Influence of Microstructure and Heat Treatment on the Corrosion Resistance of Mg-1Zn Alloy Produced by Laser Powder Bed Fusion
Abstract
1. Introduction
2. Materials and Methods
2.1. Starting Materials
2.2. Processing
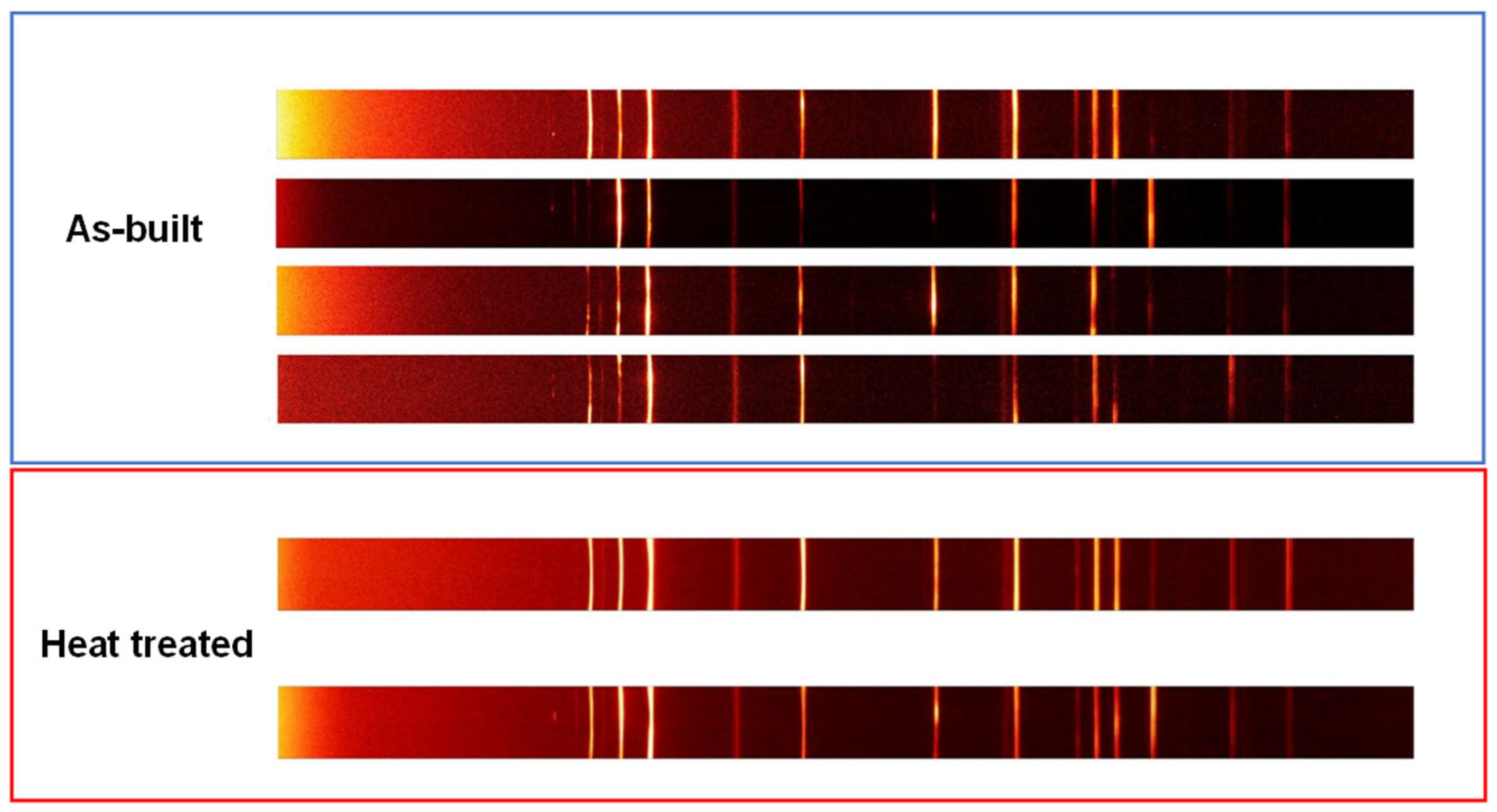
2.3. Microstructural Characterization
2.4. Electrochemical Measurements
2.5. Electrochemical Measurements
3. Results and Discussion
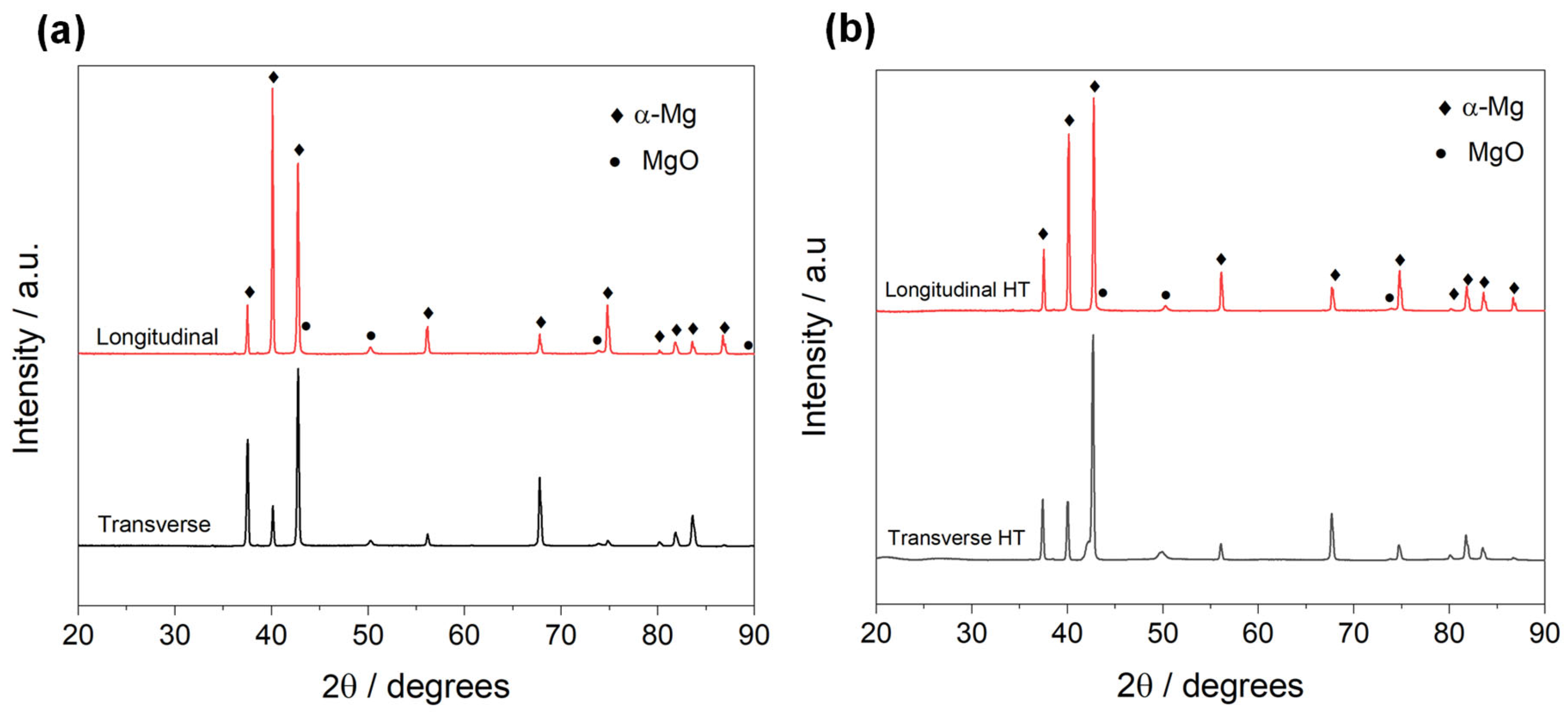
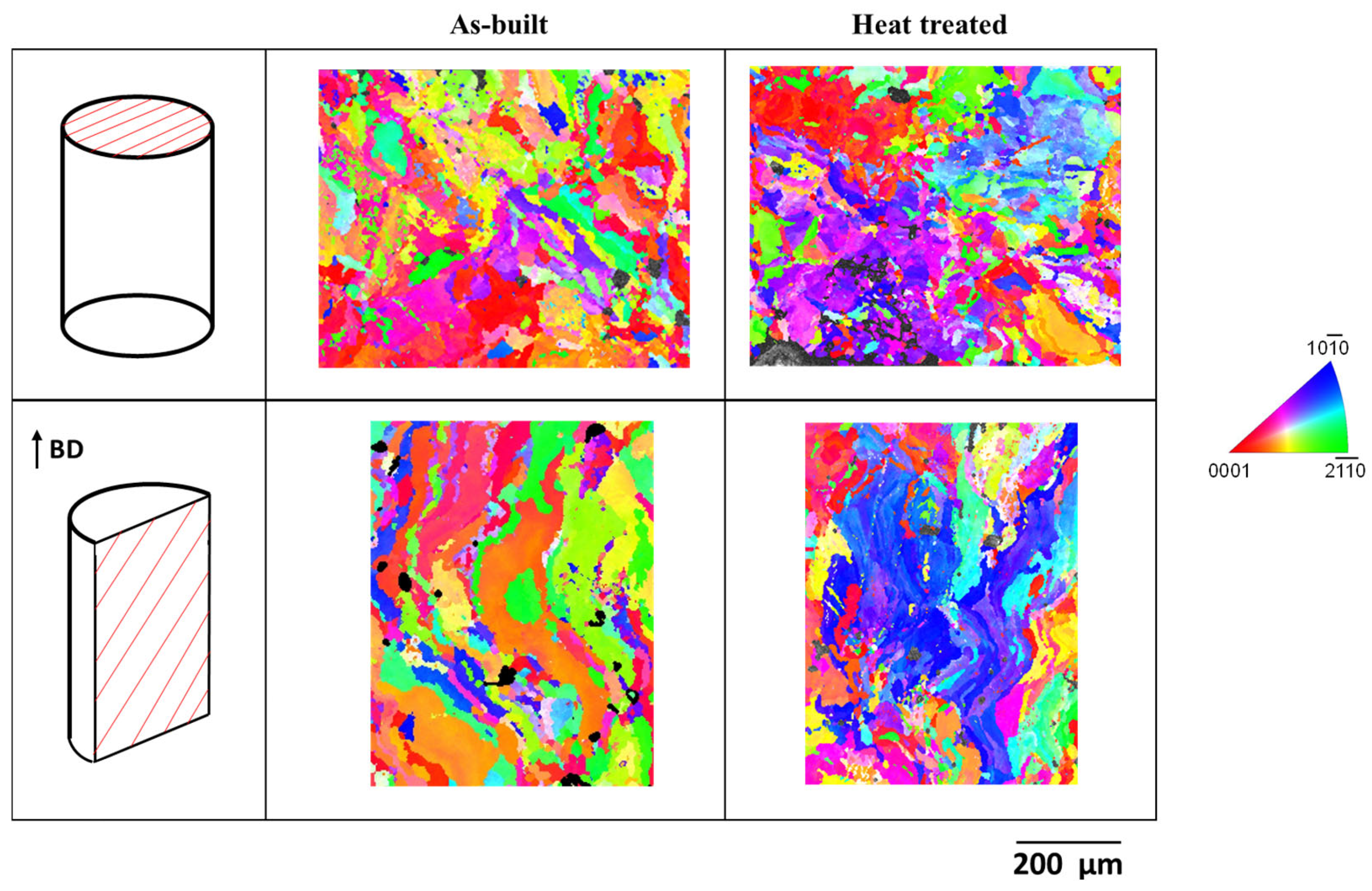
3.1. Microstructural Characterization
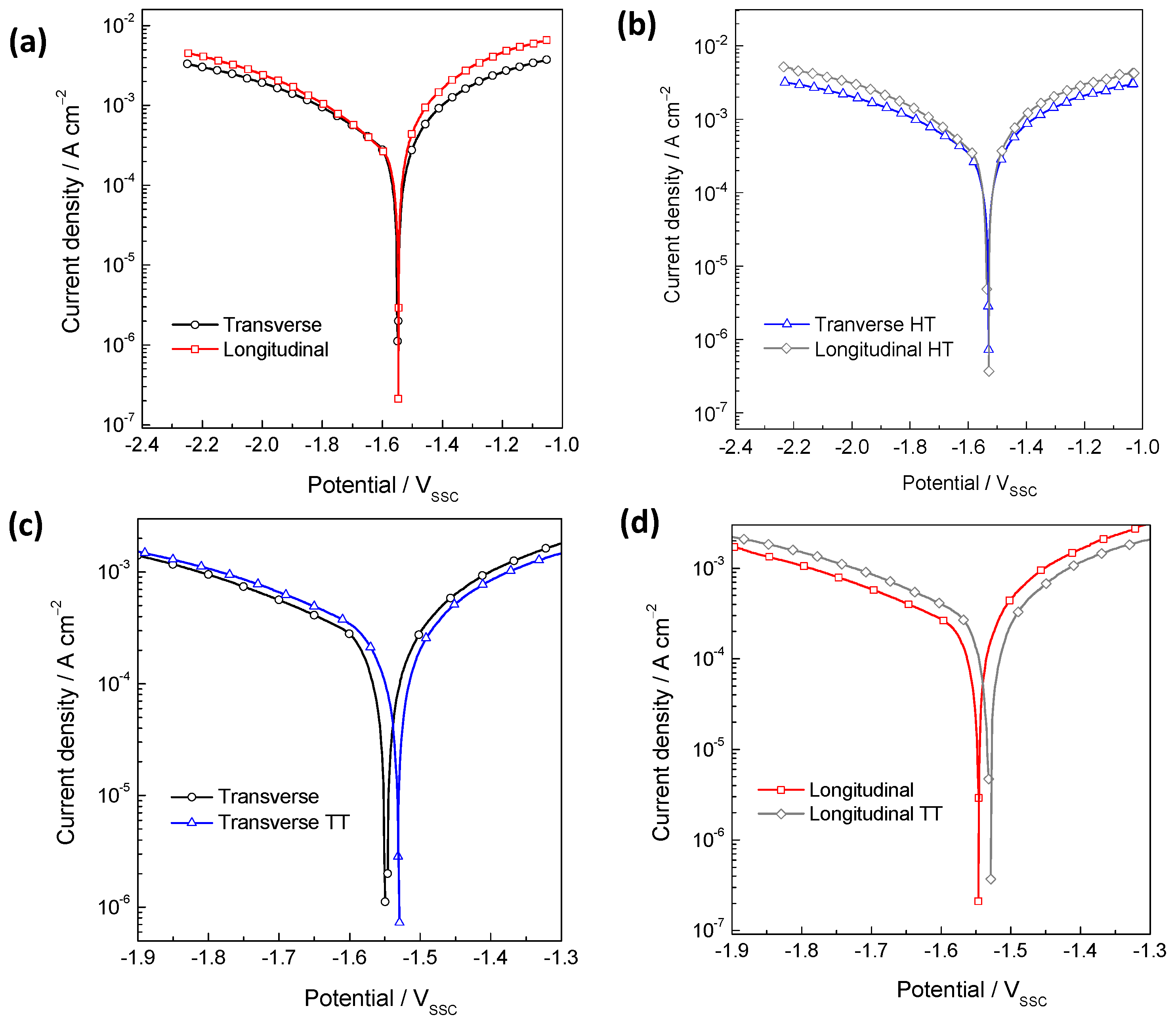
3.2. Potentiodynamic Polarization (PDP) Measurements
3.3. Gravimetric HE Collection Measurements
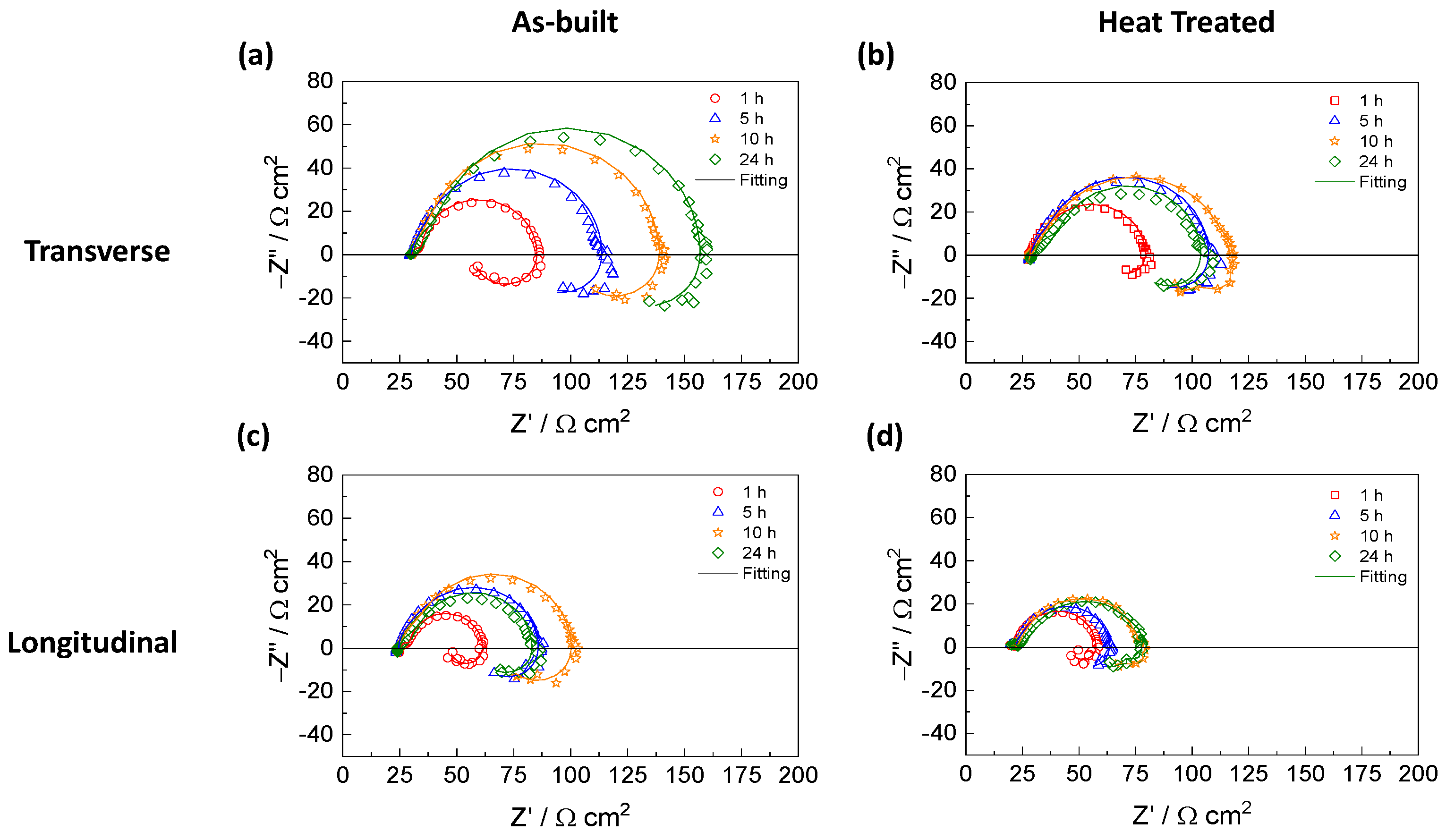
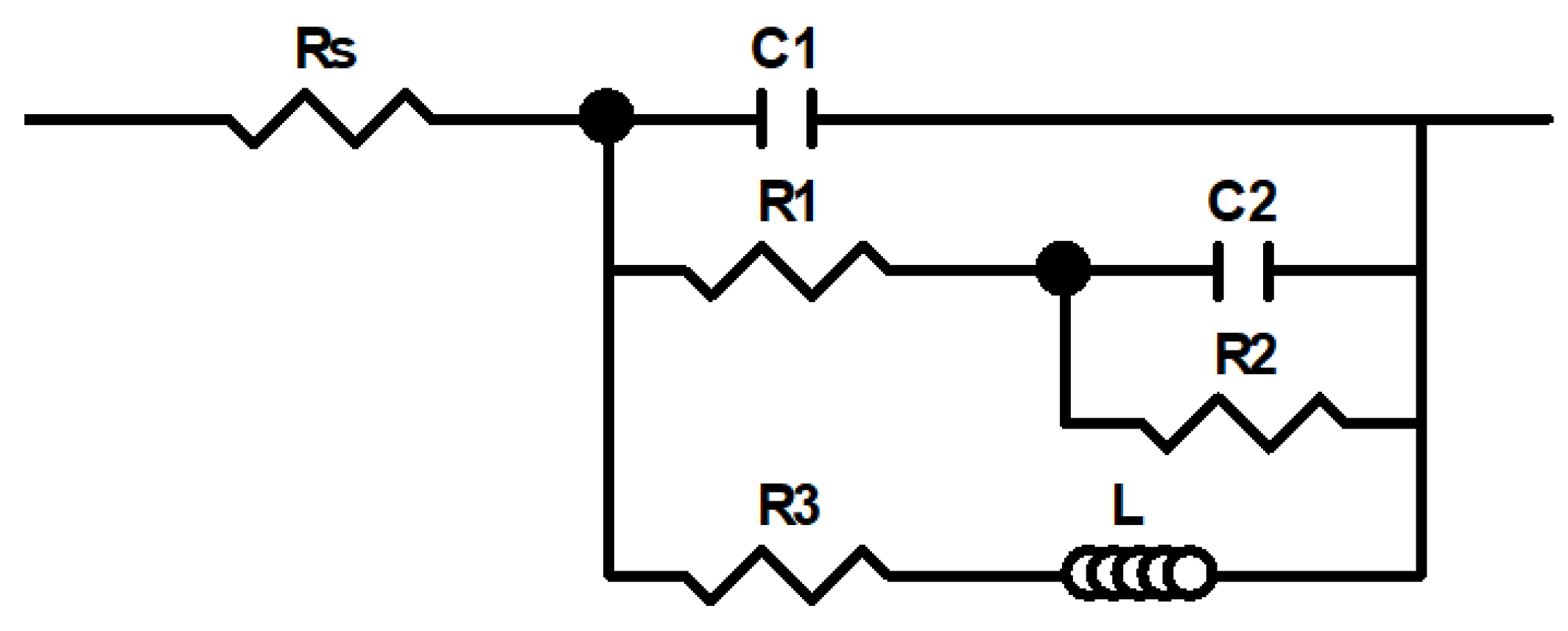
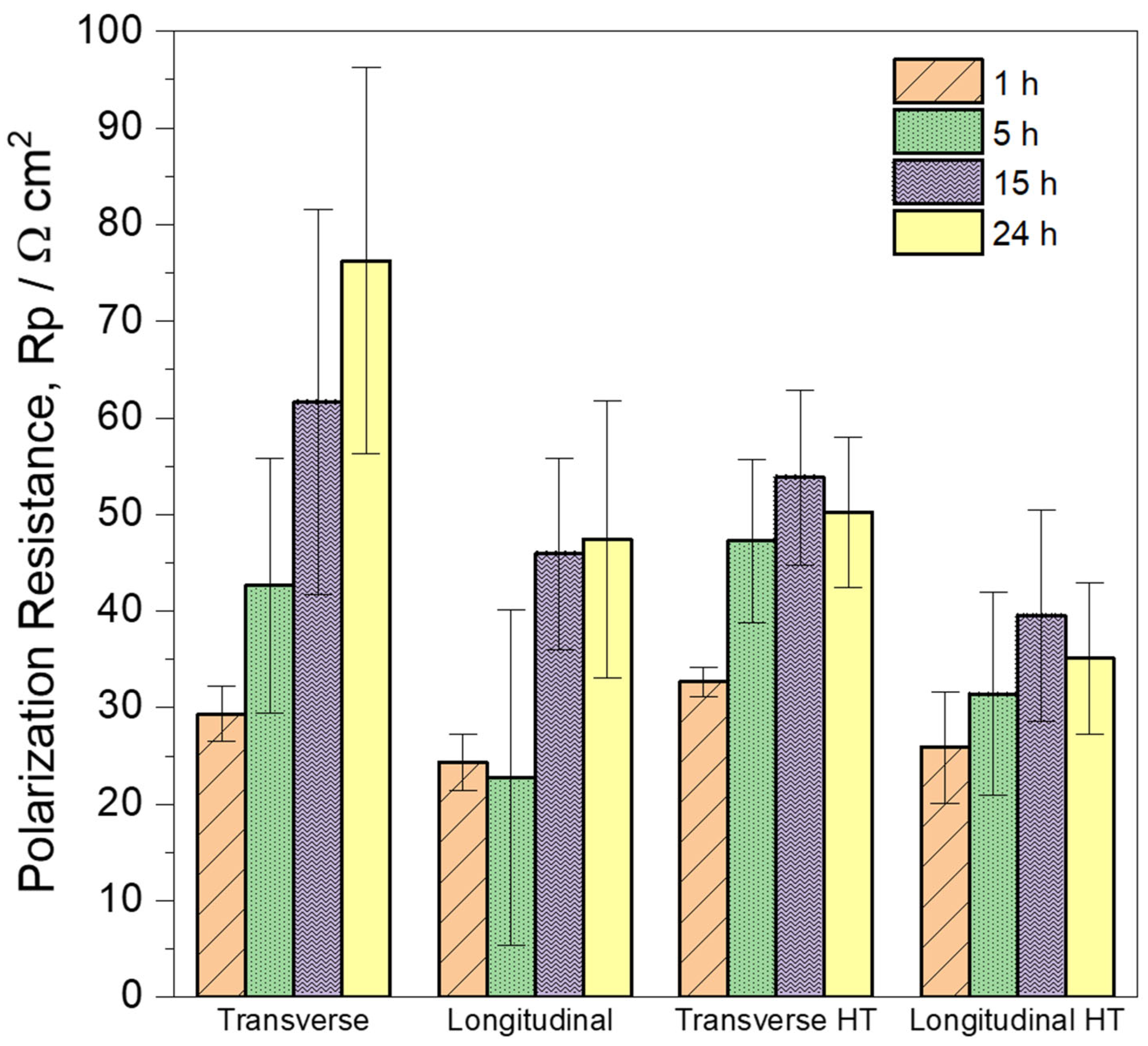
3.4. Electrochemical Impedance Spectroscopy (EIS) Measurements
4. Conclusions
- A Mg-1Zn alloy was successfully fabricated by laser powder bed fusion (LPBF), achieving density values of 97 ± 1% and 96 ± 2% for the transverse and longitudinal sections, respectively.
- The as-built Mg-1Zn specimen showed irregular and anisotropic grain shapes with columnar morphology along the build direction. Only minor changes were observed after heat treatment, involving a slightly refinement of grain size.
- No presence of intermetallic secondary phases was observed through XRD and SEM in any plane studied, but the presence of Mg oxide was detected.
- Regarding the alloy in the as-built state, the PDP curve showed that the longitudinal plane exhibited higher anodic kinetics than the transverse plane. This is consistent with the measurements obtained by EIS and H2 collection, demonstrating that the transverse plane presented greater corrosion resistance than the longitudinal plane.
- Following heat treatment, both the transverse and longitudinal sections demonstrated an increase in cathodic kinetics and a decrease in anodic kinetics. Consequently, the corrosion resistance decreased in both sections after heat treatment.
- The samples are ranked in increasing order of polarization resistance after 24 h of immersion as follows: longitudinal heat-treated, transverse heat-treated, longitudinal as-built, and transverse as-built.
- The grain size and/or orientation are determining factors in the differences observed in corrosion resistance between the studied planes.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aghion, E.; Bronfin, B.; Eliezer, D. The role of the magnesium industry in protecting the environment. J. Mater. Process. Technol. 2001, 117, 381–385. [Google Scholar] [CrossRef]
- Zhang, J.; Miao, J.; Balasubramani, N.; Cho, D.H.; Avey, T.; Chang, C.Y.; Luo, A.A. Magnesium research and applications: Past, present and future. J. Magnes. Alloy. 2023, 11, 3867–3895. [Google Scholar] [CrossRef]
- Fu, Q.; Liang, W.; Huang, J.; Jin, W.; Guo, B.; Li, P.; Xu, S.; Chu, P.K.; Yu, Z. Research perspective and prospective of additive manufacturing of biodegradable magnesium-based materials. J. Magnes. Alloy. 2023, 11, 1485–1504. [Google Scholar] [CrossRef]
- Cain, T.; Bland, L.G.; Birbilis, N.; Scully, J.R. A compilation of corrosion potentials for magnesium alloys. Corrosion 2014, 70, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Wu, Z.; Curtin, W.A. The origins of high hardening and low ductility in magnesium. Nature 2015, 526, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Yin, B.; Wu, Z.; Curtin, W.A. Designing high ductility in magnesium alloys. Acta Mater. 2019, 172, 161–184. [Google Scholar] [CrossRef]
- Zeng, Z.; Salehi, M.; Kopp, A.; Xu, S.; Esmaily, M.; Birbilis, N. Recent progress and perspectives in additive manufacturing of magnesium alloys. J. Magnes. Alloy. 2022, 10, 1511–1541. [Google Scholar] [CrossRef]
- Xu, W.; Li, J.; Zhang, Z.; Yuan, H.; An, G.; Shi, H.; Cai, C.; Jiang, W.; Li, W.; Wei, Q. Laser powder bed fusion of WE43 magnesium alloy with superior balance of strength and ductility. J. Magnes. Alloy. 2024, 13, 1275–1293. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Z.; Zhang, J.; Tang, H.; Wang, H. Additive manufacturing of magnesium matrix composites: Comprehensive review of recent progress and research perspectives. J. Magnes. Alloy. 2023, 11, 425–461. [Google Scholar] [CrossRef]
- Shuai, C.; Yang, Y.; Wu, P.; Lin, X.; Liu, Y.; Zhou, Y.; Feng, P.; Liu, X.; Peng, S. Laser rapid solidification improves corrosion behavior of Mg-Zn-Zr alloy. J. Alloy. Compd. 2017, 691, 961–969. [Google Scholar] [CrossRef]
- Zengin, H.; Hassel, A.W. Magnesium alloys with rare earth elements—A review of the recent progress on the corrosion properties. Corros. Sci. 2014, 249, 112827. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, D.; You, C.; Chen, M. Effects of grain size on the corrosion resistance of pure magnesium by cooling rate-controlled solidification. Front. Mater. Sci. 2015, 9, 247–253. [Google Scholar] [CrossRef]
- Liu, D.; Liu, Y.; Huang, Y.; Song, R.; Chen, M. Effects of solidification cooling rate on the corrosion resistance of Mg-Zn-Ca alloy. Prog. Nat. Sci. 2014, 24, 452–457. [Google Scholar] [CrossRef]
- Song, J.; Chen, J.; Xiong, X.; Peng, X.; Chen, D.; Pan, F. Research advances of magnesium and magnesium alloys worldwide in 2021. J. Magnes. Alloy. 2022, 10, 863–898. [Google Scholar] [CrossRef]
- Sames, W.J.; List, F.A.; Pannala, S.; Dehoff, R.R.; Babu, S.S. The metallurgy and processing science of metal additive manufacturing. Int. Mater. Rev. 2016, 61, 315–360. [Google Scholar] [CrossRef]
- Deng, Q.; Wu, Y.; Zhu, W.; Chen, K.; Liu, D.; Peng, L.; Ding, W. Effect of heat treatment on microstructure evolution and mechanical properties of selective laser melted Mg-11Gd-2Zn-0.4Zr alloy. Mater. Sci. Eng. A—Struct. Mater. Prop. Microstruct. Process. 2021, 829, 142139. [Google Scholar] [CrossRef]
- BNagesha, K.; Vinodh, K.; Tigga, A.K.; Barad, S.; Kumar, S.A. Influence of post-processing techniques on residual stresses of SLM processed HPNGV. J. Manuf. Process. 2021, 66, 189–197. [Google Scholar] [CrossRef]
- Cai, H.; Zhang, N.; Liu, L.; Su, J.; Li, Y.; Kang, Y.; Guo, F. Effects of cooling rate on the microstructure and properties of magnesium alloy-a review. J. Magnes. Alloy. 2024, 12, 3094–3114. [Google Scholar] [CrossRef]
- Sefene, E.M. State-of-the-art of selective laser melting process: A comprehensive review. J. Manuf. Syst. 2021, 63, 250–274. [Google Scholar] [CrossRef]
- Chowdhury, S.; Yadaiah, N.; Prakash, C.; Ramakrishna, S.; Dixit, S.; Gupta, L.R.; Buddhi, D. Laser powder bed fusion: A state-of-the-art review of the technology, materials, properties & defects, and numerical modelling. J. Mater. Res. Technol.-JMRT 2022, 20, 2109–2172. [Google Scholar] [CrossRef]
- Niu, X.; Shen, H.; Fu, J.; Yan, J.; Wang, Y. Corrosion behaviour of laser powder bed fused bulk pure magnesium in hank’s solution. Corros. Sci. 2019, 157, 284–294. [Google Scholar] [CrossRef]
- Esmaily, M.; Zeng, Z.; Mortazavi, A.N.; Gullino, A.; Choudhary, S.; Derra, T.; Benn, F.; D’Elia, F.; Müther, M.; Thomas, S.; et al. A detailed microstructural and corrosion analysis of magnesium alloy WE43 manufactured by selective laser melting. Addit. Manuf. 2019, 35, 101321. [Google Scholar] [CrossRef]
- Zumdick, N.A.; Jauer, L.; Kersting, L.C.; Kutz, T.N.; Schleifenbaum, J.H.; Zander, D. Additive manufactured WE43 magnesium: A comparative study of the microstructure and mechanical properties with those of powder extruded and as-cast WE43. Mater. Charact. 2018, 147, 384–397. [Google Scholar] [CrossRef]
- Wei, K.; Gao, M.; Wang, Z.; Zeng, X. Effect of energy input on formability, microstructure and mechanical properties of selective laser melted AZ91D magnesium alloy. Mater. Sci. Eng. A—Struct. Mater. Prop. Microstruct. Process. 2014, 611, 212–222. [Google Scholar] [CrossRef]
- Jiang, P.; Blawert, C.; Zheludkevich, M.L. The Corrosion Performance and Mechanical Properties of Mg-Zn Based Alloys—A Review. Corros. Mater. Degrad. 2020, 1, 92–158. [Google Scholar] [CrossRef]
- Yin, P.; Li, N.F.; Lei, T.; Liu, L.; Ouyang, C. Effects of Ca on microstructure, mechanical and corrosion properties and biocompatibility of Mg–Zn–Ca alloys. J. Mater. Sci.: Mater. Med. 2013, 24, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Zeng, X.; Wang, Z.; Deng, J.; Liu, M.; Huang, G.; Yuan, X. Selective laser melting of Mg-Zn binary alloys: Effects of Zn content on densification behavior, microstructure, and mechanical property. Mater. Sci. Eng. A—Struct. Mater. Prop. Microstruct. Process. 2019, 756, 226–236. [Google Scholar] [CrossRef]
- Taheri-Andani, M.; Zhang, Z.; Sundararaghavan, V.; Misra, A. Mapping the roles of scan strategy and build orientation in predicting the crystallographic texture and yield strength of 316L stainless steel produced by laser powder bed fusion. J. Mater. Res. Technol. 2024, 30, 7375–7383. [Google Scholar] [CrossRef]
- Dejene, N.D.; Tucho, W.M.; Lemu, H.G. Effects of Scanning Strategies, Part Orientation, and Hatching Distance on the Porosity and Hardness of AlSi10Mg Parts Produced by Laser Powder Bed Fusion. J. Manuf. Mater. Process. 2025, 9, 78. [Google Scholar] [CrossRef]
- Zhou, F.; Lu, S.S.; Jiang, B.; Song, R.G. Effect of annealing treatment of hot-rolled AZ31 magnesium alloy on properties and stress corrosion resistance of MAO coatings. Anti-Corros. Methods Mater. 2024, 71, 403–416. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Ramasse, Q.M.; Fan, Z. Grain refinement of Mg-Ca alloys by native MgO particles. J. Magnes. Alloy. 2024, 12, 980–996. [Google Scholar] [CrossRef]
- Garcés, G.; Pérez, P.; Medina, J.; Barea, R.; Gómez, A.; García, J.; Adeva, P. On the Influence of Precipitation on the Dynamic Strain Aging in Mg-2%Nd. JOM 2023, 75, 2385–2396. [Google Scholar] [CrossRef]
- Curioni, M. The behaviour of magnesium during free corrosion and potentiodynamic polarization investigated by real-time hydrogen measurement and optical imaging. Electrochim. Acta 2014, 120, 284–292. [Google Scholar] [CrossRef]
- Fajardo, S.; Frankel, G.S. Gravimetric Method for Hydrogen Evolution Measurements on Dissolving Magnesium. J. Electrochem. Soc. 2015, 162, C693–C701. [Google Scholar] [CrossRef]
- Wu, C.L.; Xie, W.J.; Man, H.C. Laser additive manufacturing of biodegradable Mg-based alloys for biomedical applications: A review. J. Magnes. Alloy. 2014, 10, 915–937. [Google Scholar] [CrossRef]
- Toda-Caraballo, I.; Jiménez, J.A.; Milenkovic, S.; Jimenez-Aguirre, J.; San-Martin, D. Microstructural Stability of the CoCrFe2Ni2 High Entropy Alloys with Additions of Cu and Mo. Metals 2021, 11, 1994. [Google Scholar] [CrossRef]
- Bramble, M.S.; Flemming, R.L.; McCausland, P.J.A. Grain size measurement from two-dimensional micro-X-ray diffraction: Laboratory application of a radial integration technique. Am. Miner. 2015, 100, 1899–1911. [Google Scholar] [CrossRef]
- Manjhi, S.K.; Sekar, P.; Bontha, S.; Balan, A.S.S. Additive manufacturing of magnesium alloys: Characterization and post-processing. Int. J. Lightweight Mater. Manuf. 2024, 7, 184–213. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N.; Davies, C.H.J. Revealing the relationship between grain size and corrosion rate of metals. Scr. Mater. 2010, 63, 1201–1204. [Google Scholar] [CrossRef]
- Ahmadkhaniha, D.; Fedel, M.; Sohi, M.H.; Deflorian, F. Corrosion behavior of severely plastic deformed magnesium based alloys: A review. Surf. Eng. Appl. Electrochem. 2017, 53, 439–448. [Google Scholar] [CrossRef]
- Hagihara, K.; Okubo, M.; Yamasaki, M.; Nakano, T. Crystal-orientation-dependent corrosion behaviour of single crystals of a pure Mg and Mg-Al and Mg-Cu solid solutions. Corros. Sci. 2016, 109, 68–85. [Google Scholar] [CrossRef]
- Liu, B.; Liu, X.; Wu, P.; Zhang, S.; Zhang, Y.; Zhang, Z.; Wang, H. Microstructure and corrosion behavior of a hot-rolled pure Mg after annealing at various temperatures. Mater. Today Commun. 2024, 39, 109104. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N. Effect of Grain Size on Corrosion: A Review. Corrosion 2010, 66, 075005-1–075005-13. [Google Scholar] [CrossRef]
- Liu, X.; Shan, D.; Song, Y.; Han, E. Effects of heat treatment on corrosion behaviors of Mg-3Zn magnesium alloy. Trans. Nonferrous Met. Soc. China 2010, 20, 1345–1350. [Google Scholar] [CrossRef]
- Zheng, R.; Bhattacharjee, T.; Gao, S.; Gong, W.; Shibata, A.; Sasaki, T.; Hono, K.; Tsuji, N. Change of Deformation Mechanisms Leading to High Strength and Large Ductility in Mg-Zn-Zr-Ca Alloy with Fully Recrystallized Ultrafine Grained Microstructures. Sci. Rep. 2019, 9, 11702. [Google Scholar] [CrossRef]
- Shi, D.F.; Cepeda-Jiménez, C.M.; Pérez-Prado, M.T. The relation between ductility at high temperature and solid solution in Mg alloys. J. Magnes. Alloy. 2022, 10, 224–238. [Google Scholar] [CrossRef]
- Fajardo, S.; Glover, C.F.; Williams, G.; Frankel, G.S. The Source of Anodic Hydrogen Evolution on Ultra High Purity Magnesium. Electrochim. Acta 2016, 212, 510–521. [Google Scholar] [CrossRef]
- Salleh, S.H.; Thomas, S.; Yuwono, J.A.; Venkatesan, K.; Birbilis, N. Enhanced hydrogen evolution on Mg(OH)2 covered Mg surfaces. Electrochim. Acta 2015, 161, 144–152. [Google Scholar] [CrossRef]
- Lebouil, S.; Duboin, A.; Monti, F.; Tabeling, P.; Volovitch, P.; Ogle, K. A novel approach to on-line measurement of gas evolution kinetics: Application to the negative difference effect of Mg in chloride solution. Electrochim. Acta 2014, 124, 176–182. [Google Scholar] [CrossRef]
- Gomes, M.P.; Costa, I.; Pébère, N.; Rossi, J.L.; Tribollet, B.; Vivier, V. On the corrosion mechanism of Mg investigated by electrochemical impedance spectroscopy. Electrochim. Acta 2019, 306, 61–70. [Google Scholar] [CrossRef]
- Leleu, S.; Rives, B.; Bour, J.; Causse, N.; Pébère, N. On the stability of the oxides film formed on a magnesium alloy containing rare-earth elements. Electrochim. Acta 2018, 290, 586–594. [Google Scholar] [CrossRef]
- García-Galvan, F.R.; Fajardo, S.; Barranco, V.; Feliu, S. Experimental apparent stern–geary coefficients for AZ31B Mg alloy in physiological body fluids for accurate corrosion rate determination. Metals 2021, 11, 391. [Google Scholar] [CrossRef]
- Fajardo, S.; Miguélez, L.; Arenas, M.A.; de Damborenea, J.; Llorente, I.; Feliu, S. Corrosion resistance of pulsed laser modified AZ31 Mg alloy surfaces. J. Magnes. Alloy. 2022, 10, 756–768. [Google Scholar] [CrossRef]
- Yang, Y.; Scenini, F.; Stevens, N.; Curioni, M. Relationship between the inductive response observed during electrochemical impedance measurements on aluminium and local corrosion processes. Corros. Eng. Sci. Technol. 2019, 54, 1–9. [Google Scholar] [CrossRef]
- Hernández, L.; González, J.E.; Barranco, V.; Veranes-Pantoja, Y.; Galván, J.C.; Gattorno, G.R. Biomimetic hydroxyapatite (HAp) coatings on pure Mg and their physiological corrosion behavior. Ceram. Int. 2022, 48, 1208–1222. [Google Scholar] [CrossRef]
- Bland, L.G.; King, A.D.; Birbilis, N.; Scully, J.R. Assessing the corrosion of commercially pure magnesium and commercial AZ31B by electrochemical impedance, mass-loss, hydrogen collection, and inductively coupled plasma optical emission spectrometry solution analysis. Corrosion 2015, 71, 128–145. [Google Scholar] [CrossRef]
- Curioni, M.; Scenini, F.; Monetta, T.; Bellucci, F. Correlation between electrochemical impedance measurements and corrosion rate of magnesium investigated by real-time hydrogen measurement and optical imaging. Electrochim. Acta 2015, 166, 372–384. [Google Scholar] [CrossRef]
- Wang, L.; Snihirova, D.; Deng, M.; Wang, C.; Vaghefinazari, B.; Wiese, G.; Langridge, M.; Höche, D.; Lamaka, S.V.; Zheludkevich, M.L. Insight into physical interpretation of high frequency time constant in electrochemical impedance spectra of Mg. Corros. Sci. 2021, 187, 109501. [Google Scholar] [CrossRef]
- Deng, M.; Wang, L.; Höche, D.; Lamaka, S.V.; Wang, C.; Snihirova, D.; Jin, Y.; Zhang, Y.; Zheludkevich, M.L. Approaching ‘stainless magnesium’ by Ca micro-alloying. Mater. Horiz. 2021, 8, 589–596. [Google Scholar] [CrossRef]
- Li, Y.; Lu, X.; Wu, K.; Yang, L.; Zhang, T.; Wang, F. Exploration the inhibition mechanism of sodium dodecyl sulfate on Mg alloy. Corros. Sci. 2020, 168, 108559. [Google Scholar] [CrossRef]
- Gharbi, O.; Tran, M.T.T.; Orazem, M.E.; Tribollet, B.; Turmine, M.; Vivier, V. Impedance Response of a Thin Film on an Electrode: Deciphering the Influence of the Double Layer Capacitance. ChemPhysChem 2021, 22, 1371–1378. [Google Scholar] [CrossRef]
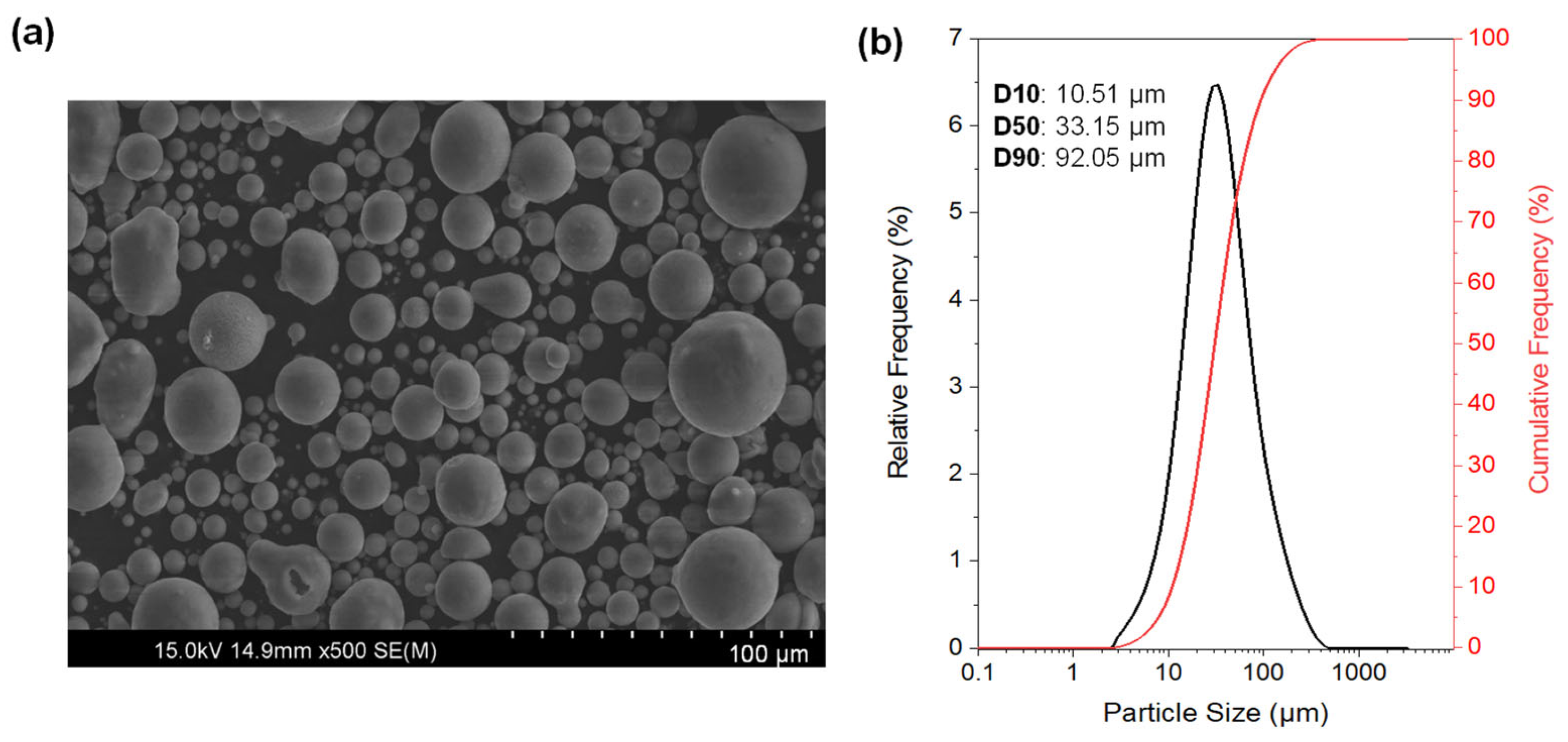
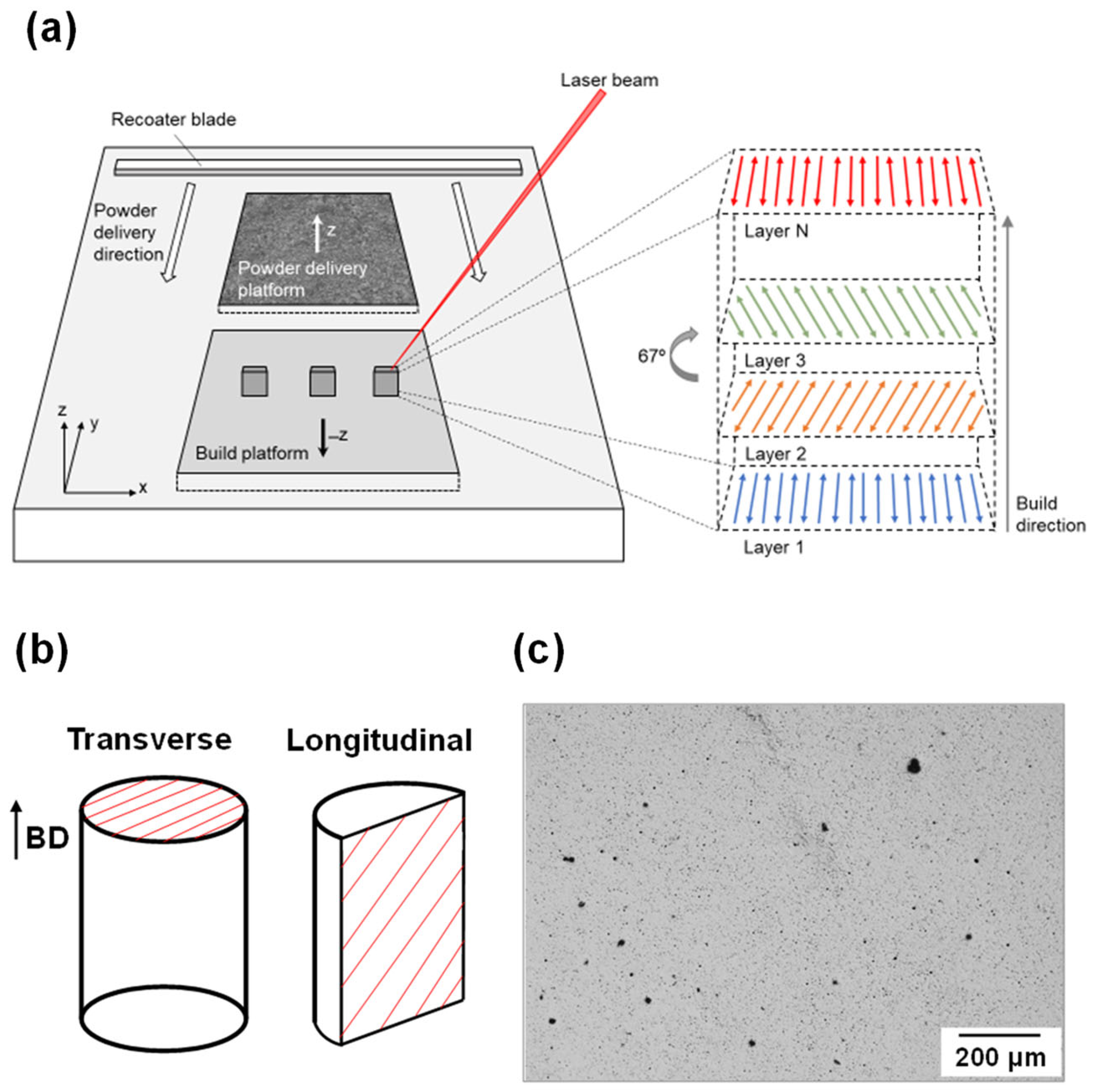
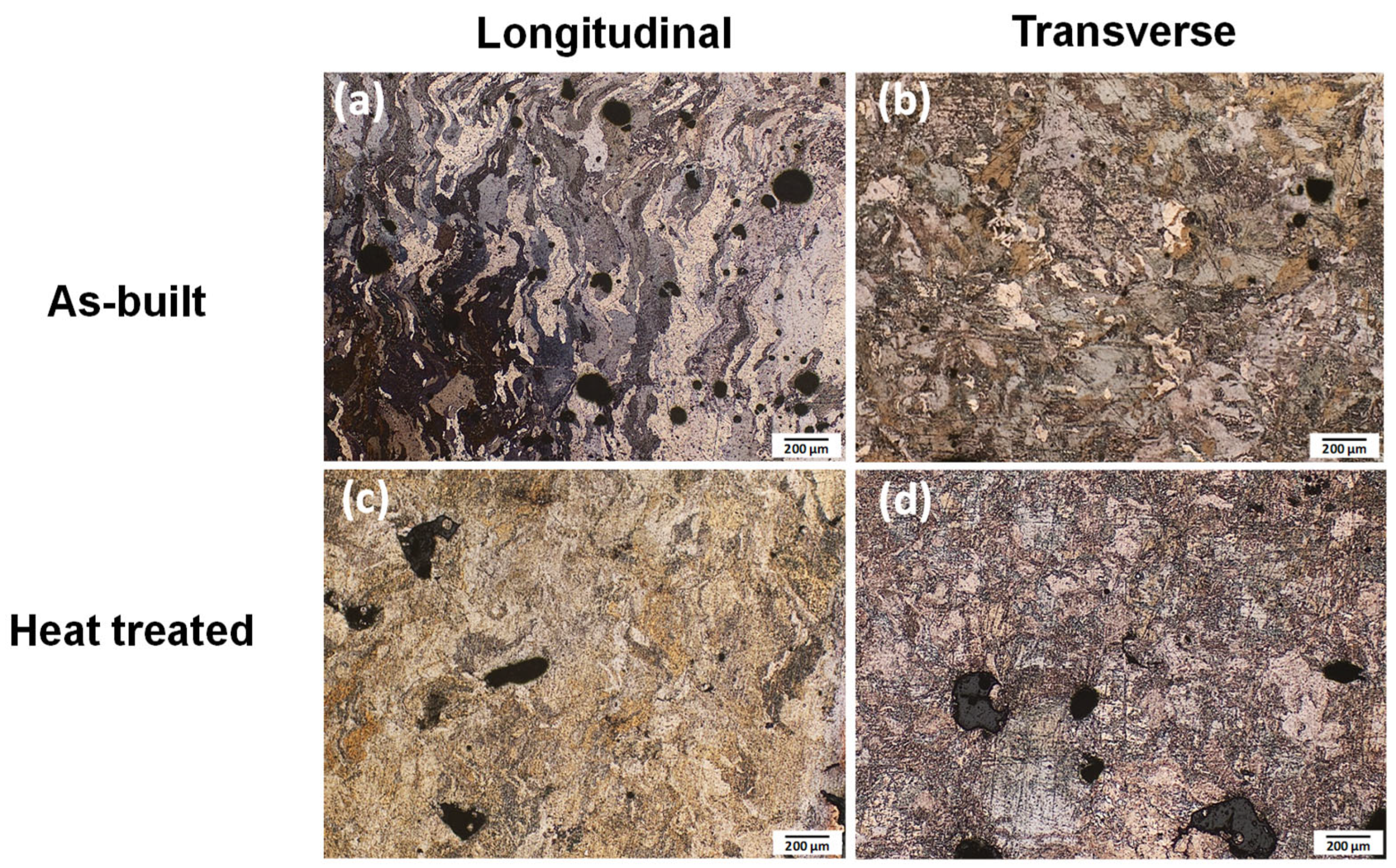

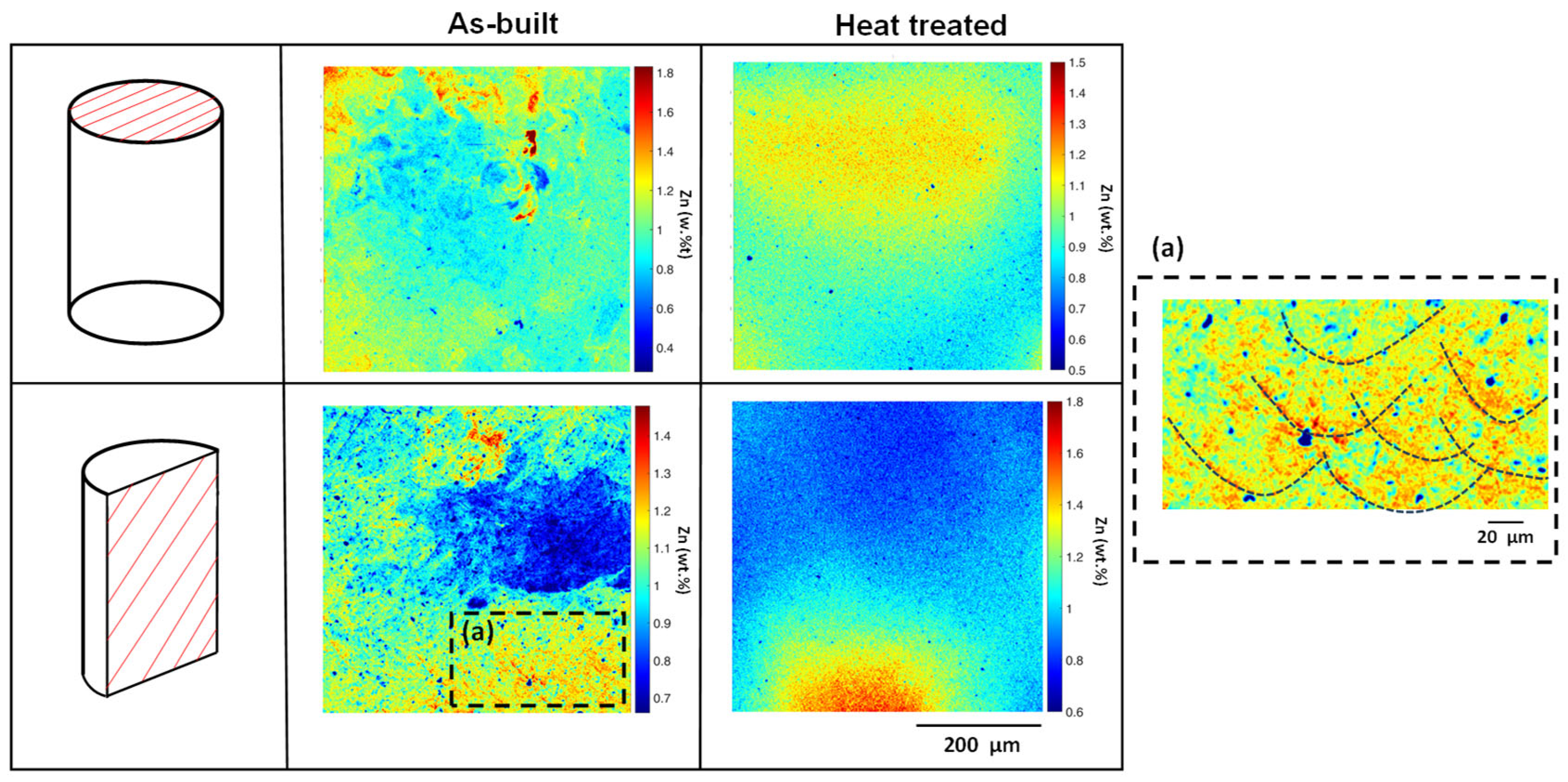


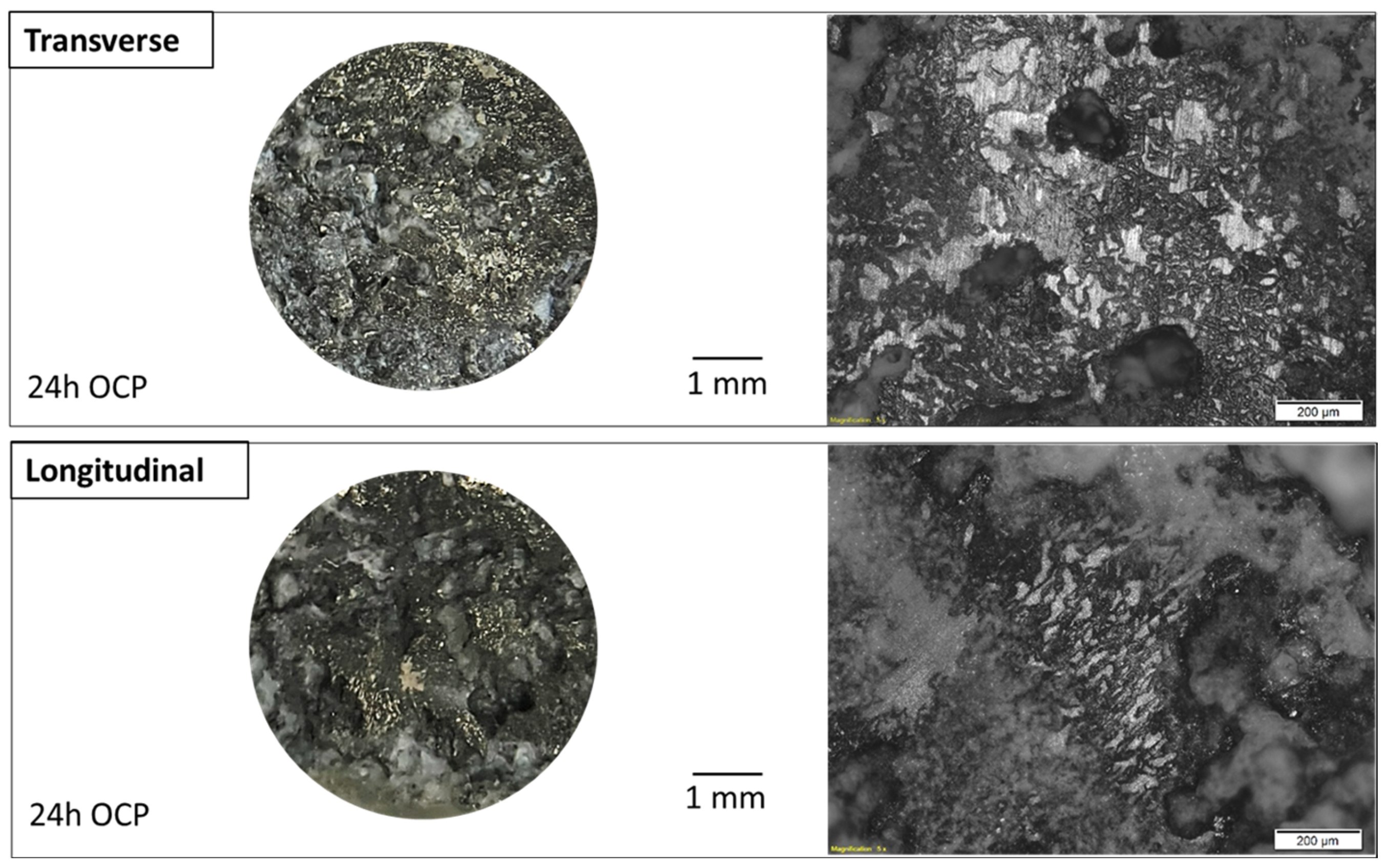
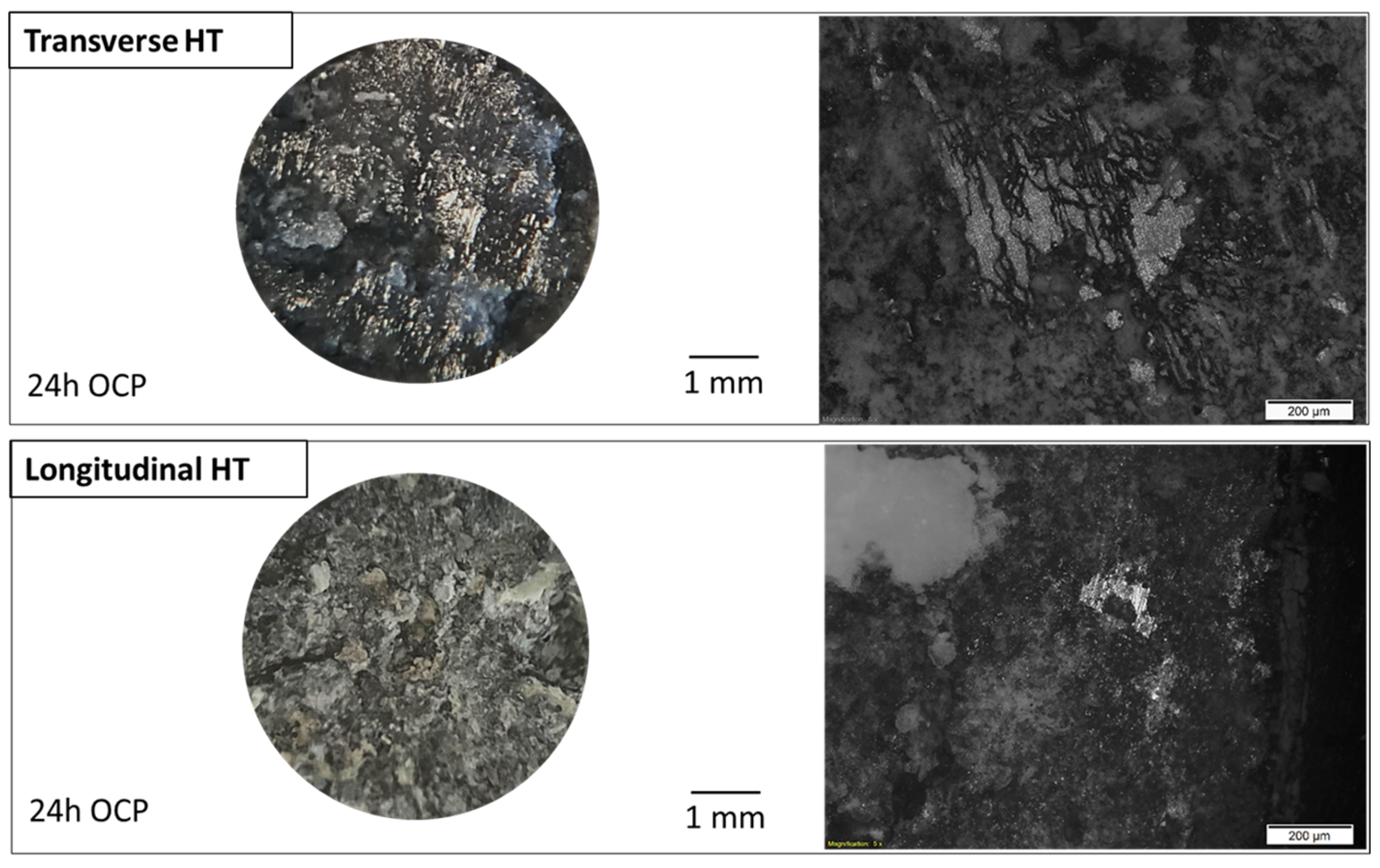


| Alloy | Zn | Al | Cu | Fe | Mn | Ni | Si | Mg |
|---|---|---|---|---|---|---|---|---|
| Mg-1Zn | 1.10 | 0.002 | 0.001 | 0.001 | 0.04 | 0.002 | 0.001 | Bal. |
| As-Built | Heat-Treated | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 h | 5 h | 10 h | 24 h | 1 h | 5 h | 10 h | 24 h | ||
| Rs | (Ω cm2) | 29.3 ± 0.9 | 30 ± 0.3 | 31 ± 0.4 | 31 ± 0.4 | 30.2 ± 1.9 | 30.4 ± 1.2 | 30.8 ± 1.0 | 31.6 ± 0.4 |
| C1 | (µF cm−2) | 14 ± 1 | 73 ± 10 | 54 ± 8 | 32 ± 6 | 108 ± 1 | 66 ± 3 | 43 ± 2 | 25 ± 1 |
| R1 | (Ω cm2) | 7 ± 1 | 34 ± 11 | 38 ± 16 | 35 ± 10 | 18 ± 1 | 21 ± 2 | 20 ± 3 | 17 ± 2 |
| C2 | (µF cm−2) | 73 ± 10 | 120 ± 73 | 87 ± 20 | 59 ± 12 | 150 ± 14 | 94 ± 6 | 81 ± 8 | 82 ± 10 |
| R2 | (Ω cm2) | 54 ± 9 | 43 ± 18 | 64 ± 25 | 93 ± 21 | 31 ± 1 | 55 ± 11 | 68 ± 14 | 67 ± 12 |
| L | (H cm2) | 149 ± 28 | 2384 ± 439 | 2538 ± 238 | 4581 ± 1349 | 1696 ± 312 | 2362 ± 679 | 2132 ± 335 | 2094 ± 511 |
| R3 | (Ω cm2) | 57 ± 2 | 97 ± 21 | 161 ± 29 | 189 ± 55 | 98 ± 6 | 125 ± 24 | 139 ± 18 | 125 ± 17 |
| As-Built | Heat-Treated | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 h | 5 h | 15 h | 24 h | 1 h | 5 h | 14 h | 24 h | ||
| Rs | (Ω cm2) | 24 ± 2 | 24 ± 1 | 25.5 ± 0.6 | 25.0 ± 0.8 | 22.0 ± 0.4 | 22.8 ± 0.3 | 23.2 ± 0.8 | 23.5 ± 0.4 |
| C1 | (µF cm−2) | 18 ± 5 | 86 ± 9 | 66 ± 8 | 47 ± 6 | 100 ± 30 | 86 ± 4 | 66 ± 4 | 49 ± 8 |
| R1 | (Ω cm2) | 5.5 ± 0.5 | 27 ± 5 | 30 ± 6 | 23 ± 8 | 10 ± 2 | 21 ± 6 | 23 ± 6 | 18 ± 3 |
| C2 | (µF cm−2) | 126 ± 11 | 150 ± 56 | 130 ± 41 | 112 ± 15 | 146 ± 22 | 163 ± 23 | 157 ± 26 | 152 ± 39 |
| R2 | (Ω cm2) | 35 ± 5 | 32 ± 5 | 43 ± 10 | 48 ± 7 | 27 ± 5 | 28 ± 6 | 37 ± 7 | 35 ± 8 |
| L | (H cm2) | 145 ± 69 | 1331 ± 326 | 2026 ± 544 | 2110 ± 696 | 581 ± 109 | 1535 ± 313 | 2281± 322 | 1415 ± 268 |
| R3 | (Ω cm2) | 61 ± 6 | 50 ± 44 | 124 ± 26 | 150 ± 58 | 87 ± 26 | 91 ± 43 | 118 ± 45 | 104 ± 26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes-Riverol, R.; Triviño-Peláez, Á.; García-Galván, F.; Lieblich, M.; Jiménez, J.A.; Fajardo, S. Influence of Microstructure and Heat Treatment on the Corrosion Resistance of Mg-1Zn Alloy Produced by Laser Powder Bed Fusion. Metals 2025, 15, 853. https://doi.org/10.3390/met15080853
Reyes-Riverol R, Triviño-Peláez Á, García-Galván F, Lieblich M, Jiménez JA, Fajardo S. Influence of Microstructure and Heat Treatment on the Corrosion Resistance of Mg-1Zn Alloy Produced by Laser Powder Bed Fusion. Metals. 2025; 15(8):853. https://doi.org/10.3390/met15080853
Chicago/Turabian StyleReyes-Riverol, Raúl, Ángel Triviño-Peláez, Federico García-Galván, Marcela Lieblich, José Antonio Jiménez, and Santiago Fajardo. 2025. "Influence of Microstructure and Heat Treatment on the Corrosion Resistance of Mg-1Zn Alloy Produced by Laser Powder Bed Fusion" Metals 15, no. 8: 853. https://doi.org/10.3390/met15080853
APA StyleReyes-Riverol, R., Triviño-Peláez, Á., García-Galván, F., Lieblich, M., Jiménez, J. A., & Fajardo, S. (2025). Influence of Microstructure and Heat Treatment on the Corrosion Resistance of Mg-1Zn Alloy Produced by Laser Powder Bed Fusion. Metals, 15(8), 853. https://doi.org/10.3390/met15080853







