Electrochemical Characterization of CO2 Corrosion Inhibition of API X100 by a Gemini Surfactant Under Static and Dynamic Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Test Solution
2.2. Electrochemical Measurements
2.3. Surface Analysis by SEM-EDS
3. Results and Discussion
3.1. Corrosion Inhibitor Performance Under Static Conditions
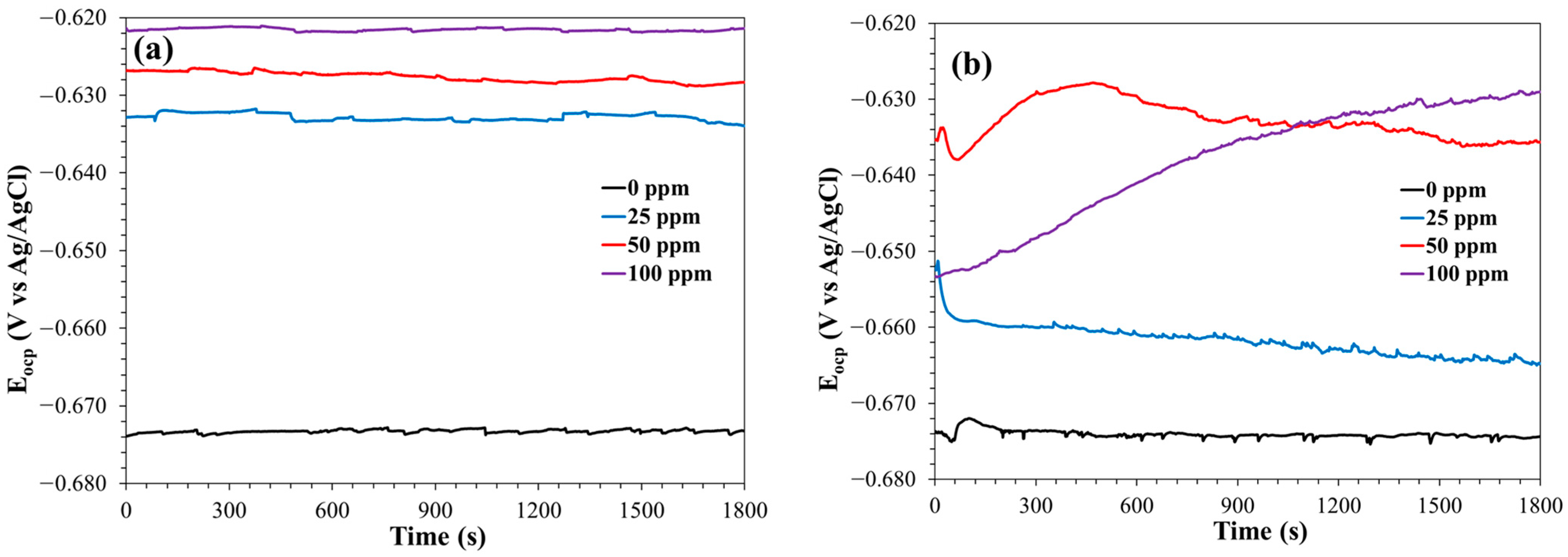
3.1.1. Open Circuit Potential (Eocp)
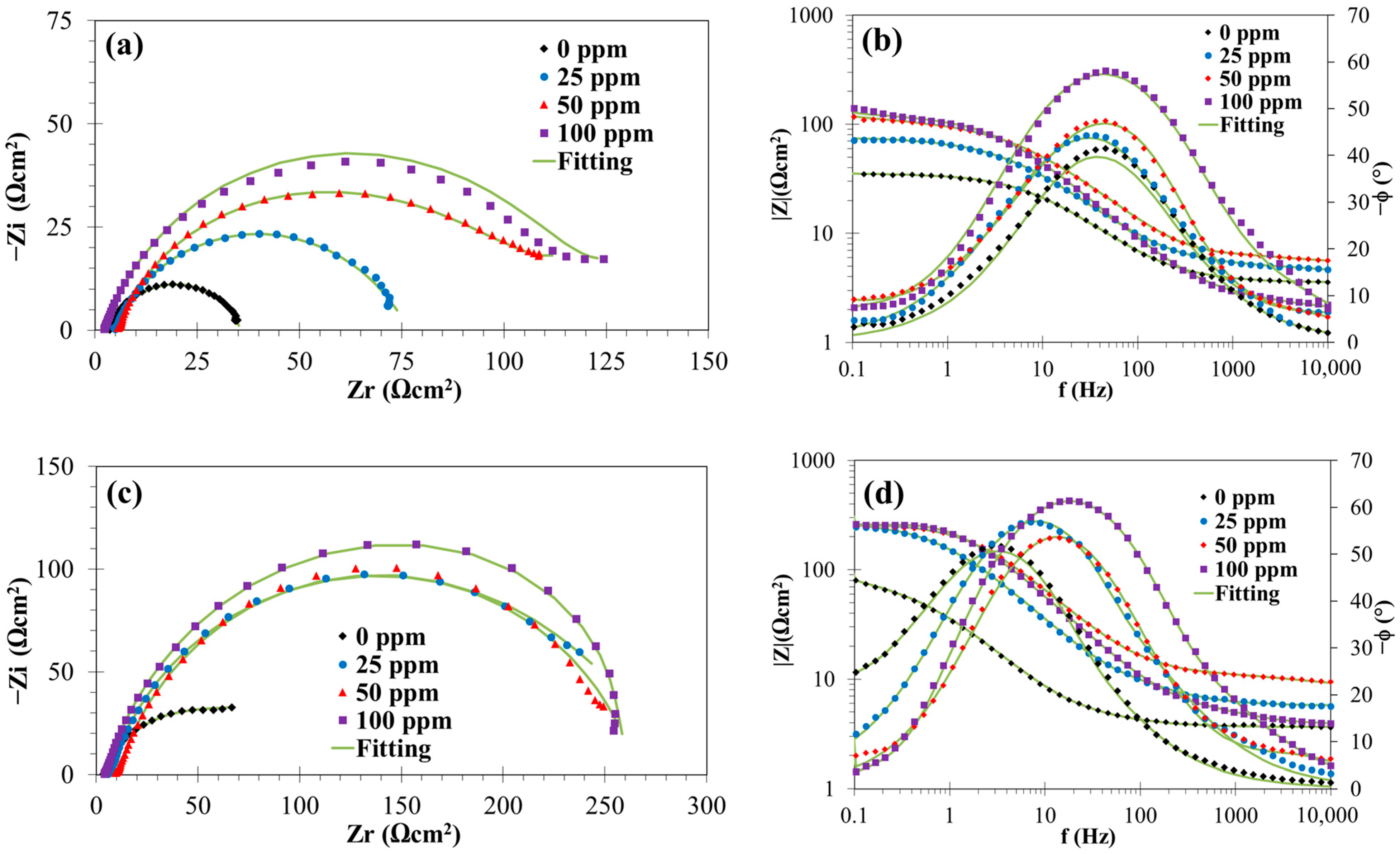
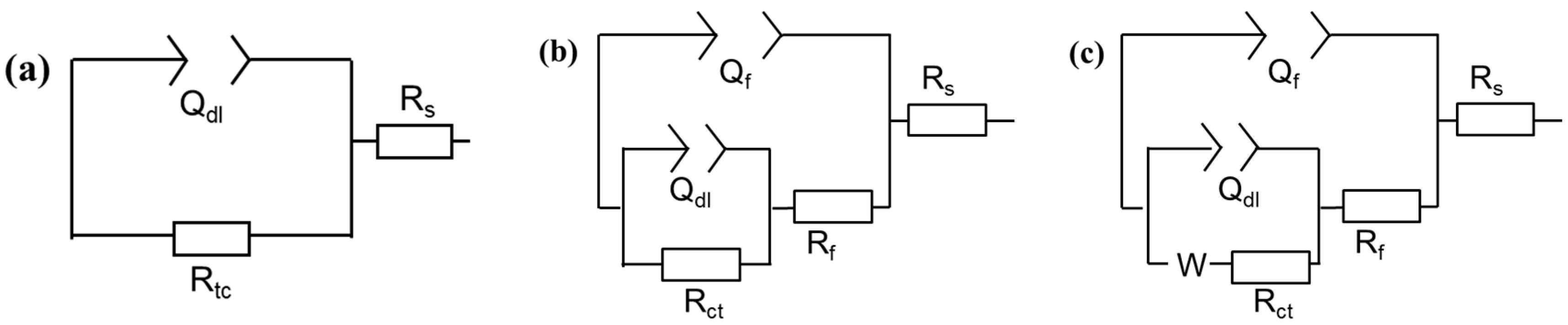
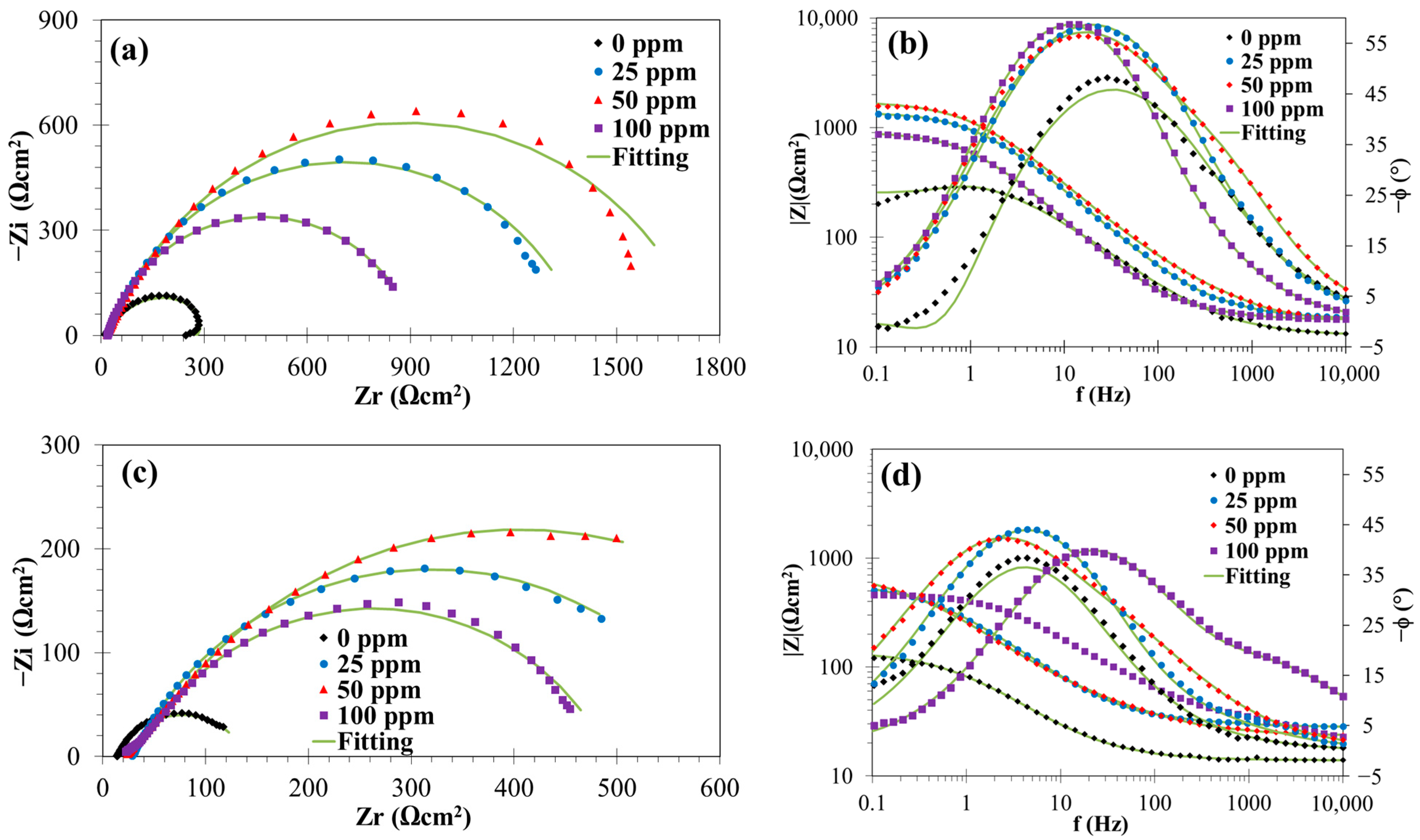
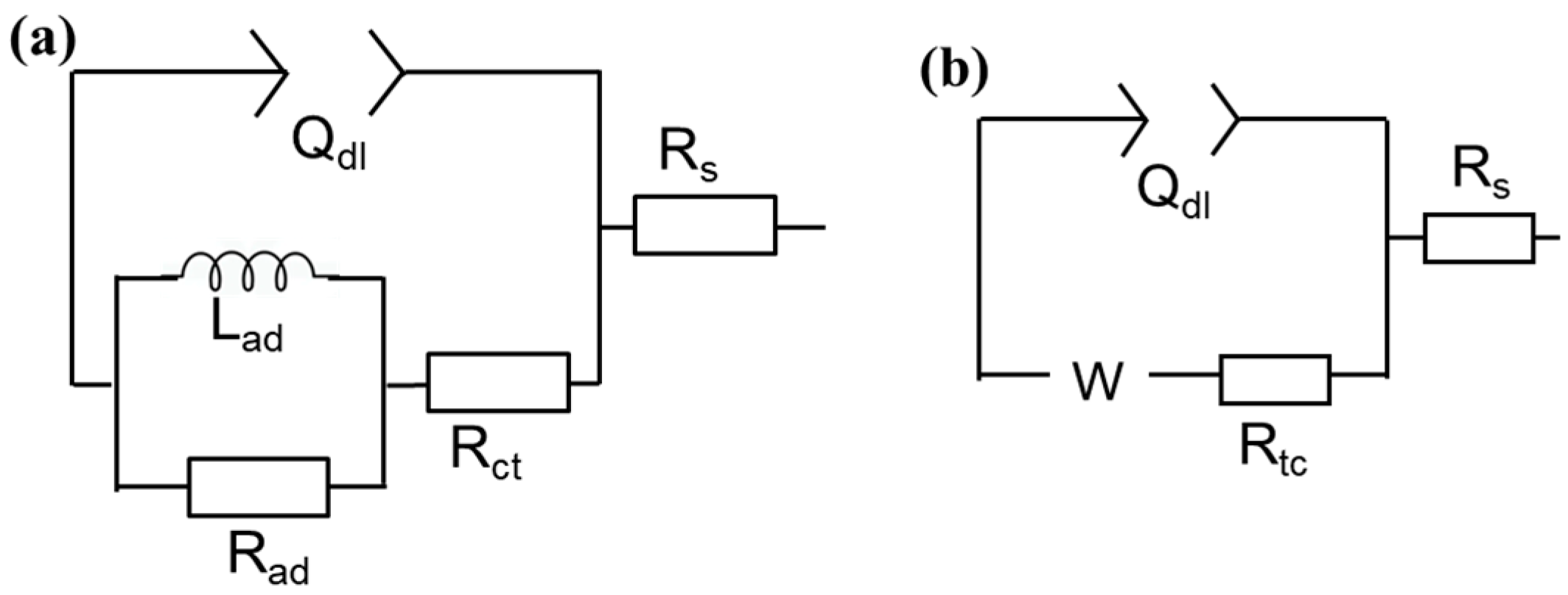
3.1.2. EIS Measurements
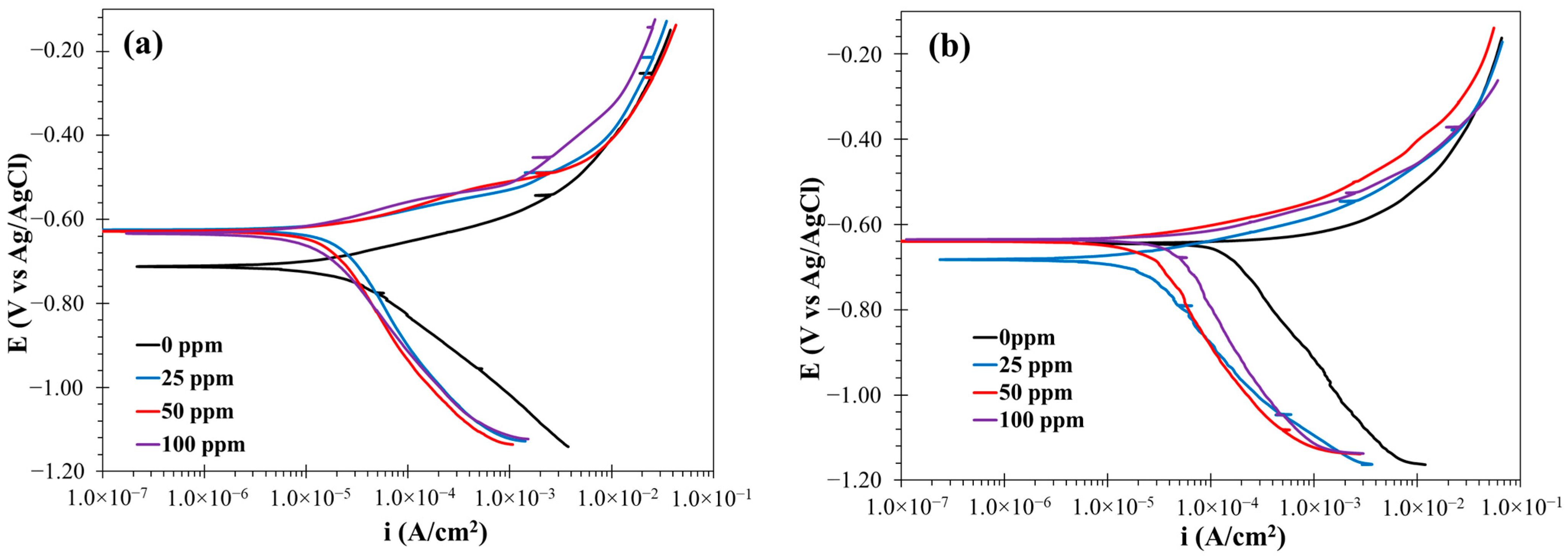
3.1.3. Polarization Curve Results
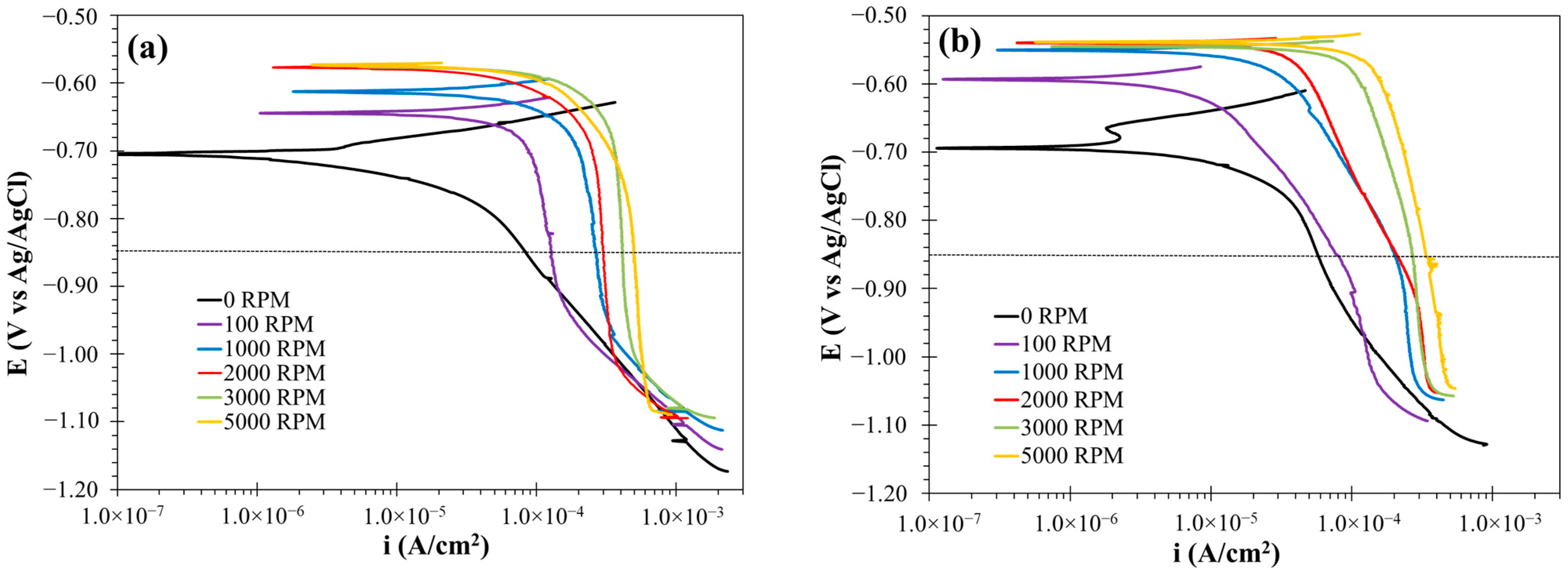
3.2. Corrosion Inhibitor Performance Under Hydrodynamic Conditions
3.2.1. Open Circuit Potential (Eocp)
3.2.2. EIS Results
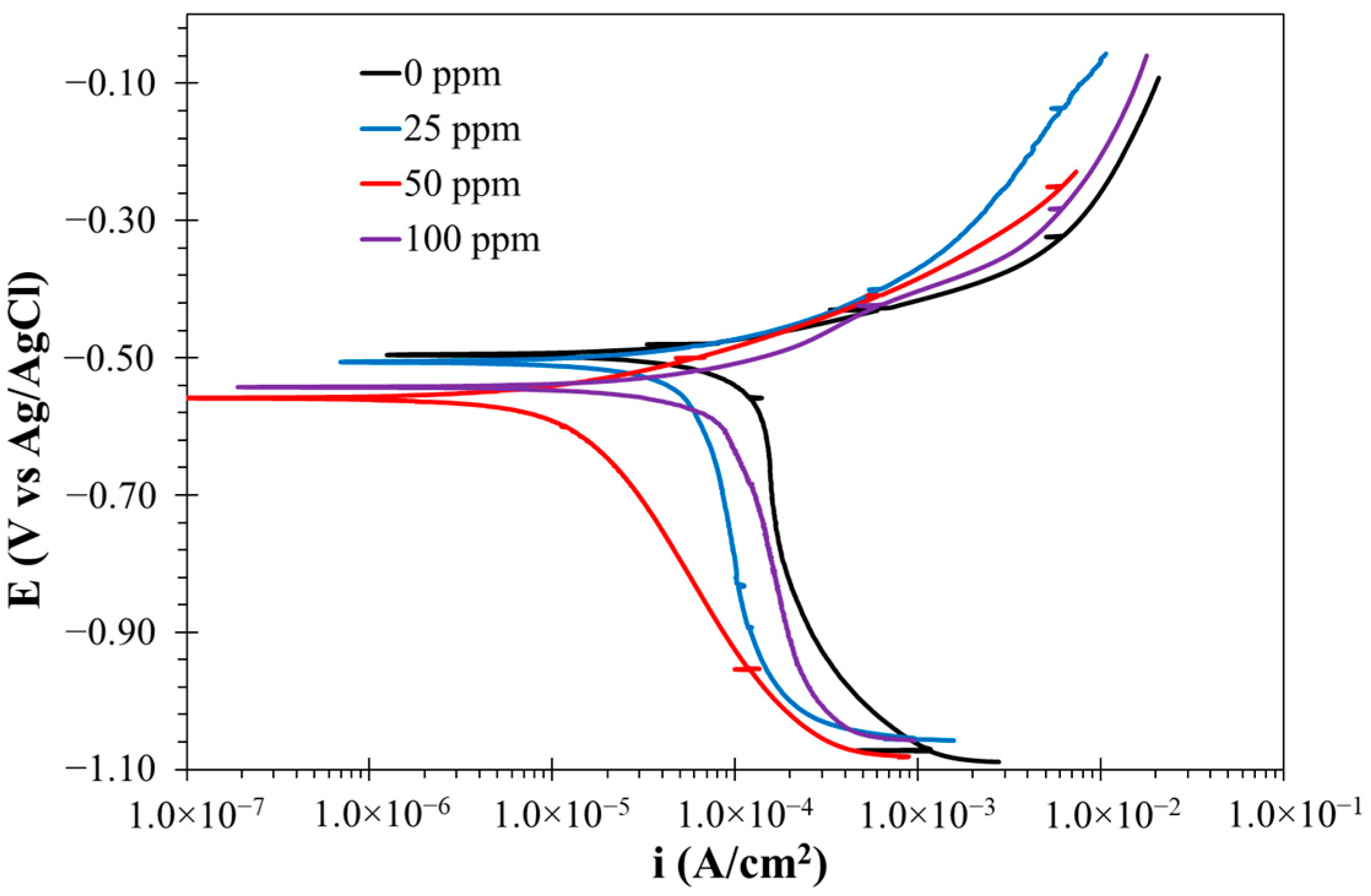
3.2.3. Polarization Curve Results
3.2.4. Cathodic Kinetics in the CO2-Saturated Brine Solution with and Without the Corrosion Inhibitor
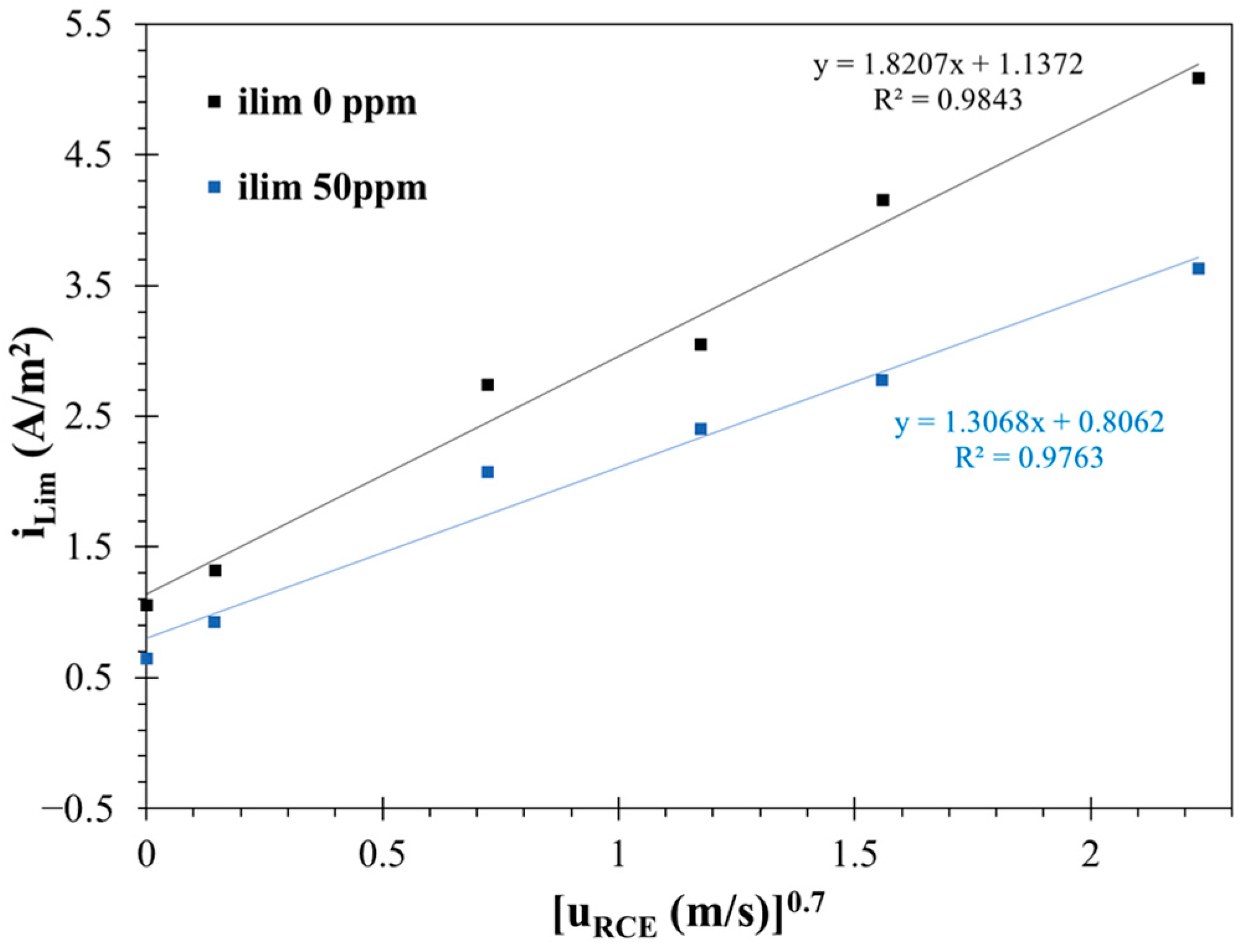
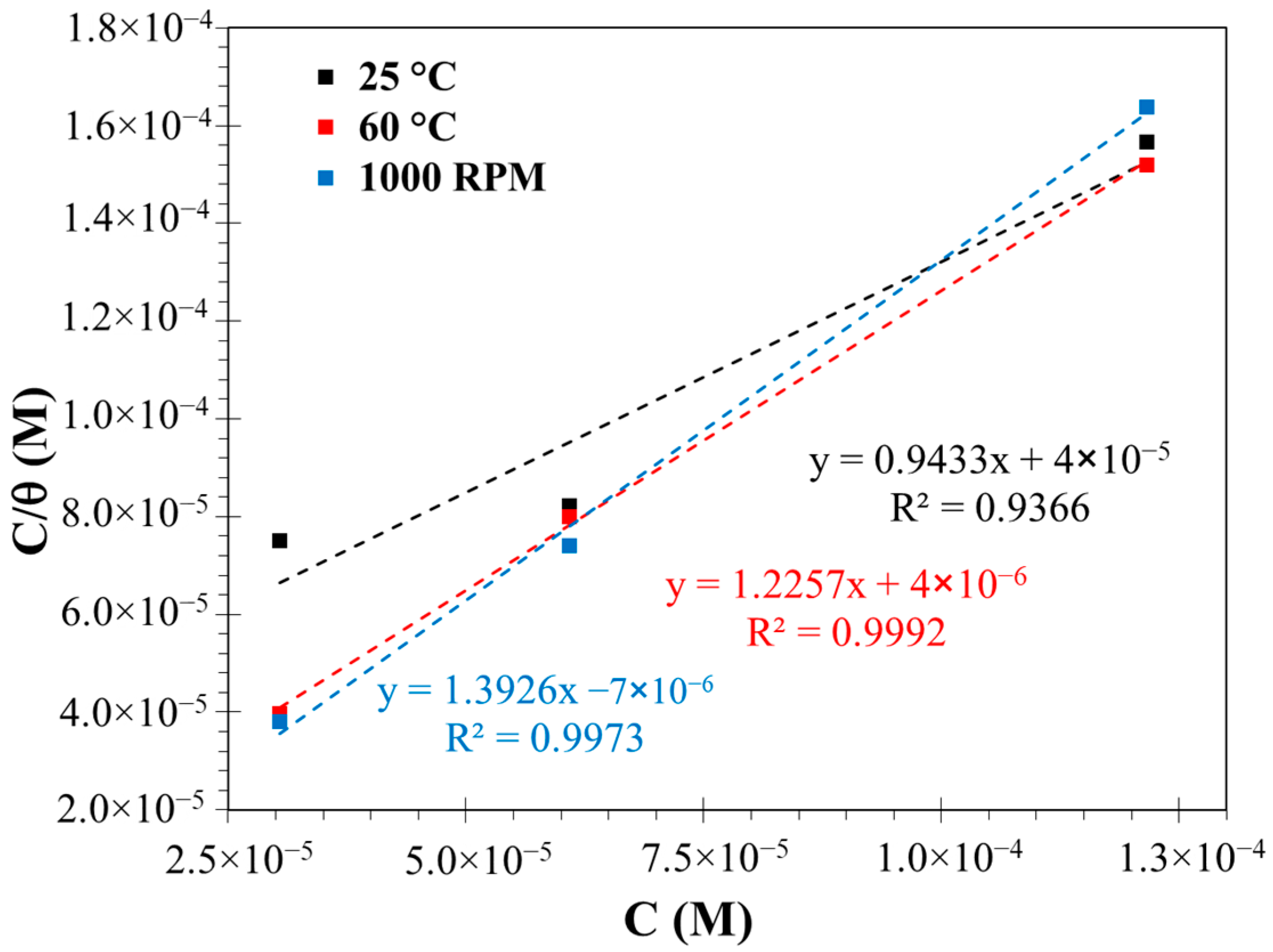
3.3. Adsorption Isotherms
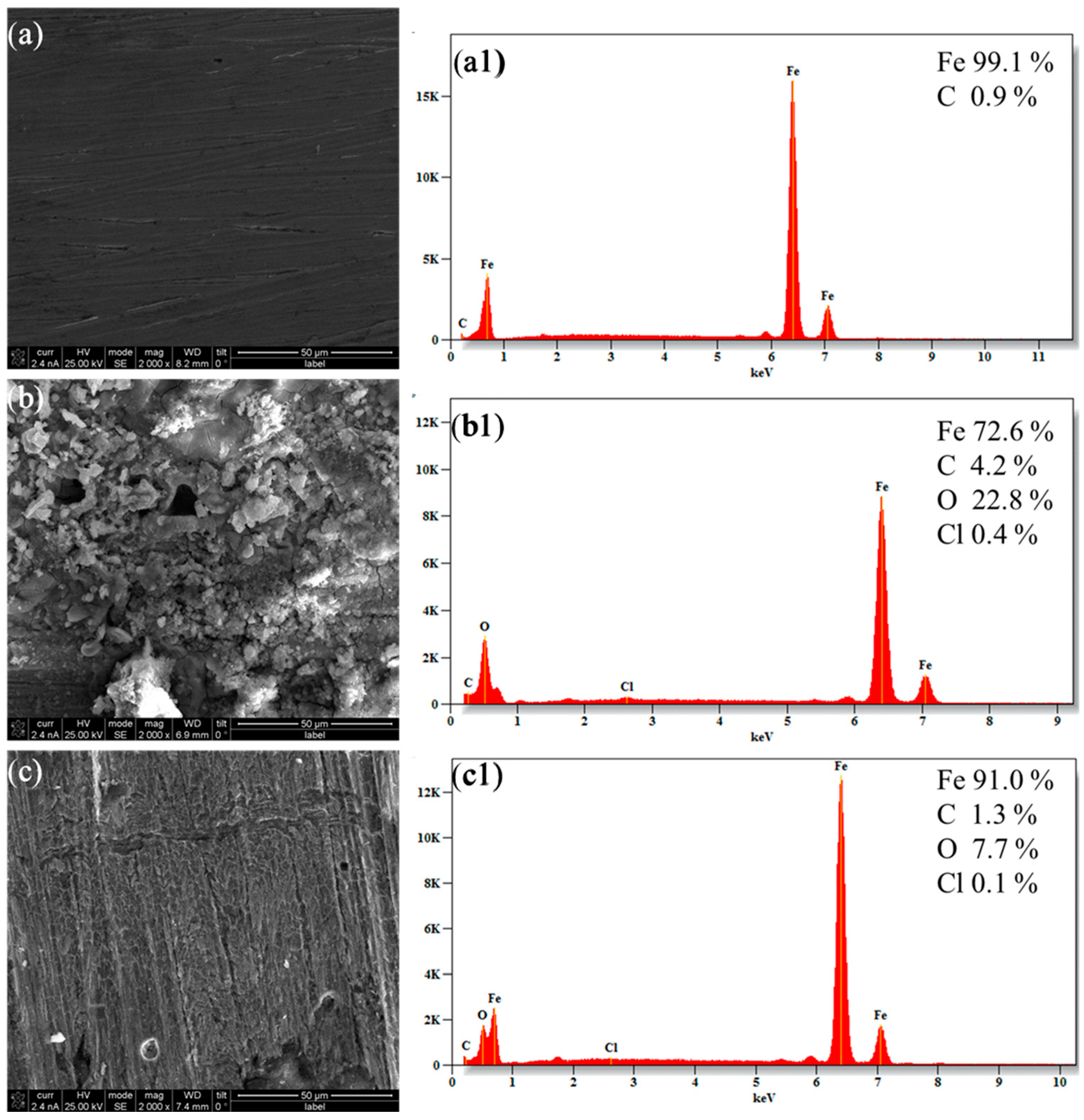
3.4. Surface Analysis by SEM-EDS
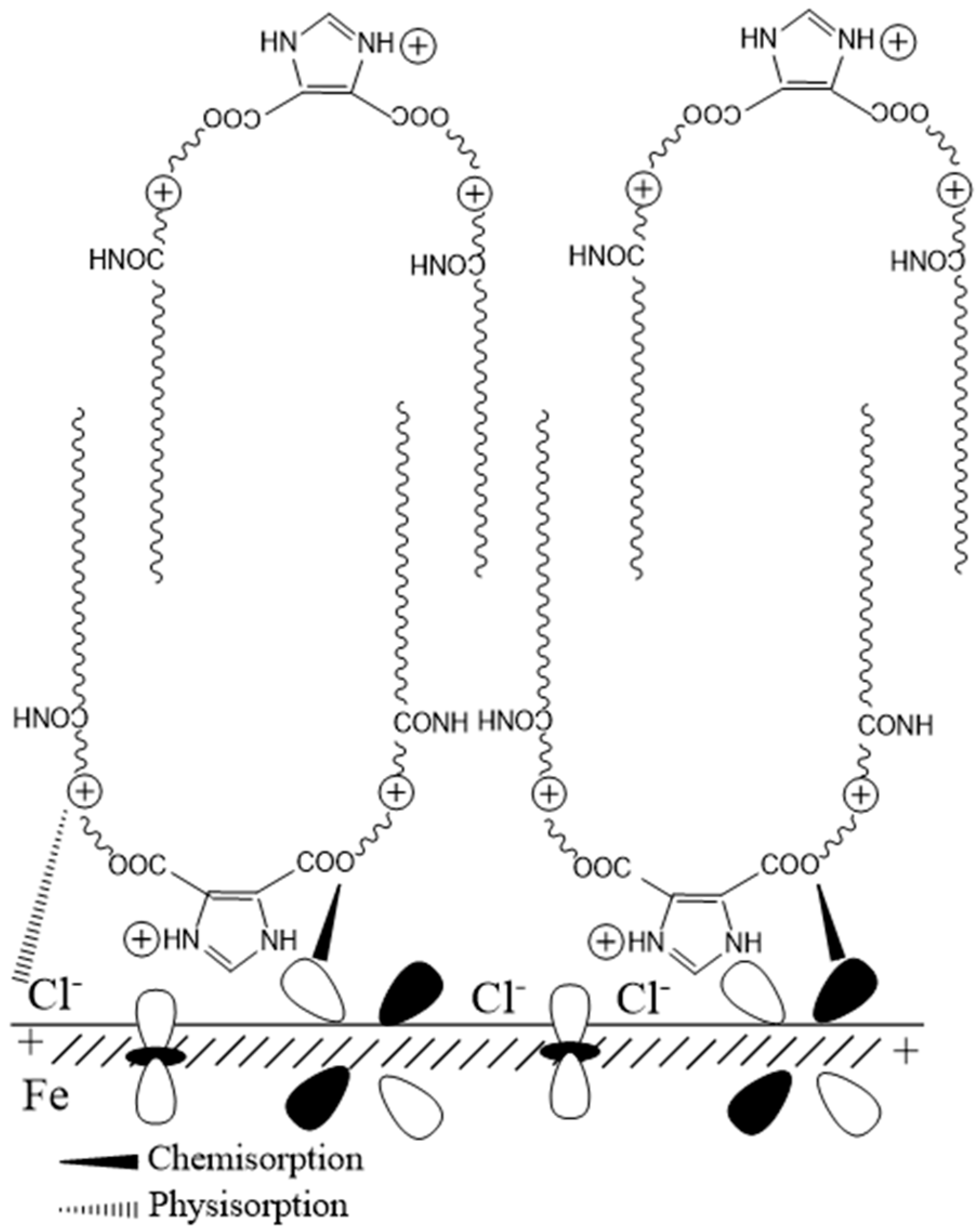
3.5. Corrosion Inhibition Mechanism of Gemini Surfactant in CO2 Environment
4. Conclusions
- The PPC results indicated that the inhibitor acted as a mixed-type inhibitor, having a predominant effect on anodic dissolution under static conditions at room temperature, and a greater influence on the cathodic reactions under static conditions at 60 °C and under turbulent flow conditions.
- The results of electrochemical measurements showed that the corrosion inhibition performance of the surfactant at 25 °C enhanced as the concentration and exposure time increased, reaching a maximum η of 80% at 100 ppm. Meanwhile, η was independent of the concentration after 24 h of exposure at 60 °C, obtaining a maximum η value of 82.3%.
- Under hydrodynamic conditions, electrochemical measurements indicated that the surfactant achieved a maximum inhibition efficiency of 91% at 50 ppm. The effectiveness of the corrosion inhibitor in suppressing the corrosion rate decreased after 24 h of exposure.
- The analysis of the adsorption isotherms revealed that the surfactant adsorption on the surface obeyed the Langmuir isotherm, with a chemisorption mechanism for the three conditions studied.
- Surface characterization by SEM and EDS confirmed the formation of a protective corrosion inhibitor film on the surface of the X100 steel.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bharatiya, U.; Gal, P.; Agrawal, A.; Shah, M.; Sircar, A. Effect of Corrosion on Crude Oil and Natural Gas Pipeline with Emphasis on Prevention by Ecofriendly Corrosion Inhibitors: A Comprehensive Review. J. Bio Tribocorros. 2019, 5, 35. [Google Scholar] [CrossRef]
- Sonke, J.; Zheng, Y.; Bos, W.M. Development of a Practical Model for H2S Corrosion Prediction for Upstream Oil and Gas Applications. In Proceedings of the CORROSION 2018, NACE International, Phoenix, AZ, USA, 15 April 2018; pp. 1–15. [Google Scholar]
- Al-Janabi, Y.T. An Overview of Corrosion in Oil and Gas Industry. In Corrosion Inhibitors in the Oil and Gas Industry; Wiley: Hoboken, NJ, USA, 2020; pp. 1–39. [Google Scholar]
- Ibrahim, A.; Hawboldt, K.; Bottaro, C.; Khan, F. Review and Analysis of Microbiologically Influenced Corrosion: The Chemical Environment in Oil and Gas Facilities. Corros. Eng. Sci. Technol. 2018, 53, 549–563. [Google Scholar] [CrossRef]
- Solovyeva, V.A.; Almuhammadi, K.H.; Badeghaish, W.O. Current Downhole Corrosion Control Solutions and Trends in the Oil and Gas Industry: A Review. Materials 2023, 16, 1795. [Google Scholar] [CrossRef] [PubMed]
- Perez, T.E. Corrosion in the Oil and Gas Industry: An Increasing Challenge for Materials. JOM 2013, 65, 1033–1042. [Google Scholar] [CrossRef]
- Kahyarian, A.; Singer, M.; Nesic, S. Modeling of Uniform CO2 Corrosion of Mild Steel in Gas Transportation Systems: A Review. J. Nat. Gas Sci. Eng. 2016, 29, 530–549. [Google Scholar] [CrossRef]
- Nešić, S. Key Issues Related to Modelling of Internal Corrosion of Oil and Gas Pipelines—A Review. Corros. Sci. 2007, 49, 4308–4338. [Google Scholar] [CrossRef]
- Qin, M.; Chen, S.; Ye, N.; Chen, Y.; Zhang, S.; Li, K.; Liao, K. Study on the Evolution Mechanism and Influencing Factors of CO2 Corrosion Product Film under Pipe Flow. Int. J. Press. Vessel. Pip. 2025, 216, 105506. [Google Scholar] [CrossRef]
- Chen, H.; Wang, X.; Shen, J. Revealing the Deterioration Effect of Scale Inhibitor on the Performance of Corrosion Inhibitor under Dynamic Conditions. J. Mater. Eng. Perform. 2025, 34, 2145–2155. [Google Scholar] [CrossRef]
- Ochoa, N.; Vega, C.; Pébère, N.; Lacaze, J.; Brito, J.L. CO2 Corrosion Resistance of Carbon Steel in Relation with Microstructure Changes. Mater. Chem. Phys. 2015, 156, 198–205. [Google Scholar] [CrossRef]
- Saddik, A.A.; Sayed, M.; Mohammed, A.A.K.; Abdel-Hakim, M.; Ahmed, M. Molecular Design and Performance of Emissive Amide-Containing Compounds as Corrosion Inhibitors: Synthesis, Electrochemical Evaluation, DFT Calculations and Molecular Dynamics Simulations. RSC Adv. 2025, 15, 15384–15396. [Google Scholar] [CrossRef]
- Hossain, N.; Asaduzzaman Chowdhury, M.; Kchaou, M. An Overview of Green Corrosion Inhibitors for Sustainable and Environment Friendly Industrial Development. J. Adhes. Sci. Technol. 2021, 35, 673–690. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Quraishi, M.A.; Qurashi, A. Recent Trends in Environmentally Sustainable Sweet Corrosion Inhibitors. J. Mol. Liq. 2021, 326, 115117. [Google Scholar] [CrossRef]
- Quraishi, M.A.; Chauhan, D.S.; Ansari, F.A. Development of Environmentally Benign Corrosion Inhibitors for Organic Acid Environments for Oil-Gas Industry. J. Mol. Liq. 2021, 329, 115514. [Google Scholar] [CrossRef]
- Zhao, J.-M.; Li, J. Corrosion Inhibition Performance of Carbon Steel in Brine Solution Containing H2S and CO2 by Novel Gemini Surfactants. Acta Phys.-Chim. Sin. 2012, 28, 623–629. [Google Scholar]
- Wang, H.; Tang, J.; Xie, J. Corrosion Inhibition of N80 Steel with the Presence of Asymmetric Gemini in a CO2-Saturated Brine Solution. Int. J. Electrochem. Sci. 2017, 12, 11017–11029. [Google Scholar] [CrossRef]
- Sanchez-Salazar, E.; Vazquez-Velez, E.; Uruchurtu, J.; Porcayo-Calderon, J.; Casales, M.; Rosales-Cadena, I.; Lopes-Cecenes, R.; Gonzalez-Rodriguez, J. Use of a Gemini-Surfactant Synthesized from the Mango Seed Oil as a CO2-Corrosion Inhibitor for X-120 Steel. Materials 2021, 14, 4206. [Google Scholar] [CrossRef] [PubMed]
- Carmona Hernandez, A.; Vazquez Velez, E.; Uruchurtu, J.; Gonzalez Rodriguez, J.G. A Corrosion Inhibition Study of a Novel Nonionic Gemini Surfactant Derived from Palm Oil for Supermartensitic Stainless Steel in Sour Solution. ECS Trans. 2019, 94, 41–51. [Google Scholar] [CrossRef]
- Mesquita, T.J.; Chauveau, E.; Mantel, M.; Bouvier, N.; Koschel, D. Corrosion and Metallurgical Investigation of Two Supermartensitic Stainless Steels for Oil and Gas Environments. Corros. Sci. 2014, 81, 152–161. [Google Scholar] [CrossRef]
- Silkin, S.A.; Belevskii, S.S.; Gradinar, A.S.; Petrenko, V.I.; Yakovets, I.V.; Tsyntsaru, N.I.; Dikusar, A.I. Electrodeposition of Nanocrystalline Co-W Coatings from Citrate Electrolytes under Controlled Hydrodynamic Conditions Part 3: The Micro- and Macrodistribution of the Deposition Rates, the Structure, and the Mechanical Properties. Surf. Eng. Appl. Electrochem. 2010, 46, 206–214. [Google Scholar] [CrossRef]
- Onyeachu, I.B.; Solomon, M.M. Benzotriazole Derivative as an Effective Corrosion Inhibitor for Low Carbon Steel in 1 M HCl and 1 M HCl + 3.5 Wt% NaCl Solutions. J. Mol. Liq. 2020, 313, 113536. [Google Scholar] [CrossRef]
- Ngobiri, N.C.; Oguzie, E.E.; Oforka, N.C.; Akaranta, O. Comparative Study on the Inhibitive Effect of Sulfadoxine–Pyrimethamine and an Industrial Inhibitor on the Corrosion of Pipeline Steel in Petroleum Pipeline Water. Arab. J. Chem. 2019, 12, 1024–1034. [Google Scholar] [CrossRef]
- Zeng, Z.; Lillard, R.S.; Cong, H. Effect of Salt Concentration on the Corrosion Behavior of Carbon Steel in CO2 Environment. CORROSION 2016, 72, 805–823. [Google Scholar] [CrossRef]
- Lasia, A. The Origin of the Constant Phase Element. J. Phys. Chem. Lett. 2022, 13, 580–589. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The Analysis of Electrode Impedances Complicated by the Presence of a Constant Phase Element. J. Electroanal. Chem. Interfacial Electrochem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Huang, J. Diffusion Impedance of Electroactive Materials, Electrolytic Solutions and Porous Electrodes: Warburg Impedance and Beyond. Electrochim. Acta 2018, 281, 170–188. [Google Scholar] [CrossRef]
- He, S.; Xue, S.; Xu, H.; Li, B.; Li, J. Hairy Bamboo Leaf Extract as an Eco-Friendly Corrosion Inhibitor for L245N Steel in CO2-Saturated Oilfield Produced Water. Corros. Rev. 2023, 41, 575–591. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, B.; Liu, J.; Zhou, J.; Liu, C. Corrosion Inhibition of Q235 and X65 Steels in CO2-Saturated Solution by 2-Phenyl Imidazoline. Arab. J. Chem. 2023, 16, 104774. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, J.; Liang, G.; Lyu, X.; Li, Z. Evaluation of Green Corrosion Inhibitors of Chitosan Oligosaccharide Derivatives for CO2 Corrosion Inhibition on 20# Carbon Steel. Process Saf. Environ. Prot. 2024, 190, 1520–1535. [Google Scholar] [CrossRef]
- Popoola, L.T. Organic Green Corrosion Inhibitors (OGCIs): A Critical Review. Corros. Rev. 2019, 37, 71–102. [Google Scholar] [CrossRef]
- Musa, A.Y.; Kadhum, A.A.H.; Mohamad, A.B.; Daud, A.R.; Takriff, M.S.; Kamarudin, S.K.; Muhamad, N. Stability of Layer Forming for Corrosion Inhibitor on Mild Steel Surface under Hydrodynamic Conditions. Int. J. Electrochem. Sci. 2009, 4, 707–716. [Google Scholar] [CrossRef]
- das Chagas Almeida, T.; Bandeira, M.C.E.; Moreira, R.M.; Mattos, O.R. New Insights on the Role of CO2 in the Mechanism of Carbon Steel Corrosion. Corros. Sci. 2017, 120, 239–250. [Google Scholar] [CrossRef]
- Belarbi, Z.; Dominguez Olivo, J.M.; Farelas, F.; Singer, M.; Young, D.; Nešić, S. Decanethiol as a Corrosion Inhibitor for Carbon Steels Exposed to Aqueous CO2. CORROSION 2019, 75, 1246–1254. [Google Scholar] [CrossRef]
- Fang, Q.; Zhao, Y.; Wei, J.; Wang, Z.; Yao, J.; Chen, S.; He, M. Flow Corrosion Simulation Study of Local Defects in CO2 Saturated Solution. Gas Sci. Eng. 2024, 131, 205460. [Google Scholar] [CrossRef]
- Olivo, J.D.; Brown, B.; Young, D.; Nešić, S. Effect of Corrosion Inhibitor Alkyl Tail Length on the Electrochemical Process Governing CO2 Corrosion of Mild Steel. CORROSION 2019, 75, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, M.; Tobias, C.W.; Wilke, C.R. Ionic Mass Transfer and Concentration Polarization at Rotating Electrodes. J. Electrochem. Soc. 1954, 101, 306. [Google Scholar] [CrossRef]
- Olvera-Martínez, M.E.; Mendoza-Flores, J.; Rodríguez-Gómez, F.J.; Durán-Romero, R.; Genesca, J. Assessment of the Effects of Acetic Acid and Turbulent Flow Conditions on the Corrosion of API 5L X52 Steel in Aqueous CO2 Solutions. Mater. Corros. 2018, 69, 376–385. [Google Scholar] [CrossRef]
- Kokalj, A. Considering the Concept of Synergism in Corrosion Inhibition. Corros. Sci. 2023, 212, 110922. [Google Scholar] [CrossRef]
- Răuță, D.-I.; Matei, E.; Avramescu, S.-M. Recent Development of Corrosion Inhibitors: Types, Mechanisms, Electrochemical Behavior, Efficiency, and Environmental Impact. Technology 2025, 13, 103. [Google Scholar] [CrossRef]
- Farelas, F.; Galicia, M.; Brown, B.; Nesic, S.; Castaneda, H. Evolution of Dissolution Processes at the Interface of Carbon Steel Corroding in a CO2 Environment Studied by EIS. Corros. Sci. 2010, 52, 509–517. [Google Scholar] [CrossRef]
- Kahyarian, A.; Nesic, S. A New Narrative for CO2 Corrosion of Mild Steel. J. Electrochem. Soc. 2019, 166, C3048–C3063. [Google Scholar] [CrossRef]
- Kahyarian, A.; Brown, B.; Nešić, S. Technical Note: Electrochemistry of CO2 Corrosion of Mild Steel: Effect of CO2 on Cathodic Currents. CORROSION 2018, 74, 851–859. [Google Scholar] [CrossRef]
- Olvera-Martínez, M.E.; Mendoza-Flores, J.; Genesca, J. CO2 Corrosion Control in Steel Pipelines. Influence of Turbulent Flow on the Performance of Corrosion Inhibitors. J. Loss Prev. Process Ind. 2015, 35, 19–28. [Google Scholar] [CrossRef]
- Xiong, Y.; Cao, M. Application of Surfactants in Corrosion Inhibition of Metals. Curr. Opin. Colloid Interface Sci. 2024, 73, 101830. [Google Scholar] [CrossRef]
- Yotapan, N.; Sriplai, N.; Ruengsangtongkul, S.; Sombatmankhong, K. Imidazoline As a Volatile Corrosion Inhibitor for Mitigation of Top- and Bottom-of-the-Line CO2 Corrosion in Carbon Steel Pipelines. Langmuir 2024, 40, 11888–11902. [Google Scholar] [CrossRef]
- Adewuyi, A.; Oderinde, R.A. Synthesis of Hydroxylated Fatty Amide from Underutilized Seed Oil of Khaya Senegalensis: A Potential Green Inhibitor of Corrosion in Aluminum. J. Anal. Sci. Technol. 2018, 9, 26. [Google Scholar] [CrossRef]
- Galvan-Martinez, R.; Fabre-Pulido, J.; Carmona-Hernández, A.; Orozco-Cruz, R.; Subramanian, V.; Contreras, A. Imidazoline Behavior as Corrosion Inhibitor in the Electrochemical Characterization of SCC Behavior of an API X70 Steel Exposed to Brine Solution. Corros. Rev. 2023, 41, 455–471. [Google Scholar] [CrossRef]
- Pakiet, M.; Kowalczyk, I.; Leiva Garcia, R.; Moorcroft, R.; Nichol, T.; Smith, T.; Akid, R.; Brycki, B. Gemini Surfactant as Multifunctional Corrosion and Biocorrosion Inhibitors for Mild Steel. Bioelectrochemistry 2019, 128, 252–262. [Google Scholar] [CrossRef] [PubMed]
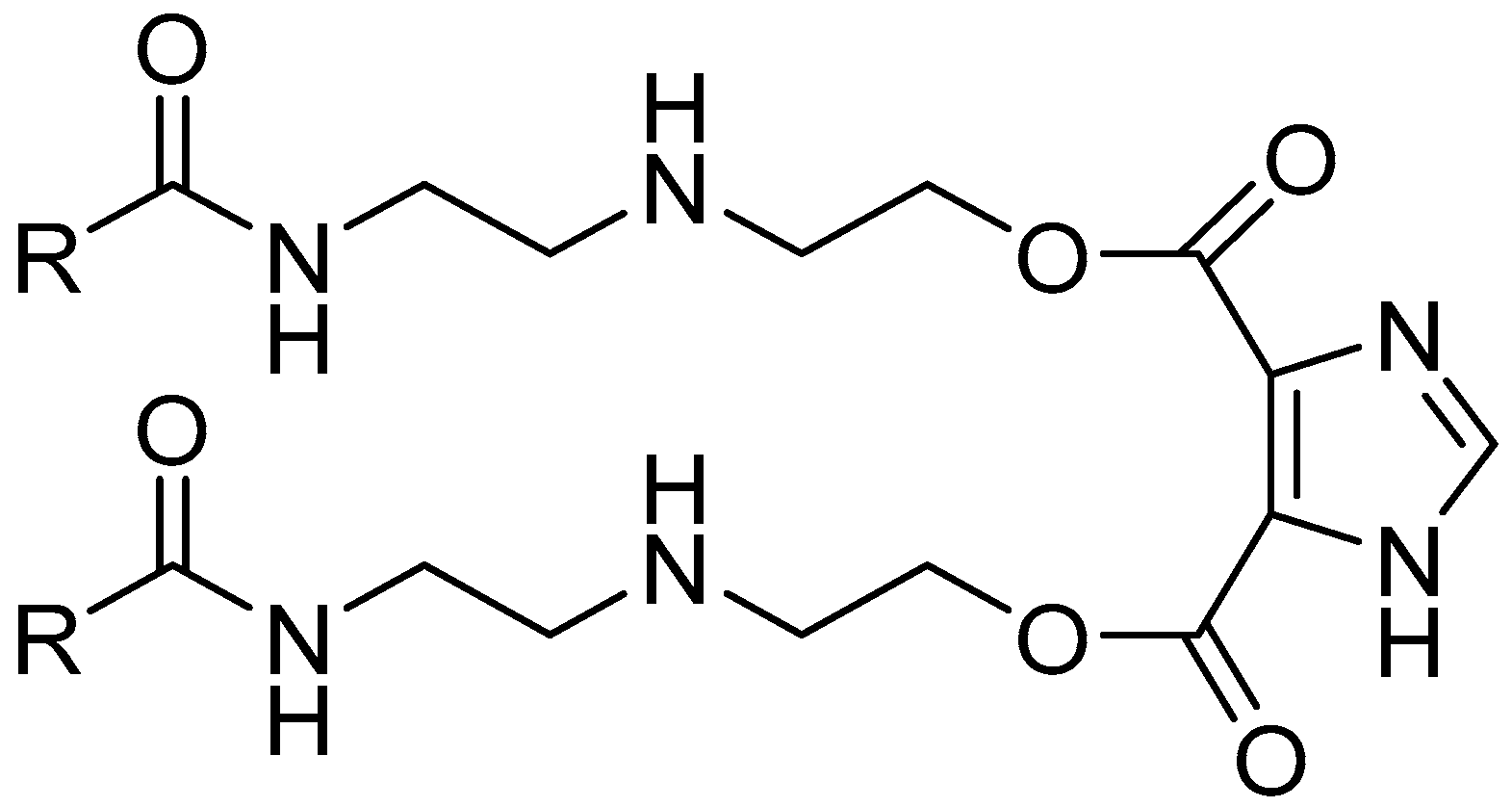


| Element (wt.%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| C | Mn | Si | Cr | Ni | Mo | Nb/V | Cu | Ti | Fe |
| 0.05 | 1.75 | 0.36 | 0.36 | 0.29 | 0.2 | 0.03 | 0.51 | 0.04 | Bal. |
| T (°C) | t (h) | Cinh (ppm) | Rs (Ωcm2) | Rf (Ωcm2) | Qf-Y0 (sn/Ωcm2) | Qf-n | Rct (Ωcm2) | CPEdl-Y0 (sn/Ωcm2) | Qdl-n | σ (Ωcm2/s0.5) | Cdl (μF/cm2) | χ2 × 104 | η (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25 | 0 | 0 | 7.20 | 119.9 | 8.54×10−4 | 0.75 | 248.39 | 0.35 | |||||
| 25 | 4.89 | 3.43 | 4.39×10−3 | 0.21 | 102.3 | 4.78×10−4 | 0.82 | 5.75 | 160.14 | 0.48 | - | ||
| 50 | 6.79 | 21.28 | 1.10×10−3 | 0.99 | 157.3 | 1.06×10−3 | 0.70 | 8.04 | 223.07 | 1.22 | 23.78 | ||
| 100 | 7.63 | 11.41 | 5.02×10−4 | 0.74 | 168.50 | 3.14×10−5 | 1.00 | 14.32 | 31.39 | 1.38 | 28.84 | ||
| 24 | 0 | 7.07 | 136.6 | 1.13×10−3 | 0.84 | 621.90 | 1.50 | ||||||
| 25 | 4.78 | 9.35 | 1.25×10−3 | 0.75 | 229.5 | 6.23×10−5 | 1.00 | 62.29 | 1.94 | 40.48 | |||
| 50 | 6.37 | 8.24 | 1.75×10−4 | 0.77 | 524.13 | 4.03×10−4 | 0.80 | 127.68 | 2.95 | 73.94 | |||
| 100 | 8.33 | 16.49 | 1.85×10−4 | 0.79 | 608.06 | 3.02×10−4 | 0.84 | 130.95 | 9.05 | 77.54 | |||
| 60 | 0 | 0 | 3.53 | 32.1 | 1.44×10−3 | 0.77 | 288.17 | 18.50 | |||||
| 25 | 3.40 | 1.98 | 6.36×10−4 | 0.56 | 71.57 | 6.90×10−4 | 0.80 | 150.10 | 6.80 | 55.15 | |||
| 50 | 5.07 | 2.87 | 8.72×10−4 | 0.65 | 102 | 6.23×10−5 | 1.00 | 9.85 | 62.28 | 10.01 | 68.53 | ||
| 100 | 1.97 | 4.58 | 7.77×10−4 | 0.74 | 116.50 | 4.34×10−5 | 1.00 | 5.81 | 43.39 | 12.80 | 72.45 | ||
| 24 | 0 | 3.64 | 9.81 | 4.94×10−3 | 0.79 | 61.29 | 4.96×10−4 | 1.00 | 15.02 | 496.30 | 4.00 | ||
| 25 | 5.54 | 3.39 | 3.51×10−4 | 0.85 | 262.3 | 7.15×10−4 | 0.79 | 230.00 | 3.28 | 76.48 | |||
| 50 | 8.54 | 3.07 | 7.22×10−5 | 0.83 | 254.3 | 4.26×10−4 | 0.83 | 188.87 | 3.91 | 75.90 | |||
| 100 | 3.78 | 6.48 | 4.92×10−4 | 0.78 | 305.20 | 1.14×10−4 | 0.92 | 64.28 | 1.51 | 79.92 |
| Temp (°C) | Cinh (ppm) | Ecorr (mV) | ba (mV/dec) | bc (mV/dec) | icorr (mA/cm2) | η (%) |
|---|---|---|---|---|---|---|
| 25 | 0 | −711.64 | 86.52 | −214.20 | 0.0344 | - |
| 25 | −618.43 | 55.11 | −391.55 | 0.0197 | 42.59 | |
| 50 | −626.50 | 59.51 | −356.51 | 0.0137 | 60.11 | |
| 100 | −635.13 | 58.46 | −286.13 | 0.0068 | 80.13 | |
| 60 | 0 | −645.00 | 28.80 | −320.10 | 0.1350 | - |
| 25 | −683.16 | 65.03 | −296.22 | 0.0239 | 82.30 | |
| 50 | −639.09 | 55.74 | −385.93 | 0.0248 | 81.63 | |
| 100 | −636.75 | 60.12 | −380.07 | 0.0364 | 73.01 |
| t (h) | Cinh (ppm) | Rs (Ωcm2) | Rf (Ωcm2) | Qf-Y0 (sn/Ωcm2) | Qf-n | Rct (Ωcm2) | CPEdl-Y0 (sn/Ωcm2) | Qdl-n | Rad (Ωcm2) | Lad (Hcm2) | σ (Ωcm2/s0.5) | Cdl (μF/cm2) | χ2 × 104 | η (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0 | 12.82 | 245.3 | 3.27×10−4 | 0.72 | 119.60 | 26.27 | 37.08 | 22.8 | |||||
| 25 | 18.07 | 47.37 | 1.03×10−4 | 0.80 | 1340.4 | 3.45×10−5 | 0.76 | 3.51 | 2.79 | 81.70 | ||||
| 50 | 17.17 | 80.73 | 6.85×10−5 | 0.80 | 1653.4 | 5.90×10−5 | 0.76 | 6.89 | 7.44 | 85.16 | ||||
| 100 | 17.72 | 27.61 | 1.68×10−4 | 0.83 | 871.76 | 6.63×10−5 | 0.81 | 13.37 | 1.37 | 71.86 | ||||
| 24 | 0 | 14.17 | 115 | 2.16×10−3 | 0.74 | 8.85 | 617.20 | 9.25 | ||||||
| 25 | 27.92 | 21.36 | 6.99×10−4 | 0.67 | 566.44 | 9.32×10−5 | 0.87 | 39.48 | 3.88 | 79.70 | ||||
| 50 | 23.25 | 122.77 | 8.09×10−4 | 0.64 | 639.66 | 2.60×10−4 | 0.78 | 61.45 | 2.87 | 82.02 | ||||
| 100 | 19.61 | 26.93 | 5.39×10−5 | 0.73 | 445.82 | 2.10×10−4 | 0.70 | 20.28 | 3.54 | 74.20 |
| Cinh (ppm) | Ecorr (mV) | ba (mV/dec) | bc (mV/dec) | icorr (mA/cm2) | η (%) |
|---|---|---|---|---|---|
| 0 | −495.37 | 85.37 | -- | 0.5179 | -- |
| 25 | −535.78 | 105.56 | -- | 0.3052 | 41.07 |
| 50 | −560.75 | 79.81 | −392.75 | 0.044 | 91.05 |
| 100 | −544.8 | 115.47 | -- | 0.2894 | 44.1 |
| Condition | Kads (L/mol) | ∆Gads (kJ/mol) |
|---|---|---|
| 25 °C | 26,456 | −35.16 |
| 60 °C | 268,442 | −40.90 |
| 1000 RPM 25 °C | 148,527 | −39.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carmona-Hernandez, A.; Sánchez-Garrido, R.A.; Palacios-González, E.; Flores-Frías, E.A.; Landa-Gómez, A.E.; Mejía-Sánchez, E.; Espinoza-Vázquez, A.; Orozco-Cruz, R.; Galván-Martínez, R. Electrochemical Characterization of CO2 Corrosion Inhibition of API X100 by a Gemini Surfactant Under Static and Dynamic Conditions. Metals 2025, 15, 918. https://doi.org/10.3390/met15080918
Carmona-Hernandez A, Sánchez-Garrido RA, Palacios-González E, Flores-Frías EA, Landa-Gómez AE, Mejía-Sánchez E, Espinoza-Vázquez A, Orozco-Cruz R, Galván-Martínez R. Electrochemical Characterization of CO2 Corrosion Inhibition of API X100 by a Gemini Surfactant Under Static and Dynamic Conditions. Metals. 2025; 15(8):918. https://doi.org/10.3390/met15080918
Chicago/Turabian StyleCarmona-Hernandez, Andres, Rolando Abraham Sánchez-Garrido, Eduardo Palacios-González, Elizabeth America Flores-Frías, Aldo Emelio Landa-Gómez, Edgar Mejía-Sánchez, Araceli Espinoza-Vázquez, Ricardo Orozco-Cruz, and Ricardo Galván-Martínez. 2025. "Electrochemical Characterization of CO2 Corrosion Inhibition of API X100 by a Gemini Surfactant Under Static and Dynamic Conditions" Metals 15, no. 8: 918. https://doi.org/10.3390/met15080918
APA StyleCarmona-Hernandez, A., Sánchez-Garrido, R. A., Palacios-González, E., Flores-Frías, E. A., Landa-Gómez, A. E., Mejía-Sánchez, E., Espinoza-Vázquez, A., Orozco-Cruz, R., & Galván-Martínez, R. (2025). Electrochemical Characterization of CO2 Corrosion Inhibition of API X100 by a Gemini Surfactant Under Static and Dynamic Conditions. Metals, 15(8), 918. https://doi.org/10.3390/met15080918











