Converting Iron-Bearing Tailings from Recycling of Urban Steel Scrap to Direct Reduced Iron via Magnetic Separation Followed by Hydrogen Reduction Under Microwave Irradiation
Abstract
1. Introduction
2. Experimental Section
2.1. Raw Materials
2.2. Methods
2.2.1. Experimental Procedure
2.2.2. Instrumental Characterizations
3. Results and Discussion
3.1. Properties of Magnetic Concentrate
3.2. Thermodynamic Analysis
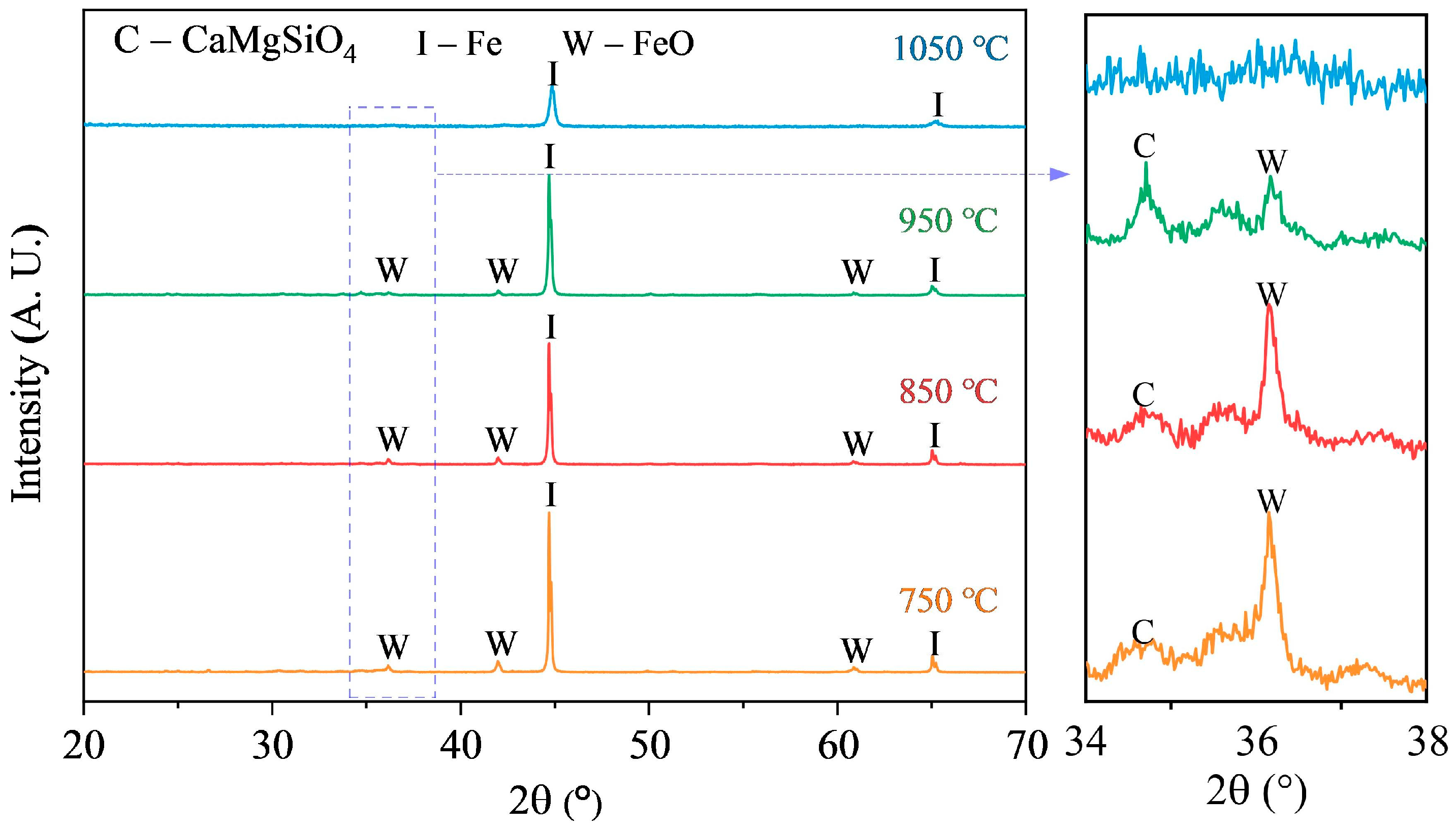
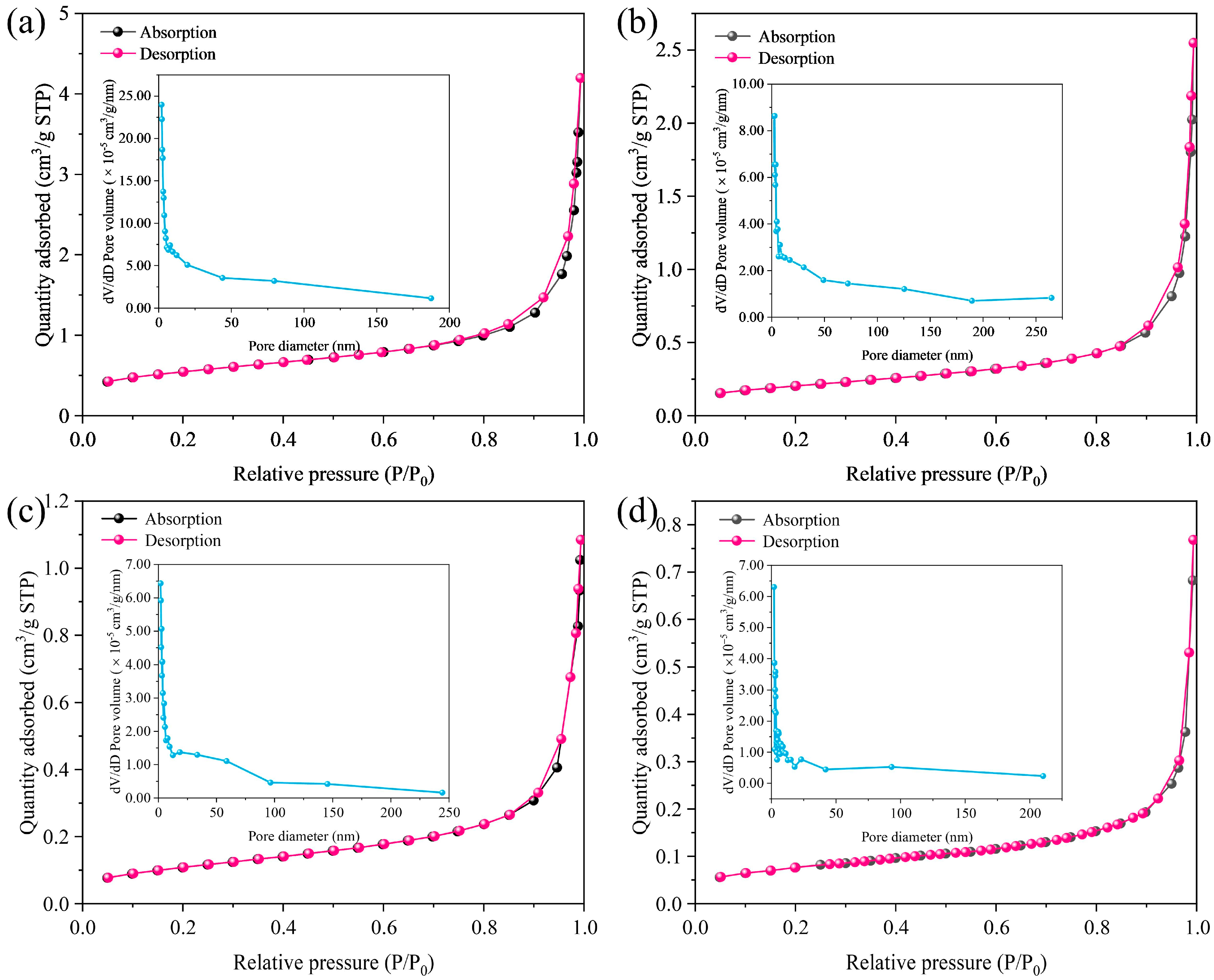
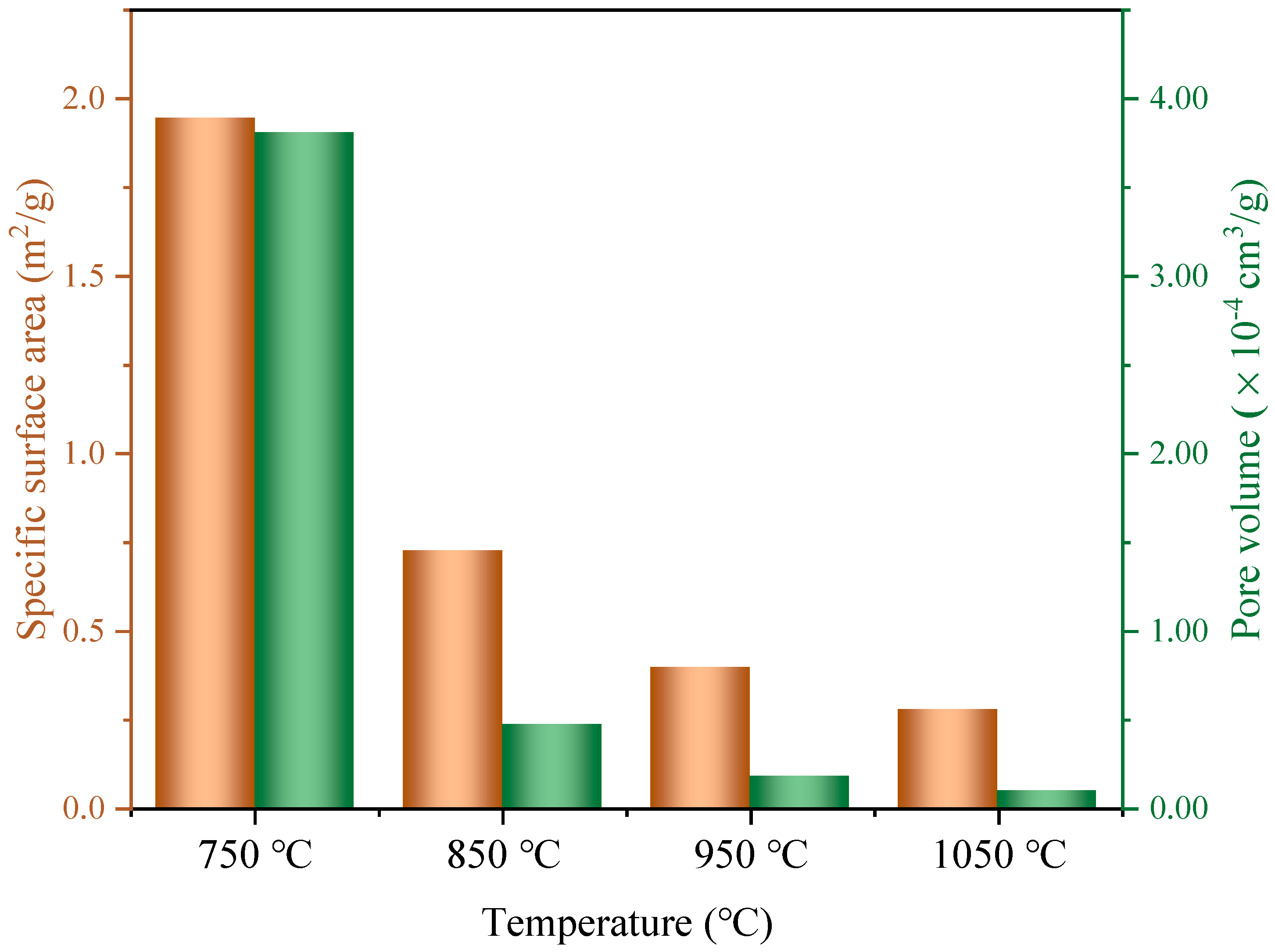
3.3. Microwave Reduction Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- He, K.; Wang, L.; Li, X. Review of the energy consumption and production structure of china’s steel industry: Current situation and future development. Metals 2020, 10, 302. [Google Scholar] [CrossRef]
- Shahabuddin, M.; Brooks, G.; Rhamdhani, M.A. Decarbonisation and hydrogen integration of steel industries: Recent development, challenges and technoeconomic analysis. J. Clean. Prod. 2023, 395, 136391. [Google Scholar] [CrossRef]
- Andrade, C.; Desport, L.; Selosse, S. Net-negative emission opportunities for the iron and steel industry on a global scale. Appl. Energy 2024, 358, 122566. [Google Scholar] [CrossRef]
- Gyllenram, R.; Arzpeyma, N.; Wei, W.; Jönsson, P.G. Driving investments in ore beneficiation and scrap upgrading to meet an increased demand from the direct reduction-EAF route. Miner. Econ. 2022, 35, 203–220. [Google Scholar] [CrossRef]
- Wei, C.; Zhang, X.; Zhang, J.; Xu, L.; Li, G.; Jiang, T. Development of direct reduced iron in China: Challenges and pathways. Engineering 2024, 41, 93–109. [Google Scholar] [CrossRef]
- Liu, W.; Zuo, H.; Wang, J.; Xue, Q.; Ren, B.; Yang, F. The production and application of hydrogen in steel industry. Int. J. Hydrogen Energy 2021, 46, 10548–10569. [Google Scholar] [CrossRef]
- Abdul Quader, M.; Ahmed, S.; Dawal, S.Z.; Nukman, Y. Present needs, recent progress and future trends of energy-efficient ultra-low carbon dioxide (CO) steelmaking (ULCOS) program. Renew. Sustain. Energy Rev. 2016, 55, 537–549. [Google Scholar] [CrossRef]
- Guo, J.; Bao, Y.; Wang, M. Steel slag in China: Treatment, recycling, and management. Waste Manag. 2018, 78, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhou, W.; Lyu, X.; Liu, X.; Su, H.; Li, C.; Wang, H. Comprehensive utilization of steel slag: A review. Powder Technol. 2023, 422, 118449. [Google Scholar] [CrossRef]
- Watari, T.; Fishman, T.; Wieland, H.; Wiedenhofer, D. Global stagnation and regional variations in steel recycling. Resour. Conserv. Recycl. 2025, 220, 108363. [Google Scholar] [CrossRef]
- Oda, J.; Akimoto, K.; Tomoda, T. Long-term global availability of steel scrap. Resour. Conserv. Recycl. 2013, 81, 81–91. [Google Scholar] [CrossRef]
- Wang, P.; Ryberg, M.; Yang, Y.; Feng, K.; Kara, S.; Hauschild, M.; Chen, W.-Q. Efficiency stagnation in global steel production urges joint supply- and demand-side mitigation efforts. Nat. Commun. 2021, 12, 2066. [Google Scholar] [CrossRef]
- Rem, P.C.; van den Broeck, F.; Bakker, M.C.M. Purification of post-consumer steel scrap. Ironmak. Steelmak. 2012, 39, 504–507. [Google Scholar] [CrossRef]
- Lucas, H.I.; Garcia Lopez, C.; Hernández Parrodi, J.C.; Vollprecht, D.; Raulf, K.; Pomberger, R.; Pretz, T.; Friedrich, B. Quality assessment of nonferrous metals recovered from landfill mining: A case study in Belgium. Detritus 2019, 8, 79–90. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Chen, J.; Ye, L.; Du, J. Research progress of vanadium extraction processes from vanadium slag: A review. Sep. Purif. Technol. 2024, 342, 127035. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Y.; Guo, C.; Li, J. Analysis of technological pathways and development suggestions for blast furnace low-carbon ironmaking. Metals 2024, 14, 1276. [Google Scholar] [CrossRef]
- Zhang, S.; Yi, B.; Guo, F.; Zhu, P. Exploring selected pathways to low and zero CO2 emissions in China’s iron and steel industry and their impacts on resources and energy. J. Clean. Prod. 2022, 340, 130813. [Google Scholar] [CrossRef]
- Shao, L.; Zhang, X.; Zhao, C.; Qu, Y.; Saxén, H.; Zou, Z. Computational analysis of hydrogen reduction of iron oxide pellets in a shaft furnace process. Renew. Energy 2021, 179, 1537–1547. [Google Scholar] [CrossRef]
- Bhaskar, A.; Assadi, M.; Nikpey Somehsaraei, H. Decarbonization of the iron and steel industry with direct reduction of iron ore with green hydrogen. Energies 2020, 13, 758. [Google Scholar] [CrossRef]
- Na, H.; Yuan, Y.; Du, T.; Zhang, T.; Zhao, X.; Sun, J.; Qiu, Z.; Zhang, L. Multi-process production occurs in the iron and steel industry, supporting ‘dual carbon’ target: An in-depth study of CO2 emissions from different processes. J. Environ. Sci. 2024, 140, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Hwang, J.-Y.; Andriese, M.; Zhang, Y.; Li, G.; Jiang, T. Electromagnetic characteristics of low-permittivity ceramics as substrates for mushroom-like high impedance surfaces. Ceram. Int. 2015, 41, 3058–3063. [Google Scholar] [CrossRef]
- Kumar, R.; Youssry, S.M.; Joanni, E.; Sahoo, S.; Kawamura, G.; Matsuda, A. Microwave-assisted synthesis of iron oxide homogeneously dispersed on reduced graphene oxide for high-performance supercapacitor electrodes. J. Energy Storage 2022, 56, 105896. [Google Scholar] [CrossRef]
- Ben Neon, L.; Lebrun, A.; Petit, E.; Julbe, A. From slag to structure: Formation of novel iron oxalate crystals via cyreneTM-driven microwave chemistry. Materialia 2025, 41, 102443. [Google Scholar] [CrossRef]
- Da Siva, L.M.; Nascimento, M.; de Oliveira, E.M.; de Queiroz, A.V.; Fernandes, M.T.; de Castro, J.A. Evaluation of the diffusional coefficient in the acid baking process using microwave energy to reduce phosphorus content in iron ore particles. Miner. Eng. 2020, 157, 106541. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, L.; Gu, F.; Tang, H.; Rao, M.; Zhang, Y.; Li, G.; Jiang, T. Recovery of chromium from ferronickel slag: A comparison of microwave roasting and conventional roasting strategies. Powder Technol. 2020, 372, 578–584. [Google Scholar] [CrossRef]
- Agrawal, S.; Rayapudi, V.; Dhawan, N. Comparison of microwave and conventional carbothermal reduction of red mud for recovery of iron values. Miner. Eng. 2019, 132, 202–210. [Google Scholar] [CrossRef]
- Ramesh, T.; Ashok, K.; Vardhan, J.N.V.; Rao, P.V. Role of dysprosium incorporation on structural and magnetic properties of yttrium iron garnet for high power microwave device applications. Ceram. Int. 2025, 51, 18129–18139. [Google Scholar] [CrossRef]
- Tang, H.; Tian, R.; Peng, Z.; Gong, Z.; Zhang, T.; Luo, G.; Zhong, Q.; Rao, M. Co-utilization of electric arc furnace dust and copper slag for preparing zinc ferrite based on microwave roasting. J. Environ. Chem. Eng. 2024, 12, 111533. [Google Scholar] [CrossRef]
- Zhou, S.; Wei, Y.; Li, B.; Wang, H.; Ma, B.; Wang, C.; Luo, X. Mineralogical characterization and design of a treatment process for Yunnan nickel laterite ore, China. Int. J. Miner. Process. 2017, 159, 51–59. [Google Scholar] [CrossRef]
- Bobicki, E.R.; Pickles, C.A.; Forster, J.; Marzoughi, O.; Hutcheon, R. High temperature permittivity measurements of selected industrially relevant ores: Review and analysis. Miner. Eng. 2020, 145, 106055. [Google Scholar] [CrossRef]
- Zhou, S.; Wei, Y.; Li, B.; Wang, H.; Ma, B.; Wang, C. Mechanism of sodium chloride in promoting reduction of high-magnesium low-nickel oxide ore. Sci. Rep. 2016, 6, 29061. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wang, Y. D-BJH: The intrinsic model for characterizing the pore size distribution of porous materials. Langmuir 2024, 40, 20368–20378. [Google Scholar] [CrossRef] [PubMed]
- Scherdel, C.; Reichenauer, G.; Wiener, M. Relationship between pore volumes and surface areas derived from the evaluation of N2-sorption data by DR-, BET- and t-plot. Microporous Mesoporous Mater. 2010, 132, 572–575. [Google Scholar] [CrossRef]
- Sarkar, A.; Schanche, T.L.; Wallin, M.; Safarian, J. Evaluating the reaction kinetics on the H2 reduction of a manganese ore at elevated temperatures. J. Sustain. Metall. 2024, 10, 2085–2103. [Google Scholar] [CrossRef]
- YB/T 4170-2008; Direct Reduced Iron for Steelmaking. Metallurgical Industry Press: Beijing, China, 2008.

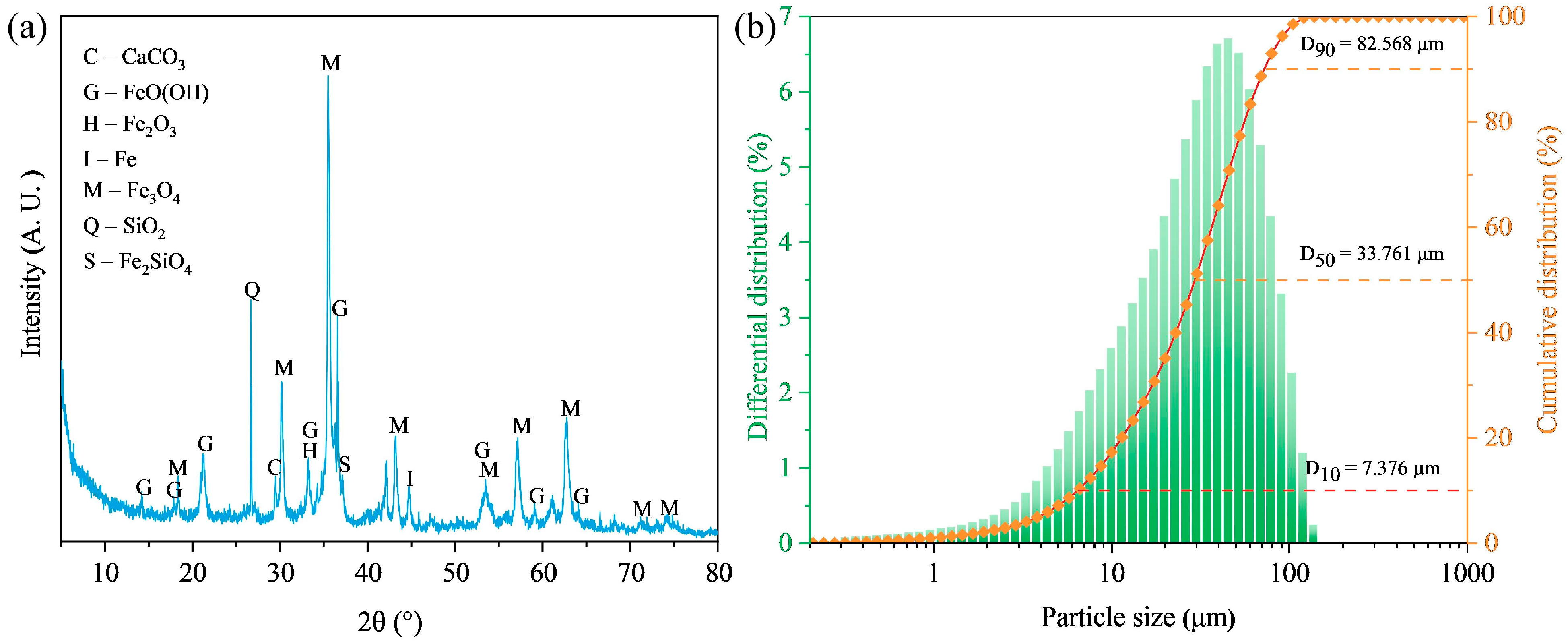
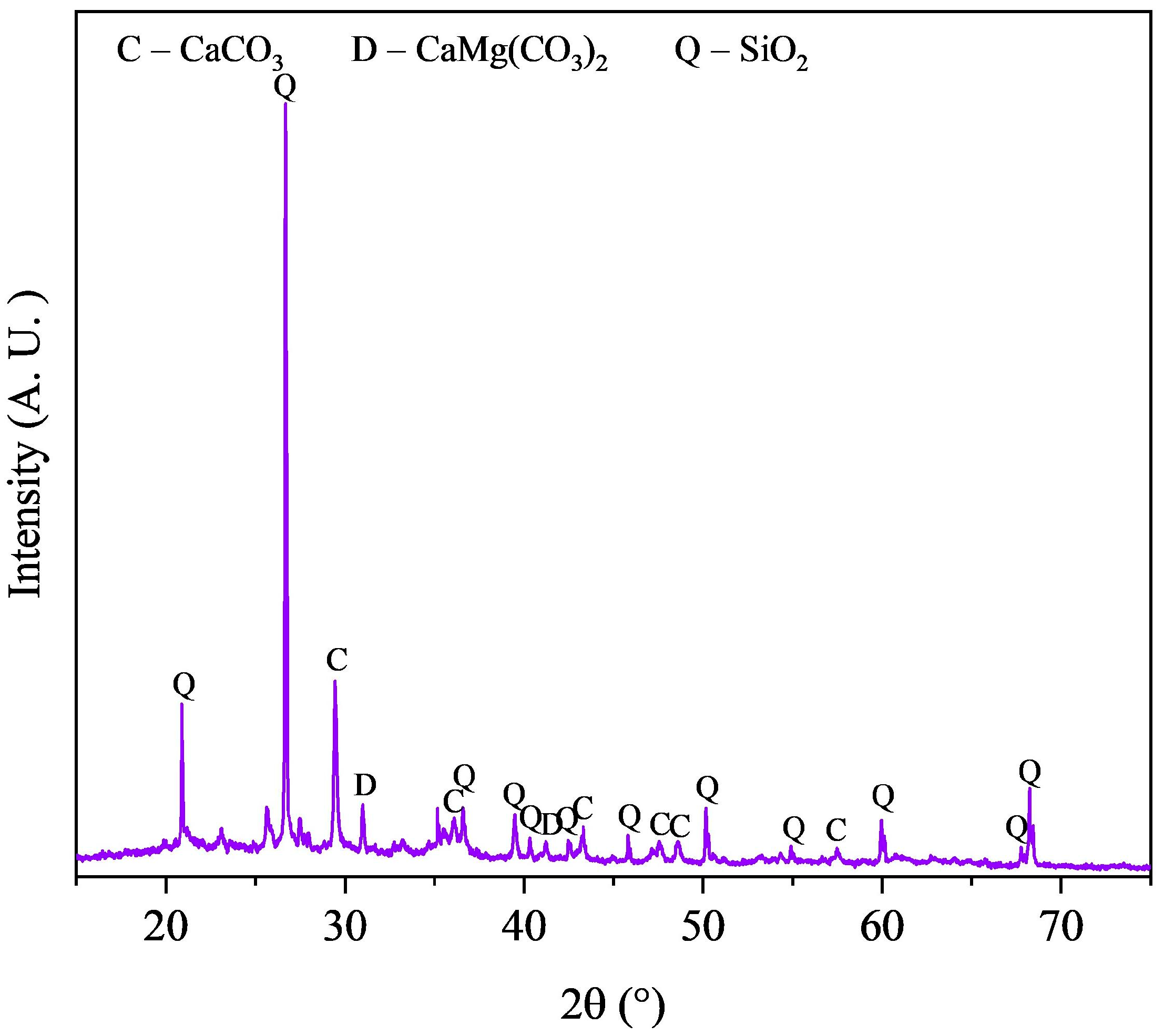
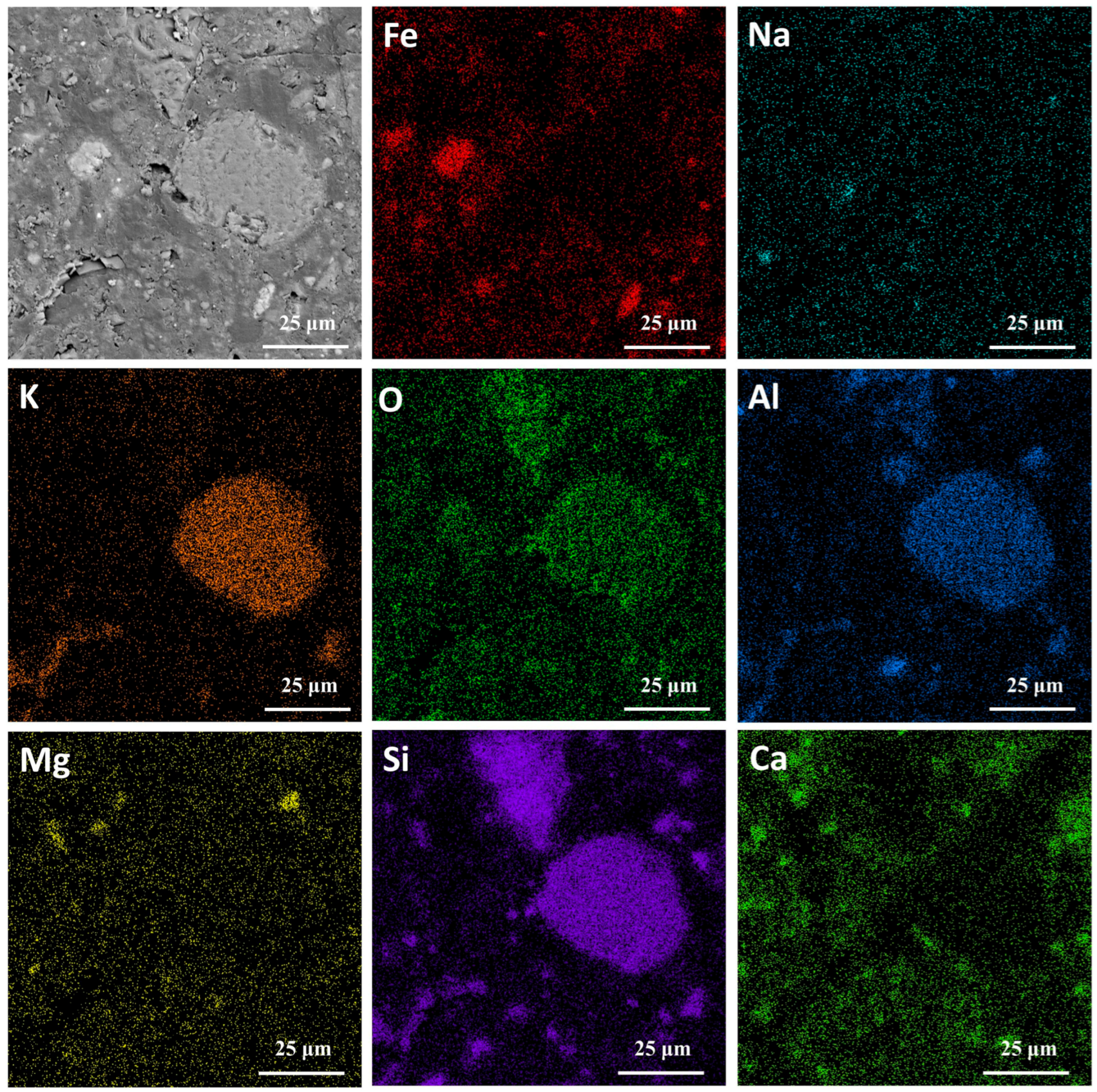
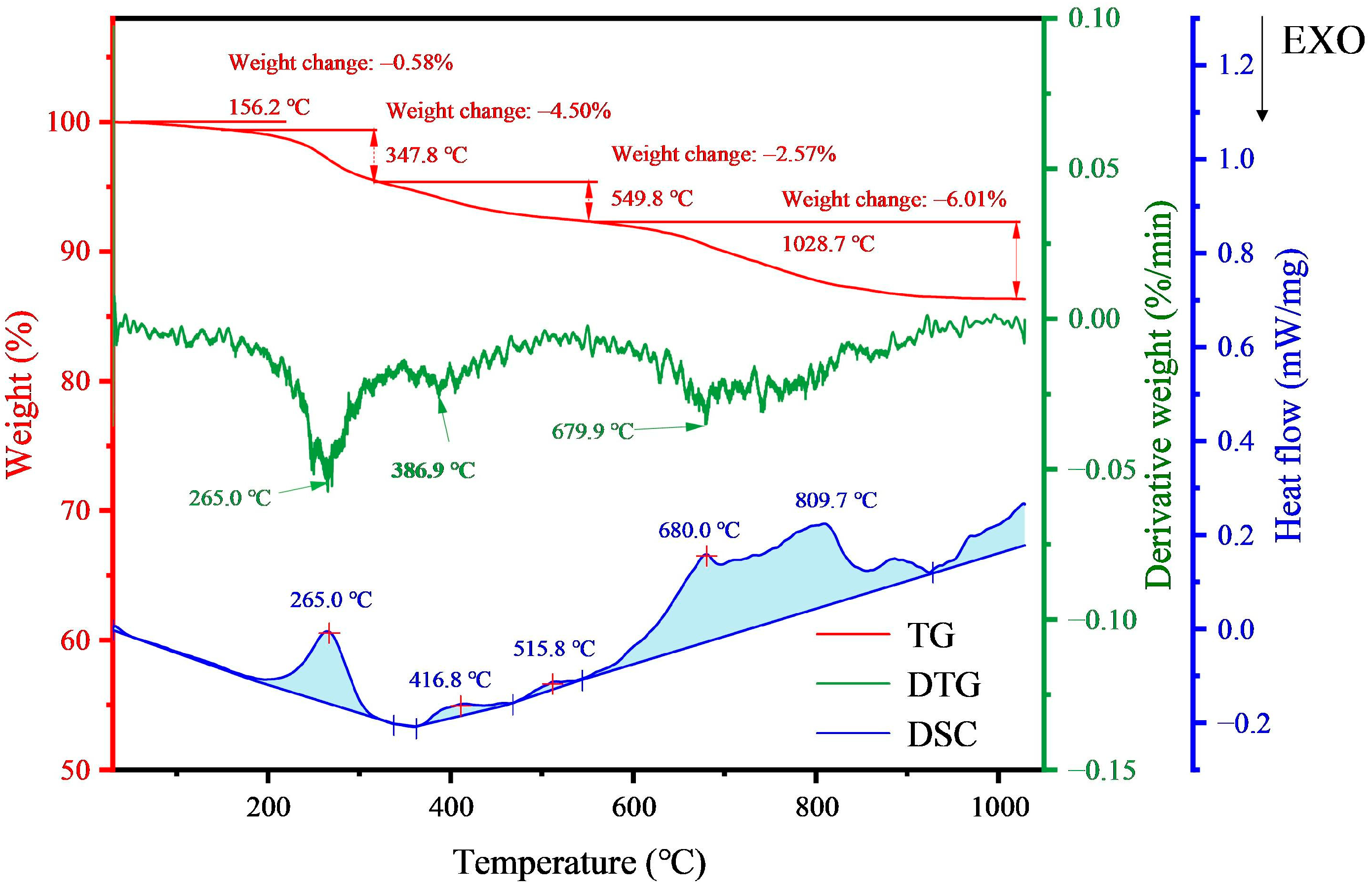
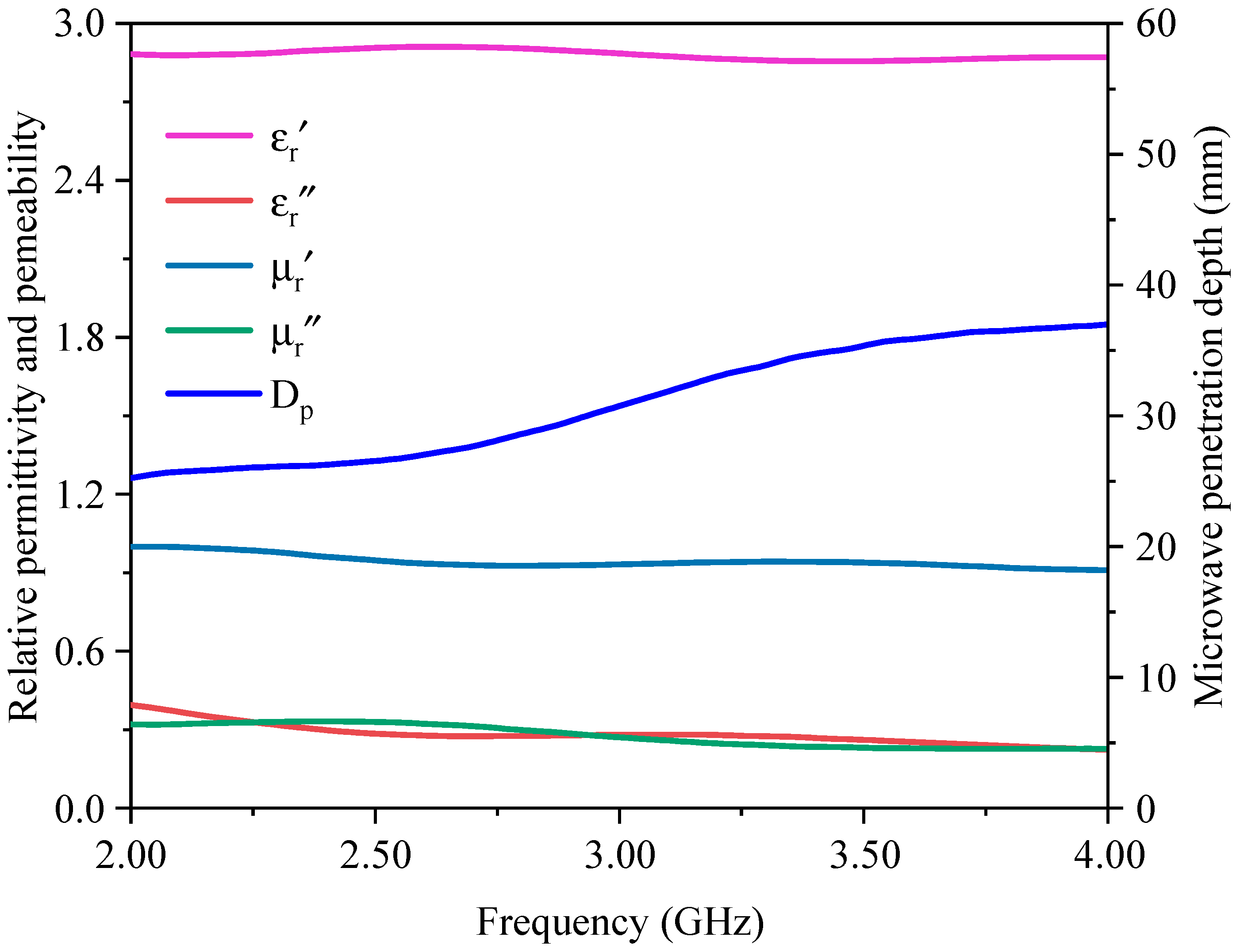
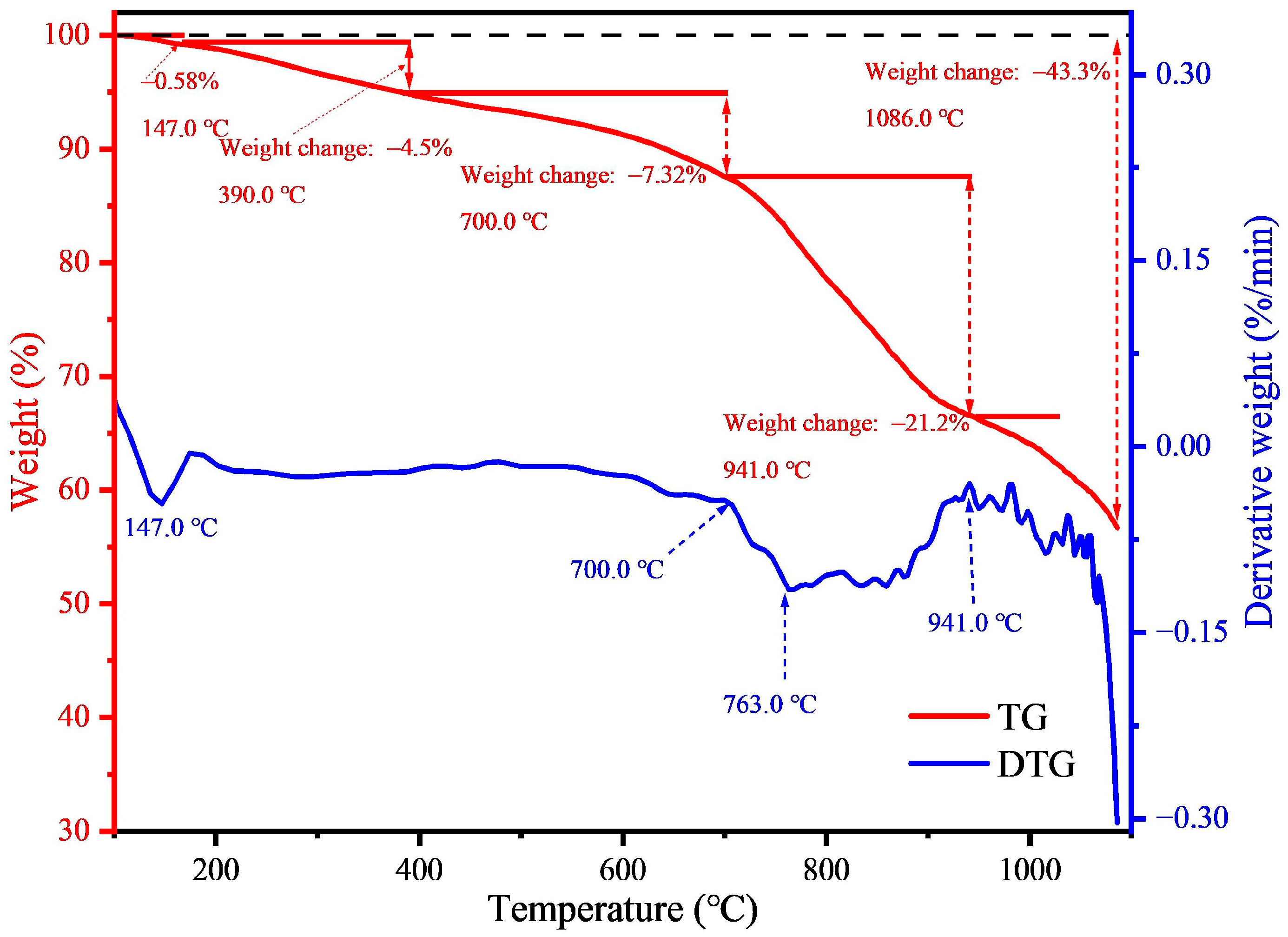
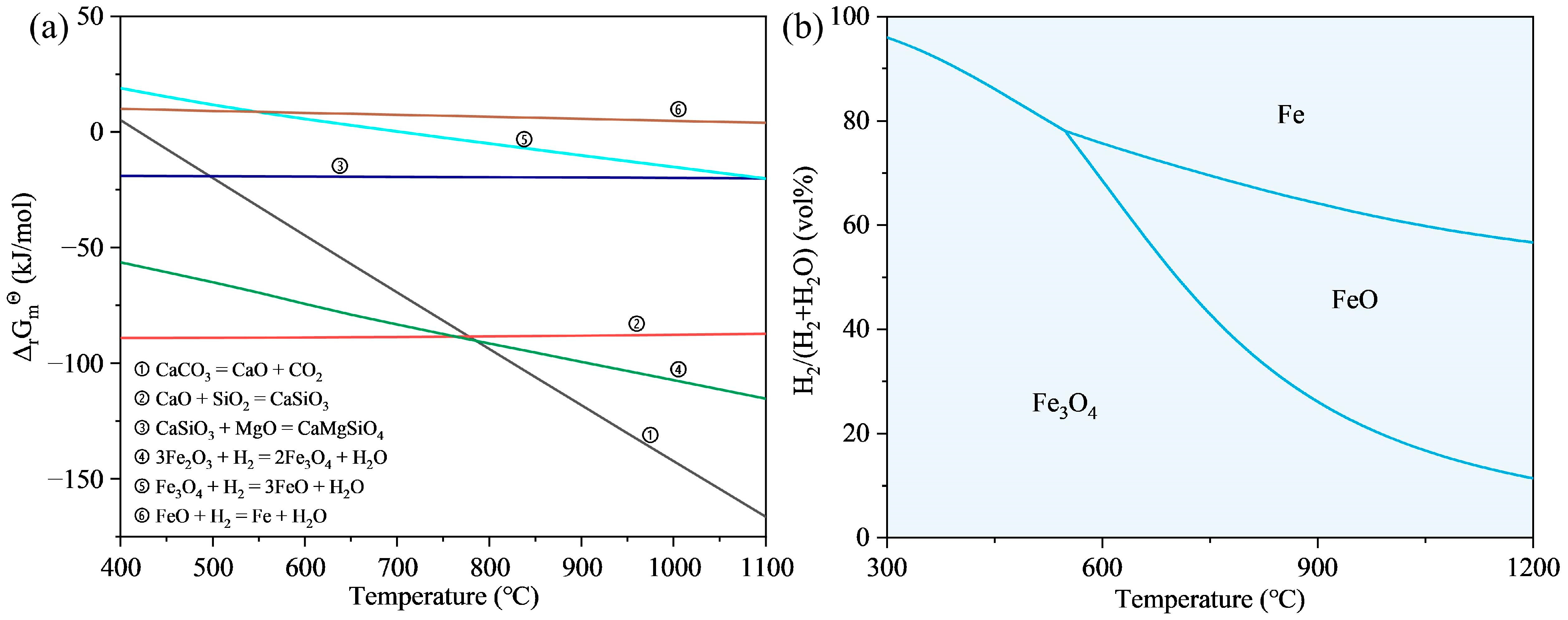
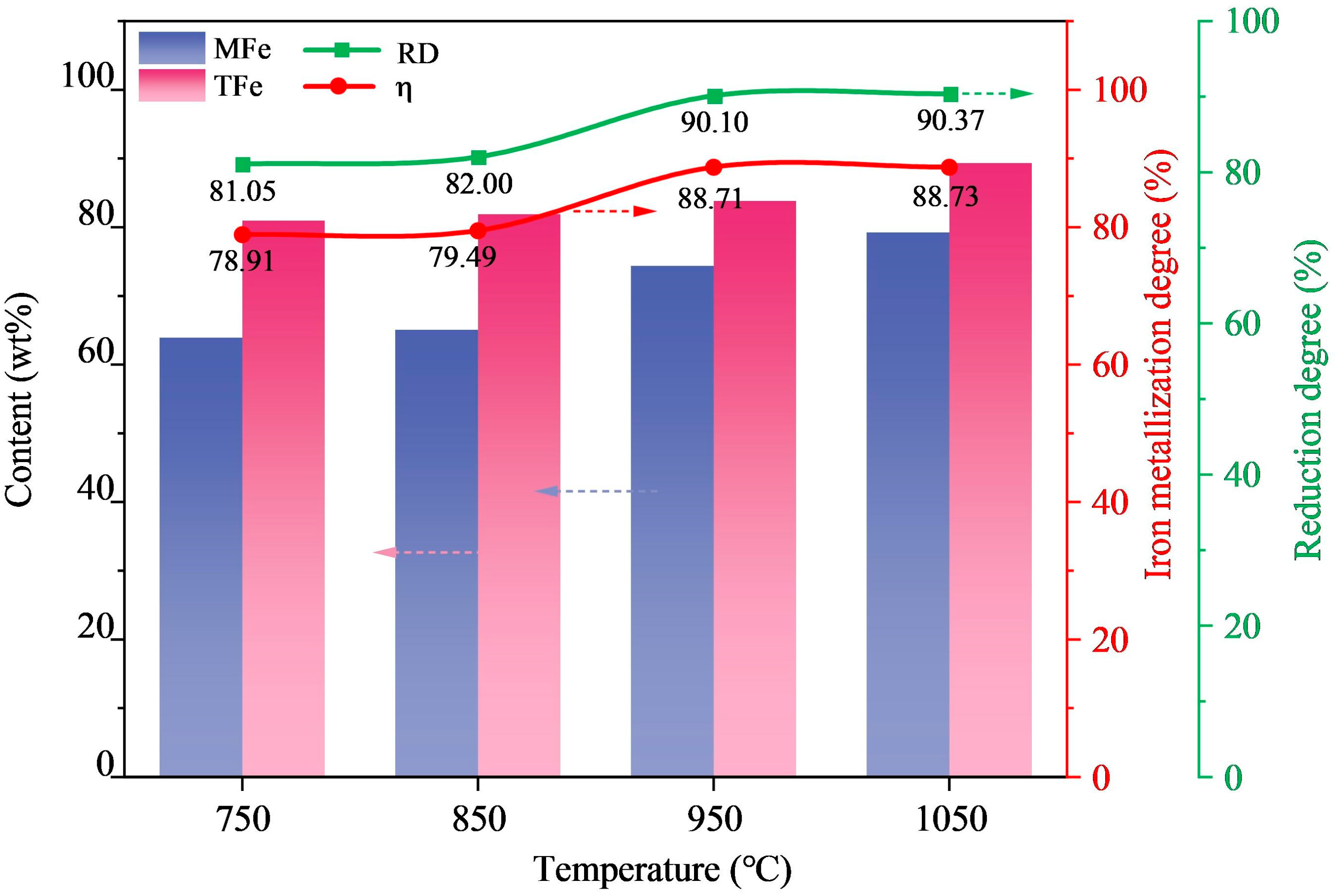



| Element | Fe | Si | C | Ca | Al | Mg | Zn |
| Content | 15.42 | 12.32 | 11.02 | 6.74 | 2.49 | 1.54 | 1.45 |
| Element | Na | K | S | P | Cr | Ni | LOI |
| Content | 1.44 | 0.56 | 0.31 | 0.16 | 0.050 | 0.031 | 25.42 |
| Existence Form | Magnetite | Martite | Hematite and Limonite | Wüstite | Silicates | Sulfides | Total |
|---|---|---|---|---|---|---|---|
| Iron content (wt%) | 5.2 | 0.65 | 7.5 | 0.64 | 1.41 | 0.024 | 15.42 |
| Proportion (%) | 33.7 | 4.2 | 48.6 | 4.2 | 9.1 | 0.2 | 100 |
| Element | Fe | Si | C | Ca | Al | Mg | Zn | Na | K | S | P |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content | 60.04 | 0.75 | 1.95 | 0.83 | 0.34 | 0.22 | 1.17 | 0.041 | 0.033 | 0.13 | 0.11 |
| Existence Form | Magnetite | Martite | Hematite and Limonite | Carbonates | Silicates | Sulfides | Total |
|---|---|---|---|---|---|---|---|
| Iron content (wt%) | 34.08 | 15.26 | 5.18 | 4.1 | 1.32 | 0.1 | 60.04 |
| Proportion (%) | 56.76 | 25.42 | 8.63 | 6.83 | 2.20 | 0.16 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, T.; Peng, Z.; Tian, W.; Fan, W.; Tang, H. Converting Iron-Bearing Tailings from Recycling of Urban Steel Scrap to Direct Reduced Iron via Magnetic Separation Followed by Hydrogen Reduction Under Microwave Irradiation. Metals 2025, 15, 924. https://doi.org/10.3390/met15080924
Yin T, Peng Z, Tian W, Fan W, Tang H. Converting Iron-Bearing Tailings from Recycling of Urban Steel Scrap to Direct Reduced Iron via Magnetic Separation Followed by Hydrogen Reduction Under Microwave Irradiation. Metals. 2025; 15(8):924. https://doi.org/10.3390/met15080924
Chicago/Turabian StyleYin, Tianle, Zhiwei Peng, Weiguang Tian, Wanlong Fan, and Huimin Tang. 2025. "Converting Iron-Bearing Tailings from Recycling of Urban Steel Scrap to Direct Reduced Iron via Magnetic Separation Followed by Hydrogen Reduction Under Microwave Irradiation" Metals 15, no. 8: 924. https://doi.org/10.3390/met15080924
APA StyleYin, T., Peng, Z., Tian, W., Fan, W., & Tang, H. (2025). Converting Iron-Bearing Tailings from Recycling of Urban Steel Scrap to Direct Reduced Iron via Magnetic Separation Followed by Hydrogen Reduction Under Microwave Irradiation. Metals, 15(8), 924. https://doi.org/10.3390/met15080924







