Phase Transformations During Heat Treatment of a CPM AISI M4 Steel
Abstract
1. Introduction
2. Materials and Methods
2.1. Numerical Methodology
2.2. Experimental Methodology
3. Results and Discussion
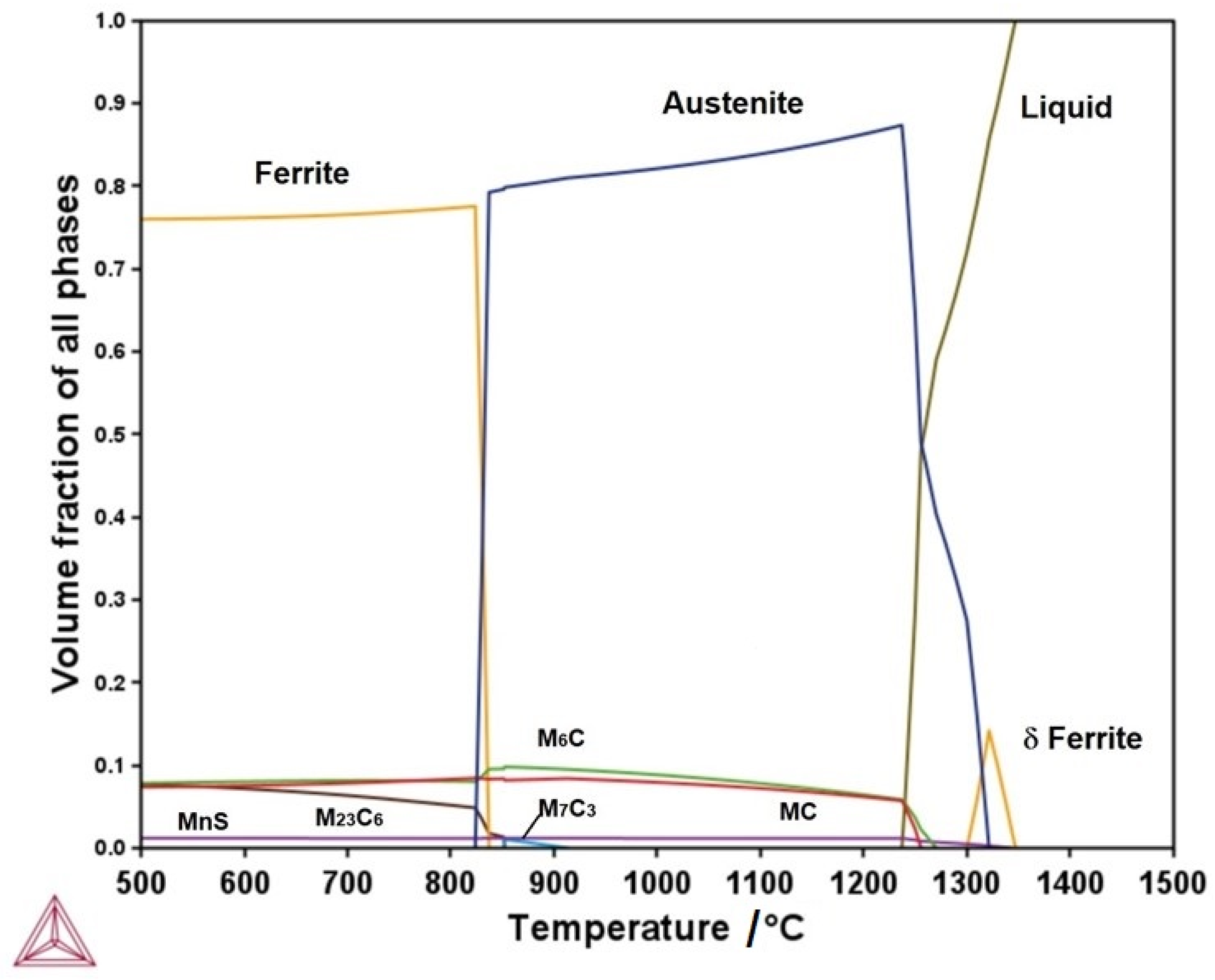
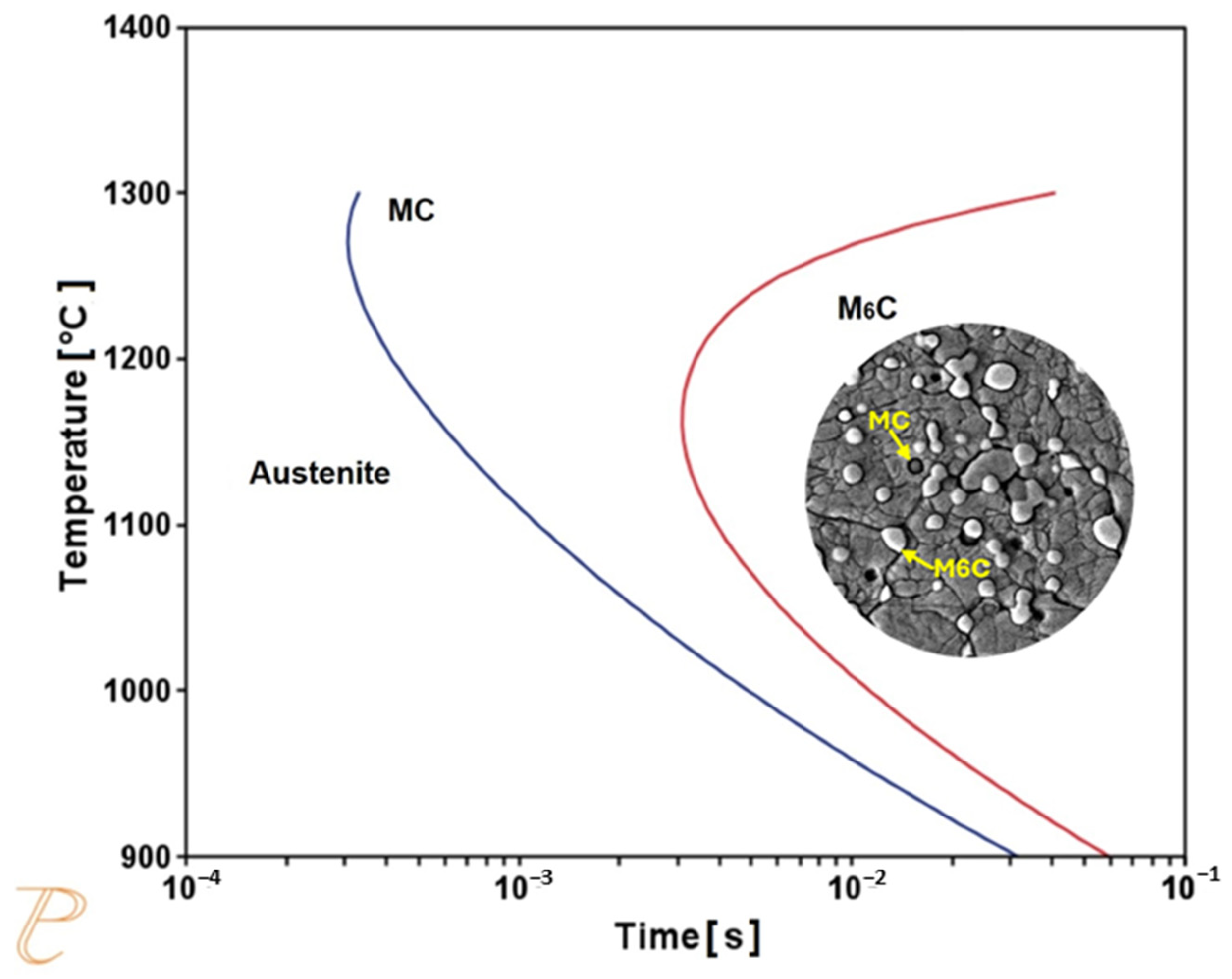
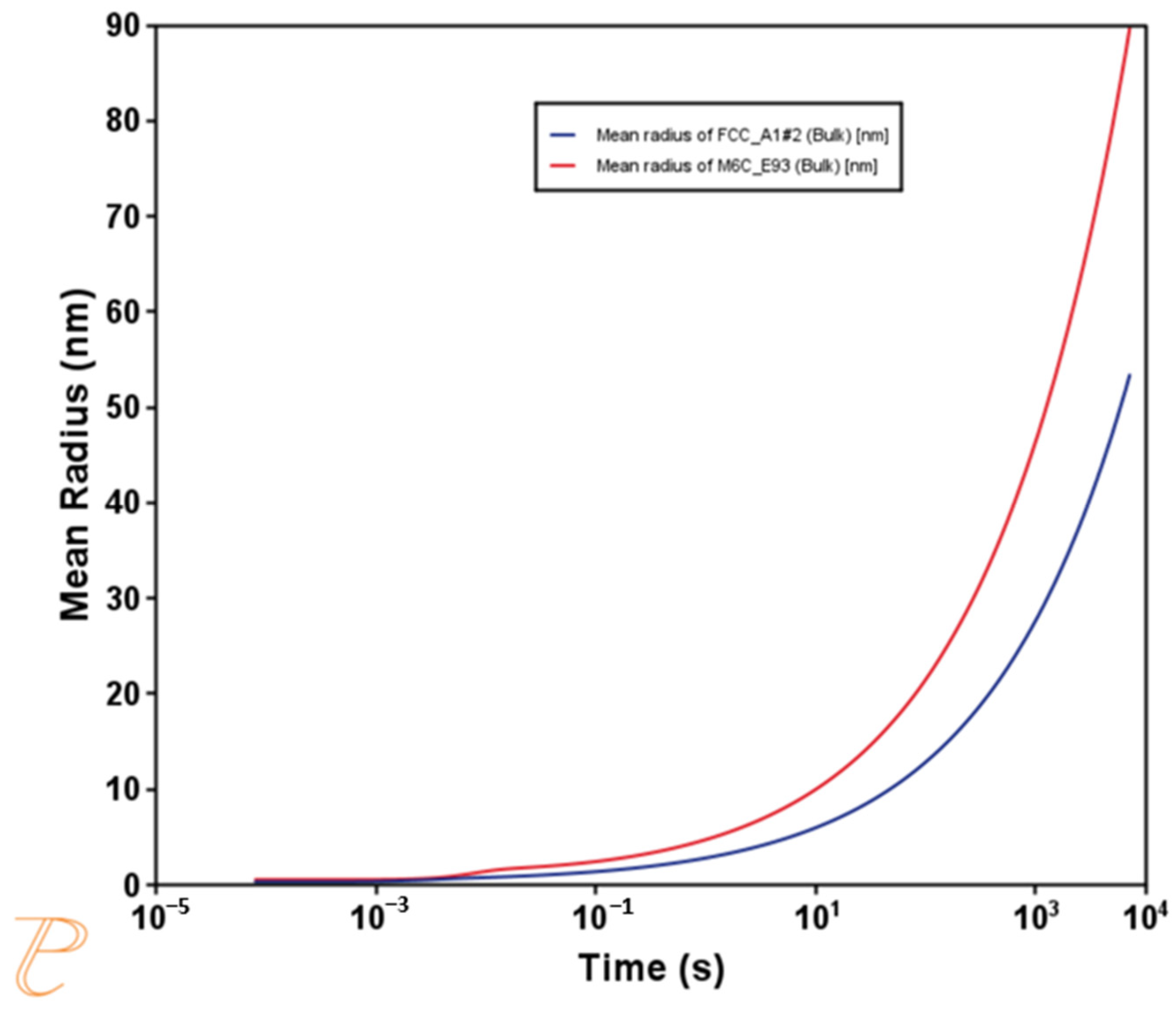
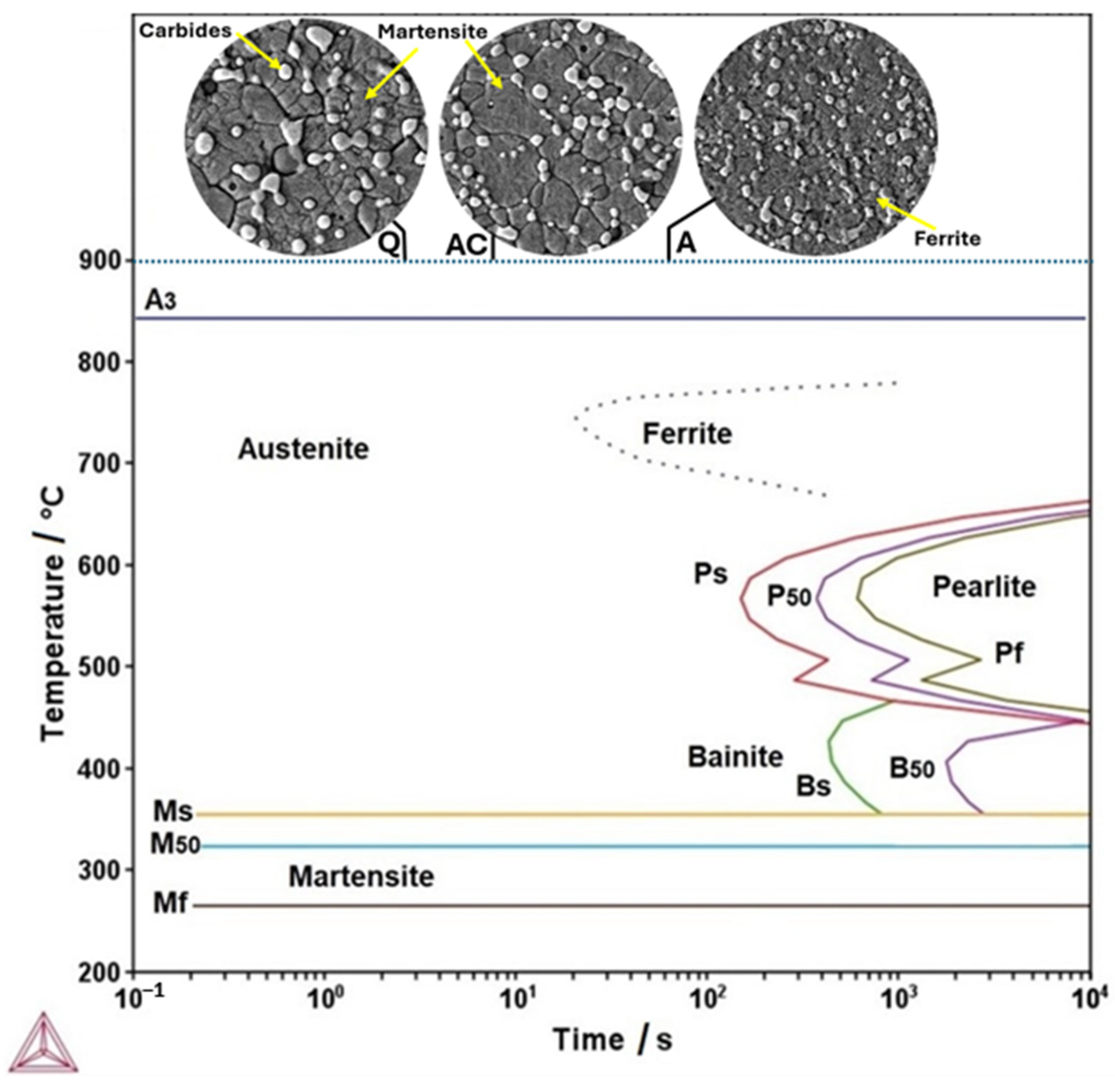
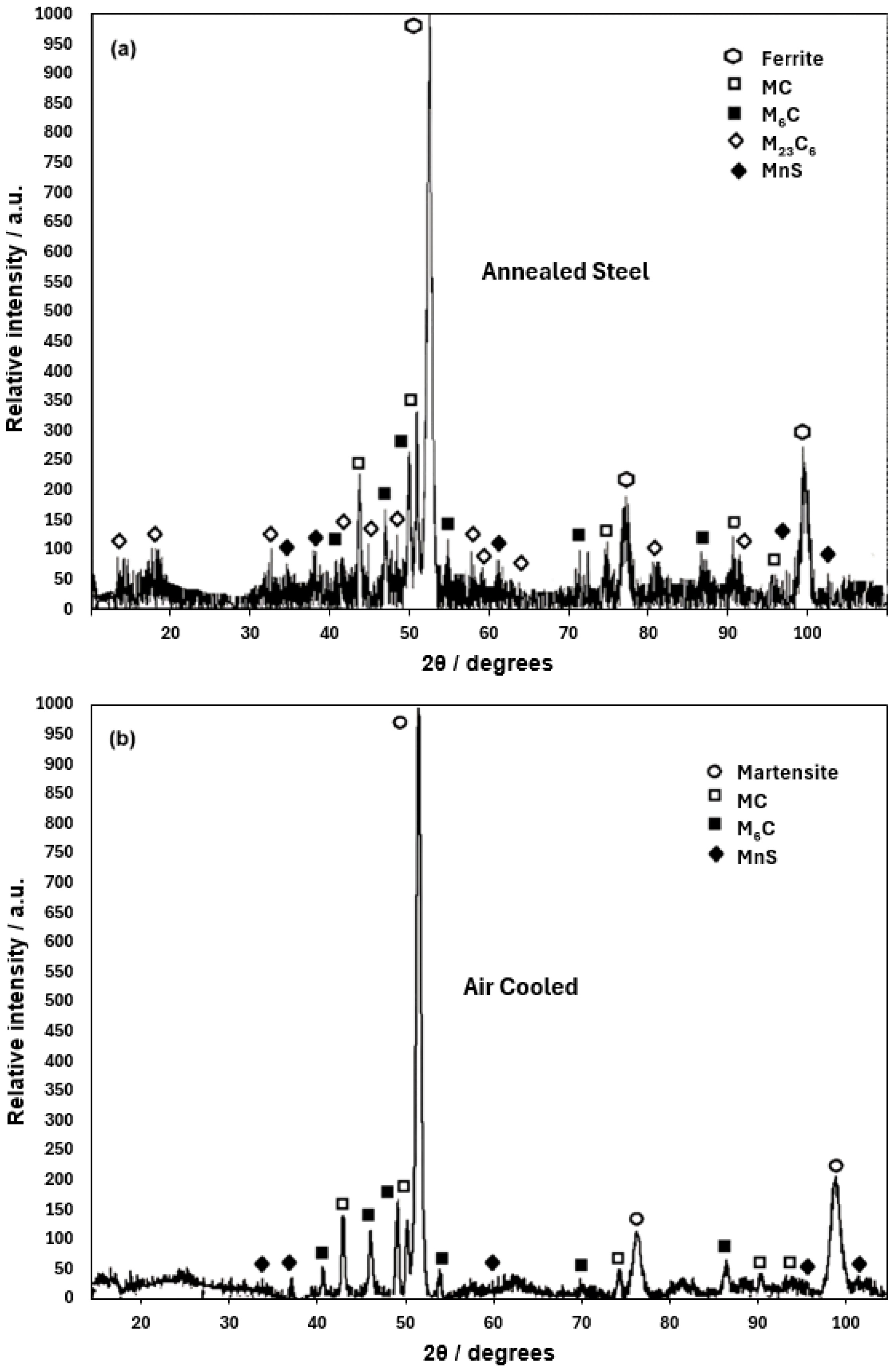
3.1. Themo-Calc Analysis of Equilibrium and Nonequilibrium Phases
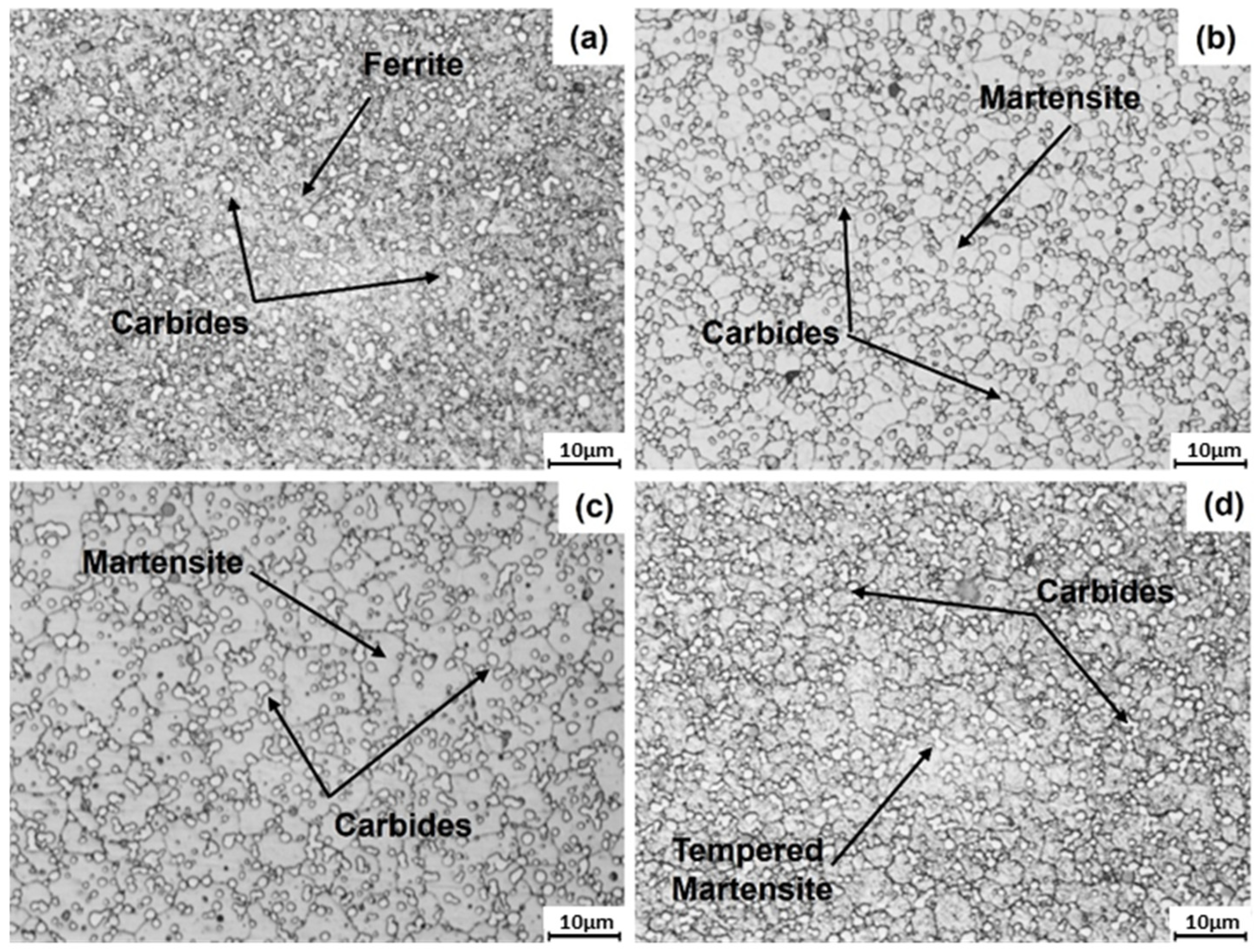
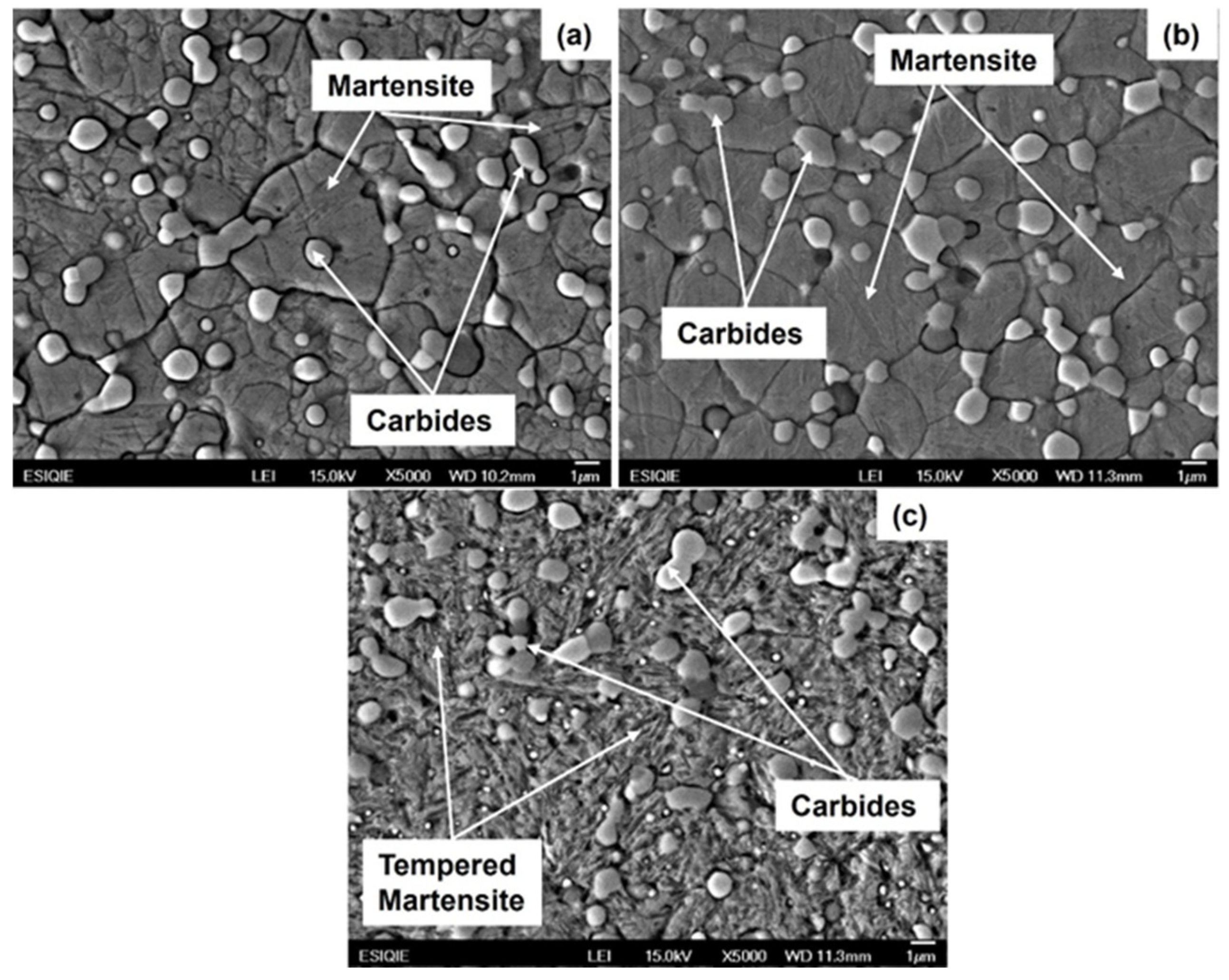
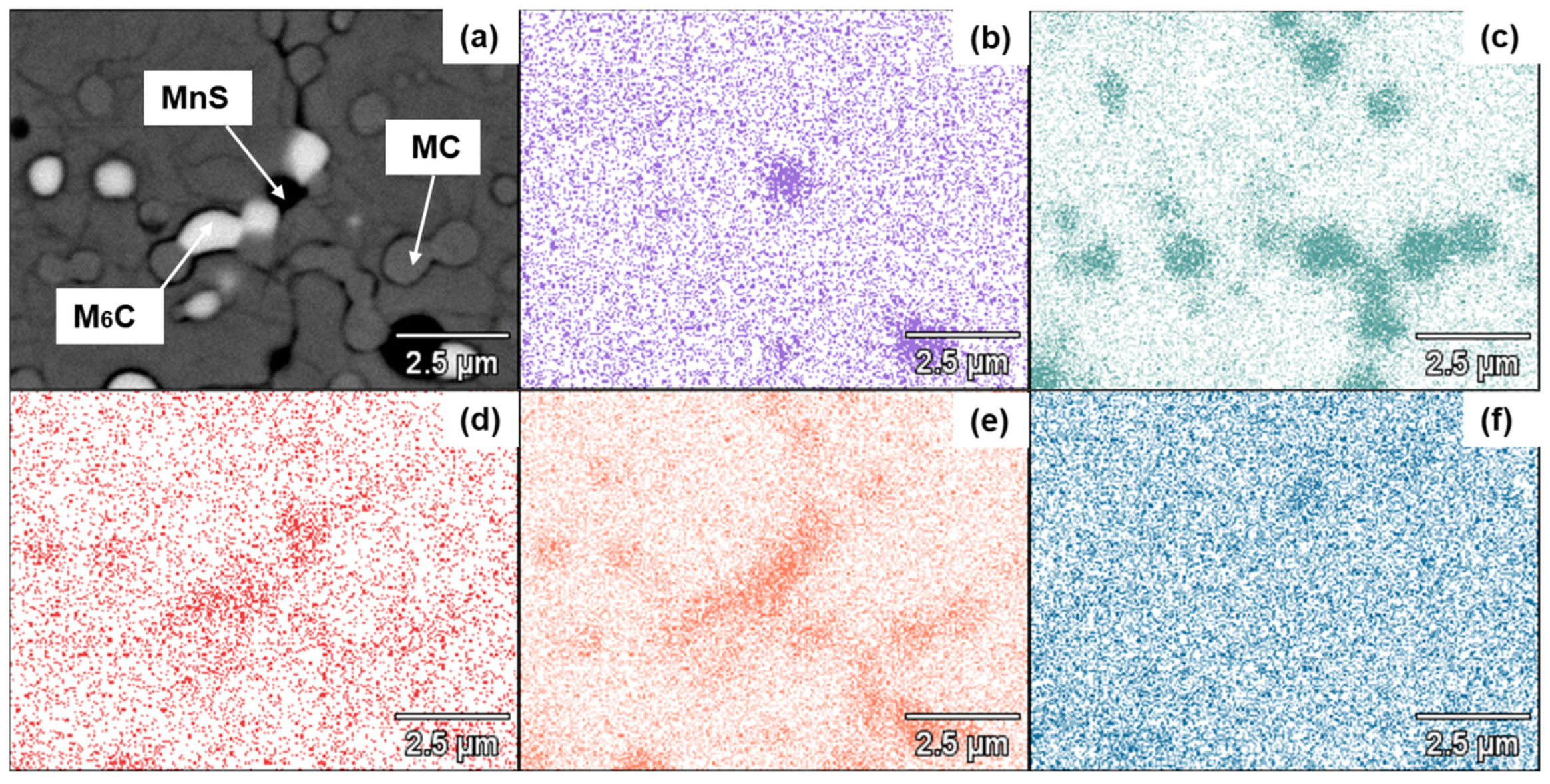
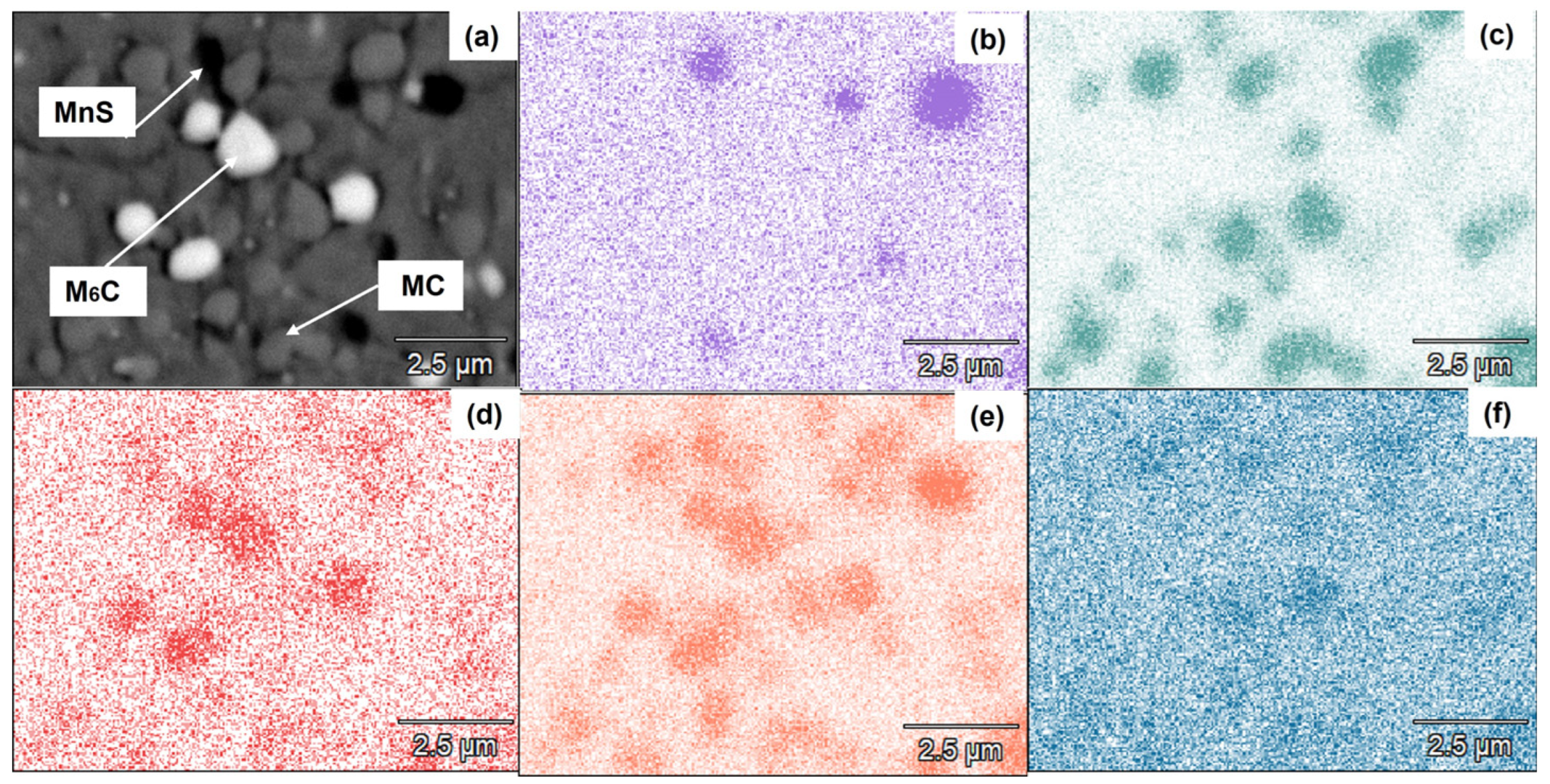
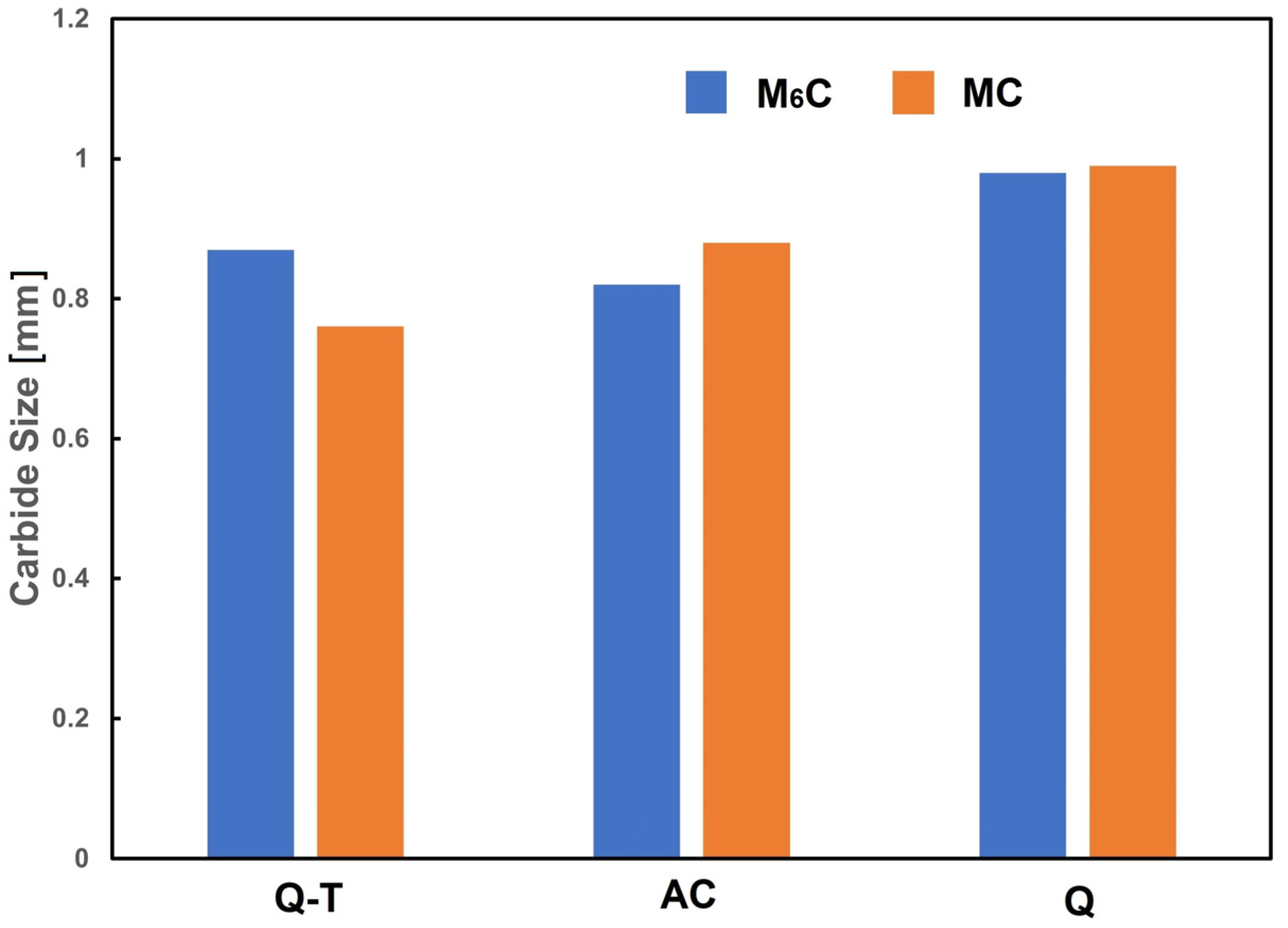
3.2. Microstructural Characterization of Heat-Treated Steel
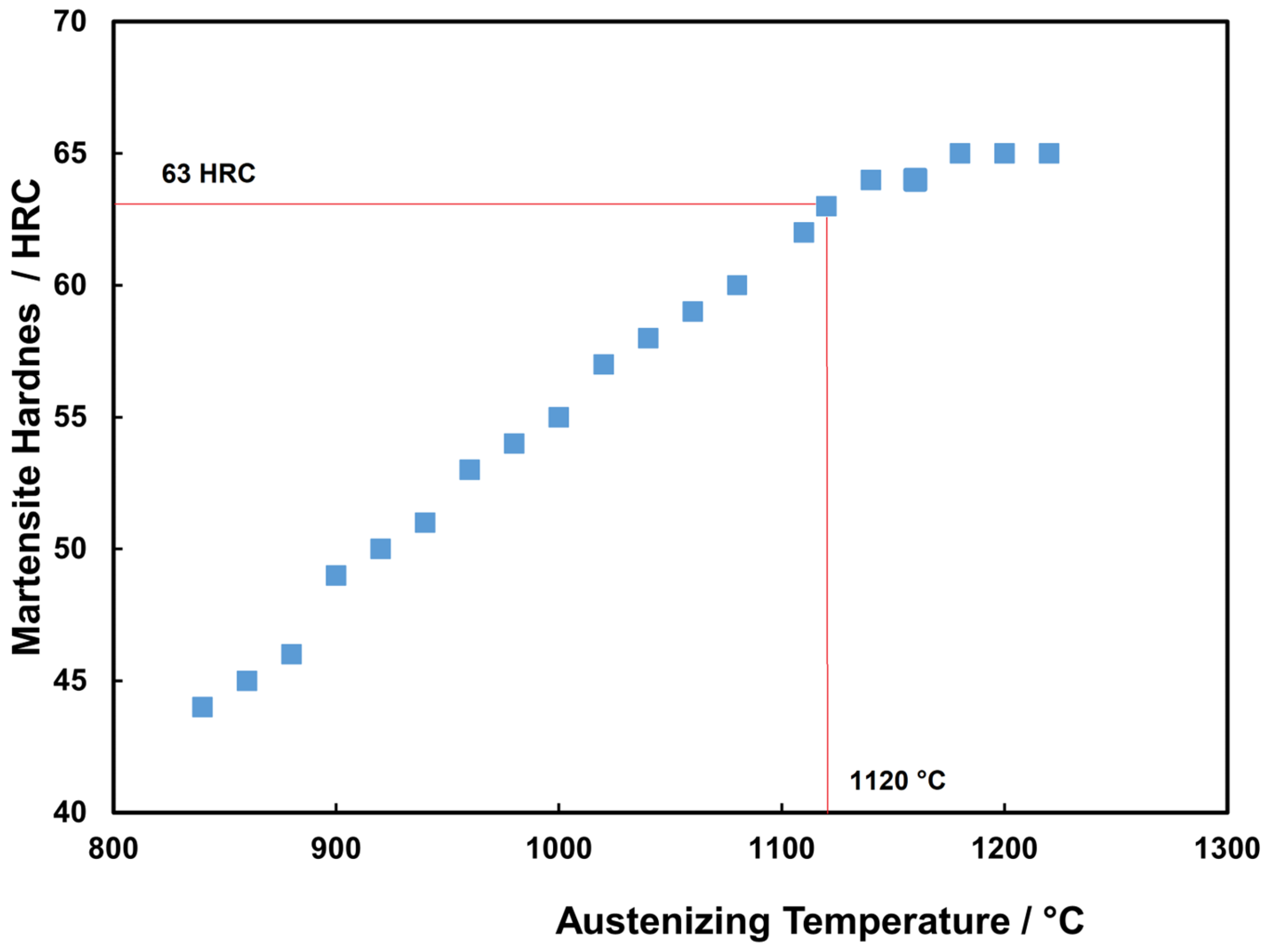
3.3. Rockwell Hardness of Heat-Treated Steels
3.4. Relationship of Microstructure to Hardness
4. Conclusions
- The ferrite and carbide phases observed in the annealed specimen are consistent with those predicted for the equilibrium condition using Thermo-Calc. The Rockwell C hardness measured for this heat treatment agrees with values expected for the observed phases.
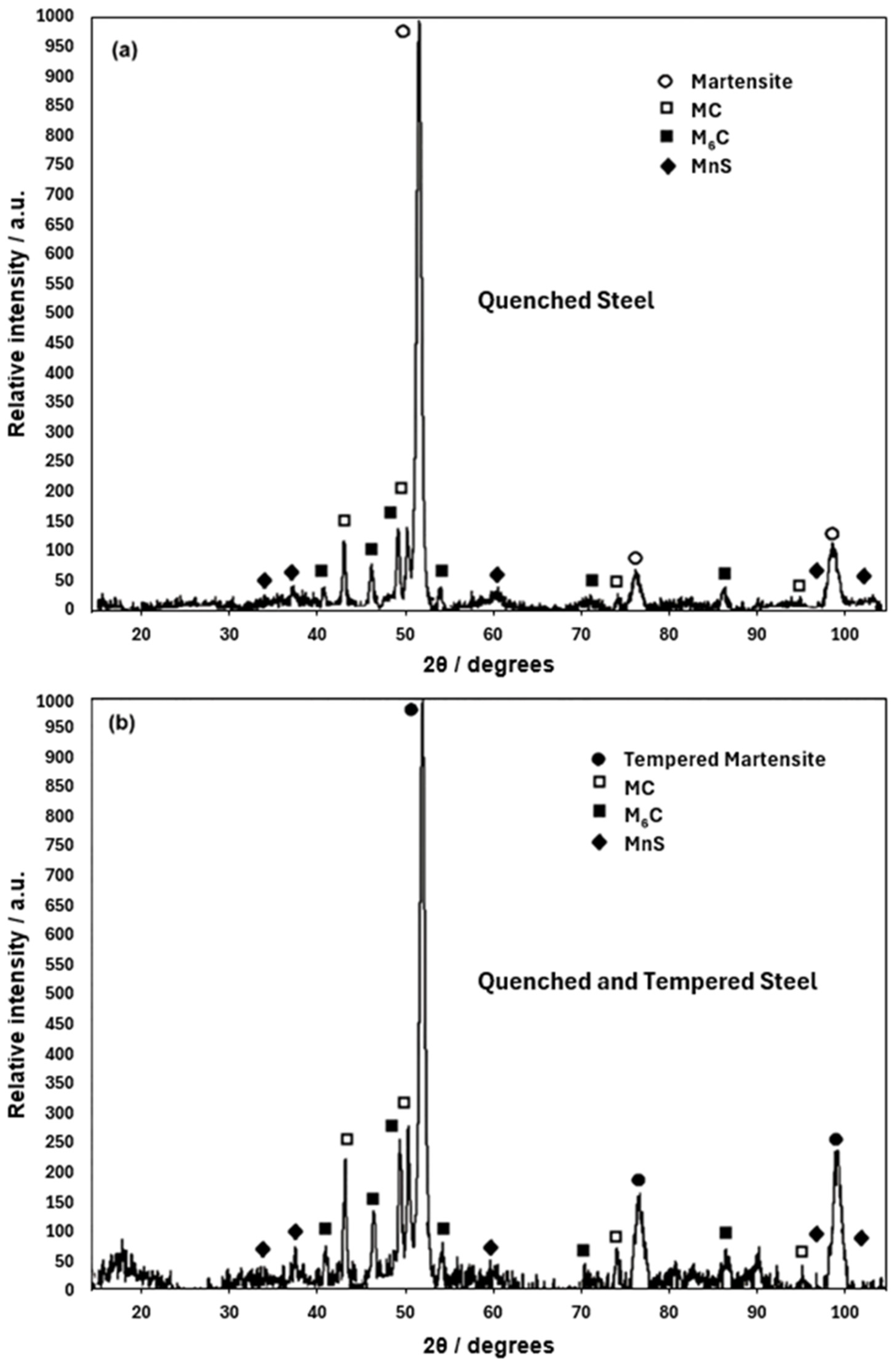
- The martensite microconstituent obtained in the air-cooled and quenched conditions aligns well with that predicted by the nonequilibrium TTT diagram calculated using Thermo-Calc. The Rockwell C hardness is consistent with the observed microconstituent.
- The austenitizing temperature and quenched hardness predicted using Themo-Calc show good agreement with the experimental values. This temperature enables the attainment of a suitable quenched Rockwell C hardness.
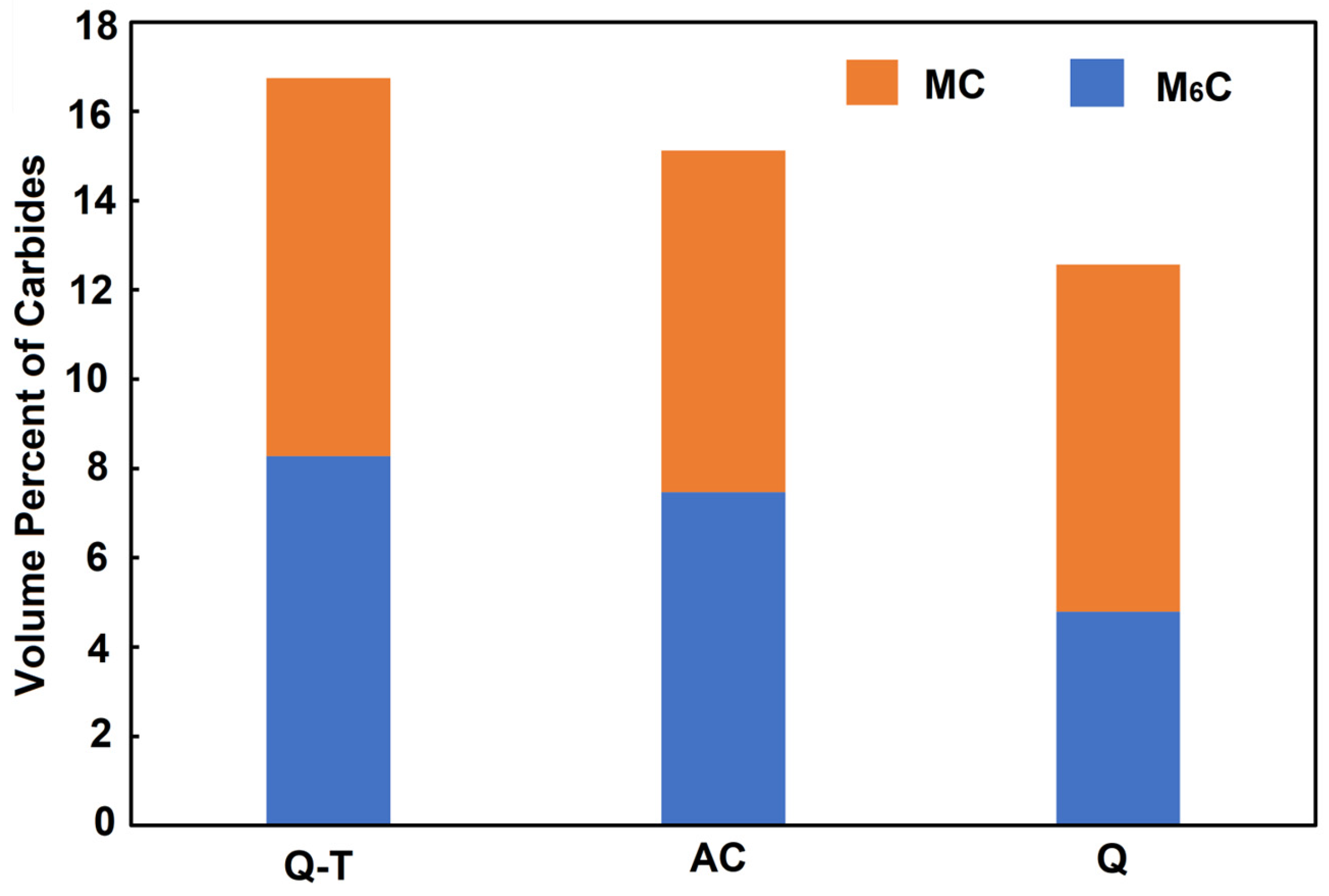
- The tempering process led to an increase in the volume fraction of finer M6C carbides, promoting secondary hardening. This finding is beneficial for producing cutting tools with better performance.
- The presence of Mo- and W-rich M6C carbides is essential if good mechanical properties are to be obtained in the quenched–tempered condition.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bryson, W.E. Heat Treatment Selection, and Application of Tool Steels, 2nd ed.; Carl Hanser Verlag GmbH & Co. KG: Munich, Germany, 2005; pp. 75–82. [Google Scholar]
- Moon, H.K.; Lee, K.B. Influences of Co addition and austenitizing temperature on secondary hardening and impact fracture behavior in P/M high speed steels of W–Mo–Cr–V(–Co) system. Mater. Sci. Eng. 2008, 474, 328–334. [Google Scholar] [CrossRef]
- Chaus, A.S.; Kryshtal, A.P. New insights into the microstructure of M2 high-speed steel. Mater. Charact. 2023, 205, 113313. [Google Scholar] [CrossRef]
- Kraus, R.G.; Kennedy, R. Tool Steels, 5th ed.; ASM: Novelty, OH, USA, 1998; pp. 46–123. [Google Scholar]
- Jurci, P.; Dlouhy, I. Tratamiento criogénico de aceros martensíticos: Fundamentos microestructurales e implicaciones para las propiedades mecánicas y el rendimiento frente al desgaste y la corrosión. Materials 2024, 17, 548. [Google Scholar] [PubMed]
- Shim, D.-S.; Baek, G.-Y.; Lee, S.-B.; Yu, J.H.; Choi, Y.-S.; Park, S.-H. Influence of heat treatment on wear behavior and impact toughness of AISI M4 coated by laser melting deposition. Surf. Coat. Technol. 2017, 328, 219–230. [Google Scholar] [CrossRef]
- Damon, J.; Schüßler, P.; Mühl, F.; Dietrich, S.; Schulz, V. Short-time induction heat treatment of high-speed steel AISI M2: Laboratory proof of and application-related component tests. Mater. Des. 2023, 230, 111991. [Google Scholar] [CrossRef]
- Fu, H.; Qu, Y.; Xing, J.; Zhi, X.; Jiang, Z.; Li, M.; Zhang, Y. Investigations on heat treatment of a high-speed steel roll. JMEPEG. 2008, 17, 535–542. [Google Scholar] [CrossRef]
- Baek, G.-Y.; Shin, G.-Y.; Lee, K.-Y.; Shim, D.-S. Effect of post-heat treatment on the AISI M4 layer deposited by directed energy deposition. Metals 2020, 10, 703. [Google Scholar] [CrossRef]
- Jardin, R.T.; Turninetti, V.; Tchuinjang, J.T.; Duchene, L.; Hashemi, N.; Tran, H.S.; Carrus, R.; Martens, A.; Habraken, A.M. Optimizing laser power directed energy by deposition process for homogeneous AISI M4 steel microstructure. Opt. Laser Technol. 2023, 163, 109426. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, M.; Chen, Y.; Li, H.; Si, Z. The effect of MC-type carbides on the microstructure and wear behavior of S390 high-speed steel produced via spark plasma sintering. Metals 2022, 12, 2168. [Google Scholar] [CrossRef]
- Shi, P.; Engstrom, A.; Hoglund, L.; Sundman, B.; Agren, J. Thermo-Calc and DICTRA enhance Materials Design and Process. Mater. Sci. For. 2005, 475–479, 3339–3346. [Google Scholar]
- Briki, J.; Slima, S.B. A new continuous cooling transformation diagram for AISI M4 high-speed tool steel. J. Mater. Eng. Perform. 2008, 17, 864–869. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, H.; Sun, X.; Mao, M.; Guo, J. Effects of austenitizing conditions on the microstructure of AISI M42 high-speed steel. Metals 2017, 7, 27. [Google Scholar] [CrossRef]
- Lopez-Hirata, V.M.; Hernandez-Santiago, F.; Saucedo-Muñoz, M.L.; Dorantes-Rosales, H.J.; Paniagua-Mercado, A.M. Growth kinetics of β’precipitation in a ferritic matrix during isothermal aging of Cu-containing Fe-10at.%Ni-15at.%Al alloys. Mater. Res. 2018, 21, 1–7. [Google Scholar]
- Thermo-Calc. Thermo-Calc, versión 2024b; PRISMA: Stockholm, Sweden, 2024.
- Cheng, O.; Wu, K.; Sterner, G.; Mason, P. Modeling Precipitation Kinetics During Heat Treatment with Calphad-Based Tools. J. Mater. Eng. Perform. 2014, 23, 4193–4196. [Google Scholar] [CrossRef]
- Yang, J.-Y.; Agren, J.; Jeppsson, J. Pearlite in multicomponent steels: Phenomenological steady-state modeling. Met. Mater. Trans. 2020, 51A, 1978–2001. [Google Scholar]
- Leach, L.; Kolmskog, P.; Höglund, L.; Hillert, M.; Borgenstam, A. Critical driving forces for formation of bainite. Met. Mater. Trans. 2018, 49A, 4509–4518. [Google Scholar] [CrossRef]
- Huyan, M.; Hedstrom, M.; Höglund, L.; Borgenstam, A. A thermodynamic-based model to predict the fraction of martensite in steels. Met. Mater. Trans. 2016, 47A, 4404–4410. [Google Scholar] [CrossRef]
- ASTM E18-22; Standard Test Methods for Rockwell Hardness of Metallic Materials. ASTM: West Conshohocken, PA, USA, 2022.
- Zhou, X.; Xu, X.Z.; Shen, Y.; Shi, T.; Huang, X. Identification of precipitate phases in an 11%Cr ferritic/martensitic steel after short-term creep. ISIJ Int. 2018, 58, 1467. [Google Scholar] [CrossRef]
- Wang, L.N.; Sun, X.F.; Guan, H.R. Effect of Melt Heat Treatment on MC Formation in Nickel Base Superalloy. Results Phys. 2017, 7, 2111–2117. [Google Scholar] [CrossRef]
- Ma, S.; Xing, J.; He, Y.; Li, Y. Microstructure and crystallography of M7C3 carbide in chromium cast iron. Mater. Chem. Phys. 2015, 161, 65–73. [Google Scholar] [CrossRef]
- Bowman, A.L.; Arnold, G.P.; Storms, E.; Nereson, N.G. The crystal structure of Cr23C6. Struct. Sci. 1972, 28, 3102–3103. [Google Scholar] [CrossRef]
- Porter, D.A.; Easterling, K.E.; Sherif, M.Y. Phase Transformations in Metals and Alloys, 4th ed.; CRC: Boca Raton, FL, USA, 2022; pp. 451–468. [Google Scholar]
- ASTM 255-20A; Standard Test Methods for Determining Hardenability of Steel. ASTM: West Conshohocken, PA, USA, 2022.
- Kraus, G. Steels: Processing, Structure, and Performance, 2nd ed.; ASM: Novelty, Ohio, USA, 2015; pp. 621–642. [Google Scholar]
- Kostorz, G. Phase Transformations in Materials, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2001; pp. 309–408. [Google Scholar]
- Brooks, C.R. Heat Treatments of Ferrous Alloys, 1st ed.; Mc Graw-Hill: New York, NY, USA, 1979; pp. 149–177. [Google Scholar]
| Element | Fe | C | Mn | Si | Cr |
| wt. % | Bal. | 1.47 | 0.57 | 0.30 | 4.42 |
| Element | Mo | V | W | S | P |
| wt. % | 5.41 | 4.05 | 5.59 | 0.22 | 0.01 |
| Temperature (°C) | C | Mn | Si | Cr | Mo | V | W |
|---|---|---|---|---|---|---|---|
| 900 | 0.17 | 0.28 | 0.34 | 4.36 | 1.27 | 0.27 | 0.0 |
| 1000 | 0.26 | 0.29 | 0.33 | 4.40 | 2.04 | 0.52 | 0.0 |
| 1120 | 0.41 | 0.30 | 0.32 | 4.40 | 3.04 | 1.05 | 0.0 |
| Heat Treatment | HRC |
|---|---|
| Annealing | 24 ± 2 |
| Air-Cooling | 65 ± 2 |
| Quenching | 63 ± 2 |
| Quenching and Tempering | 57 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saucedo-Muñoz, M.L.; Miranda-Lopez, V.; Hernandez-Santiago, F.; Ferreira-Palma, C.; Lopez-Hirata, V.M. Phase Transformations During Heat Treatment of a CPM AISI M4 Steel. Metals 2025, 15, 818. https://doi.org/10.3390/met15070818
Saucedo-Muñoz ML, Miranda-Lopez V, Hernandez-Santiago F, Ferreira-Palma C, Lopez-Hirata VM. Phase Transformations During Heat Treatment of a CPM AISI M4 Steel. Metals. 2025; 15(7):818. https://doi.org/10.3390/met15070818
Chicago/Turabian StyleSaucedo-Muñoz, Maribel L., Valeria Miranda-Lopez, Felipe Hernandez-Santiago, Carlos Ferreira-Palma, and Victor M. Lopez-Hirata. 2025. "Phase Transformations During Heat Treatment of a CPM AISI M4 Steel" Metals 15, no. 7: 818. https://doi.org/10.3390/met15070818
APA StyleSaucedo-Muñoz, M. L., Miranda-Lopez, V., Hernandez-Santiago, F., Ferreira-Palma, C., & Lopez-Hirata, V. M. (2025). Phase Transformations During Heat Treatment of a CPM AISI M4 Steel. Metals, 15(7), 818. https://doi.org/10.3390/met15070818






