Study on the Kinetics of Carbothermic Reduction of Stainless Steel Dust by Walnut Shell Biochar
Abstract
1. Introduction
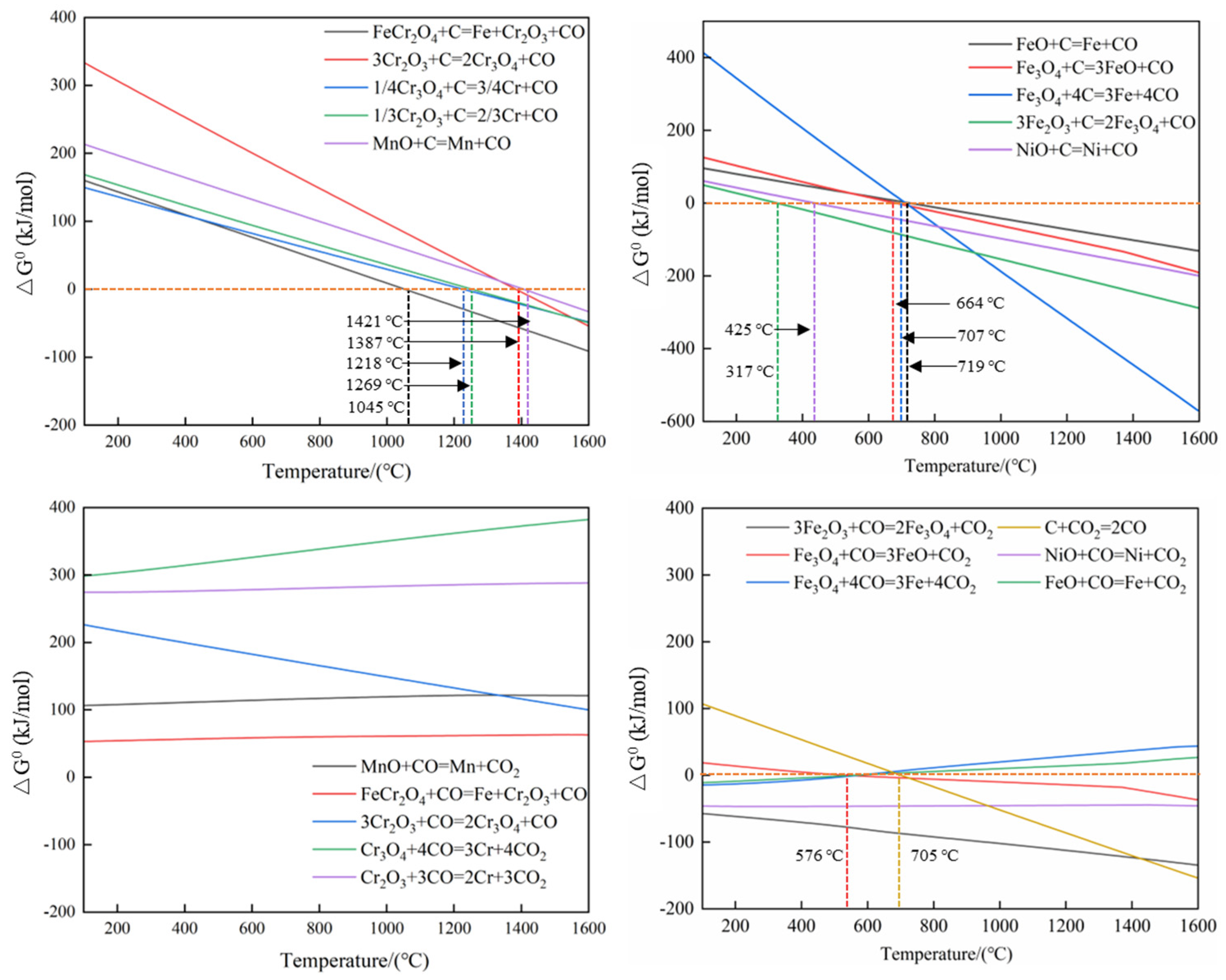
2. Thermodynamics Analysis of the Carbothermic Reduction Process of SSD
3. Experimental
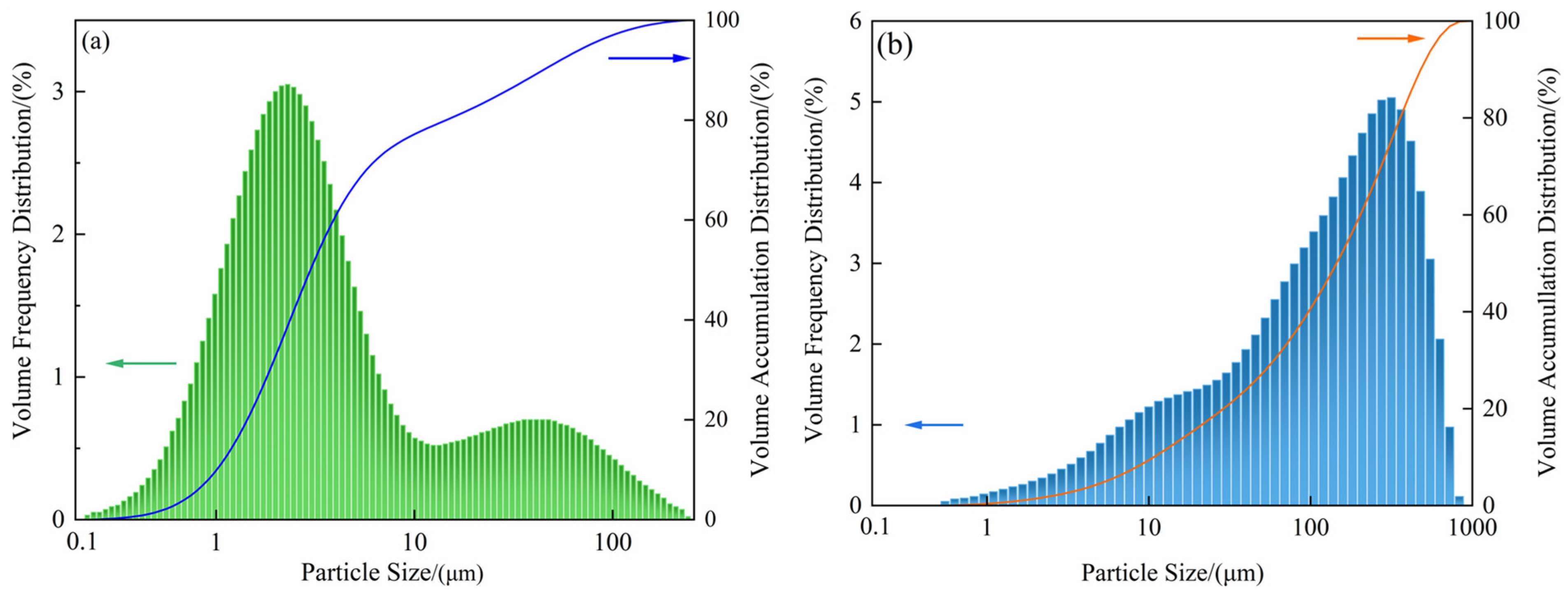
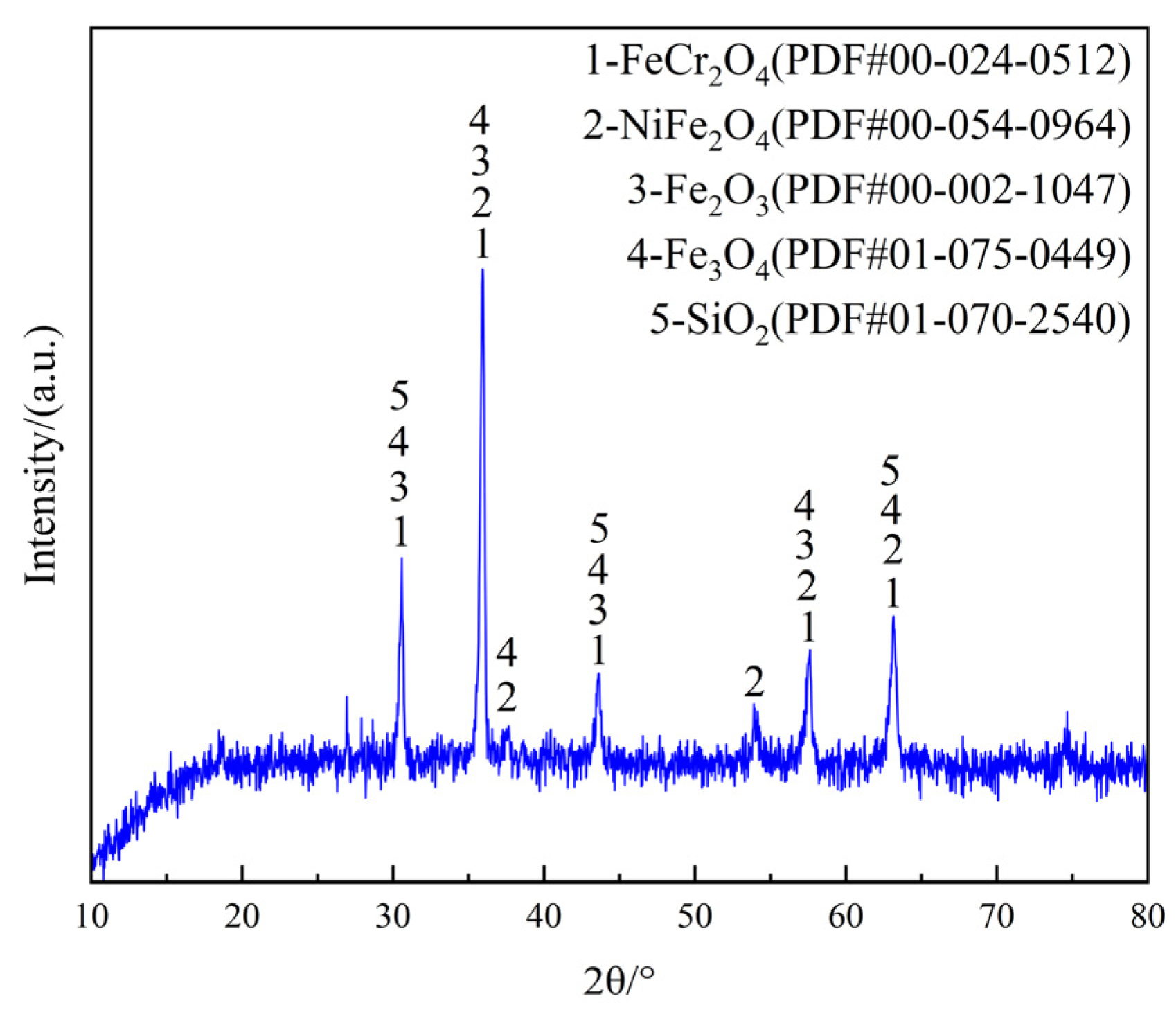
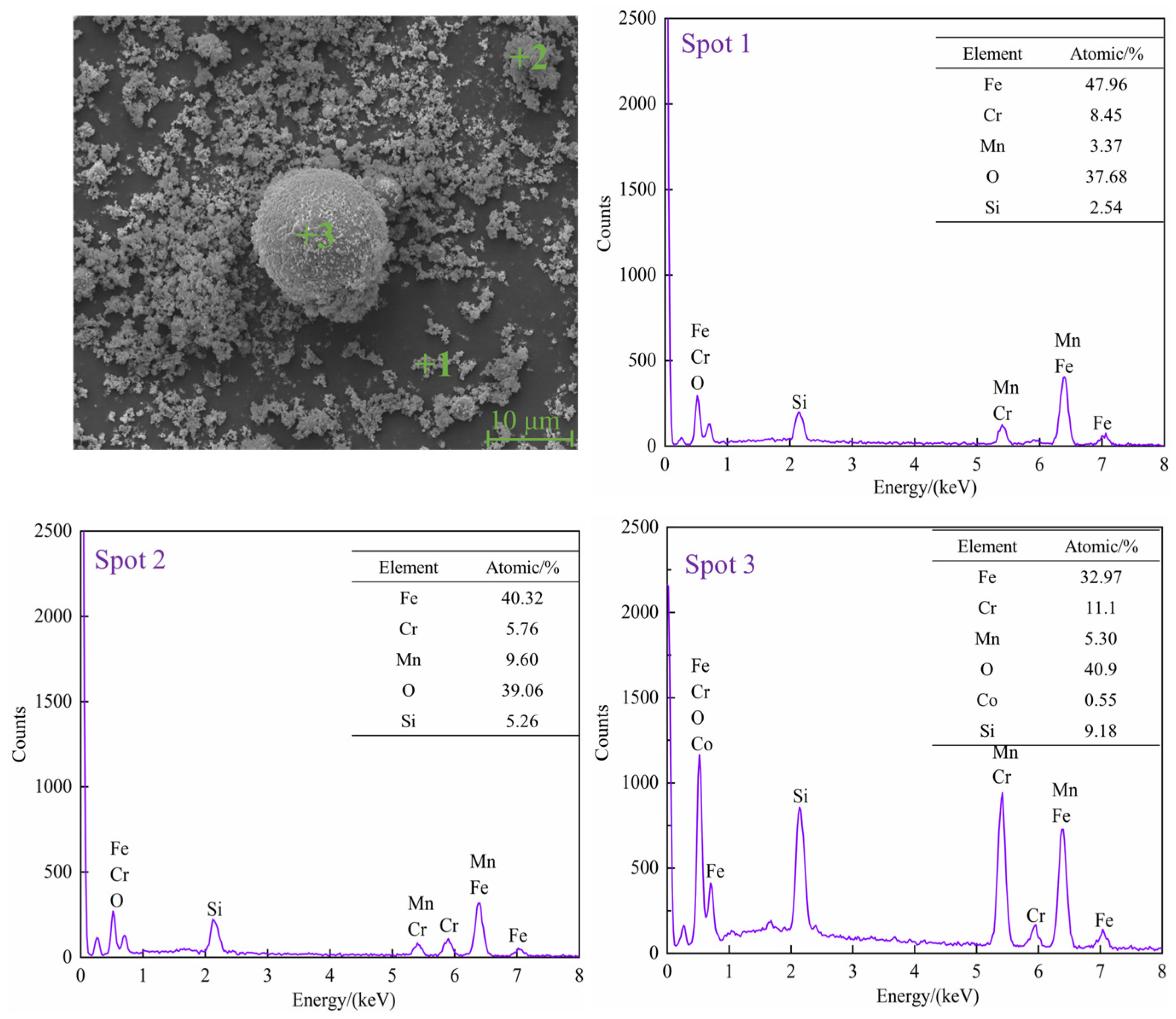
3.1. Characterization of Raw Materials
3.2. Thermogravimetric Analysis
4. Results and Discussion
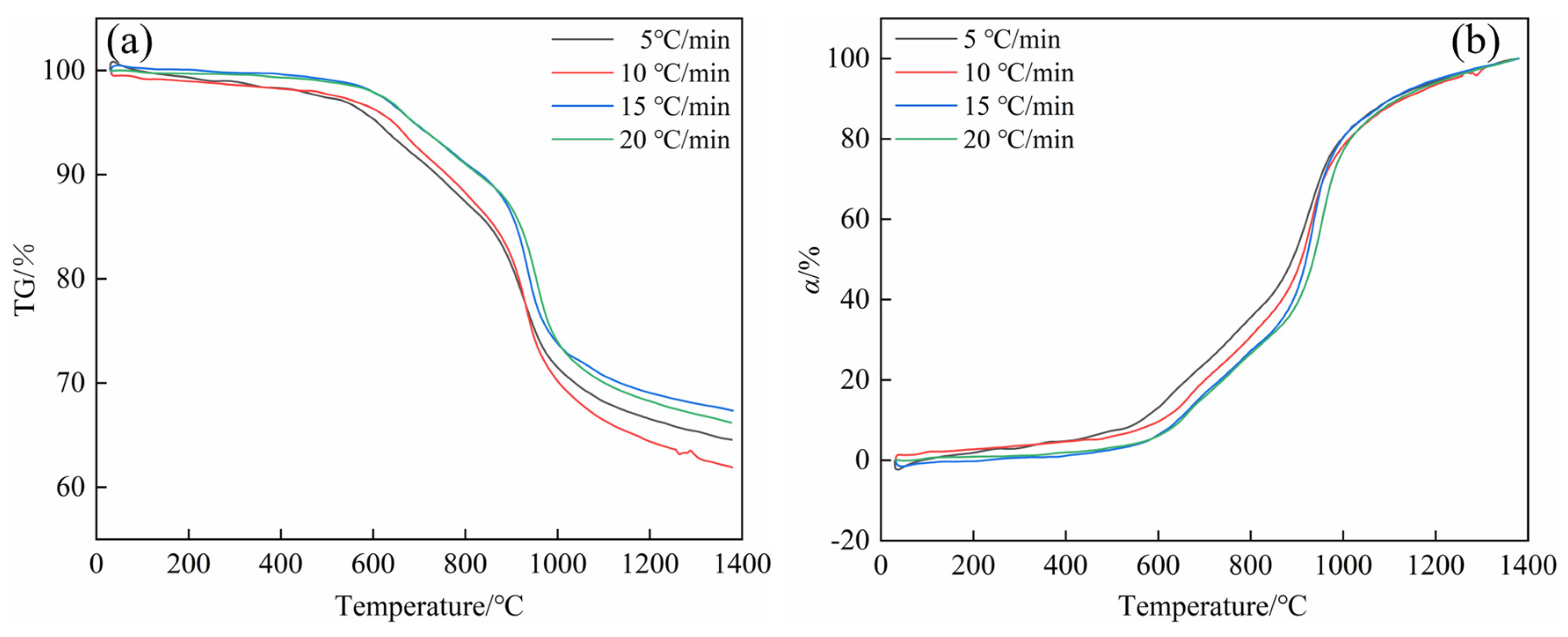
4.1. Thermogravimetric Analysis Results
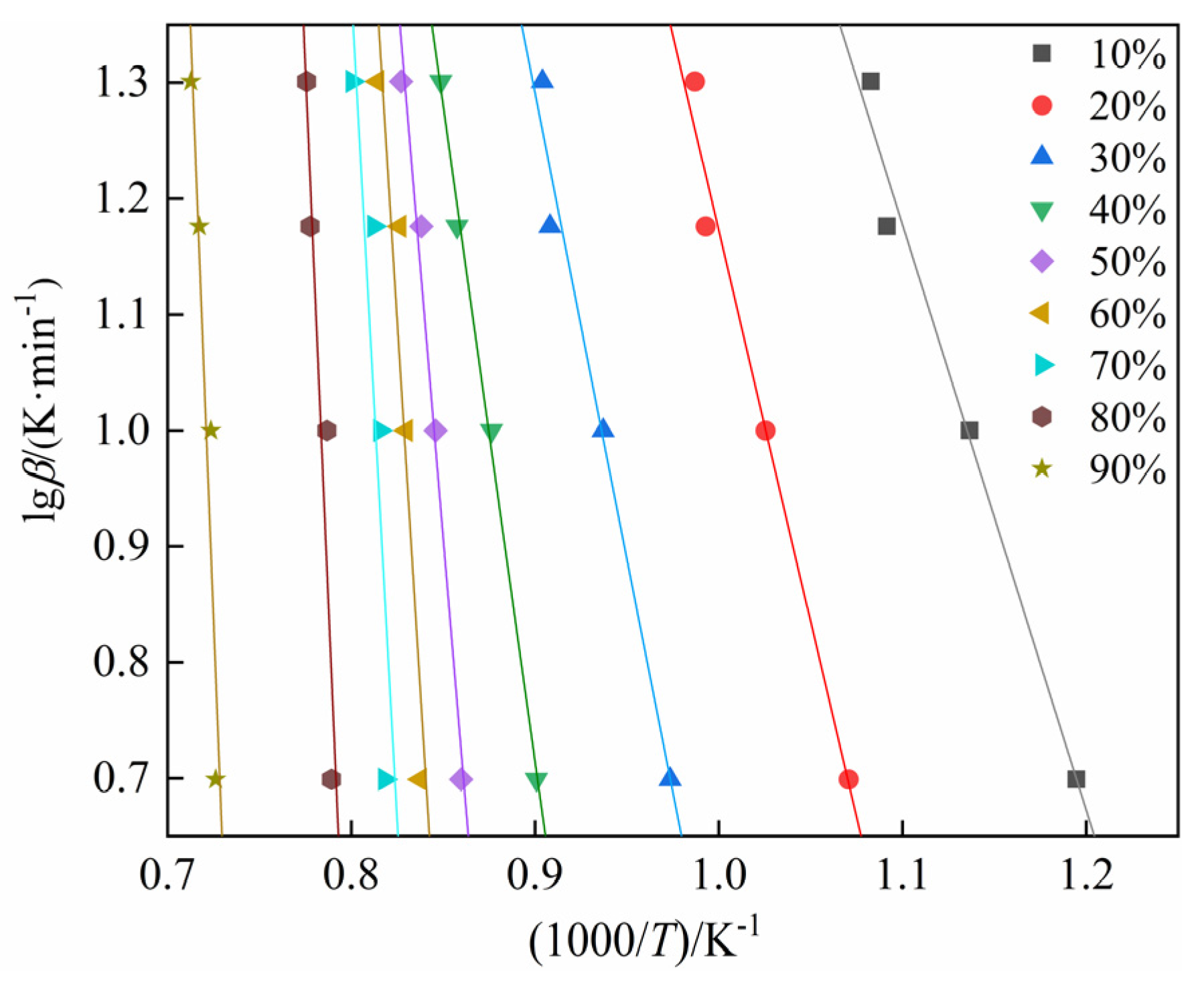
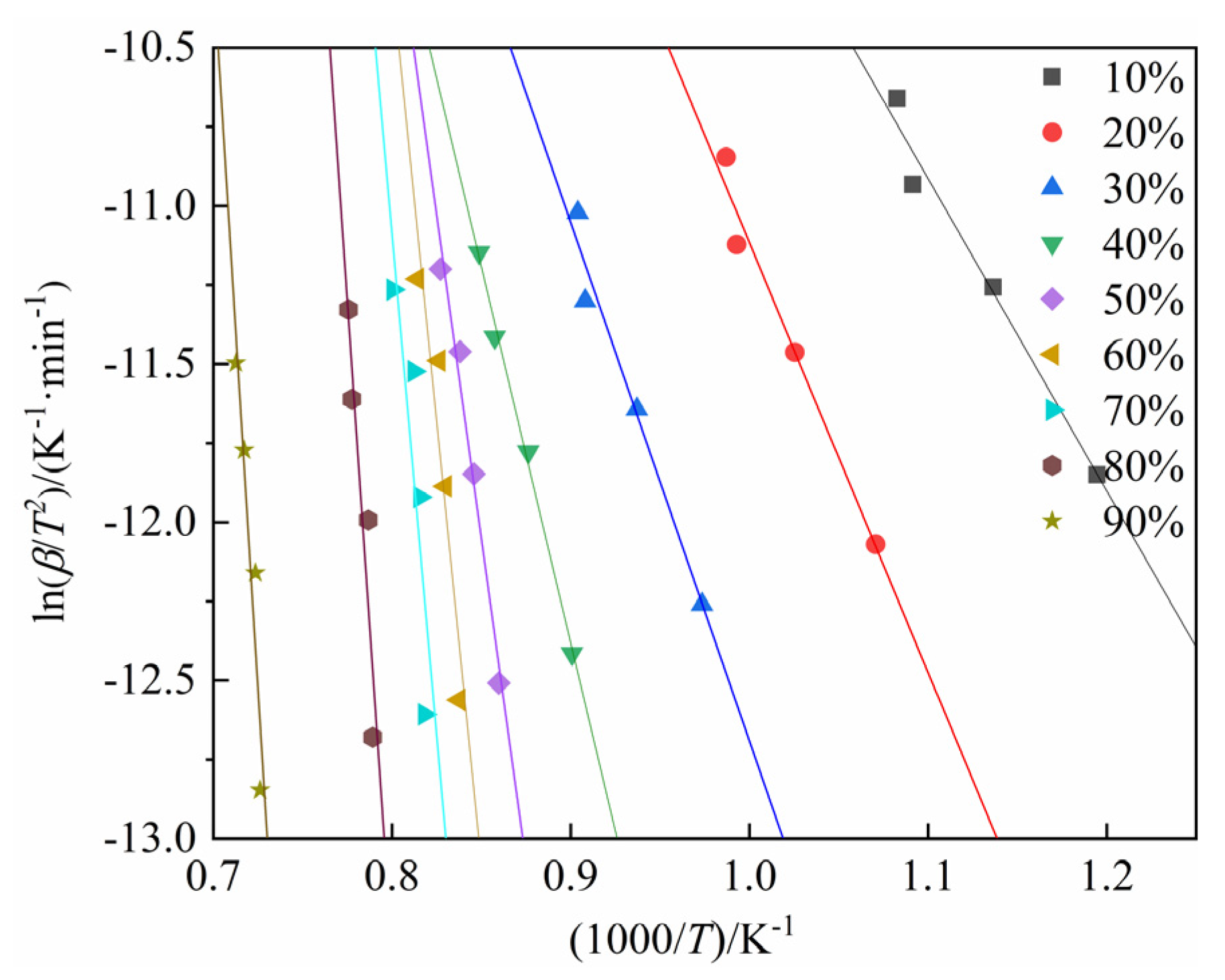
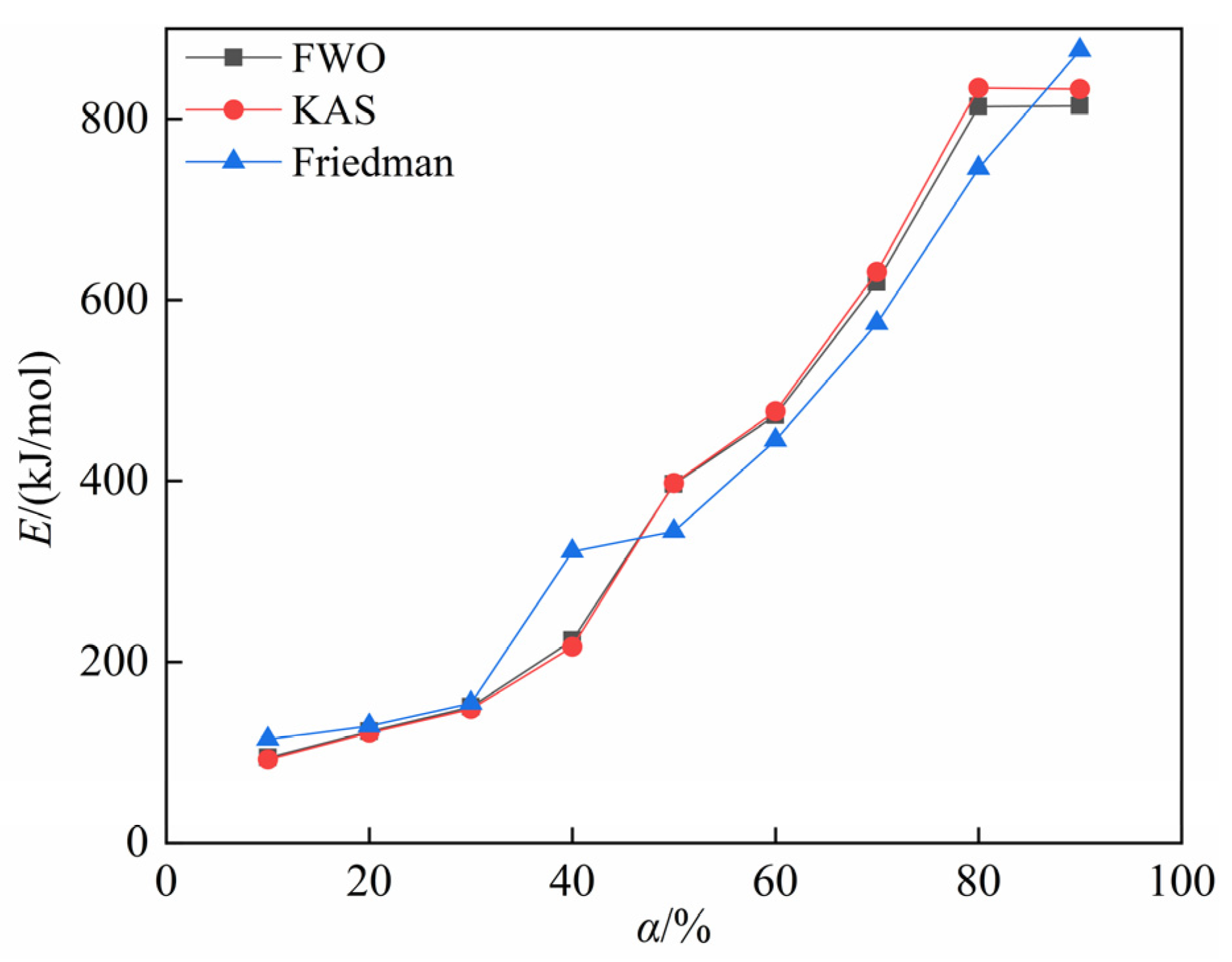
4.2. Calculation of Activation Energy for the Carbothermic Reduction Process of SSD
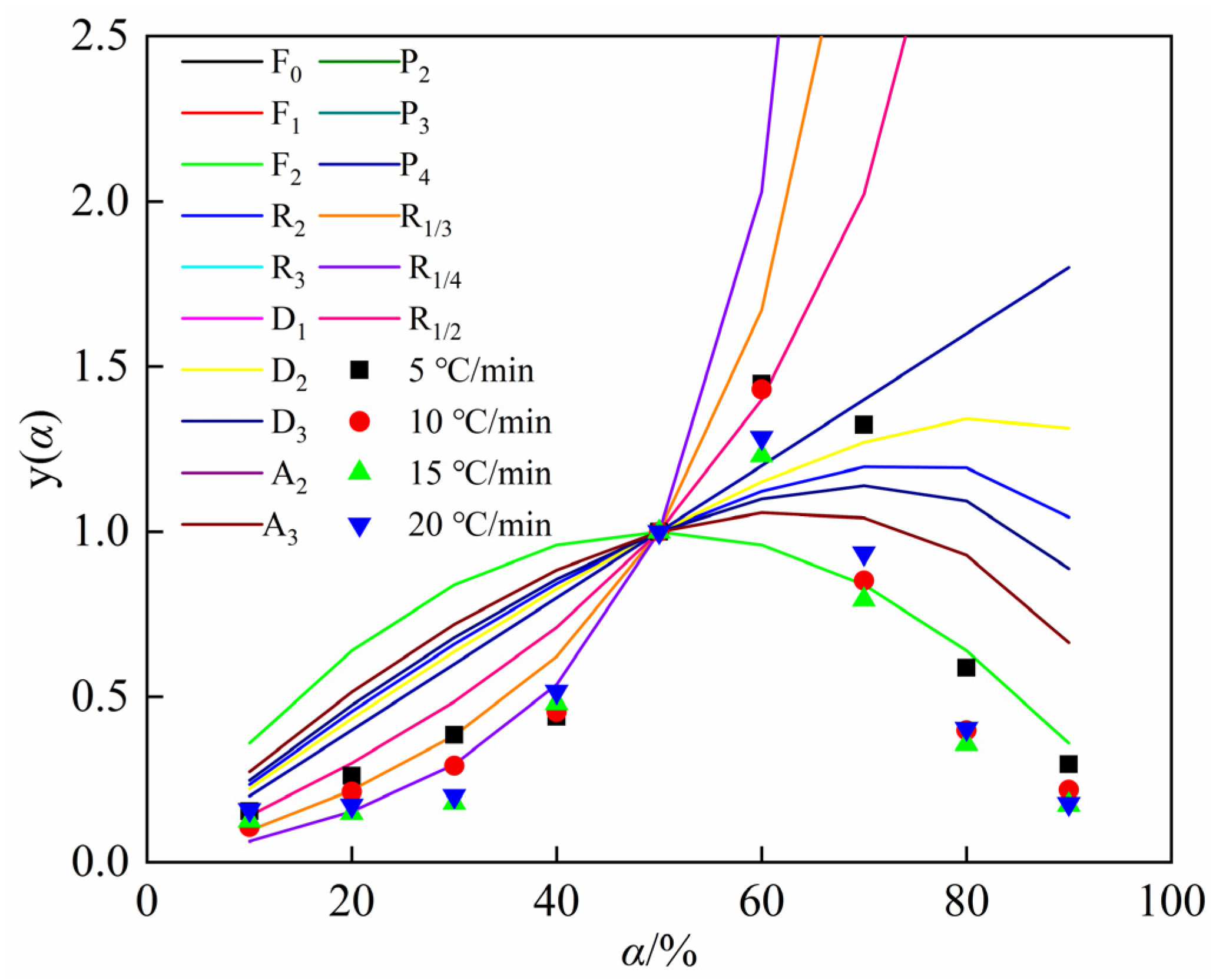
4.3. Fitting Results of Kinetic Models for the Carbothermic Reduction Process of SSD
5. Conclusions
- (1)
- Based on the thermogravimetric analysis results at different heating rates, the carbothermic reduction of stainless steel dust with biochar can be primarily divided into three stages: when the reaction temperature is below 600 °C, this stage is characterized by the direct reduction of Fe2O3 and partial direct reduction of NiO; when the reaction temperature is between 600 °C and 1200 °C, the reduction process is mainly dominated by the indirect reduction of Fe3O4 and FeO, accompanied by the direct reduction of FeCr2O4; and when the reaction temperature is between 1200 °C and 1400 °C, the primary reactions involve the stepwise reduction of chromium oxides.
- (2)
- The activation energy for the carbothermic reduction of stainless steel dust, as determined by the FWO method, ranges from 94.282 to 814.574 kJ/mol, with an average value of 412.120 kJ/mol. Utilizing the KAS method, the activation energy range is from 92.147 to 833.485 kJ/mol, with an average value of 416.930 kJ/mol. The activation energy computed using the Friedman method spans from 114.511 to 876.140 kJ/mol, with an average value of 411.778 kJ/mol. Furthermore, it is noted that the activation energy increases as the conversion rate increases.
- (3)
- Using walnut shell biochar as the reductant, SSD’s carbothermic reduction reaction is staged. In the conversion range of 10% to 50%, the carbothermic reduction reaction conforms to the shrinking core model within phase boundary reactions, coded as R1/4. In the conversion range of 50% to 60%, it conforms to the shrinking core model within phase boundary reactions, coded as R1/2, and in the conversion range of 60% to 90%, the carbothermic reduction reaction follows the second-order chemical reaction model, coded as F2.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, P.J.; Liu, Z.G.; Chu, M.S.; Yan, R.J.; Li, F.; Tang, J. Carbothermal reduction of stainless steel dust and laterite nickel ore: Slag phase behavior regulation and self-pulverization control mechanism. ISIJ Int. 2023, 63, 586–595. [Google Scholar] [CrossRef]
- Li, J.; Wu, M.L.; Wu, Z.; Liu, W.Z.; Lin, Z.; Li, X.Q. Efficient extraction of refractory Cr from stainless steel dust by sodium persulfate oxidation roasting. Waste Manag. 2024, 190, 423–431. [Google Scholar] [CrossRef]
- Liu, P.J.; Liu, Z.G.; Chu, M.S.; Yan, R.J.; Li, F.; Tang, J. A novel process for preparing Fe–Cr-Ni-C Alloy: Synergetic reduction of stainless steel dust and laterite nickel ore. Environ. Sci. Pollut. Res. 2022, 29, 65500–65520. [Google Scholar] [CrossRef]
- Li, Z.Q.; Zhang, X.; Ma, G.J.; Zheng, D.L.; Xu, J.; Xu, J. Effect of the Fe/Cr molar ratio and calcination temperature on the preparation of black ceramic pigment with stainless steel dust assisted by microwave processing. J. Clean. Prod. 2022, 372, 133751. [Google Scholar] [CrossRef]
- Li, Z.Q.; Zhang, X.; Ma, G.J.; Zheng, D.L.; Du, T.Y.; He, R.X. Effect of the nickel molar content on the preparation and properties of spinel-type black ceramic pigment by microwave processing from stainless steelmaking dust. Mater. Today Commun. 2022, 32, 104151. [Google Scholar] [CrossRef]
- Liu, P.J.; Liu, Z.G.; Chu, M.S.; Yan, R.J.; Li, F.; Tang, J. New understanding on metal recovery of Fe, Ni and Cr during carbon-thermal reduction of stainless steel dust. Adv. Powder Technol. 2021, 32, 4273–4285. [Google Scholar] [CrossRef]
- Liu, P.J.; Liu, Z.G.; Chu, M.S.; Yan, R.J.; Li, F.; Tang, J. Silicate slag system in carbothermal reduction of stainless steel dust: Strengthening mechanism and stable regulation. Mater. Chem. Phys. 2023, 304, 127850. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.Y.; Cui, K.K.; Fu, T.; Gao, J.J.; Hussain, S.; AlGarni, T.S. Pyrometallurgical recovery of zinc and valuable metals from electric arc furnace dust—A review. J. Clean. Prod. 2021, 298, 126788. [Google Scholar] [CrossRef]
- Liu, P.J.; Liu, Z.G.; Chu, M.S.; Yan, R.L.; Li, F.; Tang, J.; Feng, J.G. Efficient utilization of stainless steel dust and chromium-containing slag by carbothermal direct reduction: Synergistic mechanism and optimization analysis. J. Sustain. Metall. 2022, 8, 1877–1891. [Google Scholar] [CrossRef]
- Liu, P.J.; Liu, Z.G.; Chu, M.S.; Yan, R.J.; Li, F.; Tang, J. Effect of basicity on direct reduction and self-pulverization of stainless steel dust. J. Sustain. Metall. 2022, 8, 430–442. [Google Scholar] [CrossRef]
- Pu, G.Q.; Du, W.T.; Cheng, H.M.; Tian, M.H.; Chen, Z.; Chen, Y.Q.; Ju, D.C. Research on biomass waste utilization for synergetic reduction of stainless steel sludge and zinc-containing dust. J. Sustain. Metall. 2023, 10, 83–95. [Google Scholar] [CrossRef]
- Liu, P.J.; Liu, Z.G.; Chu, M.S.; Yan, R.J.; Li, F.; Tang, J.; Feng, J.G. Detoxification and comprehensive recovery of stainless steel dust and chromium containing slag: Synergistic reduction mechanism and process parameter optimization. Process Saf. Environ. Prot. 2022, 164, 678–695. [Google Scholar] [CrossRef]
- Zhu, R.L.; Huang, R.; Xu, A.X.; Li, B.; Zang, Y.G.; Deng, X.; Yang, J.; Li, M.; Long, X.Z. Research status and development of extraction process of zinc-bearing dust from ironmaking and steelmaking—A critical review. J. Iron Steel Res. Int. 2023, 30, 1303–1323. [Google Scholar] [CrossRef]
- Ye, Q.; Peng, Z.W.; Li, G.H.; Lee, J.; Liu, Y.; Liu, M.D.; Wang, L.C.; Rao, M.J.; Zhang, Y.B.; Jiang, T. Microwave-assisted reduction of electric arc furnace dust with biochar: An examination of transition of heating mechanism. ACS Sustain. Chem. Eng. 2019, 7, 9515–9524. [Google Scholar] [CrossRef]
- Xu, Y.J.; Xia, H.Y.; Zhang, Q.; Cai, W.C.; Jiang, G.Y.; Zhang, L.B. Green and efficient recovery of valuable metals from by-products of zinc hydrometallurgy and reducing toxicity. J. Clean. Prod. 2022, 380, 134993. [Google Scholar] [CrossRef]
- Kanagasundaram, T.; Murphy, O.; Haji, M.N.; Wilson, J.J. The Recovery and Separation of Lithium by Using Solvent Extraction Methods. Coord. Chem. Rev. 2024, 509, 215727. [Google Scholar] [CrossRef]
- Huang, X.; Liu, H.; Liu, J.; Liu, H. Synthesis of Micro Sphere CeO2 by a Chemical Precipitation Method with Enhanced Electrochemical Performance. Mater. Lett. 2017, 193, 115–118. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, S.F.; Sher, F.; Chen, J.B.; Xin, Y.T.; You, Z.X.; Wen, L.Y.; Hu, M.L.; Qiu, G.B. A review on recycling and reutilization of blast furnace dust as a secondary resource. J. Sustain. Metall. 2021, 7, 340–357. [Google Scholar] [CrossRef]
- Terrones-Saeta, J.M.; Suárez-Macías, J.; Moreno-López, E.R.; Corpas-Iglesias, F.A. Leaching of zinc for subsequent recovery by hydrometallurgical techniques from electric arc furnace dusts and utilisation of the leaching process residues for ceramic materials for construction purposes. Metals 2021, 11, 1603. [Google Scholar] [CrossRef]
- Xu, J.; Liu, M.K.; Ma, G.J.; Zheng, D.L.; Zhang, X.; Hou, Y.L. Valuable recovery technology and resource utilization of chromium-containing metallurgical dust and slag: A review. Metals 2023, 13, 1768. [Google Scholar] [CrossRef]
- Hanewald, R.H.; Munson, W.A.; Schweyer, D.L. Processing EAF Dusts and Other Nickel-Chromium Waste Materials Pyrometallurgically at INMETCO. Min. Metall. Explor. 1992, 9, 169–173. [Google Scholar] [CrossRef]
- Kim, G.; Sohn, I. Selective metal cation concentration during the solidification of stainless steel EAF dust and slag mixtures from high temperatures for increased Cr recovery. J. Hazard. Mater. 2018, 359, 174–185. [Google Scholar] [CrossRef]
- Ye, L.; Peng, Z.W.; Ye, Q.; Wang, L.C.; Augustine, R.; Perez, M.; Liu, Y.; Liu, M.D.; Tang, H.M.; Rao, M.J.; et al. Toward environmentally friendly direct reduced iron production: A novel route of comprehensive utilization of blast furnace dust and electric arc furnace dust. Waste Manag. 2021, 135, 389–396. [Google Scholar] [CrossRef]
- Zhang, H.N.; Hui, L.; Dong, J.H.; Xiong, H.H. Optimization of the stainless steel dust briquette reduction process for iron, chromium, and nickel recovery. High Temp. Mater. Process. 2018, 37, 785–791. [Google Scholar] [CrossRef]
- Zheng, L.C.; Malfliet, A.; Ren, H.Y.; Jiang, Z.H.; Blanpain, B.; Guo, M.X. Effect of reduction parameters on the size and morphology of the metallic particles in carbothermally reduced stainless steel dust. J. Sustain. Metall. 2022, 8, 297–309. [Google Scholar] [CrossRef]
- Stummann, M.Z.; Høj, M.; Gabrielsen, J.; Clausen, L.R.; Jensen, P.A.; Jensen, A.D. A perspective on catalytic hydropyrolysis of biomass. Renew. Sustain. Energy Rev. 2021, 143, 110960. [Google Scholar] [CrossRef]
- Guo, D.B.; Cui, B.H.; Chen, Z.H.; Yan, W.W.; Ji, B.; Zhang, Q.; Liu, Y.H.; Luo, S.Y.; Hu, M.; Ruan, R. Biomass enhances the reduction of oxidized pellets with carbon monoxide. Bioresour. Technol. 2021, 331, 124973. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Y.B.; Ou, Y.; Zhong, Q.; Zhang, Y.; Yi, L.Y.; Li, Q.; Huang, Z.C.; Jiang, T. In-depth study on the synergistic conversion mechanism of iron ore with waste biochar for Co-producing directly reduced iron (DRI) and syngas. Energy 2024, 290, 130250. [Google Scholar] [CrossRef]
- Deng, J.H.; Ning, X.A.; Shen, J.H.; Ou, W.X.; Chen, J.Y.; Qiu, G.Q.; Wang, Y.; He, Y. Biomass waste as a clean reductant for iron recovery of iron tailings by magnetization roasting. J. Environ. Manag. 2022, 317, 115435. [Google Scholar] [CrossRef]
- Yuan, P.; Shen, B.X.; Duan, D.P.; Adwek, G.; Mei, X.; Lu, F.J. Study on the formation of direct reduced iron by using biomass as reductants of carbon containing pellets in RHF process. Energy 2017, 141, 472–482. [Google Scholar] [CrossRef]
- Abdulyekeen, K.A.; Umar, A.A.; Patah, M.F.A.; Daud, W.M.A.W. Torrefaction of biomass: Production of enhanced solid biofuel from municipal solid waste and other types of biomass. Renew. Sustain. Energy Rev. 2021, 150, 111436. [Google Scholar] [CrossRef]
- Ben, H.X.; Wu, Z.H.; Han, G.T.; Jiang, W.; Ragauskas, A. Pyrolytic behavior of major biomass components in waste biomass. Polymers 2019, 11, 324. [Google Scholar] [CrossRef]
- Ibitoye, S.E.; Loha, C.; Mahamood, R.M.; Jen, T.C.; Alam, M.; Sarkar, I.; Das, P.; Akinlabi, E.T. An overview of biochar production techniques and application in iron and steel industries. Bioresour. Bioprocess. 2024, 11, 65. [Google Scholar] [CrossRef]
- Waqas, M.; Aburiazaiza, A.S.; Miandad, R.; Rehan, M.; Barakat, M.A.; Nizami, A.S. Development of biochar as fuel and catalyst in energy recovery technologies. J. Clean. Prod. 2018, 188, 477–488. [Google Scholar] [CrossRef]
- Ye, Q.; Peng, Z.W.; Li, G.H.; Liu, Y.; Liu, M.D.; Ye, L.; Wang, L.C.; Rao, M.J.; Jiang, T. Rapid microwave-assisted reduction of ferromanganese spinel with biochar: Correlation between phase transformation and heating mechanism. J. Clean. Prod. 2021, 286, 124919. [Google Scholar] [CrossRef]
- Du, W.T.; Pu, G.Q.; Cheng, H.M.; Wang, Q.H.; Chen, Z.; Ju, D.C. Kinetic study of biomass Co-disposal of zinc-bearing dust and chromium-bearing sludge. J. Sustain. Metall. 2024, 10, 2020–2031. [Google Scholar] [CrossRef]
- Liu, P.; Gong, S.Y.; Chao, Y.W.; Liu, B.G.; Zhang, L.B.; Wu, E.H. Kinetic study on microwave-enhanced direct reduction of titanomagnetite concentrate with coal. J. Iron Steel Res. Int. 2023, 30, 429–445. [Google Scholar] [CrossRef]
- Nagata, K.; Fukada, S. Effect of microwave irradiation to kinetics of carbothermic reduction of NiO. Mater. Trans. 2023, 64, 889–895. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, R.Q.; Ju, D.C.; Mao, R.; Ma, H.; Peng, H.B.; Du, W.T. Carbothermic kinetics and reaction mechanism of carbon-containing pellets: A combined treatment of chromium-containing sludge and zinc-bearing dust. J. Sustain. Metall. 2022, 8, 1001–1013. [Google Scholar] [CrossRef]
- Li, G.; Lv, X.W.; Ding, C.Y.; Zhou, X.G.; Zhong, D.P.; Qiu, G.B. Non-isothermal carbothermic reduction kinetics of calcium ferrite and hematite as oxygen carriers for chemical looping gasification applications. Appl. Energy 2020, 262, 114604. [Google Scholar] [CrossRef]
- Liu, Y.J.; Lv, X.M.; You, Z.X.; Lv, X.W. Kinetics study on non-isothermal carbothermic reduction of nickel laterite ore in presence of Na2SO4. Powder Technol. 2020, 362, 486–492. [Google Scholar] [CrossRef]
- Muvunyi, R.A.; Zheng, D.L.; Li, J.L.; Ma, G.J.; Owusu-Acheaw, Y.; Zhang, X. Kinetics of carbothermal reduction of electric arc furnace dust from a stainless steel plant. JOM 2023, 75, 1137–1147. [Google Scholar] [CrossRef]
- Yu, Y.; Cai, X.; Cao, Z.; Jiao, X.; Xie, W.; Yu, Y.; Feng, P. Effect of the Heating Rate on the Thermal Explosion Behavior and Oxidation Resistance of 3D-Structure Porous NiAl Intermetallic. Mater. Charact. 2022, 190, 112062. [Google Scholar] [CrossRef]
- Özgün, Ö.; Dirba, I.; Gutfleisch, O.; Ma, Y.; Raabe, D. Green ironmaking at higher H2 pressure: Reduction kinetics and microstructure formation during hydrogen-based direct reduction of hematite pellets. J. Sustain. Metall. 2024, 10, 1127–1140. [Google Scholar] [CrossRef]


| TFe | Fe2O3 | CaO | MgO | ZnO | MnO | Cr2O3 | NiO | Al2O3 | SiO2 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|
| 48.41 | 69.60 | 5.21 | 0.55 | 0.18 | 6.45 | 11.22 | 1.03 | 0.20 | 3.38 | 2.72 |
| Sample | Fixed Carbon | Volatile Matter on Air-Dried Basis | Ash Content on Air-Dried Basis | Moisture Content on Air-Dried Basis | High Heat Value |
|---|---|---|---|---|---|
| Walnut shell biochar | 86.48 | 11.10 | 1.85 | 0.56 | 7891 cal/g |
| Sample | C | H | O | N | S |
|---|---|---|---|---|---|
| Walnut shell biochar | 74.31 | 2.35 | 8.36 | 1.33 | 0.44 |
| α | FWO | R2 | KAS | R2 | Friedman | R2 |
|---|---|---|---|---|---|---|
| E/(kJ·mol−1) | E/(kJ·mol−1) | E/(kJ·mol−1) | ||||
| 10% | 94.282 | 0.9919 | 92.147 | 0.9882 | 114.511 | 0.9697 |
| 20% | 123.206 | 0.9910 | 121.625 | 0.9884 | 129.265 | 0.9897 |
| 30% | 150.169 | 0.9894 | 148.042 | 0.9866 | 154.288 | 0.9823 |
| 40% | 223.950 | 0.9924 | 216.789 | 0.9978 | 322.210 | 0.9985 |
| 50% | 396.592 | 0.9892 | 397.554 | 0.9878 | 344.382 | 0.9645 |
| 60% | 472.770 | 0.9654 | 477.024 | 0.9517 | 445.184 | 0.9487 |
| 70% | 619.718 | 0.9722 | 631.145 | 0.9456 | 574.358 | 0.9483 |
| 80% | 813.819 | 0.9384 | 834.552 | 0.9584 | 745.666 | 0.9411 |
| 90% | 814.574 | 0.9480 | 833.485 | 0.9645 | 876.140 | 0.9813 |
| Mean value | 412.120 | - | 416.930 | - | 411.778 | - |
| Model | Mechanism | Code | Differential Model f(α) | Integral Model G(α) |
|---|---|---|---|---|
| Order of reaction (n) | n = 0 | F0 | 1 | α |
| n = 1 | F1 | 1 − α | −ln(1 − α) | |
| n = 2 | F2 | (1 − α)2 | (1 − α)−1 − 1 | |
| Phase boundary reaction | Shrinking core, m = 1/4 | R1/4 | 1/4(1 − α)−3 | 1 − (1 − α)4 |
| Shrinking core, m = 1/3 | R1/3 | 1/3(1 − α)−2 | 1 − (1 − α)3 | |
| Shrinking core, m = 1/2 | R1/2 | 1/2(1 − α)−3 | 1 − (1 − α)2 | |
| Shrinking core, m = 2 | R2 | 2(1 − α)1/2 | 1 − (1 − α)1/2 | |
| Shrinking core, m = 3 | R3 | 3(1 − α)2/3 | 1 − (1 − α)1/3 | |
| Diffusion | The one-dimensional diffusion | D1 | 1/2α−1 | α2 |
| The two-dimensional diffusion | D2 | [−ln(1 − α)]−1 | α + (1 − α) ln(1 − α) | |
| The three-dimensional diffusion | D3 | (1 − α)1/2 [1 − (1 − α)1/2] − 1 | [1 − (1 − α)1/2]2 | |
| Random nucleation and nuclei growth | Two dimension | A2 | 2(1 − α) [−ln(1 − α)]1/2 | −ln(1 − α)1/2 |
| Three dimension | A3 | 3(1 − α) [−ln(1 − α)]1/3 | −ln(1 − α)1/3 | |
| Exponential nucleation | Power series law, n = 1/2 | P2 | 2α1/2 | α1/2 |
| Power series law, n = 1/3 | P3 | 3α2/3 | α1/3 | |
| Power series law, n = 1/4 | P4 | 4α3/4 | α1/4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, G.; Zhang, X.; Xu, Y.; Ma, G.; Zheng, D.; Xu, J. Study on the Kinetics of Carbothermic Reduction of Stainless Steel Dust by Walnut Shell Biochar. Metals 2025, 15, 835. https://doi.org/10.3390/met15080835
Cui G, Zhang X, Xu Y, Ma G, Zheng D, Xu J. Study on the Kinetics of Carbothermic Reduction of Stainless Steel Dust by Walnut Shell Biochar. Metals. 2025; 15(8):835. https://doi.org/10.3390/met15080835
Chicago/Turabian StyleCui, Guoyu, Xiang Zhang, Yanghui Xu, Guojun Ma, Dingli Zheng, and Ju Xu. 2025. "Study on the Kinetics of Carbothermic Reduction of Stainless Steel Dust by Walnut Shell Biochar" Metals 15, no. 8: 835. https://doi.org/10.3390/met15080835
APA StyleCui, G., Zhang, X., Xu, Y., Ma, G., Zheng, D., & Xu, J. (2025). Study on the Kinetics of Carbothermic Reduction of Stainless Steel Dust by Walnut Shell Biochar. Metals, 15(8), 835. https://doi.org/10.3390/met15080835







