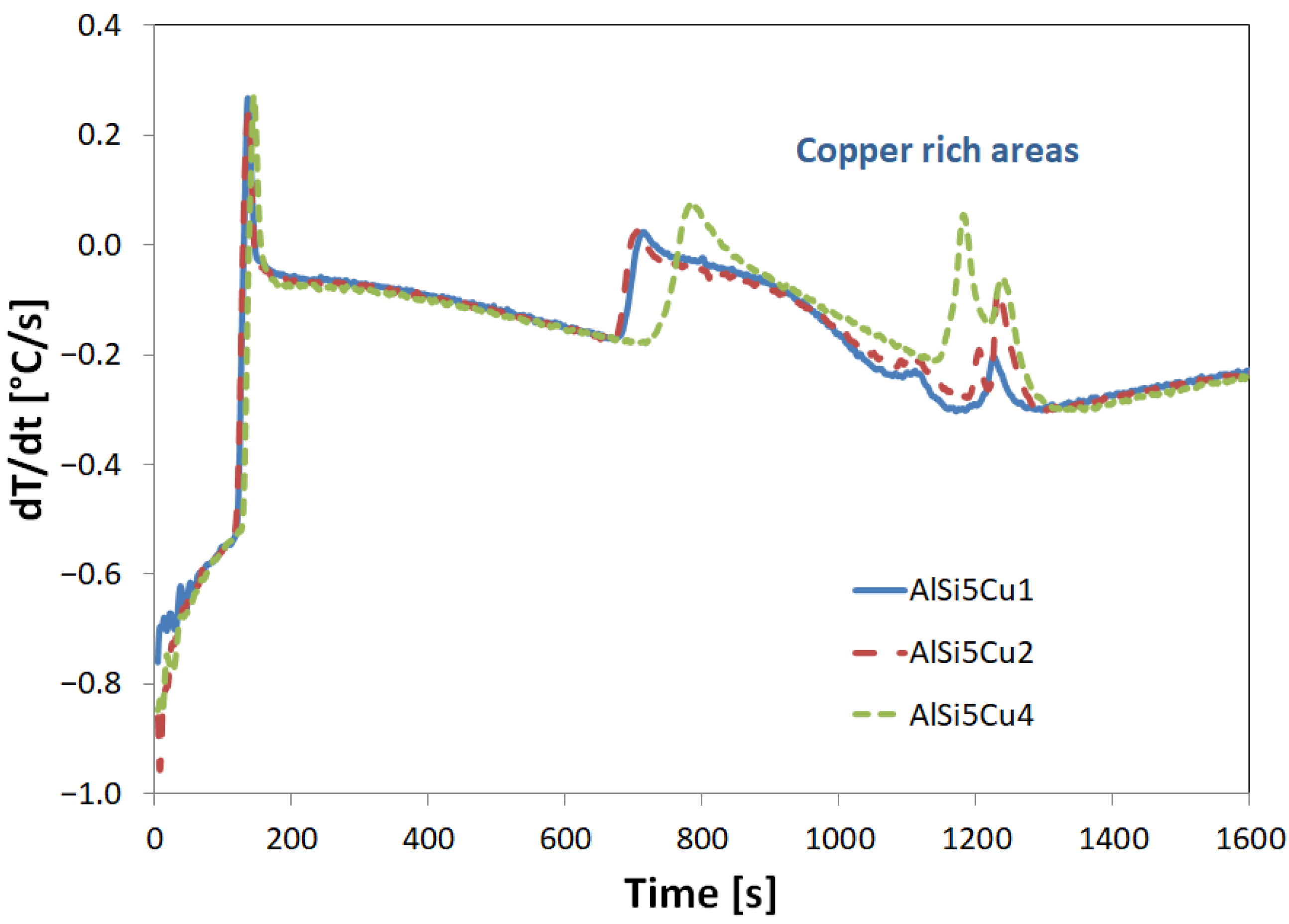
3.1. Melting Procedure
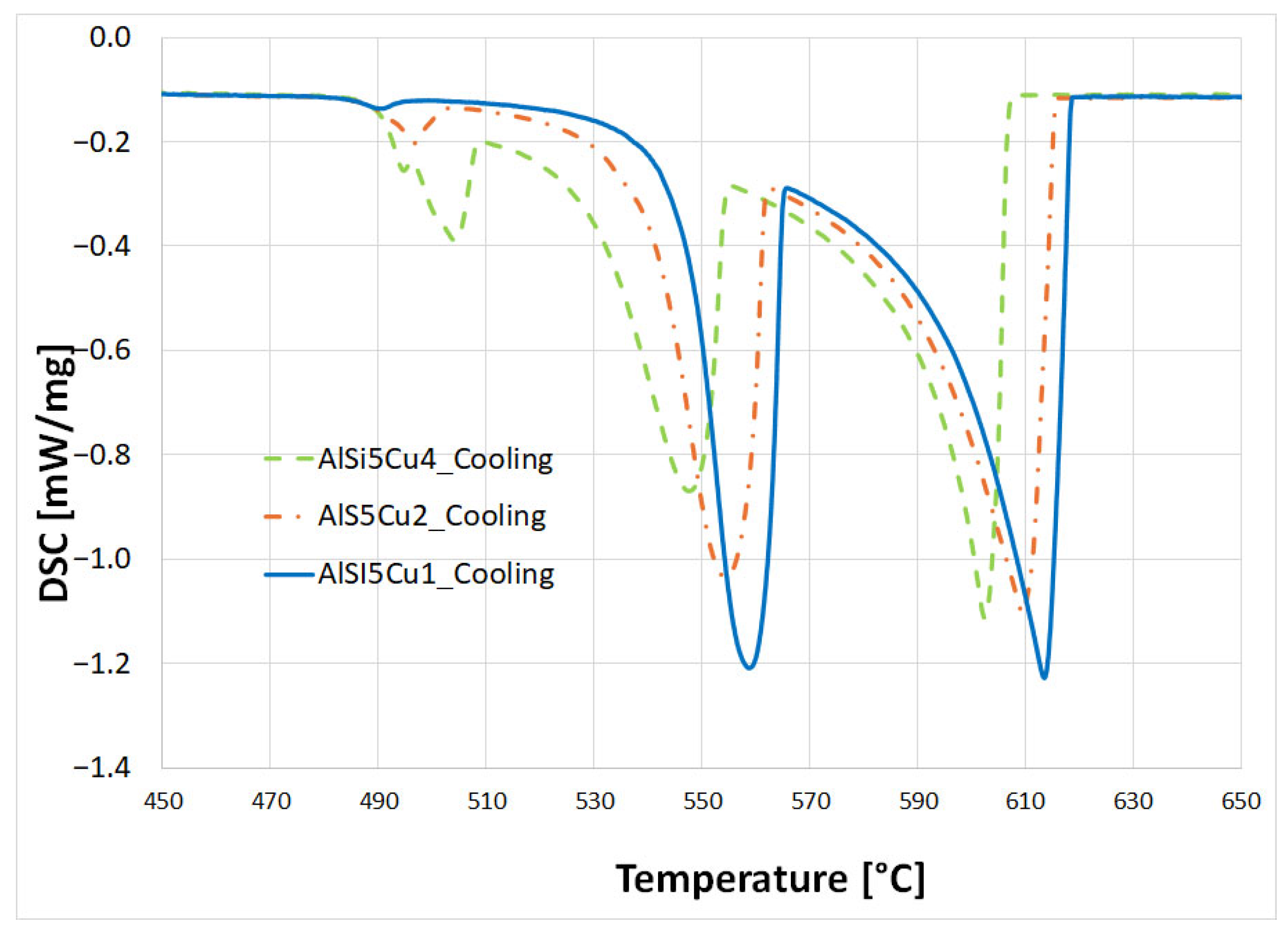
3.2. DSC Procedure
- Primary α-aluminum dendrite formation: The initial reaction marks the precipitation of primary α-aluminum dendrites, occurring between approximately 617 °C (for AlSi5Cu4) and 629 °C (for AlSi5Cu1). This temperature is primarily influenced by the copper content of the alloy. As these dendrites form, the concentration of both silicon and copper increases in the remaining liquid. Initially, these dendritic crystals are separate and freely moving within the melt. However, as cooling progresses, the tips of the growing dendrites begin to impinge upon one another, eventually forming a coherent dendritic network.
- Al-Si eutectic precipitation: Further cooling leads to the onset of primary Al-Si eutectic structure precipitation, observed between 574 °C (AlSi5Cu1) and 562 °C (AlSi5Cu4). This reaction involves the formation of a eutectic mixture of silicon and α-aluminum, which in turn causes a further localized increase in the copper content of the remaining liquid.
- Copper-rich eutectic formation: The final significant reaction involves the formation of copper-rich eutectic structures, which precipitate in the temperature range of 513 °C (AlSi5Cu1) to 507 °C (AlSi5Cu4).
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DSC | Differential Scanning Calorimetry |
| TA | Thermal Analysis |
| BLN | Newtonian baseline |
References
- Djurdjevic, M.B.; Odanović, Z.; Talijan, N. Characterization of the solidification path of AlSi5Cu(1–4 wt.%) alloys using cooling curve analysis. JOM 2011, 63, 51–57. [Google Scholar] [CrossRef]
- Davies, J.R. Aluminium and Aluminium Alloys; ASM International: Detroit, MI, USA, 1993. [Google Scholar]
- Zamani, M. AlSi Cast Alloys-Microstructure and Mechanical Properties at Ambient and Elevated Temperature. Ph.D. Thesis, Jönköping University, Jönköping, Sweden, 2015; pp. 1–87. [Google Scholar]
- ASM International. ASM Handbook Volume 2, Properties and Selection: Nonferrous Alloys and Special-Purpose Materials; ASM Internacional: Detroit, MI, USA, 1990; pp. 152–177. [Google Scholar]
- Sparkman, D.A. Microstructure by Thermal Analysis. AFS Trans. 2011, 119, 1–8. [Google Scholar]
- Chen, Z.; Liu, K.; Elgallad, E.; Breton, F.; Chen, X.G. Differential Scanning Calorimetry Fingerprints of Various Heat-Treatment Tempers of Different Aluminum Alloys. Metals 2020, 10, 763. [Google Scholar] [CrossRef]
- Saunders, N.; Li, X.; Miodownik, A.P.; Schille, J.P. Modelling of the thermo-physical and physical properties for solidification of Al-alloys. In Light Metals 2003, Proceedings of the 132th TMS Annual Conference, San Diego, CA, USA, 2–6 March 2003; The Minerals, Metals & Materials Society: Pittsburgh, PA, USA, 2003; pp. 999–1004. [Google Scholar]
- Van Ekeren, P.J.; Holl, C.M.; Witteveen, A.J. A comparative test of differential scanning Calorimeters. J. Therm. Anal. 1997, 49, 1105–1114. [Google Scholar] [CrossRef]
- JMatPro®. 2024. Available online: https://www.sentesoftware.co.uk/jmatpro (accessed on 25 July 2025).
- Thermo-Calc Software. 2024. Available online: http://www.thermocalc.com (accessed on 24 July 2025).
- Saunders, N. Applicability of the Equilibrium and ‘Scheil Model’ to Solidification in Multi-Component Alloys. In Proceedings of the 4th Decennial International Conference on Solidification Processing, Sheffield, UK, 7–10 July 1997; pp. 362–366. [Google Scholar]
- Emadi, D.; Whiting, L. Determination of solidification characteristics of Al-Si alloys by thermal analysis. AFS Trans. 2002, 110, 285–296. [Google Scholar]
- Djurdjevic, M.B.; Manasijevic, S.; Pataric, A.; Mihailovic, M. Challenges by latent heat calculation—Competition among analytical and computational methods. J. Int. Commun. Heat Mass Transf. 2024, 157, 107704. [Google Scholar] [CrossRef]
- Djurdjevic, M.B. Application of thermal analysis in ferrous and nonferrous foundries. J. Metall. Mater. Eng. 2021, 27, 457–471. [Google Scholar] [CrossRef]
- Djurdjevic, M.B.; Manasijevic, S.; Odanovic, Z.; Radisa, R. Influence of different contents of silicon and copper on the solidification pathways of cast hypoeutectic AlSi (5–9 wt.%) Cu (1–4 wt.%) alloys. Int. J. Mater. Res. 2013, 104, 865–873. [Google Scholar] [CrossRef]
- Bäckerud, L.; Chai, G.; Tamminen, J. Solidification Characteristics of Aluminum Alloys; AFS/SKANALUMINIUM: Oslo, Norway, 1986; Volume 2. [Google Scholar]
- Arnberg, L.; Backerud, L. Solidification Characteristics of Aluminum Alloys; AFS: Des Plaines, IL, USA, 1996; Volume 3. [Google Scholar]
- Fras, E.; Kapturkiewicz, W.; Burbielko, A.; Lopez, H.F. A New Concept in Thermal Analysis of Castings. AFS Trans. 1993, 101, 505–511. [Google Scholar]
- Mondolfo, L.F. Aluminum Alloys, Structure and Properties; Butterworths: London, UK, 1979; pp. 213–614. [Google Scholar]
- Crossley, P.B.; Mondolfo, L.F. The Modification of Aluminum Silicon Alloys. Mod. Cast. AFS Trans. 1966, 74, 53–64. [Google Scholar]
- Kierkus, W.T.; Sokolowski, J.H. Recent Advances in CCA: A New Method of Determining ‘Baseline’ Equation. AFS Trans. 1999, 66, 161–167. [Google Scholar]
- Joubert, J.M.; Kaplan, B.; Selleby, M. The specific heat of Al-based compounds, evaluation of the Neumann-Kopp rule and proposal for a modified Neumann-Kopp rule. Calphad 2023, 81, 1–27. [Google Scholar] [CrossRef]
- Doty, H.W.; Samuel, A.M.; Samuel, F.H. Factors controlling the type and morphology of Cu-Containing phases in the 319-aluminum alloy. In Proceedings of the 100th AFS Casting Congress, Philadelphia, PA, USA, 20–23 April 1996; pp. 1–30. [Google Scholar]
- Stanić, D.; Zovko Brodarac, Z.; Li, L. Influence of Copper Addition in AlSi7MgCu Alloy on Microstructure Development and Tensile Strength Improvement. Metals 2020, 10, 1623. [Google Scholar] [CrossRef]
| Alloy | w [%] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Si | Cu | Fe | Mg | Mn | Zn | Ti | Sr | Ni | Al | |
| AlSi5Cu1 | 4.85 | 1.03 | 0.09 | 0.14 | 0.01 | 0.01 | 0.058 | 0.000 | 0.000 | 93.812 |
| AlSi5Cu2 | 5.01 | 2.06 | 0.10 | 0.15 | 0.01 | 0.01 | 0.062 | 0.000 | 0.000 | 92.598 |
| AlSi5Cu4 | 4.89 | 3.85 | 0.09 | 0.16 | 0.01 | 0.01 | 0.057 | 0.000 | 0.000 | 90.933 |
| Temperature Segment | Temperature [°C] | Heating/Cooling Rate [6 °C/min] |
|---|---|---|
| Isothermal | 25 | 0 |
| Dynamic | 800 | 6 |
| Isothermal | 800 | 0 |
| Dynamic | 25 | −6 |
| Alloy | Latent Heat, kJ/kg | |||
|---|---|---|---|---|
| DSC Method | Thermo-Calc | JMatPro | TA-Newtonian | |
| AlSi5Cu1 | 424.10 | 455.80 | 432.06 | 422.59 |
| AlSi5Cu2 | 429.40 | 447.39 | 430.45 | 423.28 |
| AlSI5Cu4 | 437.60 | 433.78 | 428.97 | 434.15 |
| Relative Error [%] | |||
|---|---|---|---|
| Alloy | Thermo-Calc | JMatPro | TA-Newtonian |
| AlSi5Cu1 | −7.474 | −1.877 | 0.356 |
| AlSi5Cu2 | −4.188 | −0.245 | 1.424 |
| ALSi5Cu4 | 0.873 | 1.972 | 0.787 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djurdjevic, M.; Jovanovic, V.; Stopic, S. Quantifying Latent Heat in AlSi5Cu Alloys (with 1, 2, and 4% of Cu by Mass) via DSC, Thermal Analysis, and Commercial Software. Metals 2025, 15, 1045. https://doi.org/10.3390/met15091045
Djurdjevic M, Jovanovic V, Stopic S. Quantifying Latent Heat in AlSi5Cu Alloys (with 1, 2, and 4% of Cu by Mass) via DSC, Thermal Analysis, and Commercial Software. Metals. 2025; 15(9):1045. https://doi.org/10.3390/met15091045
Chicago/Turabian StyleDjurdjevic, Mile, Vladimir Jovanovic, and Srecko Stopic. 2025. "Quantifying Latent Heat in AlSi5Cu Alloys (with 1, 2, and 4% of Cu by Mass) via DSC, Thermal Analysis, and Commercial Software" Metals 15, no. 9: 1045. https://doi.org/10.3390/met15091045
APA StyleDjurdjevic, M., Jovanovic, V., & Stopic, S. (2025). Quantifying Latent Heat in AlSi5Cu Alloys (with 1, 2, and 4% of Cu by Mass) via DSC, Thermal Analysis, and Commercial Software. Metals, 15(9), 1045. https://doi.org/10.3390/met15091045







