Abstract
This study investigates the microstructural evolution, mechanical behavior, and electrochemical performance of CoCrFeNiNb and CoCrFeNiV HEAs fabricated via mechanical alloying and spark plasma sintering. Microstructural analyses reveal that the alloys have a face-centered cubic (FCC) matrix with Nb-enriched Laves and V-enriched σ phases. The CoCrFeNiNb HEA exhibits superior compressive strength and hardness than CoCrFeNiV due to uniform Laves phases distribution. Fracture surface analysis reveals that at lower sintering temperatures, the fracture is primarily caused by incomplete particle bonding, whereas at higher temperatures, brittle fracture modes dominated via transgranular cracking become predominant. The research results of potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) show that both alloys exhibited superior electrochemical stability in a 3.5 wt.% NaCl solution compared to the CoCrFeNi base alloy. X-ray photoelectron spectroscopy (XPS) analysis confirms the formation of stable oxide layers (Nb2O5 and V2O3) on the precipitated phases, acting as protective barriers against chloride ion penetration. The selective oxidation of Nb and V improves the integrity of the passive film, reducing the corrosion rates and enhancing the long-term durability. These findings highlight the critical role of precipitated phases in enhancing the corrosion resistance of HEAs, and emphasize their potential for use in extreme environments.
1. Introduction
High-entropy alloys (HEAs) have emerged as promising materials for extreme en-vironments due to their exceptional mechanical properties and resistance to wear and corrosion. Unlike conventional alloys, which rely on one or two principal elements, HEAs consist of multiple principal elements in near-equiatomic ratios, resulting in unique microstructures with high configurational entropy [1,2]. Various fabrication techniques, including arc melting, laser melting, and chemical deposition, have been employed to produce HEAs. However, many of these methods present challenges such as compositional segregation, uneven microstructures, and the volatilization of low-melting-point elements [3,4,5,6,7]. Recently, solid-state sintering processes such as mechanical alloying (MA) and spark plasma sintering (SPS) have gained increasing attention due to their ability to produce uniform compositions, refined microstructures, and enhanced material properties [8,9]. For example, CoCrFeMnNi HEAs fabricated via MA and SPS achieved a relative density of 98%, significantly higher than the 87% obtained via conventional pressureless sintering [10]. Compared to traditional casting or press sintering methods, which often suffer from longer processing times, coarser grain structures, and higher residual porosity, the SPS process offers distinct advantages. These include rapid heating rates, short sintering durations, and the application of uniaxial pressure, all of which contribute to grain refinement, densification, and the suppression of elemental segregation [11,12]. As a result, SPS-processed HEAs exhibit superior mechanical strength and corrosion resistance, making solid-state sintering an effective and reliable approach for developing high-performance materials intended for demanding service environments.
Among the various HEA systems, CoCrFeNi-based alloys have been widely studied due to their simple FCC structure and excellent ductility [13,14,15,16]. To enhance their strength, elements such as Al, Mn, Mo, Ti, and W are commonly added to form precipitate phases, contributing to both solid solution strengthening and grain boundary reinforcement [17,18,19,20]. However, improving the corrosion resistance of CoCrFeNi alloys, particularly in aggressive chloride-containing environments, remains a significant challenge. Alloying with refractory elements such as Nb and V has shown promise in addressing this issue by improving both mechanical performance and electrochemical stability through the formation of stable precipitated phases and protective oxide layers. The addition of Nb and V has been demonstrated to promote the formation of Laves and σ phases, which act as sites for the development of protective passive films, such as Nb2O5 and V2O3, on the alloy surface. The presence of these oxide layers has been demonstrated to enhance the corrosion resistance by providing a barrier against chloride ion penetration. However, the precise mechanisms through which these oxides form on different phases, such as the FCC matrix and precipitated phases, remain unclear. Furthermore, the interaction between the FCC matrix and precipitated phases during corrosion and its impact on the alloy’s electrochemical stability are still not fully understood.
Addressing these gaps is essential for the optimization of high-entropy alloys (HEAs) for utilization in aggressive environments, such as marine structures and chemical reactors, where long-term corrosion resistance is paramount. The present study investigates the synthesis, microstructure, mechanical properties, and corrosion behavior of CoCrFeNiNb and CoCrFeNiV HEAs, which were prepared using MA and SPS. The present research is focused on the understanding of the role of precipitated phases, specifically the Nb-enriched Laves phase and the V-enriched σ phase, in enhancing mechanical properties and corrosion resistance. A combination of XRD, SEM, EBSD, electrochemical tests, and XPS analysis was utilized to ascertain the formation of stable passive films, including Nb2O5 and V2O3, on these phases. These oxide layers act as protective barriers against chloride ion penetration, reducing the corrosion rate and improving the electrochemical stability of the alloys. The findings demonstrate that the selective oxidation of Nb and V significantly improves the long-term electrochemical stability of the alloys, offering valuable guidelines for the design of corrosion-resistant HEAs for industrial applications, particularly in harsh environments.
2. Materials and Methods
2.1. Synthesis of CoCrFeNiNb and CoCrFeNiV HEAs via Mechanical Alloying and Spark Plasma Sintering
The preparation process begins with the utilization of elemental powders of Co, Cr, Fe, Ni, Nb, and V with a 70 μm diameter and 99% purity, mixed in equimolar ratios. The powders were milled using a high-energy ball mill (QM-QX4L, Changsha MiQi Instrument Plant, Changsha, China) equipped with stainless steel grinding balls and 1000 mL stainless steel bottles. The milling process was conducted at a rotation speed of 300 rpm, with a ball-to-powder weight ratio of 10:1, using grinding balls with diameters of 5, 10, and 15 mm. To prevent oxidation during the milling process, the entire procedure was carried out in an argon atmosphere. After ball milling, the alloy powders were consolidated into high-entropy alloy blocks using a vacuum SPS furnace (SPS-3T-3-MIN(H), Chenhua Instrument Co., Ltd., Shanghai, China). The sintering process was conducted under a vacuum of approximately 5.0 × 10−3 Pa, with a uniaxial pressure of 37 MPa applied by using a hydraulic press. The sintering temperature was monitored and controlled via an infrared thermometer. To facilitate the process of demolding, graphite paper was placed between the alloy powder and the graphite mold. The sintering process was performed at temperatures of 800 °C, 900 °C, 1000 °C, and 1100 °C, with a holding time of 15 min at each temperature.
2.2. Microstructural Characterization
The phase evolution and phase composition of the alloy powders and sintered blocks were analyzed using X-ray diffraction (XRD, X’Pert3 Powder, PANalytical, Almelo, The Netherlands) with Cu Kα radiation (λ = 0.154 nm) at 40 kV and 30 mA. The diffraction data were collected over a 2θ range of 30° to 100° with a step size of 0.02°. The microstructure of CoCrFeNiNb and CoCrFeNiV HEA powders and their corresponding sintered blocks were examined using scanning electron microscopy (SEM, Quanta FEG-450, FEI, Hillsboro, OR, USA), and the elemental composition was determined by using an energy-dispersive spectrometer (EDS, Octane Pro, EDAX, Mahwah, NJ, USA) equipped on the SEM. Electron backscatter diffraction (EBSD, X-Max80, Oxford Instruments, Abingdon, UK) was employed to characterize the phase distribution and quantify the grain size of the sintered blocks. High-resolution transmission electron microscopy (HRTEM, JEM-F200, JEOL Ltd., Tokyo, Japan) operating at an accelerating voltage of 200 kV was utilized for detailed microstructural observation and selected area electron diffraction (SAED) analysis. For TEM sample preparation, small discs with a diameter of 3 mm were polished to a thickness of 60 μm and further thinned using an electrolytic double-jet method in a 10% HClO4 + 90% C2H5OH electrolyte at 25 V and −15 °C. Following the process of thinning, the discs were thoroughly cleaned with anhydrous ethanol and subsequently air-dried prior to analysis.
2.3. Mechanical Property Evaluation
The density of the sintered CoCrFeNiNb and CoCrFeNiV HEAs was determined at room temperature by means of the Archimedes method. Hardness measurements were conducted on the polished surface of the HEA samples using a Vickers hardness tester (HVS-1000, Laizhou Huayin Testing Instrument Co., Ltd., Laizhou, China) under a 4.9 N indentation load applied for 15 s. Each sample was tested seven times to ensure the accuracy of the results. The compressive properties of the HEAs were evaluated at room temperature using a universal testing machine (AGS-X-300kN, Shimadzu, Kyoto, Japan). Compression specimens were cylindrical in shape, with a diameter of 2.5 mm and a height of 5 mm, and were tested at a strain rate of 0.001 s−1. At least three compression tests were conducted for each alloy to obtain average values. After compression, the fractured specimens were retrieved for microstructural observation with the objective of assessing the deformation and fracture mechanisms.
2.4. Electrochemical Performance Evaluation
The evaluation of the corrosion resistance of the HEAs was conducted through the utilization of electrochemical measurements, which were performed on a CHI660E electrochemical workstation (CH Instruments, Austin, TX, USA). The corrosion tests were conducted in a 3.5 wt.% NaCl solution without prior oxygen removal. Specimens with dimensions of 5 mm × 5 mm × 2 mm were embedded in epoxy resin, with copper wires used for electrical connections, and subsequently polished to expose a working surface area of 25 mm2. This surface served as the working electrode in a three-electrode electrochemical cell, accompanied by a platinum counter electrode and a saturated Ag/AgCl reference electrode. A 3.5 wt.% NaCl solution was used as the electrolyte, and all the electrochemical measurements were conducted under ambient laboratory conditions (22–25 °C). The open circuit potential (OCP) was measured in a static solution and allowed to stabilize for a period of 30 min prior to further measurements. Subsequently, the stabilized OCP was applied as the initial potential for electrochemical impedance spectroscopy (EIS) measurements, which were performed over a frequency range of 0.01 Hz to 100 kHz with a 10 mV amplitude perturbation. Potentiodynamic polarization tests were conducted using a three-electrode setup with a scan rate of 1 mV/s.
3. Results and Discussion
3.1. Phase and Microstructural Evolution of CoCrFeNiNb and CoCrFeNiV Powders During Mechanical Alloying
Figure 1a,b show the XRD patterns of CoCrFeNiNb and CoCrFeNiV HEA powders at various milling times. At 0 h, distinct diffraction peaks corresponding to Co, Cr, Fe, Ni, Nb, and V are observed, indicating that the initial powders remain in their elemental forms without significant interdiffusion. Following a 10 h period of high-energy ball milling, there was a decrease in the intensity of the elemental peak decreases, suggesting the commencement of alloying among the constituent elements. As shown in Figure 1a,b, by 20 h, the diffraction peaks of Nb and V exhibit a substantial decline, suggesting the progression of further alloying and the formation of a face-centered cubic (FCC) solid solution phase. At 30 h, the Nb and V peaks disappear entirely, with only peaks associated with the FCC phase remaining. This confirms the complete dissolution of Nb and V into the FCC lattice. The progressive disappearance of elemental peaks and the dominance of the FCC phase suggest that the alloying process is nearly complete by this stage. Furthermore, with increasing milling time, the XRD diffraction peak has undergone significant broadening, which is attributed to crystallite size refinement, residual strain accumulation, and severe lattice distortion. These phenomena are the result of high-energy impacts during prolonged milling. The microstructural changes promote the formation of a homogeneous solid solution phase, which is consistent with previous reports in the literature [21,22].
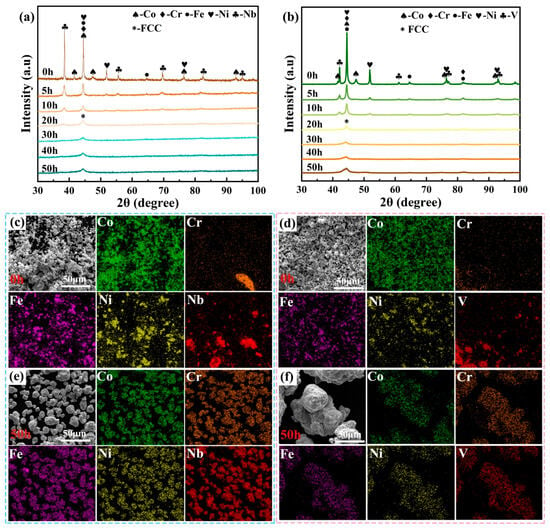
Figure 1.
XRD patterns, SEM images, and EDS elemental maps of CoCrFeNiNb (a,c,e) and CoCrFeNiV (b,d,f) HEA powders at different milling times. (a,b) XRD patterns from 0 h to 50 h; (c,d) and (e,f) microstructure and elemental distributions at 0 h and 50 h, respectively.
Figure 1c–f show the SEM images and EDS elemental mapping of CoCrFeNiNb and CoCrFeNiV alloy powders at different ball milling times (0 h and 50 h). At 0 h, the SEM images reveal that the powders have irregular shapes and varying particle sizes, which is typical for mechanically alloyed powders at the initial stage. The EDS elemental maps indicate that the alloying elements, such as Co, Cr, Fe, Ni, Nb, and V, exhibit distinct cluster-like agglomerations of varying sizes, suggesting that the elements remain in their original forms without significant interdiffusion. This finding indicates that additional milling is necessary to attain a more uniform distribution of the alloying elements. After 50 h of ball milling, SEM observations reveal that the powder particles, while retaining an irregular morphology, exhibit a more homogeneous appearance with fine particle sizes, generally in the range of several micrometers. These refined particles contribute to improved sinterability and help preserve fine grain structures during consolidation. The EDS elemental maps demonstrate a significantly more uniform distribution of elements such as Co, Cr, Fe, Ni, Nb, and V, suggesting that cold welding and crushing during the ball milling process have reached a dynamic equilibrium, resulting in a more uniform CoCrFeNiNb element distribution under mutual diffusion [23]. The dissolution of Nb and V into the FCC lattice during prolonged milling results in a reduction in microstructural heterogeneity and the formation of a stable FCC solid solution. These observations are in alignment with the XRD results, which serve to confirm that extended ball milling enhances the homogenization of alloying elements and promotes the formation of a uniform solid solution phase.
3.2. Microstructure of Sintered CoCrFeNiNb and CoCrFeNiV HEAs
Figure 2a,b show the XRD patterns of sintered CoCrFeNiNb and CoCrFeNiV HEAs at different temperatures, indicating that alloying elements significantly has a substantial impact on the phase composition and stability of the HEAs. In Figure 2a, the CoCrFeNiNb HEA exhibits a dual-phase structure, comprising a primary FCC phase and a secondary Laves phase. The addition of Nb was found to promote the formation of the Co2Nb-type Laves phase, which has a hexagonal close-packed (HCP) crystal structure with lattice parameters of a = 0.4835 nm and c = 0.7860 nm [24]. The presence of the Laves phase suggests that Nb acts as a stabilizer for the intermetallic phase, thereby enhancing the alloy’s strength [24]. In Figure 2b, the CoCrFeNiV HEA exhibits an FCC phase and a σ phase. The formation of the σ phase is attributed to the metallic V element, which has very negative enthalpies of mixing with Co, Ni, and Fe, promoting the formation of an FCC solid solution with these elements. However, Cr, which is not fully dissolved in the solid solution, preferentially forms the σ phase with V [25]. It is acknowledged that both the Laves phase and the σ phase are capable of enhancing hardness but may reduce ductility. The presence of these phases indicates that Nb and V act as strong stabilizers for the intermetallic phases, contributing to the overall strengthening of the HEAs.
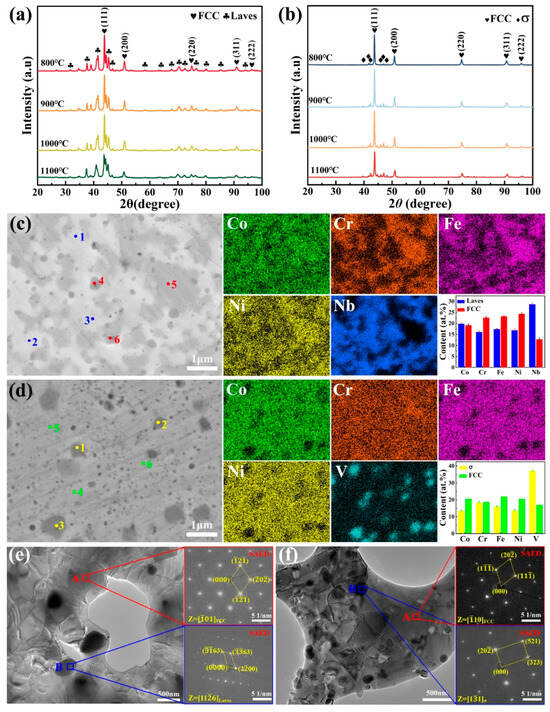
Figure 2.
XRD patterns, SEM images, EDS elemental maps, and TEM images of sintered CoCrFeNiNb (a,c,e) and CoCrFeNiV (b,d,f) HEAs at different temperatures. (a,b) XRD patterns from 800 °C to 1100 °C; (c,d) elemental distributions and (e,f) TEM and corresponding SAED patterns of samples sintered at 1100 °C. The marked points 1, 2, and 3 correspond to the Laves phase and σ-phase regions, while points 4, 5, and 6 represent the FCC phase regions (c,d). The compositional statistics of these different regions are displayed in the corresponding bar charts.
Figure 2c,d show the EDS elemental mapping and the corresponding SEM images of sintered CoCrFeNiNb and CoCrFeNiV HEAs at 1100 °C with a holding time of 15 min. In Figure 2c, the CoCrFeNiNb HEA exhibits two distinct regions in the SEM image, characterized by light gray and dark gray regions, respectively. The XRD analysis confirmed the presence of FCC and Laves phases, which is consistent with the EDS results. The light gray region is enriched in Nb, indicating the formation of the Co2Nb-type Laves phase, while the dark gray region shows higher concentrations of Cr, Fe, and Ni, corresponding to the FCC phase. The point scan analysis (bar chart) in Figure 2c further confirms these observations. In the CoCrFeNiNb system, Nb has very negative enthalpies of mixing with other metallic elements, which promotes the formation of stable intermetallic compounds. As a result, Nb tends to form the Co(Ni, Fe, Cr)2Nb-type Laves phase rather than an FCC solid solution. This observation is consistent with the findings of previous studies [26]. The CoCrFeNiV HEA exhibits a similar microstructure, as evidenced by the presence of light gray and dark gray regions observed in the SEM image. EDS mapping and the point scan results indicate that the light gray region corresponds to the FCC phase, with a uniform distribution of Cr, Co, Fe, and Ni. In contrast, the dark gray region is enriched in V, indicating the formation of the σ phase, which is consistent with the XRD pattern shown in Figure 2b. Similar to the CoCrFeNiNb HEA, the elements, V and Cr, tend to segregate and form the σ phase, while Co, Fe, and Ni remain within the FCC matrix. These observations demonstrate that the formation of intermetallic phases, including the Laves and σ phases, is driven by the segregation behavior of Nb and V, respectively.
As illustrated in Figure 2e,f, the TEM microstructure of CoCrFeNiNb and CoCrFeNiV HEAs confirms the dual-phase structures that were previously observed in SEM and XRD analyses. In Figure 2e, the bright-field TEM image of the CoCrFeNiNb HEA shows a conventional dual-phase structure, where the FCC matrix appears as light gray regions and the Laves phase manifests as darker grains. The selected area electron diffraction (SAED) pattern from Region A, along the [01]FCC zone axis, confirms the FCC structure. The SAED pattern from Region B, along the [116]Laves Laves zone axis, confirms the Co2Nb-type Laves phase. The distribution of the Laves phase within the FCC matrix provides reinforcement and contributes to a balanced combination of strength and ductility. In Figure 2f, the TEM image of the CoCrFeNiV HEA reveals a similar dual-phase structure, comprising of the FCC matrix and the σ phase. The SAED pattern from Region A, situated along the [10]FCC zone axis, confirms the FCC phase, while the pattern from Region B, positioned along the [11]σ zone axis, corroborates the presence of the σ phase. The σ phase is distributed along grain boundaries and within the FCC grains, which is consistent with the findings obtained by SEM. This phase distribution suggests that V and Cr tend to segregate and form the σ phase, while the remaining elements stabilize the FCC matrix.
To further investigate the phase distribution and grain size, EBSD characterization was performed on the bulk CoCrFeNiNb and CoCrFeNiV HEAs (Figure 3). As illustrated in Figure 3a, the phase distribution of the CoCrFeNiNb HEA is depicted, with the red areas denoting the FCC phase, the blue areas indicating the Laves phase, and the black unindexed regions corresponding to pits caused by over-etching during the sample preparation process [27]. Figure 3b shows the grain distribution map of the CoCrFeNiNb HEA, illustrating the spatial arrangement and orientation of FCC and Laves grains within the alloy microstructure. The map is consistent with the phase distribution shown in Figure 3a, in which the FCC phase is predominantly distributed throughout the microstructure with finer Laves grains found within it. This observation is further quantified in Figure 3c,d, which provide the grain size statistics for the FCC and Laves phases, showing average grain sizes of 1.81 ± 1.18 µm for the FCC phase and 1.10 ± 0.93 µm for the Laves phase, respectively. The finer and more uniform distribution of Laves grains suggests its role as a strengthening phase within the FCC matrix, contributing to enhanced mechanical properties.
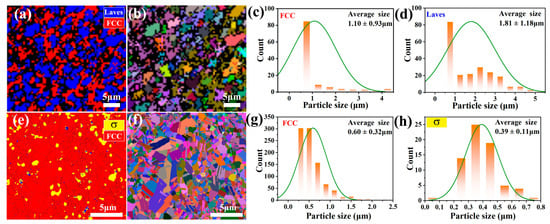
Figure 3.
Microstructural characterization of CoCrFeNiNb (a–d) and CoCrFeNiV HEAs (e–h) sintered at 1100 °C. (a,e) Phase distribution maps. (b,f) Grain size distribution maps. (c,g) FCC grain size statistics. (d,h) Laves and σ phase grain size statistics.
Figure 3e shows the phase distribution of the CoCrFeNiV HEA, where the red areas represent the FCC phase, the yellow areas indicate the σ phase, and the black unindexed regions denote areas where indexing was not possible. The dominance of the FCC phase is evident, while the σ phase manifests isolated regions distributed along grain bounda-ries and within the FCC matrix. This phase distribution aligns well with the earlier SEM and XRD analyses, thus confirming the coexistence of FCC and σ phases. Figure 3f pre-sents the grain distribution map, revealing a more heterogeneous particle size distribu-tion for FCC and σ phases compared to the CoCrFeNiNb HEA. Figure 3g,h provide further quantification of the grain sizes, showing an average grain size of 0.60 ± 0.32 µm for the FCC phase and 0.39 ± 0.11 µm for the σ phase. The finer grain size of the σ phase, despite its lower abundance, indicates its potential to strengthen the alloy locally. However, its uneven distribution and limited presence suggest that its impact on the overall mechanical properties might be minimal compared to the FCC phase, which dominates the microstructure and contributes significantly to ductility and toughness. Compared to the mechanically alloyed powders (Figure 1c–f) with particle sizes of several micrometers, the SPS-processed samples retained a significantly refined grain structure (~0.39–1.81 μm), demonstrating that the rapid heating and short sintering time effectively suppress grain growth.
In comparison with CoCrFeNiNb and CoCrFeNiV HEAs prepared by using conventional melting methods, the MA and SPS approach results in more uniform grain distribution and significantly finer grain sizes. For reference, the grain sizes of similar CoCrFeNi-based HEAs prepared via arc melting typically exceed 10 μm [28,29], whereas the SPS-processed samples in this study exhibited average grain sizes below 2 μm. The rapid heating and short holding times of SPS effectively limit grain growth, leading to a refined microstructure. It is anticipated that this refined grain structure will enhance the mechanical properties, including strength and toughness through grain boundary strengthening. These findings are consistent with earlier SEM and TEM observations, which demonstrate that mechanical alloying and SPS are effective in tailoring the microstructure to achieve superior performance in comparison to conventional melting techniques. While SPS currently encounters challenges in scaling up to large-sized components relative to traditional methods, continued advancements in large-scale SPS systems and mold engineering are anticipated to effectively address these issues and further expand the applicability of this technique in practical manufacturing.
3.3. Mechanical Properties of CoCrFeNiNb and CoCrFeNiV HEAs
Figure 4 illustrates the variation in hardness, relative density, and compressive stress–strain curves of CoCrFeNiNb and CoCrFeNiV HEAs at different sintering temperatures. The data presented in Figure 4a–d were derived from the detailed measurements of compressive strength and density in Table 1 and Table 2, which have been consolidated here for the sake of clarity.
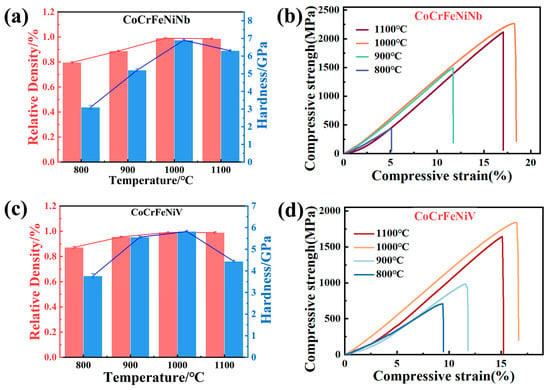
Figure 4.
Relative density and hardness of CoCrFeNiNb HEAs (a) and CoCrFeNiV HEAs (c) at different SPS sintering temperatures. The room-temperature compressive stress–strain curves of CoCrFeNiNb HEAs (b) and CoCrFeNiV HEAs (d) at different sintering temperatures.

Table 1.
Density, hardness, and compressive properties of CoCrFeNiNb HEAs at different sintering temperatures.

Table 2.
Density, hardness, and compressive properties of CoCrFeNiV HEAs at different sintering temperatures.
As shown in Figure 4a,c, the hardness and relative density exhibit a tendency to initially increase with rising temperature, reaching a maximum at 1000 °C, followed by a decline at 1100 °C. At 800 °C, the CoCrFeNiNb HEA exhibits a hardness of approximately 3.08 GPa and a relative density of 79.6%, while the CoCrFeNiV HEA achieves higher values of 3.76 GPa and 87.1%, respectively. At this temperature, the incomplete solidification of the alloy powders gives rise to the formation of alloy blocks with relatively low density and hardness. As the sintering temperature is increased to 1000 °C, the CoCrFeNiNb HEA achieves a hardness of approximately 6.89 GPa and a relative density nearing 99.0%. Similarly, the CoCrFeNiV HEA reaches a hardness of about 5.86 GPa and a relative density of 99.1%. This improvement in hardness and densification can be attributed to the accelerated solid-state diffusion and sintering kinetics at this temperature, which promote the formation of fully densified alloy blocks with minimized porosity. However, further increasing the sintering temperature to 1100 °C results in a noticeable decline in both properties for both HEAs. This decline is primarily caused by the volatilization of elements with low melting points and highly volatility, as well as the introduction of impurities during high-temperature sintering. Moreover, the process of grain coarsening, occurring as a consequence of prolonged exposure to high-temperature conditions, leads to the generation of residual porosity. This, in turn, has a detrimental effect on the density and hardness of the alloys [30].
Figure 4b,d show the room-temperature compressive stress–strain curves of CoCrFeNiNb and CoCrFeNiV HEAs at different sintering temperatures. Both alloys exhibit brittle fracture behavior under compressive loading at all sintering temperatures. At 800 °C, the ultimate compressive strength (σmax) of CoCrFeNiNb and CoCrFeNiV HEAs is approximately 396 MPa and 721 MPa, respectively, with corresponding fracture strains (εf) of about 4.8% and 9.5%, respectively. These relatively poor compressive properties are primarily due to the incomplete densification of the alloys at this temperature. This results in insufficient bonding between the alloy particles and increased porosity, which weakens the material under compressive loading. When the sintering temperature is increased to 1000 °C, both alloys demonstrate significant improvement in compressive properties. The CoCrFeNiNb HEA achieves an ultimate compressive strength (σmax) of 2201 MPa and a fracture strain (εf) of 17.5%, while the CoCrFeNiV HEA exhibits a compressive strength of 1835 MPa and a fracture strain of 16.5%. These enhancements are attributed to effective densification and solidification at this temperature, which minimize residual porosity and promote microstructural homogeneity. Based on the values reported in the literature, these strength levels are significantly higher than those of typical engineering alloys such as 316L stainless steel (~940 MPa) [31]. In addition to surpassing conventional engineering alloys, the MA + SPS-processed CoCrFeNiNb and CoCrFeNiV HEAs also exhibit superior mechanical performance compared to similar FeCoCrNi-based HEAs fabricated via arc melting. For instance, a B-free AlFeCoNi HEA synthesized via vacuum arc melting exhibited a compressive strength of ~850 MPa and a fracture strain of ~7% [32]. These results underscore the advantages of MA + SPS in achieving both high strength and ductility through improved densification and microstructural control.
The excellent compressive strength of the FeCoCrNiNb/V high-entropy alloy prepared in this study can be attributed to multiple factors. Firstly, the mutual diffusion and solid solution of different atoms contribute to the formation of a stable solid solution phase, providing substantial solid solution strengthening. Secondly, the rapid heating and cooling rates, along with the short holding times characteristic of SPS, effectively suppress grain growth, resulting in a refined microstructure with fine grains. Thirdly, the addition of elements with large atomic radii, such as Nb and V, facilitates the formation of secondary precipitate phases, including the Laves and σ phases. These precipitates serve as effective barriers to dislocation motion, thereby enhancing the alloy’s strength through a process known as precipitation hardening. Despite these enhancements, the compressive strength of the CoCrFeNiNb HEA exceeds that of the CoCrFeNiV HEA. Evidence from EBSD observations suggests that the uniform distribution of Laves phases in CoCrFeNiNb enhances its load-bearing capacity, thereby contributing to its superior compressive strength. In contrast, the CoCrFeNiV HEA exhibits a lower abundance of σ phases, and their uneven distribution limits their overall strengthening effect.
To investigate the fracture behaviors, the compressive fracture morphologies of CoCrFeNiNb and CoCrFeNiV HEAs at different sintering temperatures were analyzed (Figure 5). At 800 °C (Figure 5a,e), severe cracks and numerous unsintered powder particles are observed at the fracture site, indicating incomplete densification. As the sintering temperature increases to 900 °C (Figure 5b,f), the bonding between alloy particles is enhanced as the powders begin to solidify. However, residual porosity and incomplete densification remain evident. At 1000 °C, the alloy powders achieve a high degree of densification, as evidenced by the fracture morphologies, as shown in Figure 5c,g. While residual pores remain visible, their distribution and size vary due to differences in magnification and material composition. The fracture surfaces predominantly exhibit brittle characteristics, with flat regions and layered structures indicative of a transgranular fracture mechanism. As the temperature is increased to 1100 °C, distinct granular precipitates (highlighted as “particles” in Figure 5d) become prominent on the fracture surface of the CoCrFeNiNb HEA. These precipitates, exhibiting a weak bond to the matrix, detach under compressive stress, resulting in a localized stress concentration. Additionally, the presence of larger pores, caused by grain coarsening and volatilization of low-melting-point phases, exacerbates the process of crack initiation and propagation. For the CoCrFeNiV HEA at 1100 °C, the fracture surfaces also display brittle characteristics with layered features and limited plastic deformation, while granular precipitates similar to those observed in the CoCrFeNiNb HEA are not clearly visible at the current magnification. The observed residual porosity and brittle fracture features indicate that microstructural defects remain a dominant factor in the initiation and propagation of cracks.
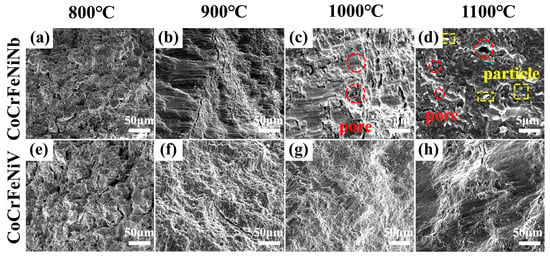
Figure 5.
Compression fracture surface morphology of CoCrFeNiNb HEA: (a) 800 °C; (b) 900 °C; (c) 1000 °C; (d) 1100 °C. Compression fracture surface morphology of CoCrFeNiV HEA: (e) 800 °C; (f) 900 °C; (g) 1000 °C; (h) 1100 °C.
3.4. Electrochemical Corrosion Behavior and Resistance Mechanisms of CoCrFeNiNb and CoCrFeNiV HEAs
Figure 6a shows the potentiodynamic polarization curves of CoCrFeNi, CoCrFeNiNb, and CoCrFeNiV HEAs in a 3.5 wt.% NaCl solution at 25 °C. The polarization behavior demonstrates that the anodic current density varies with the applied potential for all three alloys. It is evident that none of the alloys exhibit clear passivation during anodic polarization, suggesting the partial breakdown of passive films in the chloride-rich environment. Tafel polarization analysis (Table 3) provides the key corrosion resistance parameters, including corrosion current density (Icorr) and corrosion potential (Ecorr), which reflect the corrosion rate and the alloy’s tendency to resist corrosion, respectively [33]. Among the three HEAs, the CoCrFeNiNb alloy exhibits the lowest corrosion current density (3.055 μmA/cm2) and a relatively higher corrosion potential (−0.296 V), indicating its superior corrosion resistance. Conversely, the CoCrFeNi HEA demonstrates the highest corrosion current density (3.698 μmA/cm2) and the most negative corrosion potential (−0.378 V), indicating its lower resistance to corrosion. The enhanced corrosion resistance of CoCrFeNiNb and CoCrFeNiV HEAs is primarily attributed to the addition of Nb and V. These elements contribute to the formation of protective oxide films, such as Nb2O5 and V2O3, which act as stable barriers against aggressive chloride ions. Furthermore, the presence of Laves and σ phases in the microstructure plays a critical role. These precipitated phases provide localized sites for the formation of dense oxide layers, thereby further stabilizing the passive film and enhancing the alloys’ electrochemical stability. The combined effect of these factors significantly reduces the corrosion rate, thereby enhancing the resistance of the HEAs in chloride-containing environments.
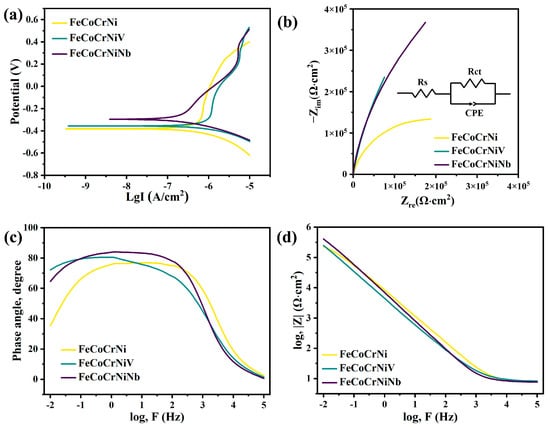
Figure 6.
Potentiodynamic polarization curves (a), Nyquist plots (b), and Bode plots (c,d) for fitting the EIS data of CoCrFeNi, CoCrFeNiNb, and CoCrFeNiV HEAs under their respective OCP conditions in the 3.5 mass% NaCl solution.

Table 3.
Corrosion resistance parameters of CoCrFeNi, CoCrFeNiNb, and CoCrFeNiV HEAs in 25 °C, 3.5wt% NaCl solution.
Figure 6b–d illustrates the potentiodynamic polarization curves, equivalent electrical circuit, Nyquist plots, and Bode plots for CoCrFeNi, CoCrFeNiNb, and CoCrFeNiV high-entropy alloys (HEAs) in a 3.5 wt.% NaCl solution under their respective open-circuit potential (OCP) conditions. The equivalent electrical circuit used to fit the EIS data is shown in the upper right corner of Figure 6b. In this model, Rs represents the solution resistance, Rct denotes the charge transfer resistance, and CPE (constant phase element) accounts for the non-ideal capacitive behavior of the electrode/electrolyte interface. This circuit is generally employed to describe the electrochemical behavior of passive films on metal surfaces in chloride-containing environments. The Nyquist plots (Figure 6b) show that CoCrFeNiNb and CoCrFeNiV HEAs exhibit larger semicircle diameters compared to CoCrFeNi, implying higher charge transfer resistance and thus improved corrosion performance. This observation is supported by quantitative fitting using the equivalent circuit model (also shown in Figure 6b). The fitted EIS parameters are presented in Table 4, where CoCrFeNiNb and CoCrFeNiV show significantly higher Rct values (2.09 × 106 Ω·cm2 and 1.53 × 106 Ω·cm2, respectively) than CoCrFeNi (6.4 × 105 Ω·cm2). These values confirm the beneficial effect of Nb and V in promoting more stable passive films. In addition, the fitted CPE parameters (Y0 and n) suggest that the oxide layers formed on Nb- and V-containing HEAs are more capacitive and uniform, further contributing to their superior electrochemical performance. The Bode plots (Figure 6c,d) provide further support for the findings of the Nyquist plot. In the low-frequency region, CoCrFeNiNb and CoCrFeNiV HEAs exhibit higher phase angles and impedance modulus values compared to the CoCrFeNi HEA. It has been demonstrated that higher phase angles in the low-frequency region are indicative of the formation of a more stable and compact passive film, which serves as an effective barrier against chloride ion penetration [34]. The larger impedance modulus values also indicate improved surface film stability, which enhances the corrosion resistance of the alloys. In summary, the EIS analysis demonstrates that the CoCrFeNiNb and CoCrFeNiV HEAs possess superior electrochemical stability and corrosion resistance in chloride-containing environments. Furthermore, it is evident that CoCrFeNiNb exhibits the most robust passive film formation and the highest charge transfer resistance.

Table 4.
Fitted EIS parameters of CoCrFeNi, CoCrFeNiNb, and CoCrFeNiV HEAs in 3.5 wt.% NaCl solution.
Figure 7 presents the EDS elemental mapping results of CoCrFeNiNb and CoCrFeNiV HEAs after electrochemical corrosion in a 3.5 wt.% NaCl solution. The results show that both alloys exhibit localized corrosion as the dominant mechanism, with no significant pitting observed on their surfaces. In Figure 7a, representing the CoCrFeNiNb HEA, the elements of Co, Cr, Fe, and Ni are distributed uniformly within the FCC matrix, while Nb is enriched in specific regions corresponding to the Laves phase. The FCC phase is observed to be more prone to corrosion compared to the Laves phase, which exhibits higher resistance due to the formation of a stable Nb2O5 oxide layer that acts as a protective barrier against chloride ions. Similarly, Figure 7b shows the EDS mapping for the CoCrFeNiV HEA, illustrating an even distribution of Co, Cr, Fe, and Ni in the matrix with V exhibiting enrichment in regions corresponding to the σ phase. The σ phase shows greater corrosion resistance in comparison to the surrounding FCC matrix, likely due to the formation of a V2O3 oxide layer that mitigates chloride ion attack.
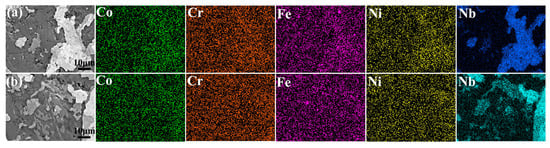
Figure 7.
EDS after electrochemical etching. (a) CoCrFeNiNb HEAs and (b) CoCrFeNiV HEAs.
Both HEAs exhibit preferential corrosion of the FCC phase, while the precipitated phases remain relatively intact. This behavior is typical of dual-phase alloys, where differences in electrochemical stability between phases create galvanic coupling, accelerating corrosion in the more active FCC matrix. The comparison between CoCrFeNiNb and CoCrFeNiV HEAs highlights that the addition of Nb or V enhances corrosion resistance by promoting the formation of stable oxide layers on the precipitated phases. These oxide films have an impact on the reduction in ion penetration and improvement of the electrochemical stability of the alloys in chloride-containing environments.
The XPS analysis confirms that the Nb-enriched Laves phase in the CoCrFeNiNb HEA and the V-enriched σ phase in the CoCrFeNiV HEA significantly enhance the corrosion resistance of the alloys by forming stable oxide layers. As shown in Figure 8a, the Nb 3d spectrum of the CoCrFeNiNb HEA displays binding energies of 202.4 eV for metallic Nb (Nb0 2d5/2) and 207.1 eV and 209.88 eV for Nb5+ peaks, indicating the formation of Nb2O5. Similarly, the V 2p spectrum of the CoCrFeNiV HEA reveals binding energies at 513.6 eV and 521.2 eV for V3+ peaks, thereby confirming the presence of V2O3 (Figure 8c). These oxides form compact and continuous passive films that act as barriers against chloride ion penetration, reducing the corrosion rate and slowing down the propagation of corrosion pits. The O 1s spectra (Figure 8b,c) further indicate that the passive films on both alloys contain a mixture of metal oxides and hydroxides. The main peaks at binding energies of approximately 530.1 eV and 531.8 eV correspond to O2− species in metal oxides (Nb2O5 and V2O3) and OH− species in hydroxides, respectively. The formation of stable oxides, such as Nb2O5 and V2O3, is critical in mitigating chloride ion attack by forming dense, protective layers that enhance the electrochemical stability of the alloys. In contrast, the FCC matrix, which is composed primarily of Fe, Co, Cr, and Ni, forms fewer protective oxides under the same conditions. Oxides such as Fe2O3, Co3O4, and NiO exhibit reduced stability in chloride-rich environments, thereby increasing the susceptibility of the FCC matrix to localized corrosion. This difference in oxide stability between the FCC phase and the precipitated phases elucidates the preferential corrosion observed in the FCC matrix, as evidenced by the surface morphology and EDS mapping results (Figure 7).
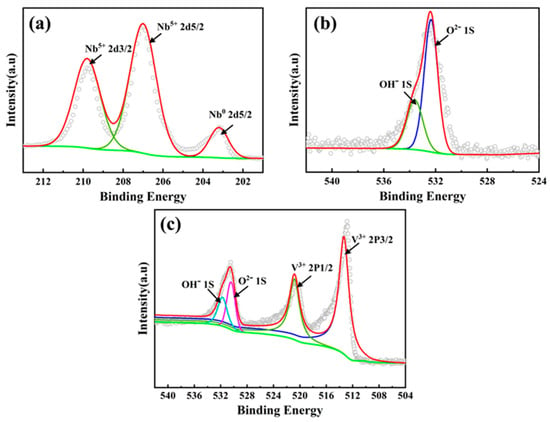
Figure 8.
XPS spectra of the surface oxide layers formed on CoCrFeNiNb and CoCrFeNiV HEAs after electrochemical corrosion in a 3.5 wt.% NaCl solution. (a) Nb 2d spectrum of CoCrFeNiNb HEA, (b) O 1s spectrum of CoCrFeNiNb HEA, (c) V 2p and O 1s spectrums of CoCrFeNiV HEA. Gray circles represent the experimental data, and red curves show the fitting results.
XPS analysis highlights the critical role of the precipitated phases in enhancing the corrosion resistance of CoCrFeNiNb and CoCrFeNiV HEAs. The Nb-enriched Laves phase and V-enriched σ phase facilitate the formation of durable oxide films, which provide effective protection against aggressive chloride ions in corrosive environments. The formation of these heterogeneous passive films, which is achieved through a process of selective oxidation, contribute significantly to the alloys’ electrochemical stability and maintain their overall corrosion resistance in chloride-containing environments.
4. Conclusions
This study systematically investigated the microstructural evolution, mechanical behavior, and corrosion resistance of CoCrFeNiNb and CoCrFeNiV high-entropy alloys (HEAs) fabricated via mechanical alloying and spark plasma sintering (SPS) at various sintering temperatures. The SPS process, characterized by its rapid heating and short holding times, effectively promoted densification and minimized grain growth, thereby enabling the formation of fine-grained microstructures. Microstructural analyses revealed an FCC matrix with Nb-enriched Laves phases and V-enriched σ phases. The distribution and composition of these phases exhibited a significant influence on both the mechanical and electrochemical properties. The uniform dispersion of Laves phases in the CoCrFeNiNb HEA contributes to its superior compressive strength and hardness across all sintering temperatures. Fracture surface analysis revealed that at lower sintering temperatures, the fracture is primarily caused by incomplete particle bonding. By contrast, at higher temperatures, brittle fracture modes dominated by transgranular cracking become pre-dominant.
Electrochemical testing and XPS analysis further demonstrated that both HEAs ex-hibited excellent corrosion resistance in a 3.5 wt.% NaCl solution compared to the CoCrFeNi base alloy. The formation of stable oxide layers, such as Nb2O5 and V2O3, on the precipitated phases enhanced the integrity of the passive film, reducing the corrosion rates and improving the long-term durability. These findings underscore the critical role of precipitated phases in concurrently optimizing the mechanical and electrochemical performance of HEAs.
Future research should aim to evaluate the performance of FeCoCrNiNb/V high-entropy alloys in complex service environments, such as marine and high-temperature industrial applications. Upcoming work will focus on the thermal stability of microstructures, corrosion behavior under cyclic or long-term exposure, and wear resistance. Additionally, further testing under simulated service conditions will help verify the structural stability and durability of these alloys, enabling a more comprehensive understanding of their application potential.
Author Contributions
Conceptualization, Y.Z. and J.T.; methodology, Y.Z. and Y.L.; software, Z.M.; validation, Z.M. and J.T.; investigation, Y.Z. and J.T.; resources, Y.Z. and Z.M.; data curation, Y.Z. and Y.L.; writing—original draft preparation, Y.L. and J.T.; writing—review and editing, Y.Z. and J.T.; visualization, Y.Z. and Z.M.; supervision, Y.L. and J.T.; funding acquisition, Z.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Guangdong Basic and Applied Basic Research Foundation, China, grant number 2023A1515111142.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- She, S.H.; Wang, C.X.; Chen, M.; Ji, V. Mechanical Properties and Strengthening Mechanisms of FCC-Based and Refractory High-Entropy Alloys: A Review. Metals 2025, 15, 247. [Google Scholar] [CrossRef]
- Ren, M.X.; Li, B.S.; Fu, H.Z. Formation condition of solid solution type high-entropy alloy. Trans. Nonferrous Met. Soc. China 2013, 23, 991–995. [Google Scholar] [CrossRef]
- Senkov, O.N.; Miracle, D.B.; Chaput, K.J.; Couzinie, J.-P. Development and exploration of refractory high entropy alloys—A review. J. Mater. Res. 2018, 33, 3092–3128. [Google Scholar] [CrossRef]
- Ye, X.Y.; Ma, M.X.; Cao, Y.X.L.; Liu, W.J.; Ye, X.H.; Gu, Y. The property research on high-entropy alloy AlxFeCoNiCuCr coating by laser cladding. Phys. Procedia 2011, 12, 303–312. [Google Scholar] [CrossRef]
- Zhang, M.N.; Zhou, X.L.; Yu, X.N.; Li, J.H. Synthesis and characterization of refractory TiZrNbWMo high-entropy alloy coating by laser cladding. Surf. Coat. Technol. 2017, 311, 321–329. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Jain, H.; Shadangi, Y.; Chakravarty, D.; Dubey, A.K.; Mukhopadhyay, N.K. High entropy steel processed through mechanical alloying and spark plasma sintering: Alloying behaviour, thermal stability and mechanical properties. Mater. Sci. Eng. A 2022, 856, 144029. [Google Scholar] [CrossRef]
- Ammar, C.B.; Khitouni, N.; Alshammari, M.; Alsawi, A.; Khitouni, M.; Suñol, J.-J.; Chemingui, M. Microstructural and Magnetic Characteristics of High-Entropy FeCoNiMnTi Alloy Produced via Mechanical Alloying. Metals 2024, 14, 1302. [Google Scholar] [CrossRef]
- Li, N.; Wu, C.B.; Wu, Z.N.; Jiang, M.Y.; Hou, J.F.; Dong, F.Y. Effect of Sintering Temperature and Time on Microstructure and Mechanical Properties of CoCrFeNiMn High-Entropy Alloys. Metals 2025, 15, 591. [Google Scholar] [CrossRef]
- Eißmann, N.; Klöden, B.; Weißgärber, T.; Kieback, B. High-entropy alloy CoCrFeMnNi produced by powder metallurgy. Powder Metall. 2017, 60, 184–197. [Google Scholar] [CrossRef]
- Lu, Y.P.; Dong, Y.; Guo, S.; Jiang, L.; Kang, H.J.; Wang, T.M.; Wen, B.; Wang, Z.J.; Jie, J.C.; Cao, Z.Q.; et al. A promising new class of high-temperature alloys: Eutectic high-entropy alloys. Sci. Rep. 2014, 4, 6200. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Tsai, C.W.; Juan, C.C.; Chuang, M.H.; Yeh, J.W.; Chin, T.-S.; Chen, S.-K. Amorphization of equimolar alloys with HCP elements during mechanical alloying. J. Alloys Compd. 2010, 506, 210–215. [Google Scholar] [CrossRef]
- Wu, W.H.; Yang, C.C.; Yeh, L.W. Industrial development of high-entropy alloys. In Annales de Chimie-Science des Materiaux; Masson: Paris, France; New York, NY, USA, 2006; p. 737. [Google Scholar]
- Yeh, J.W. Recent progress in high entropy alloys. Ann. Chim. Sci. Mat. 2006, 31, 633–648. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Yeh, J.W.; Chen, S.K.; Shun, T.T. Wear resistance and high-temperature compression strength of Fcc CuCoNiCrAl0.5Fe alloy with boron addition. Metall. Mater. Trans. A 2004, 35, 1465–1469. [Google Scholar] [CrossRef]
- Wang, X.J.; Xu, M.; Liu, N.; Liu, L.X. The formation of sigma phase in the CoCrFeNi high-entropy alloys. Mater. Res. Express 2021, 8, 076514. [Google Scholar] [CrossRef]
- Jiang, L.; Cao, Z.Q.; Jie, J.C.; Zhang, J.J.; Lu, Y.P.; Wang, T.M.; Li, T.J. Effect of Mo and Ni elements on microstructure evolution and mechanical properties of the CoFeNixVMoy high entropy alloys. J. Alloys Compd. 2015, 649, 585–590. [Google Scholar] [CrossRef]
- Shun, T.T.; Hung, W.J. Effects of Cr Content on Microstructure and Mechanical Properties of AlCoCrxFeNi High-Entropy Alloy. Adv. Mater. Sci. Eng. 2018, 2018, 5826467. [Google Scholar] [CrossRef]
- Ye, X.C.; Xu, W.Q.; Li, Z.; Xu, D.; Zhang, W.; Li, B.; Fang, D. Microstructures and mechanical properties of FeNiCrMnAl high-entropy alloys. J. Mater. Eng. Perform. 2022, 31, 7820–7829. [Google Scholar] [CrossRef]
- Ji, W.; Wang, W.M.; Wang, H.; Zhang, J.Y.; Wang, Y.C.; Zhang, F.; Fu, Z.Y. Alloying behavior and novel properties of CoCrFeNiMn high-entropy alloy fabricated by mechanical alloying and spark plasma sintering. Intermetallics 2015, 56, 24–27. [Google Scholar] [CrossRef]
- Shivam, V.; Basu, J.; Shadangi, Y.; Singh, M.K.; Mukhopadhyay, N.K. Mechano-chemical synthesis, thermal stability and phase evolution in AlCoCrFeNiMn high entropy alloy. J. Alloys Compd. 2018, 757, 87–97. [Google Scholar] [CrossRef]
- Shivam, V.; Basu, J.; Pandey, V.K.; Shadangi, Y.; Mukhopadhyay, N.K. Alloying behaviour, thermal stability and phase evolution in quinary AlCoCrFeNi high entropy alloy. Adv. Powder Technol. 2018, 29, 2221–2230. [Google Scholar] [CrossRef]
- Chen, Y.L.; Hu, Y.H.; Hsieh, C.A.; Yeh, J.W.; Chen, S.K. Competition between elements during mechanical alloying in an octonary multi-principal-element alloy system. J. Alloys Compd. 2009, 481, 768–775. [Google Scholar] [CrossRef]
- Qin, G.; Wang, S.; Chen, R.R.; Gong, X.; Wang, L.; Su, Y.Q.; Guo, J.J.; Fu, H.Z. Microstructures and mechanical properties of Nb-alloyed CoCrCuFeNi high-entropy alloys. J. Mater. Sci. Technol. 2018, 34, 365–369. [Google Scholar] [CrossRef]
- Tsai, M.H.; Fan, A.C.; Wang, H.A. Effect of atomic size difference on the type of major intermetallic phase in arc-melted CoCrFeNiX high-entropy alloys. J. Alloys Compd. 2017, 695, 1479–1487. [Google Scholar] [CrossRef]
- Jiang, H.; Jiang, L.; Qiao, D.X.; Lu, Y.P.; Wang, T.M.; Cao, Z.Q.; Li, T.J. Effect of niobium on microstructure and properties of the CoCrFeNbxNi high entropy alloys. J. Mater. Sci. Technol. 2017, 33, 712–717. [Google Scholar] [CrossRef]
- Průša, F.; Cabibbo, M.; Šenková, A.; Kučera, V.; Veselka, Z.; Školáková, A.; Vojtěch, D.; Cibulková, J.; Čapek, J. High-strength ultrafine-grained CoCrFeNiNb high-entropy alloy prepared by mechanical alloying: Properties and strengthening mechanism. J. Alloys Compd. 2020, 835, 155308. [Google Scholar] [CrossRef]
- Wu, S.W.; Wang, G.; Yi, J.; Jia, Y.D.; Hussain, I.; Zhai, Q.J.; Liaw, P.K. Strong grain-size effect on deformation twinning of an Al0.1CoCrFeNi high-entropy alloy. Mater. Res. Lett. 2016, 5, 276–283. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, C.; Zhang, J.; Wang, H.; Chen, L. Influences of Grain Size on the Deformation Behavior of a Twinning-Induced Plasticity Metastable β Titanium Alloy. J. Alloys Compd. 2022, 895, 168274. [Google Scholar]
- Yurkova, A.I.; Cherniavsky, V.V.; Bolbut, V.; Krüger, M.; Bogomol, I. Structure formation and mechanical properties of the high-entropy AlCuNiFeCr alloy prepared by mechanical alloying and spark plasma sintering. J. Alloys Compd. 2019, 786, 139–148. [Google Scholar] [CrossRef]
- Yan, X.; Lupoi, R.; Wu, H.; Ma, W.; Liu, M.; O’Donnell, G.; Yin, S. Effect of Hot Isostatic Pressing (HIP) Treatment on the Compressive Properties of Ti6Al4V Lattice Structure Fabricated by Selective Laser Melting. Mater. Lett. 2019, 255, 126537. [Google Scholar] [CrossRef]
- Li, Q.; Xu, X.; Wu, L.; Liu, D.; Ma, Y.; Song, Y. Effects of Boron Content on Microstructure and Mechanical Properties of AlFeCoNiBx High Entropy Alloy Prepared by Vacuum Arc Melting. Electrochim. Acta 2025, 483, 145933. [Google Scholar]
- Qiu, X.W.; Wu, M.J.; Liu, C.G.; Zhang, Y.P.; Huang, C.X. Corrosion performance of Al2CrFeCoxCuNiTi high-entropy alloy coatings in acid liquids. J. Alloys Compd. 2017, 708, 353–357. [Google Scholar] [CrossRef]
- Rae, C.M.F.; Reed, R.C. The precipitation of topologically close-packed phases in rhenium-containing superalloys. Acta Mater. 2001, 49, 4113–4125. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).