Abstract
Boron (B) is widely used as a neutron-absorbing nuclide and has significant applications in the nuclear industry. B4C/Al composites combine the high hardness of B4C with the ductility of Al, making them commonly used neutron-absorbing materials. Under current preparation methods, the poor wettability and low reactivity of B4C with molten Al limit its effective incorporation into the matrix, and the addition of B4C in B4C/Al composites has reached its threshold limit, making it difficult to achieve breakthrough improvements in neutron absorption performance. However, incorporating additional B elements into the B4C/Al composite can break this limit, effectively enhancing the material’s neutron absorption performance. Nevertheless, research on the impact of this addition on the mechanical properties of the composite remains unclear. The requirements for B4C/Al composites as spent fuel storage and transportation devices include high mechanical strength and certain machinability. This study fabricated B4C/Al composites with varying B contents (5 wt.%, 10 wt.%, and 15 wt.%), and the influence of B addition on the microstructure and mechanical properties of B4C/Al composites was investigated. The results demonstrate that the composites exhibit a density of approximately 99% with well-established interfacial bonds. Increasing B content leads to a higher quantity of interfacial reaction products Al3BC and AlB2, enhancing the Vickers hardness to 370.93 HV. The bending strength and fracture toughness of composites with 5 wt.% and 15 wt.% B addition decreased, whereas those with 10 wt.% B exhibited excellent resistance to crack growth and high-temperature plastic deformation due to a high content of ductile phase.
1. Introduction
The global demand for nuclear power has notably increased in recent years. However, the rapid development of the nuclear energy sector has led to significant quantities of spent fuel, which remains radioactive and poses environmental and health hazards if not properly stored [1,2]. Neutron-absorbing materials with excellent mechanical and neutron absorption properties are essential for the safe storage and transportation of spent fuel. Boron (B), with its considerable neutron capture cross-section and cost-effectiveness, is a prominent neutron absorber [3,4]. Currently, boron-based materials for neutron absorption include boron steel [5], boron-containing polymers [6], B-Al composites [7], and B4C/Al composites [8].
The neutron absorption performance of boron-containing composites is positively correlated with B content [9]. Boron steel possesses exceptional mechanical properties and corrosion resistance, but its B content typically remains below 2.25 wt.% [10], as excessive B leads to boride precipitation and poor processing performance. However, insufficient B content fails to meet the requirements of neutron-absorbing materials, thus restricting the application of boron steel. Boron-containing polymers, known for their light weight and ease of manufacturing, are commonly used in portable radiation shielding equipment. However, many irradiation studies have indicated that the polymer is prone to aging, thereby limiting its service life and application [11,12]. The solubility of B in Al alloys is low, and costly enrichment with 10B is commonly employed to enhance surface density. B can be incorporated into Al alloys by powder metallurgy, but it results in the formation of boron-rich borides and affects the mechanical properties of the composite [13]. Spent fuel storage and transportation devices are subject to high mechanical stress and are exposed to high temperatures, so the devices should combine exceptional structural and high-temperature mechanical properties with high neutron absorption capacity [14].
B4C/Al composites are common neutron-absorbing materials with a wide range of applications in the field of nuclear protection [15,16]. They are composed of B4C particles incorporated into the Al matrix, combining the high strength and high elastic modulus characteristics of B4C with the lightweight and easily processable nature of the Al matrix. The pressureless infiltration method is advantageous for preparing B4C/Al composites with high B4C content, potentially exceeding 50 wt.% [17]. Currently, we have prepared B4C/Al composites with a B4C content of up to 52 wt.%, and the mechanical properties are in accordance with the standards for neutron-absorbing materials [18]. However, the intense covalent bonding [19] in B4C impedes particle consolidation during pressureless sintering [20], thereby constraining boron enrichment in B4C/Al composites. It can be achieved by introducing neutron absorption nuclides in the B4C/Al composite to improve the neutron absorption.
Neutron-absorbing nuclides such as Gd and B can be added to B4C/Al composites to improve their neutron-absorbing properties. Xu et al. [21] prepared (15 wt.% B4C + 1 wt.% Gd)/Al composites by vacuum hot press sintering; the addition of Gd can partially replace B4C content in the composite, which can increase the plasticity of the composite and keep the neutron shielding property simultaneously. However, Gd is a toxic high-valent heavy metal and emits excessive secondary γ-rays during thermal neutron capture reactions, which limits its application in neutron absorption [22,23]. In contrast, incorporating B into the composites significantly enhances neutron absorption, as B produces low-energy secondary γ-rays (0.48 MeV) that are easily shielded after neutron capture [22,24]. To increase B content and enhance sinterability, some studies have utilized Ni2B-coated B4C particles, which generate a liquid phase during sintering and facilitate densification through B diffusion [25]. However, this approach is not suitable for the present study, as the introduction of NiB may lead to complex interfacial reactions with molten Al and compromise microstructural stability. In this study, B powder was directly added to the B4C/Al composites to effectively increase the boron content while avoiding the formation of undesired secondary phases. However, the effect of B addition on the mechanical properties of B4C/Al composites remains unclear and will be systematically investigated in this study.
To improve the neutron absorption capacity of the composites, this study fabricated B4C/Al composites with varying B addition contents through pressureless infiltration, and their mechanical properties were evaluated. The form of B and the phase distribution of the composite were characterized and analyzed using XRD and SEM. Furthermore, the mechanical properties at room temperature and high-temperature deformation capacity of the composite were investigated to evaluate the effect of B addition on it and its suitability as a structural material. This will provide an experimental basis for the design of B4C/Al composites with enhanced neutron absorption capabilities.
2. Experimental Procedures
2.1. Fabrication Process
Commercially available powders of B4C (purity ≥ 99.9%, 3.5 μm, Shimian Bosen Technology Abrasives Co., Ltd., Ya’an, China), B powder (purity ≥ 99.9%, 1 μm, Hebei Casting and Research Metal Material Co., Ltd., Xingtai, China), and Al particles (purity ≥ 99.9%, 1 mm, Beijing Guantai Metal Materials Co., Ltd., Beijing, China) were used as raw materials. The morphology of B4C and B powders is depicted in Figure 1. The B4C powder displays an irregular polyhedral morphology with sharp edges, indicative of its crystalline nature, whereas the B powder exhibits an approximately spherical shape with clear fine particle agglomerations or clusters, suggesting an amorphous or low-crystallinity structure.
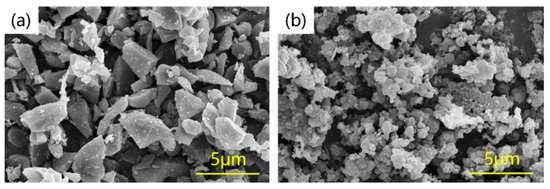
Figure 1.
The micromorphology of raw powders (a) B4C (b) B.
B4C mixed powders added with varying B contents (0 wt.%, 5 wt.%, 10 wt.%, 15 wt.%) were weighed and mixed with 4 wt.% PVA as a binder. The mixture was then ball-milled using an Omni-directional Planetary Ball Mill (QXQM-4, Suzhou Haton Technology Co., Ltd., Suzhou, China) at 180 r/min for 4 h with agate balls. The suspension was transferred to a metal container and subsequently dried at 80 °C for 10 h in an air blast drying oven. The dried powder block was pulverized, sieved through a 100-mesh sieve, and stored in a glass jar for future utilization.
The green billets were prepared by cold pressing the dry mixed powder into an alloy mold at 373 MPa for 3 min. Afterward, the billet was sintered in a tube furnace (GSL-1750X, Hefei Kejing Material Technology Co., Ltd., Hefei, China) and preheated to 1500 °C for 2 h. The sintering atmosphere consists of 99.9% Ar, with a flow rate of 80 sccm to prevent oxidation of the B4C preform. The preforms were coated with Al particles and subjected to infiltration at 1200 °C for 0.5 h in the tube furnace. During heating, the Al powder melted, and the resulting liquid Al spontaneously infiltrated the porous preform due to capillary action. In addition, the fluidity of the molten Al alloy further facilitated its penetration into the porous network. Finally, the composite was obtained after cooling it to room temperature.
2.2. Characterization
X-ray diffraction (XRD, PANalytical, X’pert, Almelo, The Netherlands) under 45 kV and 40 mA Cu-kα radiation was used to investigate the phase composition of B4C/Al composites added with varying B contents, with a 2θ ranging from 20° to 80°. The backscattered electron (BSE) mode of the field emission scanning electron microscope (SEM, JSM-7800F, JEOL, Tokyo, Japan) was utilized to investigate the phase distribution of the composites. The relative density of the composites was determined through the Archimedean drainage method according to the ASTM B962-2023 standard.
The automatic polishing machine was employed to polish the surfaces of samples with defects caused by wire cutting. The Vickers hardness test was conducted using a micro-Vickers hardness tester (HV-1000A, Laizhou Huayin Test Instrument Co., Ltd., Laizhou, China) with a 1 kg load for 10 s, and each sample was tested nine times to ensure accuracy. The bending strength was carried out according to the standard GB/T 6569-2006 [26] using an electronic universal testing machine (E43.104, MTS Industrial System Co., Ltd., Shanghai, China) with a strain rate of 0.05 mm/min, employing the three-point bending method. The specimen size was 25 mm × 4 mm × 3 mm with a lower span of 16 mm. The flexural strength of the composites was determined from the stress–strain curve, and the fracture morphology of the specimens was analyzed. Additionally, the fracture toughness was assessed using the single-edge precracked beam (SEPB) method on the electronic universal testing machine according to the standard GB/T 23806-2009 [27]. The sample dimensions were 25 mm × 5 mm × 2.5 mm with an opening height of 2.5 mm, and the lower span was 20 mm. The ability of the composite to be against crack propagation is contingent upon the calculated fracture toughness value.
To gain insight into the thermal deformation process of the material, high-temperature compression experiments were conducted on a sample sized φ8 × 12 mm using a Gleeble thermal simulation tester (Gleeble-3500, Dynamic Systems Inc., El Segundo, CA, USA). The strain rate was 0.001 s−1, and the temperature was 500 °C, with a preset compression of 20%. The heating rate was set at 10 °C/s, and samples were held for 3 min after reaching the deformation temperature to remove the temperature gradient. The compression samples were analyzed macroscopically to assess their morphology, and flow stress profiles were evaluated to assess their plastic deformability at elevated temperatures.
3. Results and Discussion
3.1. Microstructure
The XRD patterns of the B4C/Al composites with varying B contents are presented in Figure 2. The diffraction data show that the B-added composites mainly consist of Al, B4C, AlB2, and Al3BC phases, where AlB2 and Al3BC are reaction products. In addition, the diffraction peak for B has not been detected. Due to the strong metallic nature of A, both boron (B) and carbon (C) atoms exhibit high chemical reactivity with molten Al during the infiltration process. Within the binary B-Al system, elemental B readily reacts with molten Al to form AlB2, which is recognized as the most thermodynamically stable intermetallic phase below 980 °C [28], as described by Equation (1) [29,30]. In the more complex ternary B-C-Al system, B4C can undergo partial thermal decomposition at elevated temperatures, releasing free B and C atoms, as shown in Equation (2) [31]. These liberated atoms can further interact with the molten Al at the interface, leading to the concurrent formation of AlB2 and Al3BC phases, as represented by Equation (3) [32]. Moreover, under conditions where excess Al and C are present, AlB2 may progressively transform into Al3BC phase, following the reaction outlined in Equation (4) [33].
B (s) + 2Al (l) → AlB2 (s)
B4C (s) → 4B (s) + C (s)
2B4C (s) + 9Al (l) → 2Al3BC (s) + 3AlB2 (s)
AlB2 (s) + 2Al (s) + 2C (s) → Al3BC (s)
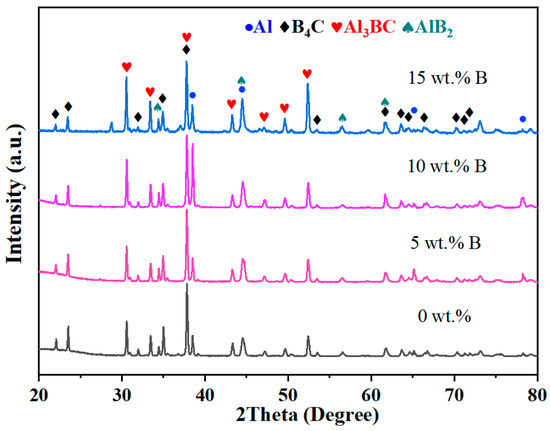
Figure 2.
XRD patterns of B4C/Al composites with different B contents.
The diffraction peak intensity of Al increases with the increase in B addition content and reaches a peak at 10 wt.% B addition, which suggests that the content of Al in the B4C/Al composites is the highest when the B addition is 10 wt.%. Molten Al continuously infiltrates the pores of the preform at high temperatures, and the addition of an appropriate amount of B can enhance the wettability between Al and the preform, thereby facilitating more effective infiltration. Meanwhile, suitable B addition promotes beneficial interfacial reactions without causing excessive formation of reaction products, thus minimizing the consumption of Al. However, when the B addition increases to 15 wt.%, excessive B reacts with molten Al to form a large quantity of intermediate phases such as AlB2 and Al3BC, which significantly deplete the Al phase and lead to a notable reduction in its overall content. Additionally, the diffraction peaks of Al3BC and AlB2 show significant enhancement, with Al3BC exhibiting a more pronounced trend. Mei Y et al. [33] synthesized Al3BC/Al composites through in situ solid phase reaction and observed that with prolonged reaction time, the production of Al3BC slightly increased, while the amount of AlB2 phase decreased. At elevated temperatures, B4C decomposes and releases free B and C [34], which subsequently participate in reactions with the molten Al matrix. Initially, amorphous B reacts with Al to form the AlB2 phase. Simultaneously, the free C released from B4C decomposition may also react with both AlB2 and the excess molten Al, further promoting the conversion of AlB2 into Al3BC. Therefore, with increasing B addition, a decrease in the AlB2 phase and a corresponding increase in the Al3BC phase are observed.
Characterization via BSE imaging elucidates the phase distribution at the interfaces in the composites. The morphology of B4C/Al composites with different B contents is presented in Figure 3. It can be observed that B4C exhibits an angular and irregular particle shape with a uniform dispersion in the Al matrix, and a well-defined boundary between B4C and the matrix indicates no obvious agglomeration of the composites. The localized interfacial products Al3BC and AlB2 were formed around the B4C particles; the quantity of Al3BC shows an increasing trend, while that of AlB2 shows a decreasing trend, both of which are consistent with the XRD results. Furthermore, no significant defects were observed within the composites.
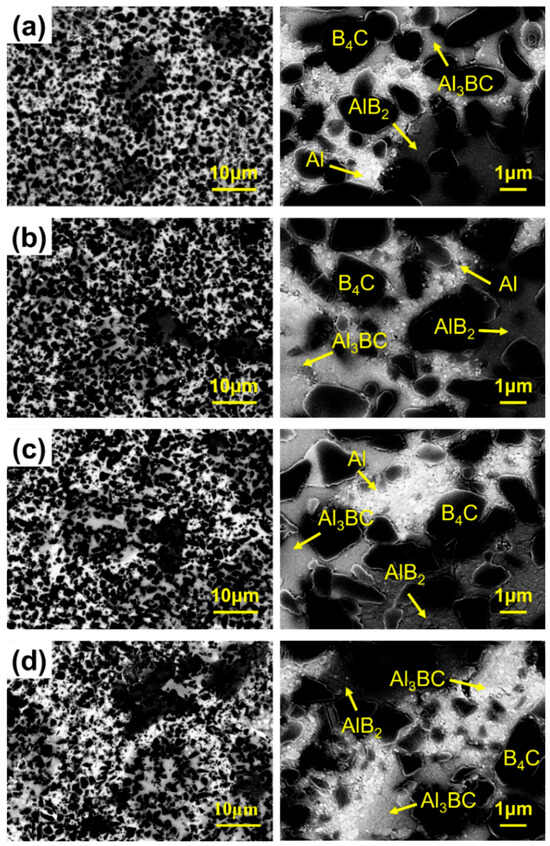
Figure 3.
BSE images of B4C/Al composites with different B contents. (a) 0 wt.%, (b) 5 wt.%, (c) 10 wt.%, (d) 15 wt.%.
The relative density results of the composites in different liquids measured by the Archimedean drainage method are presented in Table 1. The relative densities, denoted as e1, e2, and e3, are ascertained using the conventional vacuum method in different liquids based on Archimedes’ principle. The liquids are pure water (density: 1.0 g/cm3), anhydrous ethanol (density: 0.79 g/cm3), and sonication in anhydrous ethanol for 10 min, respectively.

Table 1.
The relative density results of B4C/Al composites with different B contents.
The results show a near-complete density of approximately 99%, corroborating the BSE imaging observations and indicating the absence of significant defects. Notably, the relative density of the composite increases with higher B addition content, peaking at 10 wt.% B, where optimal interfacial bonding between the reinforcement phase and aluminum matrix is achieved. When B addition reaches 15 wt.%, excessive intermediate reaction byproducts are formed at the interface of Al and B4C, which may prevent further interfacial reactions and lead to defects inside the composite.
3.2. Mechanical Properties at Room Temperature
The Vickers hardness of B4C/Al composites with varying B contents is presented in Figure 4. It demonstrates a positive correlation with B addition, with the average hardness values of the composite escalating from 283.38 HV to 370.93 HV as the B content increases from 0 to 15 wt.%. XRD analysis showed that with the increase in B addition, the content of Al3BC ceramic phase in the composites increased significantly. The hard phase effectively enhanced the deformation resistance of the composites by blocking the dislocation sliding mechanism, thus increasing the tensile strength and hardness of the composites [35]. Therefore, B addition is helpful to increase the Vickers hardness of B4C/Al composite to withstand the impact that may be suffered during the storage and transportation of spent fuel.
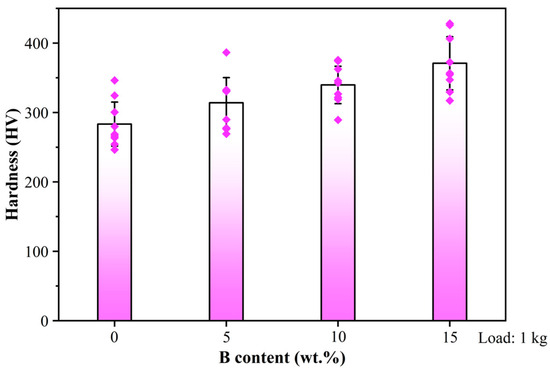
Figure 4.
Vickers hardness of B4C/Al composites with different B contents.
Figure 5 presents the results of the three-point bending and fracture toughness tests. Figure 5a,b display the stress–strain curves derived from three-point bending tests and the bending strength and flexural modulus values of the B4C/Al composite, respectively. The average flexural modulus manifests an initial increment followed by a decrement as the B addition increases, peaking at 10 wt.% B addition. The mean values of the flexural modulus for B4C/Al composites with different B addition contents are 111.00 GPa, 110.19 GPa, 116.50 GPa, and 110.38 GPa, respectively. This finding is congruent with the bending strength results, indicating superior fracture resistance at the 10 wt.% B addition thresholds.
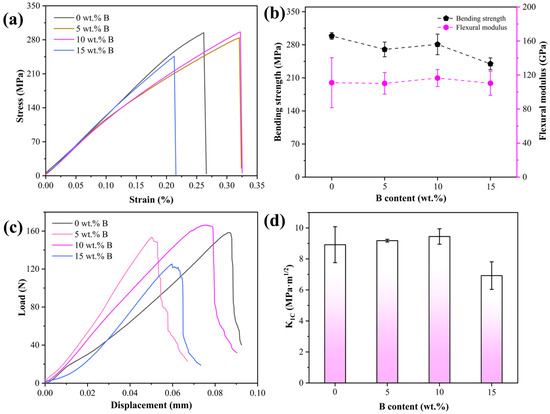
Figure 5.
(a) Bending stress–strain curves, (b) bending strength and flexural modules, (c) load–displacement curves, and (d) fracture toughness values of B4C/Al composites with different B contents.
When added with 10 wt.% B, B4C/Al composites demonstrate peak elongation values and exhibit superior flexural strength. This enhanced ductility is directly correlated with their increased metallic Al content compared to other boron-added variants of the composite, where higher proportions of the Al matrix significantly improve plastic deformation capability. Additionally, composites with higher relative density exhibit stronger interfacial bonding between the reinforcing phase and matrix, enabling efficient stress transfer and reduced stress concentration, thereby enhancing the overall material strength. As the B addition increases to 15 wt.%, the composites exhibit a higher fraction of brittle phases combined with lower relative density. This microstructural heterogeneity promotes stress concentration at pore sites during mechanical testing, which triggers crack nucleation and propagation [36], accelerates fracture, and consequently causes a significant reduction in elongation. Moreover, in B4C/Al composites with high ceramic-phase content, interfacial delamination between the matrix and ceramic particles is prone to occur during testing [37], leading to a decrease in material strength. This phenomenon accounts for the observed decline in flexural strength at 15 wt.% B addition.
Figure 5c,d show the load–displacement curves and fracture toughness values of B4C/Al composites with varying B addition levels, highlighting changes in fracture toughness. The fracture load of B4C/Al composites shows an initial increase followed by a subsequent decrease, which parallels the trend observed in bending strength. The fracture loads of the composites added with different B contents are 158.48 N, 153.52 N, 166.40 N, and 125.25 N, respectively, with the 10 wt.% B sample showing the highest fracture resistance. Furthermore, the slight bending lines of the curves with increasing displacement indicate that the Al matrix undergoes minor plastic deformation before fracturing. Notably, the 10 wt.% B-added composite displays a distinct load–displacement platform at peak load (highlighted by arrows), characteristic of ductile behavior [38]. This plateau suggests prolonged energy dissipation through plastic deformation, thereby improving fracture toughness.
Figure 5d illustrates that the peak value of 9.45 MPa·m1/2 is achieved at 10 wt.% B addition. This indicates that the composites exhibit the greatest resistance to crack propagation at this addition level. The enhanced fracture toughness arises because the higher proportion of the ductile Al phase in the composites at 10 wt.% B addition promotes crack deflection [39], which increases the crack propagation resistance. Furthermore, the three-point bending fracture morphology and post-fracture elongation measurements show that the B4C/Al composites with high Al content demonstrate exceptional plastic deformation capacity. The increased Al content at this B level contributes to the enhanced fracture toughness, correlated with the material’s ductility. However, when the B addition increases to 15 wt.%, a noticeable decrease in fracture toughness is observed, dropping to 6.92 MPa·m1/2. Excessive B addition leads to the formation of more brittle intermetallic phases while simultaneously reducing the volume fraction of the Al phase, thereby decreasing the toughness of the composite. SEM observations also indicate the presence of interfacial debonding and microvoids in the 15 wt.% B composites, which serve as crack initiation sites and facilitate rapid crack propagation [40]. Consequently, these factors synergistically reduce the fracture toughness of the composites. Therefore, while an appropriate amount of B addition can improve ductility, excessive B content may restrict plastic deformation, leading to a brittle fracture upon crack propagation.
In summary, the composite with 10 wt.% B exhibits optimal flexural strength, modulus, elongation, and fracture toughness. These improvements are attributed to the higher metallic Al content and better densification, which enhances plastic deformation and stress transfer. However, further B addition (15 wt.%) leads to increased brittle phases, reduced Al content and density, and more pronounced interfacial defects, all contributing to lower mechanical performance. The results indicate that moderate B addition optimizes strength and toughness, while excessive B deteriorates composite ductility and fracture resistance.
The fracture morphology characteristics of B4C/Al composites with varying B contents after the three-point bending test are illustrated in Figure 6. All samples exhibit distinct mixed fracture characteristics. The unadded B4C/Al composite demonstrates ductile fracture features, including dimples and tearing edge patterns of varying sizes and depths, alongside brittle fracture features like river patterns. The dimples are microvoids formed through the material’s plastic deformation, originating from nucleation, growth, aggregation, and eventual interconnection [40]. The term “tearing ridge” describes the white lines encircling the dimples, a feature of ductile failure. In contrast, flat regions with river patterns and cleavage steps are associated with the brittle fracture of the ceramic phase.
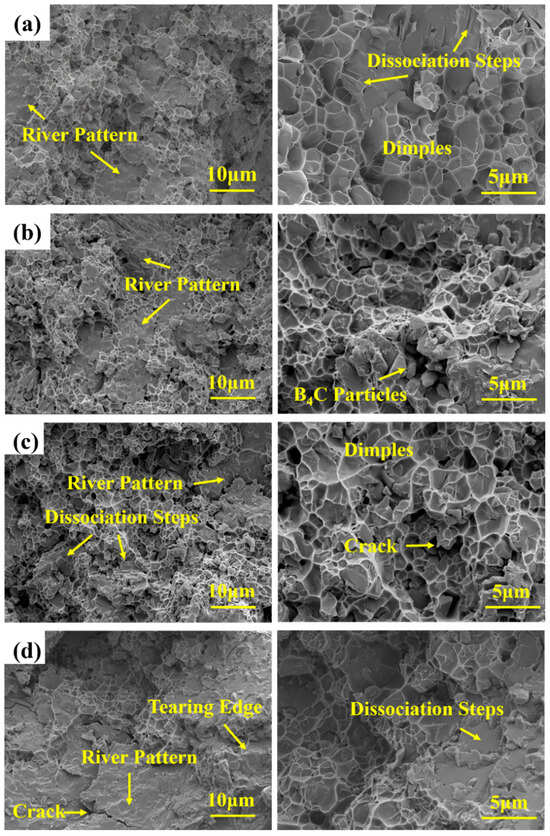
Figure 6.
Fracture morphology of B4C/Al composites with different B contents after three-bending test (a) 0 wt.%, (b) 5 wt.%, (c) 10 wt.%, (d) 15 wt.%.
For the sample with 5 wt.% B addition, the fracture surface exhibits a mixed mode, with observable B4C particles of near-complete morphology, suggesting weak interfacial bonding and particle debonding [41]. Increasing the B addition to 10 wt.% significantly increases the number and size of dimples, with a concurrent reduction in river patterns, indicating enhanced composite plasticity [42]. This improvement is attributed to the higher content of Al, which possesses excellent plasticity. Conversely, at 15 wt.% B addition, the fracture surface displays brittle characteristics, including numerous cleavage steps and river patterns, alongside tearing ridges and dimples. The presence of cracks [43] may be ascribed to the higher interfacial strength relative to the matrix, leading to microvoid nucleation, growth, and coalescence within the matrix as the primary fracture mechanisms [44]. In conclusion, appropriate B addition can improve the toughness of B4C/Al composites. Nevertheless, an overly high addition concentration may make the material brittle, thus exerting a negative influence on its overall performance.
3.3. Mechanical Properties Under High Temperature
The macroscopic morphology of B4C/Al composites after quasi-static high-temperature compression is illustrated in Figure 7. The unadded composites exhibit a “drum shape”, which is a transverse plastic deformation induced by the force applied to the sample to reach its yield strength.
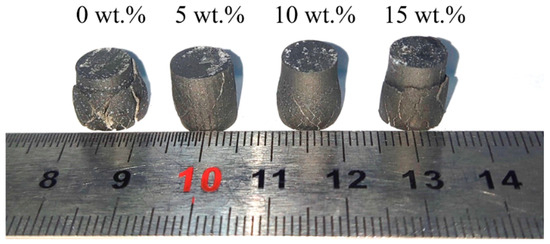
Figure 7.
Photograph of B4C/Al composites with different B contents after high-temperature compression.
In addition, shear fracture cracks at 45° angles parallel to the loading direction are observed on the surface of the composites, indicating that the sample undergoes simultaneous shear stress [45] and normal stress. At 5 wt.% B addition, both the swelling degree and oblique crack dimensions of the sample are reduced. However, for 10 wt.% B addition, the oblique cracks appear shallower and sparser, with the swelling degree intermediate between those of 0 wt.% and 5 wt.%. When the B content reaches 15 wt.%, the swelling degree is similar to that at 10 wt.% addition, but the deeper and wider cracks suggest a significant reduction in plastic deformation capacity.
Figure 8 illustrates the stress–strain behavior of B4C/Al composites at a quasi-static strain rate during high-temperature compression. The high sensitivity of the testing equipment leads to oscillatory fluctuations in the stress–strain curves, which arise from even minor internal defects in the specimens. Notably, the peak flow stress of the composites decreases progressively with increasing boron content, reaching a minimum value of 151.65 MPa at 10 wt.% B accompanied by a pronounced flattening of the curves. At elevated temperatures, the increased Al content in the sample enhances its plastic flow capability [46], promoting homogeneous stress distribution across extensive strain ranges and mitigating localized stress concentration. In contrast, when the boron content is elevated to 15 wt.%, the strain at peak flow stress sharply reduces to approximately 2% with a significantly higher stress value of 285 MPa. This is due to excessive ceramic-phase particles that immobilize grain boundaries, hindering the plastic flow of the Al matrix and inducing localized stress concentration, ultimately resulting in poor hot workability [46].
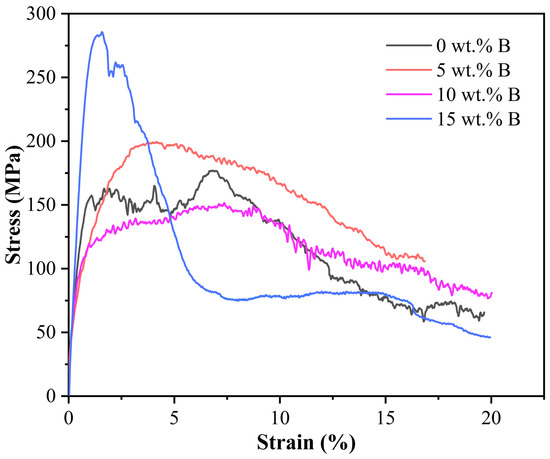
Figure 8.
Stress–strain curves of B4C/Al composites with different B contents during high-temperature compression.
At 500 °C, the Al component softens, predisposing particles to roll or slide, thereby enhancing matrix plasticity. The optimum performance of the composite at 10 wt.% B addition is attributed to its high Al content and appropriate interface reaction degree, which collectively reduce flow stress and augment fracture strain, indicating superior mechanical properties at elevated temperatures.
4. Conclusions
The objective of this study is to improve the neutron absorption capacity of B4C/Al composites while studying their mechanical properties. B4C/Al composites featuring diverse B contents (0 wt.%, 5 wt.%, 10 wt.%, 15 wt.%) were prepared through pressureless infiltration. Subsequently, an in-depth investigation was conducted to explore the influence of B addition on the microstructure and mechanical properties of these composites. The composites primarily consist of Al, B4C, Al3BC, and AlB2 phases, with the formation of Al3BC increasing linearly with B content. Uniform phase distribution and near-full density were observed. The B4C/Al composite with 10 wt.% B addition exhibits the highest Al content, promoting crack deflection during fracture and reducing interfacial stress concentrations. This enhances its resistance to crack propagation and improves room-temperature plastic deformability. At elevated temperatures, the plastic flow capability of the Al phase facilitates uniform distribution within the composite and mitigates localized stress concentrations. Finally, the 10 wt.% B-added B4C/Al composite demonstrates the lowest flow stress and most stable high-temperature stress–strain behavior. However, excessive B addition increases ceramic phase content and internal defects of the B4C/Al composites, which promote stress concentration under external loading while simultaneously hindering the plastic flow capability of the Al matrix at high temperatures, ultimately reducing the composite’s overall plastic deformation capability.
Author Contributions
Conceptualization, D.M. and Y.L. (Yongxiang Leng); methodology, Y.L. (Yao Liu) and D.M.; validation, Y.L. (Yongxiang Leng); formal analysis, Y.L. (Yao Liu); investigation, Y.L. (Yao Liu), H.P. and C.L.; resources, D.M.; data curation, Y.L. (Yao Liu), J.X., H.P. and C.L.; writing—original draft preparation, Y.L. (Yao Liu); writing—review and editing, Y.L. (Yao Liu), J.X., D.M. and Y.L. (Yongxiang Leng); supervision, J.X., H.P. and Y.L. (Yongxiang Leng); funding acquisition, Y.L. (Yongxiang Leng). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Sichuan Science and Technology Program, grant number 2025YFHZ0189.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank the Analytical and Testing Center of Southwest Jiaotong University for the SEM tests.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vislov, I.S.; Pischulin, V.P.; Kladiev, S.N.; Slobodyan, S.M. Current state of nuclear fuel cycles in nuclear engineering and trends in their development according to the environmental safety requirements. Therm. Eng. 2016, 63, 581–586. [Google Scholar] [CrossRef]
- Yamashita, S.; Suzuki, S.; Suzuki, S.; Shimura, H.; Saenko, V. Lessons from Fukushima: Latest Findings of Thyroid Cancer After the Fukushima Nuclear Power Plant Accident. Thyroid 2017, 28, 11–22. [Google Scholar] [CrossRef]
- Gan, B.; Liu, S.; He, Z.; Chen, F.; Niu, H.; Cheng, J.; Tan, B.; Yu, B. Research Progress of Metal-Based Shielding Materials for Neutron and Gamma Rays. Acta Metall. Sin. 2021, 34, 1609–1617. [Google Scholar] [CrossRef]
- Raja, S.W.; Acharya, R.; Pujari, P.K. Application of PIGE method for quantification of total boron in neutron absorbers and shielding materials and isotopic composition in in-house prepared enriched boron carbide samples. J. Radioanal. Nucl. Chem. 2020, 323, 1359–1366. [Google Scholar] [CrossRef]
- Qi, Z.-D.; Yang, Z.; Meng, X.-F.; Yang, X.-G.; Liang, M.-X.; Li, C.-Y.; Dai, Y. Microstructure, thermophysical properties and neutron shielding properties of Gd/316L composites for spent nuclear fuel transportation and storage. Mater. Today Commun. 2023, 37, 107315. [Google Scholar] [CrossRef]
- Saltan, F.; Şirin, K.; Aydın, S.; Taşköprü, C.; Yıldırım, Y. Boron containing polyvinyl alcohol/polyethylene oxide/polyvinyl pyrrolidone composites: Preparation, characterization, gamma radiation shielding and gamma radiation effect on it’s thermal properties. Radiat. Phys. Chem. 2024, 214, 111261. [Google Scholar] [CrossRef]
- Abenojar, J.; Martinez, M.A.; Velasco, F. Effect of the boron content in the aluminium/boron composite. J. Alloys Compd. 2006, 422, 67–72. [Google Scholar] [CrossRef]
- Lin, J.; Ran, G.; Lei, P.; Ye, C.; Huang, S.; Zhao, S.; Li, N. Microstructure Analysis of Neutron Absorber Al/B4C Metal Matrix Composites. Metals 2017, 7, 567. [Google Scholar] [CrossRef]
- Li, C.; Xia, X.; Cai, J.; Zhang, Z.; Wang, J.; Qian, Z.; Wang, X.; Dai, Y. Influence analysis of B4C content on the neutron shielding performance of B4C/Al. Radiat. Phys. Chem. 2023, 204, 110684. [Google Scholar] [CrossRef]
- Qi, Z.; Yang, Z.; Li, J.; Guo, Y.; Yang, G.; Yu, Y.; Zhang, J. The Advancement of Neutron-Shielding Materials for the Transportation and Storage of Spent Nuclear Fuel. Materials 2022, 15, 3256. [Google Scholar] [CrossRef]
- Avcioğlu, S. LDPE matrix composites reinforced with dysprosium-boron containing compounds for radiation shielding applications. J. Alloys Compd. 2022, 927, 166900. [Google Scholar] [CrossRef]
- Li, X.; Wu, J.; Tang, C.; He, Z.; Yuan, P.; Sun, Y.; Lau, W.-M.; Zhang, K.; Mei, J.; Huang, Y. High temperature resistant polyimide/boron carbide composites for neutron radiation shielding. Compos. Part B Eng. 2019, 159, 355–361. [Google Scholar] [CrossRef]
- Moldovan, P.; Popescu, G. The grain refinement of 6063 aluminum using Al-5Ti-1B and Al-3Ti-0.15C grain refiners. J. Miner. Met. Mater. Soc. 2004, 56, 59–61. [Google Scholar] [CrossRef]
- Cong, S.; Li, Y.; Ran, G.; Zhou, W.; Feng, Q. Microstructure and its effect on mechanical and thermal properties of Al-based Gd2O3 MMCs used as shielding materials in spent fuel storage. Ceram. Int. 2020, 46, 12986–12995. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Wang, W.; Gao, Z.; Wang, B. The design, fabrication and properties of B4C/Al neutron absorbers. J. Nucl. Mater. 2013, 437, 350–358. [Google Scholar] [CrossRef]
- Gaylan, Y.; Avar, B.; Panigrahi, M.; Aygün, B.; Karabulut, A. Effect of the B4C content on microstructure, microhardness, corrosion, and neutron shielding properties of Al-B4C composites. Ceram. Int. 2023, 49, 5479–5488. [Google Scholar] [CrossRef]
- Yao, Y.T.; Chen, L.Q. B4C/Al Composites Processed by Metal-assisted Pressureless Infiltration Technique and its Characterization. Mater. Manuf. Process. 2016, 31, 1286–1291. [Google Scholar] [CrossRef]
- Liu, Y.; Peng, H.; Wei, L.; Peng, H.; Ma, D.; Leng, Y. Influence of B4C Particle Size on the Microstructure and Mechanical Properties of B4C/Al Composites Fabricated by Pressureless Infiltration. Metals 2023, 13, 1358. [Google Scholar] [CrossRef]
- Xie, K.Y.; Yang, Q.; Christopher, J.M.; He, M.-R.; LaSalvia, J.C.; Harmer, M.P.; Hwang, C.; Haber, R.A.; Hemker, K.J. Experimental observations of amorphization in multiple generations of boron carbide. J. Am. Ceram. Soc. 2021, 105, 3008–3029. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, J.; Li, X.; Miao, Z.; Hou, J.; Wang, P.; Lv, E.; Yao, Y.; Zhang, K. Sintering, toughening mechanism and amorphization of B4C-based ceramic composites: A review. J. Eur. Ceram. Soc. 2025, 45, 117075. [Google Scholar] [CrossRef]
- Xu, Z.G.; Jiang, L.T.; Zhang, Q.; Qiao, J.; Gong, D.; Wu, G.H. The design of a novel neutron shielding B4C/Al composite containing Gd. Mater. Des. 2016, 111, 375–381. [Google Scholar] [CrossRef]
- Wang, K.; Ma, L.; Yang, C.; Bian, Z.; Zhang, D.; Cui, S.; Wang, M.; Chen, Z.; Li, X. Recent Progress in Gd-Containing Materials for Neutron Shielding Applications: A Review. Materials 2023, 16, 4305. [Google Scholar] [CrossRef]
- Jing, H.; Geng, L.; Qiu, S.; Zou, H.; Liang, M.; Deng, D. Research progress of rare earth composite shielding materials. J. Rare Earths 2023, 40, 32–41. [Google Scholar] [CrossRef]
- Yeon, J.-W.; Hwang, J.; Jung, Y.-J.; Boo, B.H.; Song, K. Dispersion properties of B4C microparticles as emergency neutron absorbers in Spent-Fuel pool water. Nucl. Sci. Eng. 2012, 172, 202–207. [Google Scholar]
- Lu, K.; Zhu, X.; Nagarathnam, K. Nickel-Boron Nanolayer-Coated Boron Carbide Pressureless Sintering. J. Am. Ceram. Soc. 2009, 92, 1500–1505. [Google Scholar] [CrossRef]
- GB/T 6569-2006; Fine Ceramics (Advanced Ceramics, Advances Technical Ceramics)—Test Method for Flexural Strength of Monolithic Ceramics at Room Temperature. Standards Press of China: Beijing, China, 2006.
- GB/T 23806-2009; Fine Ceramics (Advanced Ceramics, Advances Technical Ceramics)—Test Method for Fracture Toughness of Monolithic Ceramics at Room Temperature by Single Edge Precracked Beam (SEPB) Method. Standards Press of China: Beijing, China, 2009.
- Duschanek, H.; Rogl, P. The Al-B (aluminum-boron) system. J. Phase Equilibria 1994, 15, 543–552. [Google Scholar] [CrossRef]
- Auradi, V.; Kori, S.A. Influence of reaction temperature for the manufacturing of Al-3Ti and Al-3B master alloys. J. Alloys Compd. 2008, 453, 147–156. [Google Scholar] [CrossRef]
- Wang, X. Boride phase formation in the production of Al-B master alloys. J. Alloys Compd. 2017, 722, 302–306. [Google Scholar] [CrossRef]
- Guo, R.-F.; Chen, S.-M.; Shen, P. Influence of Si, Ti, and Cu as alloying elements on the wettability and reactivity of an Al/B4C system. J. Mater. Res. Technol. 2023, 27, 6104–6116. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, X.; Sun, H.; Zhang, Y.; Fang, Y.; Shu, R. Microstructures and mechanical properties of Al nanocomposites hybrid-reinforced with B4C, carbon nanotubes and graphene nanoplatelets. Mater. Sci. Eng. B 2023, 293, 116457. [Google Scholar] [CrossRef]
- Mei, Y.; Li, H.; Yang, W.; Wu, J.; Li, X.; Xiu, Z.; Fu, J.; Hussain, M.; Chen, G.; Wu, G. In-situ synthesis of Al3BC/Al composites from amorphous boron and graphene nanoplates by solid reaction. J. Alloys Compd. 2020, 832, 154912. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, G.; Xu, K.; Wu, H.; Li, Q.; Wu, J.; Wang, Z. Phase transformation and mechanical properties of B4C/Al composites. J. Mater. Res. Technol. 2020, 9, 2116–2126. [Google Scholar] [CrossRef]
- Zhao, Y.; Qian, Z.; Liu, X. Identification of novel dual-scale Al3BC particles in Al based composites. Mater. Des. 2016, 93, 283–290. [Google Scholar] [CrossRef]
- Selahshorrad, E.; Zeynali, E.; Houmani, A.; Zangeneh-Madar, K.; Samadi, M.R.; Afshari, M.; Afshari, H. Simultaneous optimization of the tensile strength, bending strength, hardness and wear resistance of WCu composite produced by sintering process. Int. J. Refract. Met. Hard Mater. 2023, 117, 106430. [Google Scholar] [CrossRef]
- Yan, Y.-F.; Kou, S.-Q.; Yang, H.-Y.; Dong, B.-X.; Shu, S.-L.; Chen, L.-Y.; Qiu, F.; Zhang, L.-C. Manipulating interface bonding and microstructure via tuning interfacial reaction for enhancing mechanical property of in-situ TiC/Al cermets. J. Mater. Process. Technol. 2023, 317, 117995. [Google Scholar] [CrossRef]
- Chen, M.W.; Qiu, H.P.; Chen, Y.; Zhang, Q.; Liu, S. Study on fracture toughness and impact toughness of SiCf/SiC composites. J. Aust. Ceram. Soc. 2023, 59, 325–332. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Qian, M.; Zhou, J.; Geng, L. Tailoring microstructure and mechanical properties of nacre-architecture (TiBw-TiB2p)/Al hybrid composites by particle grading. Mater. Sci. Eng. A 2025, 928, 148071. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, M.W.; Ramesh, K.T.; Ye, J.; Schoenung, J.M.; Chin, E.S.C. Tensile behavior and dynamic failure of aluminum 6092/B4C composites. Mater. Sci. Eng. A 2006, 433, 70–82. [Google Scholar] [CrossRef]
- Chen, H.S.; Wang, W.X.; Li, Y.L.; Zhang, P.; Nie, H.H.; Wu, Q.C. The design, microstructure and tensile properties of B4C particulate reinforced 6061Al neutron absorber composites. J. Alloys Compd. 2015, 632, 23–29. [Google Scholar] [CrossRef]
- Fang, J.; Zhu, Z.; Zhang, X.; Xie, L.; Huang, Z. Tensile Deformation and Fracture Behavior of AA5052 Aluminum Alloy under Different Strain Rates. J. Mater. Eng. Perform. 2021, 30, 9403–9411. [Google Scholar] [CrossRef]
- Li, Y.L.; Wang, W.X.; Zhou, J.; Chen, H.S.; Zhao, J.C.; Wang, B.D. Fatigue Crack Growth and Fracture of 30 wt% B4C/6061Al Composites. Fatigue Fract. Eng. Mater. Struct. 2017, 40, 1378–1388. [Google Scholar] [CrossRef]
- Gao, M.; Chen, Z.; Li, L.; Guo, E.; Kang, H.; Xu, Y.; Wang, T. Microstructure and enhanced mechanical properties of hybrid-sized B4C particle-reinforced 6061Al matrix composites. Mater. Sci. Eng. A 2021, 802, 140453. [Google Scholar] [CrossRef]
- Jiang, N.; Li, D.S.; Yao, Q.Q.; Duan, H.-W.; Ko, F. Experimental study on compression properties and failure mechanism of 3D MWK carbon/epoxy composites at elevated temperatures. Polym. Compos. 2018, 39, E1987–E1999. [Google Scholar] [CrossRef]
- Tang, B.; Wang, H.; Jin, P.; Jiang, X. Constitutive flow behavior and microstructural evolution of 17 vol% SiCp/7055Al composite during compression at elevated temperature. J. Mater. Res. Technol. 2020, 9, 6386–6396. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).