Nanoparticle Architecture Governing Antibacterial and Osteoinductive Responses in Bone-Integrating Implants
Abstract
1. Introduction
2. Methodology
3. Fundamental Aspects of Bioactivity in Nanoparticles: A Comprehensive Approach
4. Interaction with Biological Systems: Factors and Mechanisms Influencing Bioactivity
4.1. Antibacterial Mechanisms: The Interplay of Nanoparticle Properties and Bacterial Target
4.2. Other Bioactivity Mechanisms: Induction of Cell Growth and Interaction with Tissues
4.3. The Protein Corona: Protein Adsorption on Titanium Surfaces
5. Bioactive Characteristics According to the Type of Nanomaterial and Application Type
| Metallic Nanoparticle | Assays | Value (μg/mL) | Exposure Time (h) | Medium | Environment/Setting | Observed Effects | Ref. |
|---|---|---|---|---|---|---|---|
| Ag NPs (Silver Nanoparticles) | OECD | 0, 250, 500, 1000, 5000 | 3 | 10% FBS trisodium citrate and sodium lauryl sulfate | In vitro (human keratinocyte HaCat cells) | Significant decline in viable cell number at high concentrations | [135] |
| Fluorescein-diacetate (FDA)/ethidium bromide (Et-Br) test | 0, 250, 500, 1000, 5000 | 24 | RPMI + 10% FBS | In vitro (murine dendritic cells) | Alteration in gene expression; 1000+ genes affected | [136] | |
| MTT assay | 12,100 | 24 and 48 | DMEM + 10% FBS | In vitro (human lung epithelial cell line A549) | Intracellular production of ROS but did not induce either apoptosis or necrosis | [137] | |
| MTT and resazurin reduction assay | 1, 6, and 12 | 24 | DMEM + 10% FBS | In vitro (human lung epithelial cell line A549) | Decreased cell viability, changes in cell morphology and confluence | [138] | |
| Lactate dehydrogenase release assay (LDH) | 0, 10, 20, 50, and 100 | 12 and 24 | RPMI 1640 + 10% FBS | In vitro (human lung epithelial cell line A549) | Time and dose-dependent toxicity, induction of cell necrosis | [139] | |
| MTT, lipid peroxidation assay, ROS detection | 0, 0.31, 0.62, 1.25, 2.50, 5.00 | 72 | Hams F12 basal media + 10 mM HEPES + 5% FBS | In vitro (breast carcinoma cell line SUM159 cells) | Induction of cell death by lipid peroxidation, proteotoxic stress, and necrotic cell death | [140] | |
| Au NPs (Gold Nanoparticles) | Cytotoxicity (cell impedance), genotoxicity (micronucleus assay) | 0.5, 1, 2 and 5 nM | 72 | DMEM + 10%FBS | In vitro (Caco-2 cells) | Dose-dependent genotoxicity observed for all Au NPs tested | [134] |
| Au NPs (Gold Nanoparticles) | Flowmetry with An-nexin V and propidium iodide | 10, 50, and 100 | 24 | DMEM + 10% FBS | In vitro (MG-63 cells) | High cell viability (>90%), with less than 3% early apoptosis, 6% late apoptosis, and 1% necrosis | [141] |
6. Challenges and Future Trends
7. Translational Readiness: Challenges and Pathway
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NPs | Nanoparticles |
| MNPs | Metallic Nanoparticles |
| ROS | Reactive Oxygen Species |
| LPS | Lipopolysaccharides |
| DNA | Deoxyribonucleic Acid |
| PDGF | Platelet-Derived Growth Factor |
| FGF | Fibroblast Growth Factor |
| HAp | Hydroxyapatite |
| CNTs | Carbon Nanotubes |
| C60 | Fullerenes or Buckminsterfullerenes |
| GO | Graphene Oxide |
| CNT | Carbon Nanotube |
| CNFs | Carbon Nanofibers |
| iNPs | Inorganic Nanoparticles |
| ZnONPs | Zinc Oxide Nanoparticles |
References
- Allain, J.; Echeverry-Rendón, M.; Pavón, J.; Arias, S. Nanostructured Biointerfaces. In Nanopatterning and Nanoscale Devices for Biological Applications; CRC Press: Boca, Raton, FL, USA, 2017; pp. 41–72. [Google Scholar]
- Ordikhani, F.; Mohandes, F.; Simchi, A. Nanostructured coatings for biomaterials. In Nanobiomaterials Science, Development and Evaluation; Elsevier: Amsterdam, The Netherlands, 2017; p. 1. [Google Scholar]
- Hall, D.; Urban, R.; Pourzal, R.; Turner, T.; Skipor, A.; Jacobs, J. Nanoscale Surface Modification by Anodic Oxidation Increased Bone Ingrowth and Reduced Fibrous Tissue in the Porous Coating of Titanium–Alloy Femoral Hip Arthroplasty Implants. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Gallo, J.; Holinka, M.; Moucha, C. Antibacterial Surface Treatment for Orthopaedic Implants. Int. J. Mol. Sci. 2014, 15, 13849–13880. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elaziem, W.; Darwish, M.A.; Hamada, A.; Daoush, W.M. Titanium-Based Alloys and Composites for Orthopedic Implants Applications: A Comprehensive Review. Mater. Des. 2024, 241, 112850. [Google Scholar] [CrossRef]
- Shi, Z.; Neoh, K.G.; Kang, E.T.; Poh, C.; Wang, W. Bacterial Adhesion and Osteoblast Function on Titanium with Surface-grafted Chitosan and Immobilized RGD Peptide. J. Biomed. Mater. Res. A 2008, 86A, 865–872. [Google Scholar] [CrossRef]
- Ordikhani, F.; Tamjid, E.; Simchi, A. Characterization and Antibacterial Performance of Electrodeposited Chitosan–Vancomycin Composite Coatings for Prevention of Implant-Associated Infections. Mater. Sci. Eng. C 2014, 41, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Barberi, J.; Spriano, S. Titanium and Protein Adsorption: An Overview of Mechanisms and Effects of Surface Features. Materials 2021, 14, 1590. [Google Scholar] [CrossRef]
- Liang, W.; Zhou, C.; Bai, J.; Zhang, H.; Long, H.; Jiang, B.; Wang, J.; Huang, X.; Zhang, H.; Zhao, J. Prospective Applications of Bioactive Materials in Orthopedic Therapies: A Review. Heliyon 2024, 10, e36152. [Google Scholar] [CrossRef] [PubMed]
- Iwuji, C.; Saha, H.; Ghann, W.; Dotson, D.; Bhuiya, M.A.K.; Parvez, M.S.; Jahangir, Z.S.; Rahman, M.M.; Chowdhury, F.I.; Uddin, J. Synthesis and Characterization of Silver Nanoparticles and Their Promising Antimicrobial Effects. Chem. Phys. Impact 2024, 9, 100758. [Google Scholar] [CrossRef]
- Akhter, M.; Rahman, M.; Ripon, R.; Mubarak, M.; Akter, M.; Mahbub, S.; Al Mamun, F.; Sikder, M. A Systematic Review on Green Synthesis of Silver Nanoparticles Using Plants Extract and Their Bio-Medical Applications. Heliyon 2024, 10, e29766. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bodón, J.; Andrade del Olmo, J.; Alonso, J.; Moreno-Benítez, I.; Vilas-Vilela, J.; Pérez-Álvarez, L. Bioactive Coatings on Titanium: A Review on Hydroxylation, Self-Assembled Monolayers (SAMs) and Surface Modification Strategies. Polymers 2021, 14, 165. [Google Scholar] [CrossRef]
- Che, Z.; Sun, Q.; Zhao, Z.; Wu, Y.; Xing, H.; Song, K.; Chen, A.; Wang, B.; Cai, M. Growth Factor-Functionalized Titanium Implants for Enhanced Bone Regeneration: A Review. Int. J. Biol. Macromol. 2024, 274, 133153. [Google Scholar] [CrossRef]
- Aparicio, C.; Padrós, A.; Gil, F. In Vivo Evaluation of Micro-Rough and Bioactive Titanium Dental Implants Using Histometry and Pull-out Tests. J. Mech. Behav. Biomed. Mater. 2011, 4, 1672–1682. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Cazzola, M.; Peretti, V.; Stella, B.; Spriano, S. Zeta Potential Measurements on Solid Surfaces for in Vitro Biomaterials Testing: Surface Charge, Reactivity Upon Contact With Fluids and Protein Absorption. Front. Bioeng. Biotechnol. 2018, 6, 60. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Li, X.; Tu, Y.; Wang, Q.; Ågren, H. On the Mechanism of Protein Adsorption onto Hydroxylated and Nonhydroxylated TiO 2 Surfaces. J. Phys. Chem. C 2010, 114, 14496–14502. [Google Scholar] [CrossRef]
- Mao, C.M.; Sampath, J.; Sprenger, K.G.; Drobny, G.; Pfaendtner, J. Molecular Driving Forces in Peptide Adsorption to Metal Oxide Surfaces. Langmuir 2019, 35, 5911–5920. [Google Scholar] [CrossRef]
- Zhao, A.; Wang, Z.; Zhou, S.; Xue, G.; Wang, Y.; Ye, C.; Huang, N. Titanium Oxide Films with Vacuum Thermal Treatment for Enhanced Hemocompatibility. Surf. Eng. 2015, 31, 898–903. [Google Scholar] [CrossRef]
- Zhang, H.-P.; Lu, X.; Fang, L.-M.; Weng, J.; Huang, N.; Leng, Y. Molecular Dynamics Simulation of RGD Peptide Adsorption on Titanium Oxide Surfaces. J. Mater. Sci. Mater. Med. 2008, 19, 3437–3441. [Google Scholar] [CrossRef]
- Gold, K.; Slay, B.; Knackstedt, M.; Gaharwar, A.K. Antimicrobial Activity of Metal and Metal-Oxide Based Nanoparticles. Adv. Ther. 2018, 1, 11700033. [Google Scholar] [CrossRef]
- Yang, F.; Deng, D.; Pan, X.; Fu, Q.; Bao, X. Understanding Nano Effects in Catalysis. Natl. Sci. Rev. 2015, 2, 183–201. [Google Scholar] [CrossRef]
- Yusuf, A.; Almotairy, A.; Henidi, H.; Alshehri, O.; Aldughaim, M. Nanoparticles as Drug Delivery Systems: A Review of the Implication of Nanoparticles’ Physicochemical Properties on Responses in Biological Systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Mitra, I.; Goodman, S.; Kumar, M.; Bose, S. Improving Biocompatibility for next Generation of Metallic Implants. Prog. Mater. Sci. 2023, 133, 101053. [Google Scholar] [CrossRef]
- Paul, W.; Sharma, C.P. Inorganic Nanoparticles for Targeted Drug Delivery. In Biointegration of Medical Implant Materials; Elsevier: Amsterdam, The Netherlands, 2020; p. 333. [Google Scholar] [CrossRef]
- Juan, L.; Zhimin, Z.; Anchun, M.; Lei, L.; Jingchao, Z. International Journal of Nanomedicine Dovepress Deposition of Silver Nanoparticles on Titanium Surface for Antibacterial Effect. 2010. Available online: https://www.tandfonline.com/doi/full/10.2147/IJN.S8810 (accessed on 29 July 2025).
- Zolnik, B.S.; González-Fernández, A.; Sadrieh, N.; Dobrovolskaia, M.A. Minireview: Nanoparticles and the Immune System. Endocrinology 2010, 151, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef] [PubMed]
- Martínez, G.; Merinero, M.; Pérez-Aranda, M.; Pérez-Soriano, E.; Ortiz, T.; Villamor, E.; Begines, B.; Alcudia, A. Environmental Impact of Nanoparticles’ Application as an Emerging Technology: A Review. Materials 2020, 14, 166. [Google Scholar] [CrossRef]
- Iwamoto, T. Clinical Application of Drug Delivery Systems in Cancer Chemotherapy: Review of the Efficacy and Side Effects of Approved Drugs. Biol. Pharm. Bull. 2013, 36, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Silindir Gunay, M.; Yekta Ozer, A.; Chalon, S. Drug Delivery Systems for Imaging and Therapy of Parkinson’s Disease. Curr. Neuropharmacol. 2016, 14, 376–391. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The Effect of Nanoparticle Size on In Vivo Pharmacokinetics and Cellular Interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, J.; Ramge, P.; Petrov, V.; Hamm, S.; Gelperina, S.E.; Engelhardt, B.; Alyautdin, R.; von Briesen, H.; Begley, D.J. Direct Evidence That Polysorbate-80-Coated Poly(Butylcyanoacrylate) Nanoparticles Deliver Drugs to the CNS via Specific Mechanisms Requiring Prior Binding of Drug to the Nanoparticles. Pharm. Res. 2003, 20, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Vargason, A.M.; Anselmo, A.C.; Mitragotri, S. The Evolution of Commercial Drug Delivery Technologies. Nat. Biomed. Eng. 2021, 5, 951–967. [Google Scholar] [CrossRef] [PubMed]
- Petros, R.A.; DeSimone, J.M. Strategies in the Design of Nanoparticles for Therapeutic Applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kumar, A.; Bhardwaj, S.; Sikarwar, M.; Sriwastaw, S.; Sharma, G.; Gupta, M. Nanotoxicity Unveiled: Evaluating Exposure Risks and Assessing the Impact of Nanoparticles on Human Health. J. Trace Elem. Miner. 2025, 13, 100252. [Google Scholar] [CrossRef]
- Woźniak, A.; Malankowska, A.; Nowaczyk, G.; Grześkowiak, B.F.; Tuśnio, K.; Słomski, R.; Zaleska-Medynska, A.; Jurga, S. Size and Shape-Dependent Cytotoxicity Profile of Gold Nanoparticles for Biomedical Applications. J. Mater. Sci. Mater. Med. 2017, 28, 92. [Google Scholar] [CrossRef] [PubMed]
- Duta, L.; Popescu, A.C.; Zgura, I.; Preda, N.; Mihailescu, I.N. Wettability of Nanostructured Surfaces. In Wetting and Wettability; IntechOpen: Rijeka, Croatia, 2015. [Google Scholar] [CrossRef]
- Souza, J.C.M.; Sordi, M.B.; Kanazawa, M.; Ravindran, S.; Henriques, B.; Silva, F.S.; Aparicio, C.; Cooper, L.F. Nano-Scale Modification of Titanium Implant Surfaces to Enhance Osseointegration. Acta Biomater. 2019, 94, 112–131. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Yılmaz, G.; Göktürk, I.; Ovezova, M.; Yılmaz, F.; Kılıç, S.; Denizli, A. Antimicrobial Nanomaterials: A Review. Hygiene 2023, 3, 269–290. [Google Scholar] [CrossRef]
- Romaniuk, J.; Cegelski, L. Bacterial Cell Wall Composition and the Influence of Antibiotics by Cell-Wall and Whole-Cell NMR. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20150024. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal Nanoparticles: Understanding the Mechanisms behind Antibacterial Activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Eckhard, U.; Delgado, L.M.; de Roo Puente, Y.J.D.; Hoyos-Nogués, M.; Gil, F.J.; Perez, R.A. Antibacterial Approaches in Tissue Engineering Using Metal Ions and Nanoparticles: From Mechanisms to Applications. Bioact. Mater. 2021, 6, 4470–4490. [Google Scholar] [CrossRef]
- Epand, R.; Walker, C.; Epand, R.; Magarvey, N. Molecular Mechanisms of Membrane Targeting Antibiotics. Biochim. Biophys. Acta (BBA)—Biomembr. 2016, 1858, 980–987. [Google Scholar] [CrossRef]
- Wada, A.; Kono, M.; Kawauchi, S.; Takagi, Y.; Morikawa, T.; Funakoshi, K. Rapid Discrimination of Gram-Positive and Gram-Negative Bacteria in Liquid Samples by Using NaOH-Sodium Dodecyl Sulfate Solution and Flow Cytometry. PLoS ONE 2012, 7, e47093. [Google Scholar] [CrossRef]
- Hummels, K.; Berry, S.; Li, Z.; Taguchi, A.; Min, J.; Walker, S.; Marks, D.; Bernhardt, T.G. Coordination of Bacterial Cell Wall and Outer Membrane Biosynthesis. Nature 2023, 615, 300–304. [Google Scholar] [CrossRef]
- Vázquez Olmos, A.; Vega Jiménez, A.; Paz Díaz, B. Mecanosíntesis y Efecto Antimicrobiano de Óxidos Metálicos Nanoestructurados. Mundo Nano. Rev. Interdiscip. En Nanociencia Y Nanotecnología 2018, 11, 29–44. [Google Scholar] [CrossRef]
- Beveridge, T. Structures of Gram-Negative Cell Walls and Their Derived Membrane Vesicles. J. Bacteriol. 1999, 181, 4725–4733. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.; Felisberto-Rodrigues, C.; Meir, A.; Prevost, M.; Redzej, A.; Trokter, M.; Waksman, G. Secretion Systems in Gram-Negative Bacteria: Structural and Mechanistic Insights. Nat. Rev. Microbiol. 2015, 13, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Pandey, P.; Gupta, M.; Narayan, R. Nano–Bio Interaction and Antibacterial Mechanism of Engineered Metal Nanoparticles: Fundamentals and Current Understanding. J. Clust. Sci. 2025, 36, 5. [Google Scholar] [CrossRef]
- Jacobson, K.; Gunsolus, I.; Kuech, T.; Troiano, J.; Melby, E.; Lohse, S.; Hu, D.; Chrisler, W.; Murphy, C.; Orr, G.; et al. Lipopolysaccharide Density and Structure Govern the Extent and Distance of Nanoparticle Interaction with Actual and Model Bacterial Outer Membranes. Environ. Sci. Technol. 2015, 49, 10642–10650. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef] [PubMed]
- Kohler, T.; Xia, G.; Kulauzovic, E.; Peschel, A. Teichoic Acids, Lipoteichoic Acids and Related Cell Wall Glycopolymers of Gram-Positive Bacteria. In Microbial Glycobiology; Elsevier: Amsterdam, The Netherlands, 2010; p. 75. [Google Scholar] [CrossRef]
- Weidenmaier, C.; Peschel, A. Teichoic Acids and Related Cell-Wall Glycopolymers in Gram-Positive Physiology and Host Interactions. Nat. Rev. Microbiol. 2008, 6, 276–287. [Google Scholar] [CrossRef]
- Ramalingam, B.; Parandhaman, T.; Das, S.K. Antibacterial Effects of Biosynthesized Silver Nanoparticles on Surface Ultrastructure and Nanomechanical Properties of Gram-Negative Bacteria Viz. Escherichia Coli and Pseudomonas Aeruginosa. ACS Appl. Mater. Interfaces 2016, 8, 4963–4976. [Google Scholar] [CrossRef]
- Masters, E.; Ricciardi, B.; Bentley, K.; Moriarty, T.; Schwarz, E.; Muthukrishnan, G. Skeletal Infections: Microbial Pathogenesis, Immunity and Clinical Management. Nat. Rev. Microbiol. 2022, 20, 385–400. [Google Scholar] [CrossRef]
- Anuj, S.; Gajera, H.; Hirpara, D.; Golakiya, B. Bacterial Membrane Destabilization with Cationic Particles of Nano-Silver to Combat Efflux-Mediated Antibiotic Resistance in Gram-Negative Bacteria. Life Sci. 2019, 230, 178–187. [Google Scholar] [CrossRef]
- Frei, A.; Zuegg, J.; Elliott, A.; Baker, M.; Braese, S.; Brown, C.; Chen, F.; Dowson, C.G.; Dujardin, G.; Jung, N.; et al. Metal Complexes as a Promising Source for New Antibiotics. Chem. Sci. 2020, 11, 2627–2639. [Google Scholar] [CrossRef] [PubMed]
- Ivask, A.; ElBadawy, A.; Kaweeteerawat, C.; Boren, D.; Fischer, H.; Ji, Z.; Chang, C.H.; Liu, R.; Tolaymat, T.; Telesca, D.; et al. Toxicity Mechanisms in Escherichia Coli Vary for Silver Nanoparticles and Differ from Ionic Silver. ACS Nano 2014, 8, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, L.; Zapata, P.; Vejar, N.; Azócar, M.; Gulppi, M.; Zhou, X.; Thompson, G.; Rabagliati, F.; Páez, M. Release of Silver and Copper Nanoparticles from Polyethylene Nanocomposites and Their Penetration into Listeria Monocytogenes. Mater. Sci. Eng. C 2014, 40, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.; Koo, H.; Kim, K.; Shin, S.; Kim, S.; Park, Y. Antibacterial Activity and Mechanism of Action of the Silver Ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef] [PubMed]
- Mughal, T.; Ali, S.; Hassan, A.; Kazmi, S.; Saleem, M.; Shakir, H.; Nazer, S.; Farooq, M.; Awan, M.; Khan, M.; et al. Phytochemical Screening, Antimicrobial Activity, in Vitro and in Vivo Antioxidant Activity of Berberis Lycium Royle Root Bark Extract. Braz. J. Biol. 2024, 84, e249742. [Google Scholar] [CrossRef] [PubMed]
- Yadid, M.; Feiner, R.; Dvir, T. Gold Nanoparticle-Integrated Scaffolds for Tissue Engineering and Regenerative Medicine. Nano Lett. 2019, 19, 2198–2206. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S.; Sheng, J.; Hosseinian, F.; Willmore, W. Nanoparticle Effects on Stress Response Pathways and Nanoparticle–Protein Interactions. Int. J. Mol. Sci. 2022, 23, 7962. [Google Scholar] [CrossRef]
- Engin, A.B.; Nikitovic, D.; Neagu, M.; Henrich-Noack, P.; Docea, A.O.; Shtilman, M.I.; Golokhvast, K.; Tsatsakis, A.M. Mechanistic Understanding of Nanoparticles’ Interactions with Extracellular Matrix: The Cell and Immune System. Part. Fibre Toxicol. 2017, 14, 22. [Google Scholar] [CrossRef]
- Selim, M.M.; El-Safty, S.; Tounsi, A.; Shenashen, M. A Review of Magnetic Nanoparticles Used in Nanomedicine. APL Mater. 2024, 12, 010601. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Z.; Pan, Z.; Liu, Y. Advanced Bioactive Nanomaterials for Biomedical Applications. Exploration 2021, 1, 20210089. [Google Scholar] [CrossRef] [PubMed]
- Bodelón, G.; Costas, C.; Pérez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzán, L.M. Gold Nanoparticles for Regulation of Cell Function and Behavior. Nano Today 2017, 13, 40–60. [Google Scholar] [CrossRef]
- Palanisamy, C.; Poompradub, S.; Sansanaphongpricha, K.; Jayaraman, S.; Subramani, K.; Sonsudin, F. Biosynthesis of Silver Nanoparticles (AgNPs) Using Ethanolic Extract of Nigella sativa (L.) Seeds Promotes Wound Healing via PDGF and VEGF Signalling Pathways Activation. Biocatal. Agric. Biotechnol. 2023, 54, 102970. [Google Scholar] [CrossRef]
- Zhang, S.; Uludağ, H. Nanoparticulate Systems for Growth Factor Delivery. Pharm. Res. 2009, 26, 1561–1580. [Google Scholar] [CrossRef] [PubMed]
- Angelakeris, M. Magnetic Nanoparticles: A Multifunctional Vehicle for Modern Theranostics. Biochim. Et. Biophys. Acta (BBA)—Gen. Subj. 2017, 1861, 1642–1651. [Google Scholar] [CrossRef]
- Shi, Z.; Huang, X.; Cai, Y.; Tang, R.; Yang, D. Size Effect of Hydroxyapatite Nanoparticles on Proliferation and Apoptosis of Osteoblast-like Cells. Acta Biomater. 2009, 5, 338–345. [Google Scholar] [CrossRef]
- Afzal, O.; Altamimi, A.S.A.; Nadeem, M.S.; Alzarea, S.I.; Almalki, W.H.; Tariq, A.; Mubeen, B.; Murtaza, B.N.; Iftikhar, S.; Riaz, N.; et al. Nanoparticles in Drug Delivery: From History to Therapeutic Applications. Nanomaterials 2022, 12, 4494. [Google Scholar] [CrossRef]
- Parvin, N.; Joo, S.W.; Mandal, T.K. Nanomaterial-Based Strategies to Combat Antibiotic Resistance: Mechanisms and Applications. Antibiotics 2025, 14, 207. [Google Scholar] [CrossRef]
- Romero-Gavilán, F.; Gomes, N.C.; Ródenas, J.; Sánchez, A.; Azkargorta, M.; Iloro, I.; Elortza, F.; García Arnáez, I.; Gurruchaga, M.; Goñi, I.; et al. Proteome Analysis of Human Serum Proteins Adsorbed onto Different Titanium Surfaces Used in Dental Implants. Biofouling 2017, 33, 98–111. [Google Scholar] [CrossRef]
- Svendsen, I.E.; Lindh, L. The Composition of Enamel Salivary Films Is Different from the Ones Formed on Dental Materials. Biofouling 2009, 25, 255–261. [Google Scholar] [CrossRef]
- Zuanazzi, D.; Xiao, Y.; Siqueira, W.L. Evaluating Protein Binding Specificity of Titanium Surfaces through Mass Spectrometry–Based Proteomics. Clin. Oral. Investig. 2021, 25, 2281–2296. [Google Scholar] [CrossRef]
- Anbazhagan, E.; Rajendran, A.; Natarajan, D.; Kiran, M.S.; Pattanayak, D.K. Divalent Ion Encapsulated Nano Titania on Ti Metal as a Bioactive Surface with Enhanced Protein Adsorption. Colloids Surf. B Biointerfaces 2016, 143, 213–223. [Google Scholar] [CrossRef]
- Shi, X.; Nakagawa, M.; Kawachi, G.; Xu, L.; Ishikawa, K. Surface Modification of Titanium by Hydrothermal Treatment in Mg-Containing Solution and Early Osteoblast Responses. J. Mater. Sci. Mater. Med. 2012, 23, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Joudeh, N.; Linke, D. Nanoparticle Classification, Physicochemical Properties, Characterization, and Applications: A Comprehensive Review for Biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef] [PubMed]
- Grimsdale, A.; Müllen, K. The Chemistry of Organic Nanomaterials. Angew. Chem. Int. Ed. 2005, 44, 5592–5629. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Gonelimali, F.; Máté, M.; Sharma, P. Characteristics, Composition, and Structure of Organic Nanomaterials. In Organic-Based Nanomaterials in Food Packaging; Springer Nature: Cham, Switzerland, 2024; p. 15. [Google Scholar] [CrossRef]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.-H.; Qoronfleh, M.W. Therapeutic Efficacy of Nanoparticles and Routes of Administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef] [PubMed]
- Sanità, G.; Carrese, B.; Lamberti, A. Nanoparticle Surface Functionalization: How to Improve Biocompatibility and Cellular Internalization. Front. Mol. Biosci. 2020, 7, 587012. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.; Singh, R.; Bashambu, L. Carbon-Based Nanomaterials: Synthesis and Prospective Applications. Mater. Today Proc. 2021, 44, 608–614. [Google Scholar] [CrossRef]
- Dhall, S.; Nathawat, R.; Sood, K. Carbon-Based Nanomaterials. In Carbon Nanomaterials and their Nanocomposite-Based Chemiresistive Gas Sensors; Elsevier: Amsterdam, The Netherlands, 2023; pp. 3–39. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Li, Y.; Feng, Y.; Feng, W. Carbon-Based Functional Nanomaterials: Preparation, Properties and Applications. Compos. Sci. Technol. 2019, 179, 10–40. [Google Scholar] [CrossRef]
- Ayanda, O.S.; Mmuoegbulam, A.O.; Okezie, O.; Durumin Iya, N.I.; Mohammed, S.E.; James, P.H.; Muhammad, A.B.; Unimke, A.A.; Alim, S.A.; Yahaya, S.M.; et al. Recent Progress in Carbon-Based Nanomaterials: Critical Review. J. Nanopart. Res. 2024, 26, 106. [Google Scholar] [CrossRef]
- Armano, A.; Agnello, S. Two-Dimensional Carbon: A Review of Synthesis Methods, and Electronic, Optical, and Vibrational Properties of Single-Layer Graphene. C 2019, 5, 67. [Google Scholar] [CrossRef]
- Gupta, N.; Gupta, S.M.; Sharma, S.K. Carbon Nanotubes: Synthesis, Properties and Engineering Applications. Carbon. Lett. 2019, 29, 419–447. [Google Scholar] [CrossRef]
- Zhou, Z. Liposome Formulation of Fullerene-Based Molecular Diagnostic and Therapeutic Agents. Pharmaceutics 2013, 5, 525–541. [Google Scholar] [CrossRef] [PubMed]
- Kazemzadeh, H.; Mozafari, M. Fullerene-Based Delivery Systems. Drug Discov. Today 2019, 24, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, D.; Seke, M.; Srdjenovic, B.; Djordjevic, A. Applications of Anti/Prooxidant Fullerenes in Nanomedicine along with Fullerenes Influence on the Immune System. J. Nanomater. 2015, 2015, 565638. [Google Scholar] [CrossRef]
- Minami, K.; Song, J.; Shrestha, L.K.; Ariga, K. Nanoarchitectonics for Fullerene Biology. Appl. Mater. Today 2021, 23, 100989. [Google Scholar] [CrossRef]
- Liu, J.; Cui, L.; Losic, D. Graphene and Graphene Oxide as New Nanocarriers for Drug Delivery Applications. Acta Biomater. 2013, 9, 9243–9257. [Google Scholar] [CrossRef]
- Singh, D.P.; Herrera, C.E.; Singh, B.; Singh, S.; Singh, R.K.; Kumar, R. Graphene Oxide: An Efficient Material and Recent Approach for Biotechnological and Biomedical Applications. Mater. Sci. Eng. C 2018, 86, 173–197. [Google Scholar] [CrossRef]
- Menaa, F.; Abdelghani, A.; Menaa, B. Graphene Nanomaterials as Biocompatible and Conductive Scaffolds for Stem Cells: Impact for Tissue Engineering and Regenerative Medicine. J. Tissue Eng. Regen. Med. 2015, 9, 1321–1338. [Google Scholar] [CrossRef]
- Sonowal, L.; Gautam, S. Advancements and Challenges in Carbon Nanotube-Based Drug Delivery Systems. Nano-Struct. Nano-Objects 2024, 38, 101117. [Google Scholar] [CrossRef]
- Banihashemi Jozdani, S.; Hashemian, Z.; Ebrahim Damavandi, S.; Elyasigorji, Z.; Vosough, M. Emerging Trends in the Biomedical Application of Carbon-Based Nanomaterials. Nano Biomed. Eng. 2024, 16, 357–369. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.; Gong, C.; Liu, B.; Wei, G. Production, Structural Design, Functional Control, and Broad Applications of Carbon Nanofiber-Based Nanomaterials: A Comprehensive Review. Chem. Eng. J. 2020, 402, 126189. [Google Scholar] [CrossRef]
- Gaur, M.; Misra, C.; Yadav, A.B.; Swaroop, S.; Maolmhuaidh, F.Ó.; Bechelany, M.; Barhoum, A. Biomedical Applications of Carbon Nanomaterials: Fullerenes, Quantum Dots, Nanotubes, Nanofibers, and Graphene. Materials 2021, 14, 5978. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zheng, Z.; Yu, S.; Gao, Y.; Ma, J.; Huang, L.; Yang, L. Nanofiber Scaffolds as Drug Delivery Systems Promoting Wound Healing. Pharmaceutics 2023, 15, 1829. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, R.; Barhoum, A.; Bechelany, M.; Dufresne, A. Nanofibers for Biomedical and Healthcare Applications. Macromol. Biosci. 2019, 19, e1800256. [Google Scholar] [CrossRef]
- Jha, M.; Joshi, S.; Sharma, R.; Kim, A.; Pant, B.; Park, M.; Pant, H.R. Surface Modified Activated Carbons: Sustainable Bio-Based Materials for Environmental Remediation. Nanomaterials 2021, 11, 3140. [Google Scholar] [CrossRef] [PubMed]
- Van Wagenen, R.A.; Steggall, M.; Lentz, D.J.; Andrade, J.D. Activated Carbons for Medical Applications. In Vitro Microparticle Characterization and Solute Adsorption. Biomater. Med. Devices Artif. Organs 1975, 3, 319–364. [Google Scholar] [CrossRef] [PubMed]
- Abe, I.; Kamaya, H.; Ueda, I. Activated Carbon as a Biological Model: Comparison between Activated Carbon Adsorption and Oil–Water Partition Coefficient for Drug Activity Correlation. J. Pharm. Sci. 1988, 77, 166–168. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.; Akhavan, B.; Wise, S.G.; Bilek, M.M.M. A Review of Biomimetic Surface Functionalization for Bone-Integrating Orthopedic Implants: Mechanisms, Current Approaches, and Future Directions. Prog. Mater. Sci. 2019, 106, 100588. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, Z.; Wang, Z.; Yan, X.; Duan, X.; Sun, H. Advanced Surface Modification Techniques for Titanium Implants: A Review of Osteogenic and Antibacterial Strategies. Front. Bioeng. Biotechnol. 2025, 13, 1549439. [Google Scholar] [CrossRef]
- Gao, W.; Chen, Y.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-Based Local Antimicrobial Drug Delivery. Adv. Drug Deliv. Rev. 2018, 127, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Banche-Niclot, F.; Taraballi, F. Advantages of Nanoencapsulation in the Delivery of Therapeutics for Bone Regeneration. Nanomedicine 2025, 20, 773–775. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, Y.; Cao, Q.; Yu, T.; Zhang, J.; Liu, Q.; Yang, X. Growth Factors Enhanced Angiogenesis and Osteogenesis on Polydopamine Coated Titanium Surface for Bone Regeneration. Mater. Des. 2020, 196, 109162. [Google Scholar] [CrossRef]
- Butler, J.; Handy, R.; Upton, M.; Besinis, A. Review of Antimicrobial Nanocoatings in Medicine and Dentistry: Mechanisms of Action, Biocompatibility Performance, Safety, and Benefits Compared to Antibiotics. ACS Nano 2023, 17, 7064–7092. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Su, J.; Zhang, J.; Tang, Y.; Shi, H.; Su, J.; Luo, J.; Huang, J.; Li, S.; Cao, W.; et al. Hydrophobic Cu/Zn-MOFs Nanocage with Synergistic Drug-Metal Ion Design for Dual Anticoagulant and Antibacterial Titanium Implants. Chem. Eng. Sci. 2025, 317, 122098. [Google Scholar] [CrossRef]
- Amengual-Peñafiel, L.; Córdova, L.A.; Constanza Jara-Sepúlveda, M.; Brañes-Aroca, M.; Marchesani-Carrasco, F.; Cartes-Velásquez, R. Osteoimmunology Drives Dental Implant Osseointegration: A New Paradigm for Implant Dentistry. Jpn. Dent. Sci. Rev. 2021, 57, 12–19. [Google Scholar] [CrossRef]
- Cooper, L.; Shirazi, S. Osseointegration—The Biological Reality of Successful Dental Implant Therapy: A Narrative Review. Front. Oral. Maxillofac. Med. 2022, 4, 39. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, S.; Xie, W.; Xue, Y.; Qiao, M.; Yang, X.; Zhang, X.; Wan, Q.; Wang, J.; Chen, J.; et al. Surface Modification of the Ti Surface with Nanoscale Bio-MOF-1 for Improving Biocompatibility and Osteointegration in Vitro and in Vivo. J. Mater. Chem. B 2022, 10, 8535–8548. [Google Scholar] [CrossRef]
- Kim, T.; Hyeon, T. Applications of Inorganic Nanoparticles as Therapeutic Agents. Nanotechnology 2014, 25, 012001. [Google Scholar] [CrossRef]
- Khan, S.; Hossain, M. Classification and Properties of Nanoparticles. In Nanoparticle-Based Polymer Composites; Elsevier: Amsterdam, The Netherlands, 2022; pp. 15–54. [Google Scholar] [CrossRef]
- Moeinzadeh, S.; Jabbari, E. Nanoparticles and Their Applications. In Springer Handbook of Nanotechnology; Springer: Berlin/Heidelberg, Germany, 2017; p. 335. [Google Scholar] [CrossRef]
- Park, M.; Neigh, A.; Vermeulen, J.; de la Fonteyne, L.; Verharen, H.; Briedé, J.; van Loveren, H.; de Jong, W. The Effect of Particle Size on the Cytotoxicity, Inflammation, Developmental Toxicity and Genotoxicity of Silver Nanoparticles. Biomaterials 2011, 32, 9810–9817. [Google Scholar] [CrossRef]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef]
- Anik, M.; Hossain, M.; Hossain, I.; Mahfuz, A.; Rahman, M.; Ahmed, I. Recent Progress of Magnetic Nanoparticles in Biomedical Applications: A Review. Nano Sel. 2021, 2, 1146. [Google Scholar] [CrossRef]
- Abbas, R.; Luo, J.; Qi, X.; Naz, A.; Khan, I.A.; Liu, H.; Yu, S.; Wei, J. Silver Nanoparticles: Synthesis, Structure, Properties and Applications. Nanomaterials 2024, 14, 1425. [Google Scholar] [CrossRef] [PubMed]
- Karnwal, A.; Kumar Sachan, R.; Devgon, I.; Devgon, J.; Pant, G.; Panchpuri, M.; Ahmad, A.; Alshammari, M.; Hossain, K.; Kumar, G. Gold Nanoparticles in Nanobiotechnology: From Synthesis to Biosensing Applications. ACS Omega 2024, 9, 29966–29982. [Google Scholar] [CrossRef] [PubMed]
- França, E.L.T.; Santos, A.R.; Assis, L.K.C.S.; Castro-Lopes, S.; Oliveira, D.M.; Carvalho, A.S.; Padrón Hernández, E. Exploring Electrodeposited Iron and Iron Oxide Nanostructures on Porous Alumina Membrane for Enhanced EMI Shielding. J. Magn. Magn. Mater. 2024, 605, 172310. [Google Scholar] [CrossRef]
- Ghasempour, A.; Dehghan, H.; Ataee, M.; Chen, B.; Zhao, Z.; Sedighi, M.; Guo, X.; Shahbazi, M.-A. Cadmium Sulfide Nanoparticles: Preparation, Characterization, and Biomedical Applications. Molecules 2023, 28, 3857. [Google Scholar] [CrossRef]
- Ren, C.; Chen, S.; Yuan, Z.; Fu, R.; Cui, Y.; Ma, Z.; Li, W.; Li, X. Cobalt Nanoparticles Catalyzed Heterocycles Synthes Acceptorless Dehydrogenative Coupling. Chem.—A Eur. J. 2024, 30, e202402168. [Google Scholar] [CrossRef]
- Congreve, R.; Quezada, C.; Kokkarachedu, V. Aluminum Oxide Nanoparticles: Properties and Applications Overview. In Nanoparticles in Modern Antimicrobial and Antiviral Applications; Springer: Cham, Switzerland, 2024; p. 265. [Google Scholar] [CrossRef]
- Bakhet, S.; Tamulevičienė, A.; Vasiliauskas, A.; Andrulevičius, M.; Meškinis, Š.; Tamulevičius, S.; Kašėtienė, N.; Malakauskas, M.; Lelešius, R.; Zienius, D.; et al. Antiviral and Antibacterial Efficacy of Nanocomposite Amorphous Carbon Films with Copper Nanoparticles. Appl. Surf. Sci. 2024, 670, 160642. [Google Scholar] [CrossRef]
- Kosti, S. Nanomaterials and Nanocomposites Thermal and Mechanical Properties Modelling. In Research Anthology on Synthesis, Characterization, and Applications of Nanomaterials; IGI Global: Hershey, PE, USA, 2021; p. 180. [Google Scholar] [CrossRef]
- Gupta, S.; Tripathi, M. An Overview of Commonly Used Semiconductor Nanoparticles in Photocatalysis. High. Energy Chem. 2012, 46, 1–9. [Google Scholar] [CrossRef]
- Tripathi, A.; Singh, M.; Mathpal, M.; Mishra, S.; Agarwal, A. Study of Structural Transformation in TiO2 Nanoparticles and Its Optical Properties. J. Alloys Compd. 2013, 549, 114–120. [Google Scholar] [CrossRef]
- Shard, A.; Schofield, R.; Minelli, C. Ultraviolet–visible spectrophotometry. In Characterization of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2020; p. 185. [Google Scholar] [CrossRef]
- Magogotya, M.; Vetten, M.; Roux-van der Merwe, M.; Badenhorst, J.; Gulumian, M. In Vitro Toxicity and Internalization of Gold Nanoparticles (AuNPs) in Human Epithelial Colorectal Adenocarcinoma (Caco-2) Cells and the Human Skin Keratinocyte (HaCaT) Cells. Mutat. Res. /Genet. Toxicol. Environ. Mutagen. 2022, 883–884, 503556. [Google Scholar] [CrossRef] [PubMed]
- Pinzaru, I.; Coricovac, D.; Dehelean, C.; Moacă, E.-A.; Mioc, M.; Baderca, F.; Sizemore, I.; Brittle, S.; Marti, D.; Calina, C.D.; et al. Stable PEG-Coated Silver Nanoparticles—A Comprehensive Toxicological Profile. Food Chem. Toxicol. 2018, 111, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Muñoz, R.; Borrego, B.; Juárez-Moreno, K.; García-García, M.; Mota Morales, J.D.; Bogdanchikova, N.; Huerta-Saquero, A. Toxicity of Silver Nanoparticles in Biological Systems: Does the Complexity of Biological Systems Matter? Toxicol. Lett. 2017, 276, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Foldbjerg, R.; Irving, E.; Hayashi, Y.; Sutherland, D.; Thorsen, K.; Autrup, H.; Beer, C. Global Gene Expression Profiling of Human Lung Epithelial Cells After Exposure to Nanosilver. Toxicol. Sci. 2012, 130, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Iskuzhina, L.; Batasheva, S.; Kryuchkova, M.; Rozhin, A.; Zolotykh, M.; Mingaleeva, R.; Akhatova, F.; Stavitskaya, A.; Cherednichenko, K.; Rozhina, E. Advances in the Toxicity Assessment of Silver Nanoparticles Derived from a Sphagnum Fallax Extract for Monolayers and Spheroids. Biomolecules 2024, 14, 611. [Google Scholar] [CrossRef]
- Li, L.; Bi, Z.; Hu, Y.; Sun, L.; Song, Y.; Chen, S.; Mo, F.; Yang, J.; Wei, Y.; Wei, X. Silver Nanoparticles and Silver Ions Cause Inflammatory Response through Induction of Cell Necrosis and the Release of Mitochondria in Vivo and in Vitro. Cell Biol. Toxicol. 2021, 37, 177–191. [Google Scholar] [CrossRef]
- Rohde, M.; Snyder, C.; Sloop, J.; Solst, S.; Donati, G.; Spitz, D.; Furdui, C.; Singh, R. The Mechanism of Cell Death Induced by Silver Nanoparticles Is Distinct from Silver Cations. Part. Fibre Toxicol. 2021, 18, 37. [Google Scholar] [CrossRef]
- Garrigós, M.; de Oliveira, F.; Costa, C.; Rodrigues, L.; Nucci, M.; Alves, A.; Mamani, J.; Rego, G.; Munoz, J.; Gamarra, L. Assessing the Toxicity of One-Step-Synthesized PEG-Coated Gold Nanoparticles: In Vitro and in Vivo Studies. Einstein 2024, 22, eAO0764. [Google Scholar] [CrossRef]
- Issa, B.; Obaidat, I.; Albiss, B.; Haik, Y. Magnetic Nanoparticles: Surface Effects and Properties Related to Biomedicine Applications. Int. J. Mol. Sci. 2013, 14, 21266–21305. [Google Scholar] [CrossRef]
- Rezaei, B.; Yari, P.; Sanders, S.M.; Wang, H.; Chugh, V.K.; Liang, S.; Mostufa, S.; Xu, K.; Wang, J.; Gómez-Pastora, J.; et al. Magnetic Nanoparticles: A Review on Synthesis, Characterization, Functionalization, and Biomedical Applications. Small 2024, 20, e2304848. [Google Scholar] [CrossRef]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic Nanoparticles: Design and Characterization, Toxicity and Biocompatibility, Pharmaceutical and Biomedical Applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.; Parekh, K.; Pandey, B. Influence of Crystallite Size on the Magnetic Properties of Fe3O4 Nanoparticles. J. Alloys Compd. 2016, 678, 478–485. [Google Scholar] [CrossRef]
- Ma, Z.; Mohapatra, J.; Wei, K.; Liu, J.P.; Sun, S. Magnetic Nanoparticles: Synthesis, Anisotropy, and Applications. Chem. Rev. 2023, 123, 3904–3943. [Google Scholar] [CrossRef]
- Yoon, H.; Kang, Y.-G.; Chang, Y.-S.; Kim, J.-H. Effects of Zerovalent Iron Nanoparticles on Photosynthesis and Biochemical Adaptation of Soil-Grown Arabidopsis Thaliana. Nanomaterials 2019, 9, 1543. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Álvarez, F.; Caro, C.; García-García, G.; García-Martín, M.L.; Arias, J.L. Engineering of Stealth (Maghemite/PLGA)/Chitosan (Core/Shell)/Shell Nanocomposites with Potential Applications for Combined MRI and Hyperthermia against Cancer. J. Mater. Chem. B 2021, 9, 4963–4980. [Google Scholar] [CrossRef] [PubMed]
- Ran, Q.; Xiang, Y.; Liu, Y.; Xiang, L.; Li, F.; Deng, X.; Xiao, Y.; Chen, L.; Chen, L.; Li, Z. Eryptosis Indices as a Novel Predictive Parameter for Biocompatibility of Fe3O4 Magnetic Nanoparticles on Erythrocytes. Sci. Rep. 2015, 5, 16209. [Google Scholar] [CrossRef] [PubMed]
- Marcus, M.; Karni, M.; Baranes, K.; Levy, I.; Alon, N.; Margel, S.; Shefi, O. Iron Oxide Nanoparticles for Neuronal Cell Applications: Uptake Study and Magnetic Manipulations. J. Nanobiotechnol. 2016, 14, 37. [Google Scholar] [CrossRef] [PubMed]
- Predoi, S.-A.; Iconaru, S.L.; Predoi, D. In Vitro and In Vivo Biological Assays of Dextran Coated Iron Oxide Aqueous Magnetic Fluids. Pharmaceutics 2023, 15, 177. [Google Scholar] [CrossRef]
- Mejías, R.; Gutiérrez, L.; Salas, G.; Pérez-Yagüe, S.; Zotes, T.M.; Lázaro, F.J.; Morales, M.P.; Barber, D.F. Long Term Biotransformation and Toxicity of Dimercaptosuccinic Acid-Coated Magnetic Nanoparticles Support Their Use in Biomedical Applications. J. Control. Release 2013, 171, 225–233. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Guo, S.; Zhang, D.; Qi, J.; Wang, Y. Recent Progress in the Fabrication Strategies and Toughening Mechanism of Flexible Ceramics and Their Applications. J. Mater. Chem. C Mater. 2024, 12, 17742–17788. [Google Scholar] [CrossRef]
- Raja, G.B. An Industrial Perspective on Nanomaterials in the Semiconductor Industry. In Handbook of Emerging Materials for Semiconductor Industry; Springer Nature: Singapore, 2024; p. 25. [Google Scholar] [CrossRef]
- Radu (Dușman), R.; Drăgănescu, D. Present and Future of ZrO2 Nanostructure as Reservoir for Drug Loading and Release. Coatings 2023, 13, 1273. [Google Scholar] [CrossRef]
- Kareem, P.A.; Salh, K.K.; Ali, F.A. ZnO, TiO2 and Ag Nanoparticles Impact against Some Species of Pathogenic Bacteria and Yeast. Cell Mol. Biol. 2021, 67, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liao, C.; Tjong, S.C. Recent Advances in Zinc Oxide Nanostructures with Antimicrobial Activities. Int. J. Mol. Sci. 2020, 21, 8836. [Google Scholar] [CrossRef]
- Pino, P.; Bosco, F.; Mollea, C.; Onida, B. Antimicrobial Nano-Zinc Oxide Biocomposites for Wound Healing Applications: A Review. Pharmaceutics 2023, 15, 970. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Wang, C.; Zare, E.N.; Borzacchiello, A.; Niu, L.; Tay, F.R. Metal-Based Nanomaterials in Biomedical Applications: Antimicrobial Activity and Cytotoxicity Aspects. Adv. Funct. Mater. 2020, 30, 1910021. [Google Scholar] [CrossRef]
- Liang, S.; Sun, K.; Wang, Y.; Dong, S.; Wang, C.; Liu, L.; Wu, Y. Role of Cyt-C/Caspases-9,3, Bax/Bcl-2 and the FAS Death Receptor Pathway in Apoptosis Induced by Zinc Oxide Nanoparticles in Human Aortic Endothelial Cells and the Protective Effect by Alpha-Lipoic Acid. Chem. Biol. Interact. 2016, 258, 40–51. [Google Scholar] [CrossRef]
- Chen, G.; Shen, Y.; Li, X.; Jiang, Q.; Cheng, S.; Gu, Y.; Liu, L.; Cao, Y. The Endoplasmic Reticulum Stress Inducer Thapsigargin Enhances the Toxicity of ZnO Nanoparticles to Macrophages and Macrophage-Endothelial Co-Culture. Environ. Toxicol. Pharmacol. 2017, 50, 103–110. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, J.; Wang, B.; Xu, G.; Yang, X.; Zou, Z.; Yu, C. Ferritinophagy Is Involved in the Zinc Oxide Nanoparticles-Induced Ferroptosis of Vascular Endothelial Cells. Autophagy 2021, 17, 4266–4285. [Google Scholar] [CrossRef]
- Chinnathambi, A.; Alahmadi, T. Zinc Nanoparticles Green-Synthesized by Alhagi Maurorum Leaf Aqueous Extract: Chemical Characterization and Cytotoxicity, Antioxidant, and Anti-Osteosarcoma Effects. Arab. J. Chem. 2021, 14, 103083. [Google Scholar] [CrossRef]
- Poier, N.; Hochstöger, J.; Hackenberg, S.; Scherzad, A.; Bregenzer, M.; Schopper, D.; Kleinsasser, N. Effects of Zinc Oxide Nanoparticles in HUVEC: Cyto- and Genotoxicity and Functional Impairment After Long-Term and Repetitive Exposure in Vitro. Int. J. Nanomed. 2020, 15, 4441–4452. [Google Scholar] [CrossRef]
- Chuang, K.; Lee, K.; Pan, C.; Lai, C.; Lin, L.; Ho, S.; Ho, K.; Chuang, H. Effects of Zinc Oxide Nanoparticles on Human Coronary Artery Endothelial Cells. Food Chem. Toxicol. 2016, 93, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, L.; Thangavelu, I.; Suriyaprakash, J.; Arulselvan, P.; Salmen, S.H.; Chinnathambi, A.; Boopathi, T.S. Bougainvillea Glabra-Mediated Synthesis of Zr3O and Chitosan-Coated Zirconium Oxide Nanoparticles: Multifunctional Antibacterial and Anticancer Agents with Enhanced Biocompatibility. Int. J. Biol. Macromol. 2025, 300, 139609. [Google Scholar] [CrossRef] [PubMed]
- Wahid, F.; Zhong, C.; Wang, H.; Hu, X.; Chu, L. Recent Advances in Antimicrobial Hydrogels Containing Metal Ions and Metals/Metal Oxide Nanoparticles. Polymers 2017, 9, 636. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-Y.; Mahmoud, Z.H.; Abdullaev, S.; Ali, F.K.; Ali Naeem, Y.; Mzahim Mizher, R.; Morad Karim, M.; Abdulwahid, A.S.; Ahmadi, Z.; Habibzadeh, S.; et al. Nano Titanium Oxide (Nano-TiO2): A Review of Synthesis Methods, Properties, and Applications. Case Stud. Chem. Environ. Eng. 2024, 9, 100626. [Google Scholar] [CrossRef]
- Jafari, S.; Mahyad, B.; Hashemzadeh, H.; Janfaza, S.; Gholikhani, T.; Tayebi, L. Biomedical Applications of TiO Nanostructures: Recent Advances. Int. J. Nanomed. 2020, 15, 3447–3470. [Google Scholar] [CrossRef]
- Calabrese, G.; Franco, D.; Petralia, S.; Monforte, F.; Condorelli, G.G.; Squarzoni, S.; Traina, F.; Conoci, S. Dual-Functional Nano-Functionalized Titanium Scaffolds to Inhibit Bacterial Growth and Enhance Osteointegration. Nanomaterials 2021, 11, 2634. [Google Scholar] [CrossRef]
- Montiel-Dávalos, A.; Silva Sánchez, G.J.; Huerta-García, E.; Rueda-Romero, C.; Soca Chafre, G.; Mitre-Aguilar, I.B.; Alfaro-Moreno, E.; Pedraza-Chaverri, J.; López-Marure, R. Curcumin Inhibits Activation Induced by Urban Particulate Material or Titanium Dioxide Nanoparticles in Primary Human Endothelial Cells. PLoS ONE 2017, 12, 0188169. [Google Scholar] [CrossRef]
- Gholinejad, Z.; Khadem Ansari, M.H.; Rasmi, Y. Titanium Dioxide Nanoparticles Induce Endothelial Cell Apoptosis via Cell Membrane Oxidative Damage and P38, PI3K/Akt, NF-ΚB Signaling Pathways Modulation. J. Trace Elem. Med. Biol. 2019, 54, 27–35. [Google Scholar] [CrossRef]
- Zeng, C.; Feng, Y.; Wang, W.; Zhou, F.; Liao, F.; Liu, Y.; Feng, S. The Size-dependent Apoptotic Effect of Titanium Dioxide Nanoparticles on Endothelial Cells by the Intracellular Pathway. Environ. Toxicol. 2018, 33, 1221–1228. [Google Scholar] [CrossRef]
- Liao, F.; Chen, L.; Liu, Y.; Zhao, D.; Peng, W.; Wang, W.; Feng, S. The Size-dependent Genotoxic Potentials of Titanium Dioxide Nanoparticles to Endothelial Cells. Environ. Toxicol. 2019, 34, 1199–1207. [Google Scholar] [CrossRef]
- [2008/2208 (INI)] de 24.4.2009; Resolución del Parlamento Europeo Sobre los Aspectos Reglamentarios de los Nanomateriales. Comisión de Medio Ambiente, Salud Pública y Seguridad Alimentaria: Brussels, Belgium, 2009.
- ISO 10993-1:2018; Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing Within a Risk Management Process. International Organization for Standardization: Geneva, Switzerland, 2018.
- ISO 10993-1:2009; Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing Within a Risk Management Process. International Organization for Standardization: Geneva, Switzerland, 2009.
- ISO 10993-1:2020; Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing Within a Risk Management Process. British Standards Institution: London, UK, 2020.
- ISO 19227:2018; Implants for Surgery—Cleanliness of Orthopaedic Implants—General Requirements. International Organization for Standardization: Geneva, Switzerland, 2018.
- COM(2012) 572 Final, SWD(2012) 288 Final; Communication from the Commission to the European Parliament, the Council and the European Economic and Social Committee: Second Regulatory Review on Nanomaterials. European Commission: Brussels, Belgium, 2012.
- Park, J.; Olivares-Navarrete, R.; Baier, R.E.; Meyer, A.E.; Tannenbaum, R.; Boyan, B.D.; Schwartz, Z. Effect of Cleaning and Sterilization on Titanium Implant Surface Properties and Cellular Response. Acta Biomater. 2012, 8, 1966–1975. [Google Scholar] [CrossRef]
- ASTM G199-09; Standard Guide for Electrochemical Noise Measurement. ASTM International: West Conshohocken, PA, USA, 2014.
- INT/456 de 25.2.2009, 5 SWD(2012) 288 Final; Dictamen del Comité Económico y Social Europeo Sobre los Nanomateriales. European Union: Brussels, Belgium, 2009.
- Tao, B.; Lan, H.; Zhou, X.; Lin, C.; Qin, X.; Wu, M.; Zhang, Y.; Chen, S.; Guo, A.; Li, K.; et al. Regulation of TiO2 Nanotubes on Titanium Implants to Orchestrate Osteo/Angio-Genesis and Osteo-Immunomodulation for Boosted Osseointegration. Mater. Des. 2023, 233, 112268. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of New Metallic Alloys for Biomedical Applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yu, Y.; Dai, K.; Ma, Z.; Liu, Y.; Wang, J.; Liu, C. Improved Osteogenesis and Angiogenesis of Magnesium-Doped Calcium Phosphate Cement via Macrophage Immunomodulation. Biomater. Sci. 2016, 4, 1574–1583. [Google Scholar] [CrossRef] [PubMed]
- Diomede, F.; Marconi, G.; Fonticoli, L.; Pizzicanella, J.; Merciaro, I.; Bramanti, P.; Mazzon, E.; Trubiani, O. Functional Relationship between Osteogenesis and Angiogenesis in Tissue Regeneration. Int. J. Mol. Sci. 2020, 21, 3242. [Google Scholar] [CrossRef]
- Gomes, Y.; Tavares, A.; Barbosa, R.; Tomaz, A.; Sousa, W.; Oliveira, L.; Silva, S.; Fook, M. Biological Responses to Biomaterials: A Review. Braz. J. Med. Biol. Res. 2025, 58, e14599. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Salem-Bekhit, M.M.; Khan, F.; Alshehri, S.; Khan, A.; Ghoneim, M.M.; Wu, H.-F.; Taha, E.I.; Elbagory, I. Unique Properties of Surface-Functionalized Nanoparticles for Bio-Application: Functionalization Mechanisms and Importance in Application. Nanomaterials 2022, 12, 1333. [Google Scholar] [CrossRef]
- Asadi Tokmedash, M.; Kim, C.; Chavda, A.P.; Li, A.; Robins, J.; Min, J. Engineering Multifunctional Surface Topography to Regulate Multiple Biological Responses. Biomaterials 2025, 319, 123136. [Google Scholar] [CrossRef]
- Hossain, A.; Shuvo, M.R.H.; Khan, S.; Sayem, A.S.M.; Islam, S.; Hossain, N. Functional Nanoparticle Developments for 3D-Printed Biodegradable Implants- A Comprehensive Review. Results Surf. Interfaces 2025, 19, 100541. [Google Scholar] [CrossRef]
- Khursheed, R.; Dua, K.; Vishwas, S.; Gulati, M.; Jha, N.; Aldhafeeri, G.; Alanazi, F.; Goh, B.; Gupta, G.; Paudel, K.; et al. Biomedical Applications of Metallic Nanoparticles in Cancer: Current Status and Future Perspectives. Biomed. Pharmacother. 2022, 150, 112951. [Google Scholar] [CrossRef]
- Malik, S.; Muhammad, K.; Waheed, Y. Emerging Applications of Nanotechnology in Healthcare and Medicine. Molecules 2023, 28, 6624. [Google Scholar] [CrossRef]
- Truong, T.; Mondal, S.; Doan, V.; Tak, S.; Choi, J.; Oh, H.; Nguyen, T.; Misra, M.; Lee, B.; Oh, J. Precision-Engineered Metal and Metal-Oxide Nanoparticles for Biomedical Imaging and Healthcare Applications. Adv. Colloid. Interface Sci. 2024, 332, 103263. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, G.; Feng, L.; Chen, S. Metallic Nanomaterials with Biomedical Applications. Metals 2022, 12, 2133. [Google Scholar] [CrossRef]
- Yaqoob, A.; Ahmad, H.; Parveen, T.; Ahmad, A.; Oves, M.; Ismail, I.; Qari, H.; Umar, K.; Mohamad Ibrahim, M. Recent Advances in Metal Decorated Nanomaterials and Their Various Biological Applications: A Review. Front. Chem. 2020, 8, 341. [Google Scholar] [CrossRef] [PubMed]
- Naik, G.; Shalaev, V.; Boltasseva, A. Alternative Plasmonic Materials: Beyond Gold and Silver. Adv. Mater. 2013, 25, 3264–3294. [Google Scholar] [CrossRef] [PubMed]



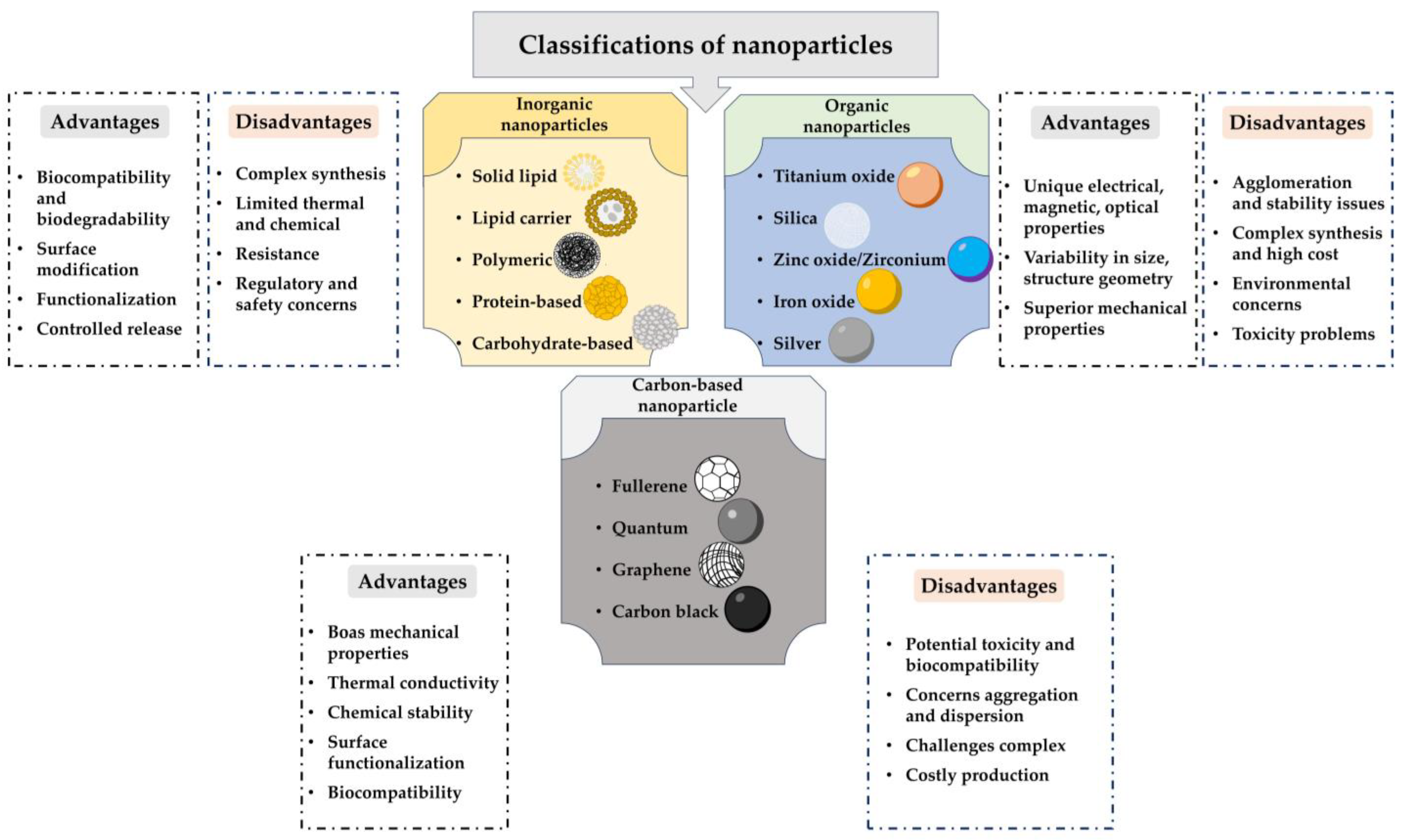
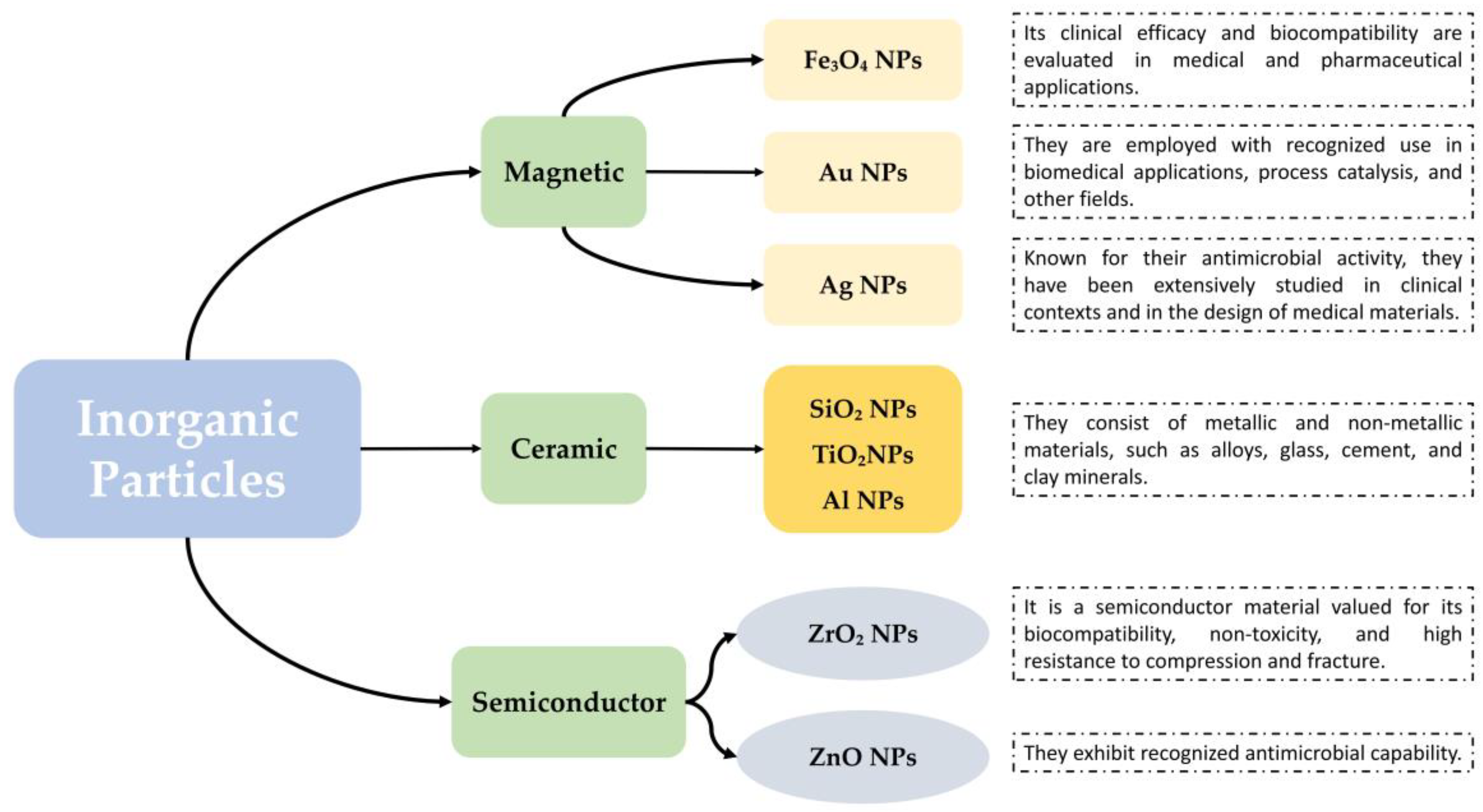
| Structural Factor | Physicochemical Change | Biological Mechanism | Outcome (Bactericidal/Osteoinductive) | Effect Direction/Strength | Ref. |
|---|---|---|---|---|---|
| Size | Increased surface area to volume ratio/higher reactivity. | ROS generation, membrane disruption. | Enhanced bactericidal activity; modulation of osteogenic differentiation. | Positive/strong | [29,30] |
| Shape (e.g., spheres, rods, wires) | Altered contact points and surface energy; differential protein adsorption | Membrane disruption; altered cell adhesion mechanics. | Variable bactericidal activity (e.g., sharp, round); dictates osteoblast adhesion and proliferation. | Depends on geometry/moderate to strong | [22] |
| Crystallinity | altered surface energy and defect density; electron–hole pair separation efficiency. | Enhanced catalytic ROS generation; controlled ion release kinetics. | Affects bacterial killing efficiency; influences osteogenic gene expression and bone matrix formation. | Positive/moderate | [8] |
| Composition (type of metal ions, alloys) | Type of metal/alloys. | Interaction with microbial enzymes/DNA; protein corona formation. | Bactericidal effect (e.g., Ag, Zn); osteoinduction via signaling pathways. | Positive/strong | [6,7] |
| Surface Hydroxylation (OH-group density) | Surface-OH density. | Enhanced protein adsorption (fibronectin), improved cell adhesion, and may reduce bacterial adhesion. | Supports osteoblast adhesion and maturation; potential for selective bioactivity (osteogenic vs. bacterial). | Positive/moderate | [12] |
| Nanotopography | Surface roughness, patterning | Influences focal adhesion formation; affects biofilm mechanism. | Modulates bacterial attachment; promotes osteogenic differentiation via contact. | Topography-dependent/moderately strong | [9] |
| Type of Magnetic Nanoparticle (MNP) | Assays | Value (μg/mL) | Exposure Time (h) | Medium | Environment/Setting | Observed Effects | Ref. |
|---|---|---|---|---|---|---|---|
| Fe3O4 (magnetite) | Intracellular ROS, Ca2+, 2, 3-DPG, ATP, and RBC deformability | 25,000,000 | 12 | N/A | In vitro (erythrocyte cells) | Increased production, ROS, cell membrane changes, and erythrocyte apoptosis. | [149] |
| ROS, phosphatidylserine exposure, hematology analysis, blood serum biochemistry and hemorheology analysis | 12 mg/kg | 144 | N/A | In vivo (rats) | Erythrocyte apoptosis, oxidative stress, and cellular function disruption. | [149] | |
| Fe3O4 (magnetite, uncoated) | XTT assay | 0.1100000 | 24 and 72 | RPMI medium + 10% Horse serum + 5% FPS | In vitro (PC12 cells—tumor origin | No significant cellular interaction. No cytotoxic effects up to 0.1 mg/mL. | [150] |
| Fe3O4 (Na-oleate-coated Fe3O4) | XTT assay | 250 | 24 and 72 | RPMI medium + 10% Horse serum + 5% FPS | In vivo (PC12 cells—tumor origin | Cell viability reduced to 70% at 0.1 mg/mL after 72 h of exposure. | [150] |
| Fe3O4 (dextran-coated magnetite) | Hematological and biochemical analysis | 62, 5–125–250–500 | 24–72 h and 21–28 days | N/A | In vivo (male brown Norway rats) | Hematological tests showed a significant increase in leukocytes, red blood cells, hemoglobin, and hematocrit compared to the values obtained for the control group for the group exposed to concentrations of (250 and 500 μg/mL). | [151] |
| Fluorescein diacetate (FDA) | 62, 5–125–250–500 | 24 h, 72 h, and 7 days | DMEM + 10 FBS | In vitro (HeLa cells) | There were no representative differences in cell viability values compared to the control cell culture and the cell culture at 0 d for cells incubated in suspensions of 62.5 and 125 μg/mL, at all-time intervals tested. | [151] | |
| CoFe2O4 (cobalt ferrite) | ROS, catalase (CAT), glutathione S-transferase (GST), and acid phosphatase (AP) | 10–500 µM | 96 | N/A | In vivo (zebrafish larvae) | Hatching delay, membrane damage, severe apoptosis in head, heart, and tail, and oxidative stress due to increased ROS. | [152] |
| Fe3O4 (DMSA-coated) | AlamarBlue assay, ROS, Caspase-3 | 0.5 | 24 | DMEM + 10% HS | In vitro (hepatocytic cells) | No significant effects on cell viability or cell cycle. | [152] |
| Metallic Nanoparticle Types by Size (nm) | Assays | Value (μg/mL) | Exposure Time (h) | Medium | Environment/Setting | Observed Effects | Ref. |
|---|---|---|---|---|---|---|---|
| ZnO (Zinc oxide 70 nm) | MTT + LDH | 8, 15, 25, and 50 | 12 | DMEM + 10% FBS | Human aortic endothelial cells (HAECs) | ZnO NPs decrease cell viability, induce necrosis and apoptosis, and activate the Fas pathway in a dose- and time-dependent manner. | [160] |
| ZnO (Zinc oxide 100 nm) | WST-1 assay + LDH assay | 2, 4, 8, 16, and 32 | 24 | NM110 + 2% FBS | Cardiovascular endothelial cells | The oxidative stress and inflammatory response triggered by ZnO NPs were not linked to ER stress. Exposure Time: 24 h. | [161] |
| ZnO (Zinc oxide < 50 nm) | MTS assay | 0, 5, 10, 15, 20, 25, 30, and 50 | 3, 6, 12, and 24 | DMEM + 10% FBS | Human umbilical vein endothelial cells (HUVECs) | Ferroptosis in HUVEC in a dose- and time-dependent manner, with clear biomarkers of oxidative stress and ionic overload. | [162,163] |
| ZnO (Zinc oxide 45–55 nm) | MTT + comet assay + | 10, 20, and 50 | 24 | EGCM/FBS | HUVECs | The subcellular toxicity of ZnO NPs results in DNA damage and loss of cell function. | [164] |
| ZnO (Zinc oxide 20 nm and 90–210 nm) | Trypan blue dye exclusion assay + ELISA | 0, 20, 50 and 150 | 4 | N/A- | HCAECs | Exposure to ZnONPs promoted a decrease in cell viability and an increase in 8-OHdG and IL-6 levels. | [165] |
| ZnO (Zinc oxide 21.46 nm) | MTT assay | 0–1000 | 24 | 90% RPMI-1640 | HUVECs | Exposure to ZnONPs does not present cytotoxicity in HUVEC lines. | [163] |
| Metal oxide Nanoparticle Types by Size (nm) | Assays | Value (μg/mL) | Exposure Time (h) | Medium | Environment/Setting | Observed Effects | Ref. |
|---|---|---|---|---|---|---|---|
| TiO2 (Titanium Dioxide 50 nm) | Oxidative stress and ROS production | 50 and 200 | 1 | H2DCFDA | Human umbilical vein endothelial cells (HUVECs) | TiO2 NPs can increase the expression of adhesion molecules in HUVECs. Exposure Time: 3 and 24 h. | [171] |
| TiO2 (Titanium Dioxide 10–30 nm) | Cell viability assay (WST-1 tetrazolium salts) | 0, 1, 5, 25, 50, and 100 | 24 | 1% PBS + 10% DMSO | HUVECs | For TiO2, oxidative stress plays a key role in toxicity, and the total antioxidant capacity tends to increase with longer exposure. Exposure Time: 24 h. | [172] |
| TiO2 (Titanium Dioxide 10, 30, 50, and 100 nm) | CCK-8 assay | 1, 5, and 25 | 24 | MEM with Earle’s salts + 10% FBS+ 1% HEPES | HUVECs | Prolonged exposure to high levels of nano-TiO2 may pose a significant risk to human cardiovascular health by inducing apoptosis in cardiovascular endothelial cells. Exposure Time: 24 h. | [173] |
| TiO2 (Titanium Dioxide 10, 30, 50, and 100 nm) | Comet assay | 1, 5, and 25 | 4 | MEM with Earle’s salts + 10% FBS+ 1% HEPES | HUVECs | TiO2 NPs are capable of causing DNA damage and an increase in the micronucleus with a positive dose-dependent and negative size-dependent effect. | [174] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaviria, J.; Gaviria, V.; Silva, K.V.R.A.; Alcudia, A.; Padrón-Hernández, E.; Torres, Y. Nanoparticle Architecture Governing Antibacterial and Osteoinductive Responses in Bone-Integrating Implants. Metals 2025, 15, 1026. https://doi.org/10.3390/met15091026
Gaviria J, Gaviria V, Silva KVRA, Alcudia A, Padrón-Hernández E, Torres Y. Nanoparticle Architecture Governing Antibacterial and Osteoinductive Responses in Bone-Integrating Implants. Metals. 2025; 15(9):1026. https://doi.org/10.3390/met15091026
Chicago/Turabian StyleGaviria, Juliana, Veronica Gaviria, Kamilla V. R. A. Silva, Ana Alcudia, Eduardo Padrón-Hernández, and Yadir Torres. 2025. "Nanoparticle Architecture Governing Antibacterial and Osteoinductive Responses in Bone-Integrating Implants" Metals 15, no. 9: 1026. https://doi.org/10.3390/met15091026
APA StyleGaviria, J., Gaviria, V., Silva, K. V. R. A., Alcudia, A., Padrón-Hernández, E., & Torres, Y. (2025). Nanoparticle Architecture Governing Antibacterial and Osteoinductive Responses in Bone-Integrating Implants. Metals, 15(9), 1026. https://doi.org/10.3390/met15091026








