Selective Oxidation Depression of Copper-Activated Sphalerite by H2O2 During Chalcopyrite Flotation
Abstract
1. Introduction
2. Materials and Methods
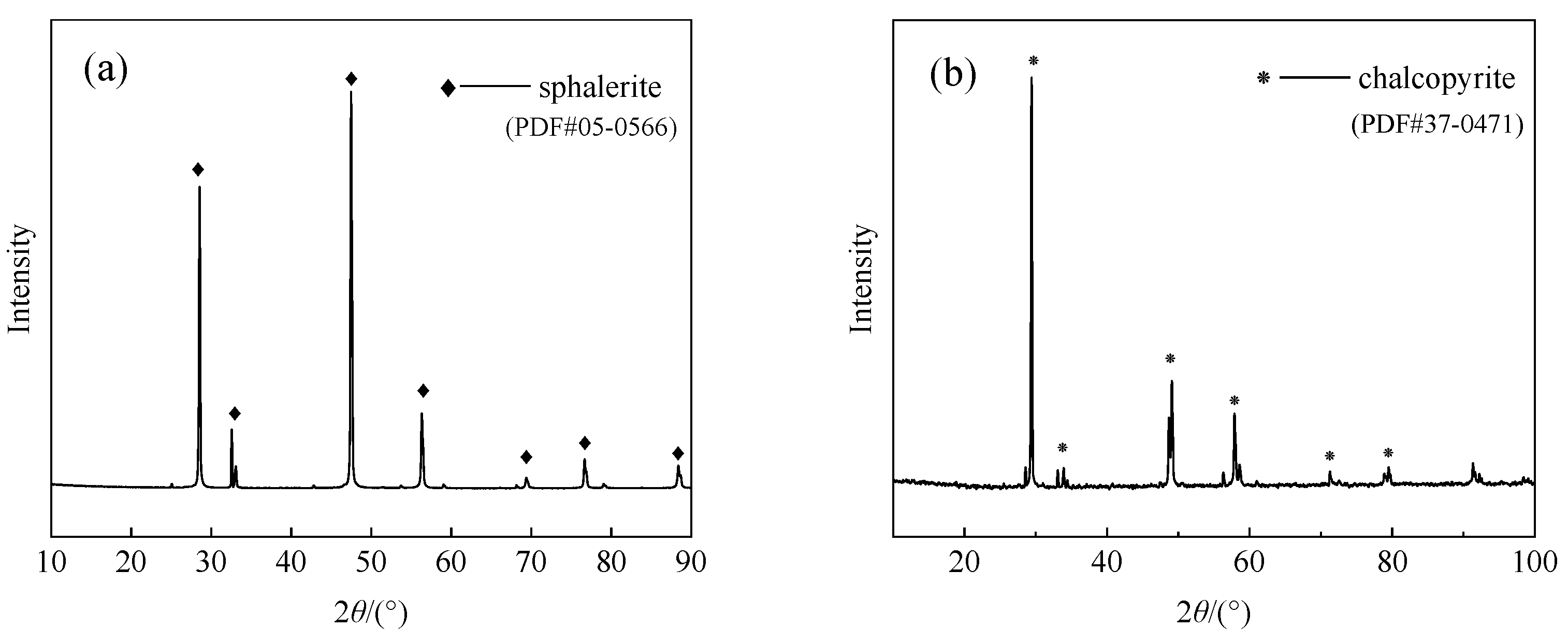
2.1. Materials
2.2. Micro-Flotation Experiments
2.3. Contact Angle Measurements
2.4. Zeta Potential Measurements
2.5. Surface Adsorption Measurements
2.6. XPS Measurements
3. Results and Discussion
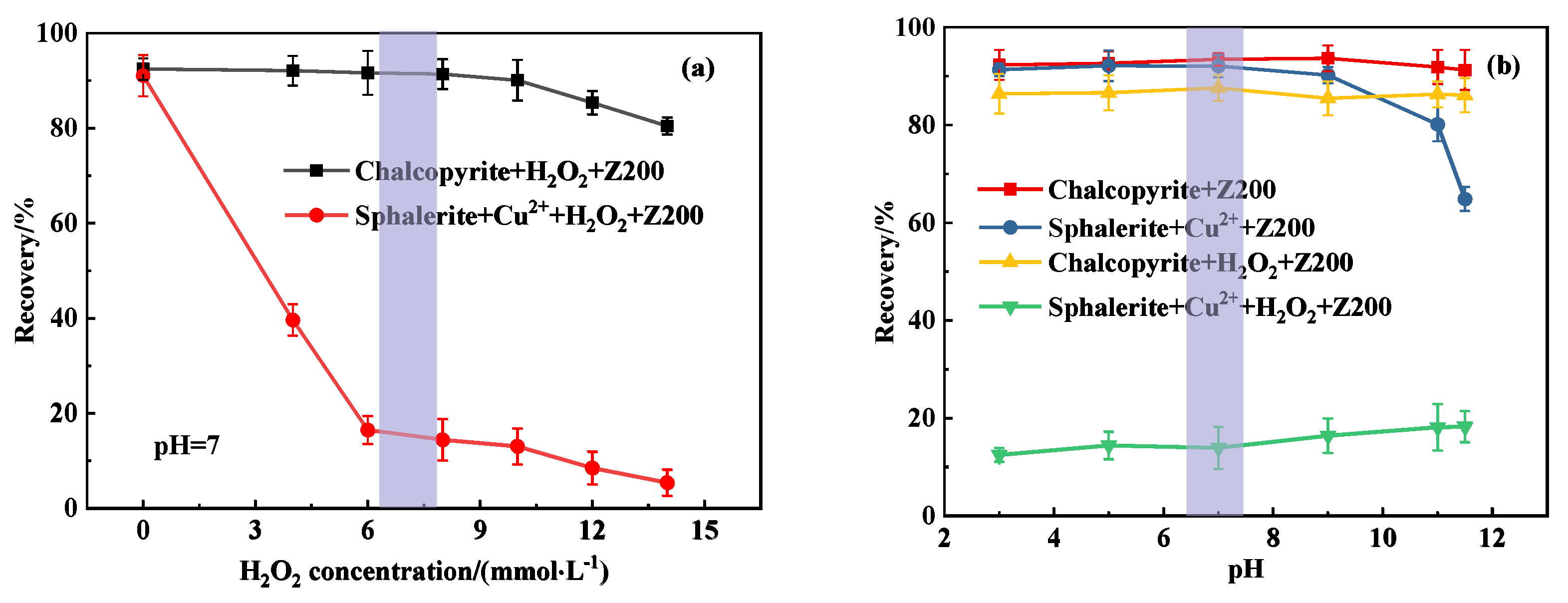
3.1. Single-Mineral Flotation Results
3.2. Flotation Results of Artificially Mixed Minerals
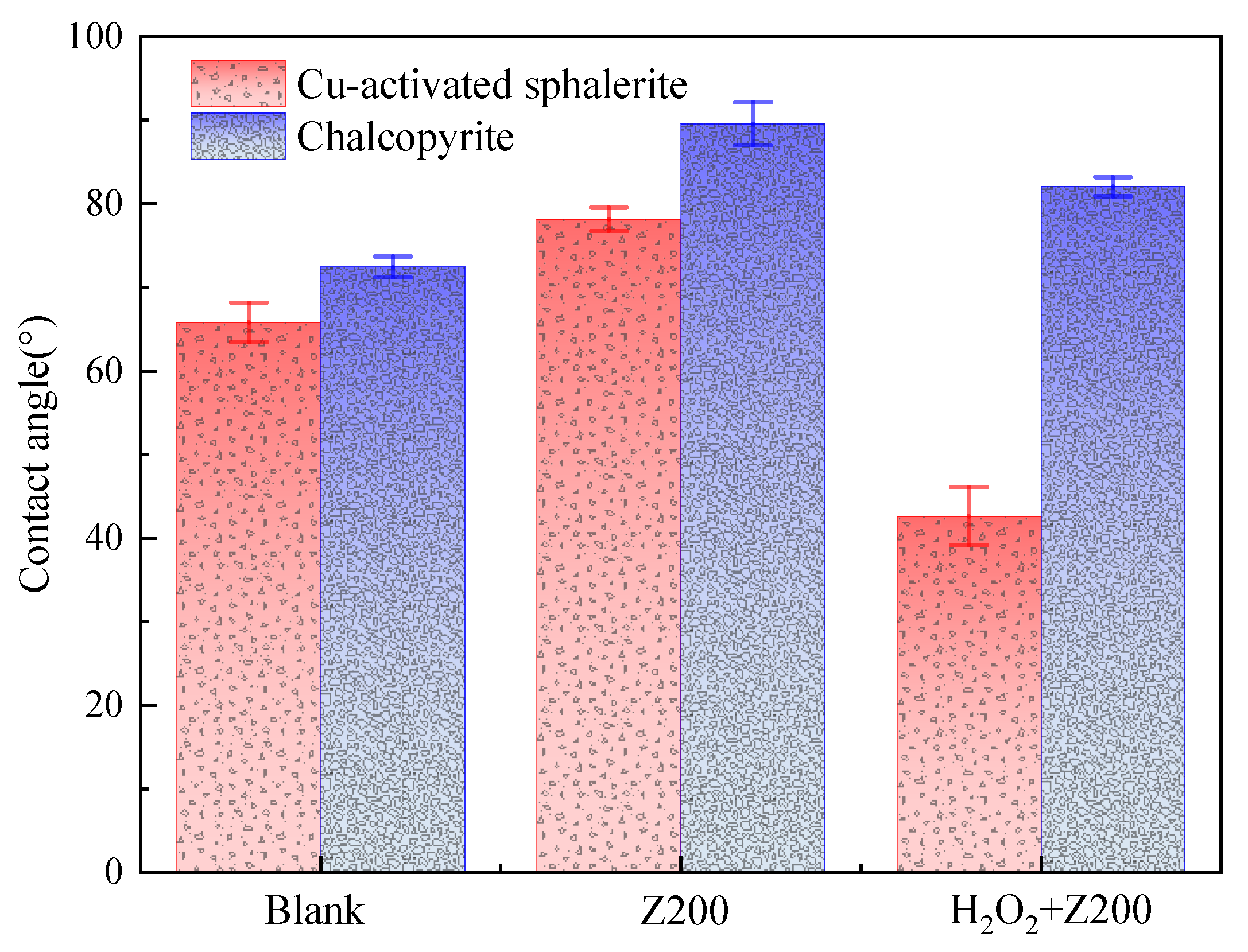
3.3. Contact Angle Test Results
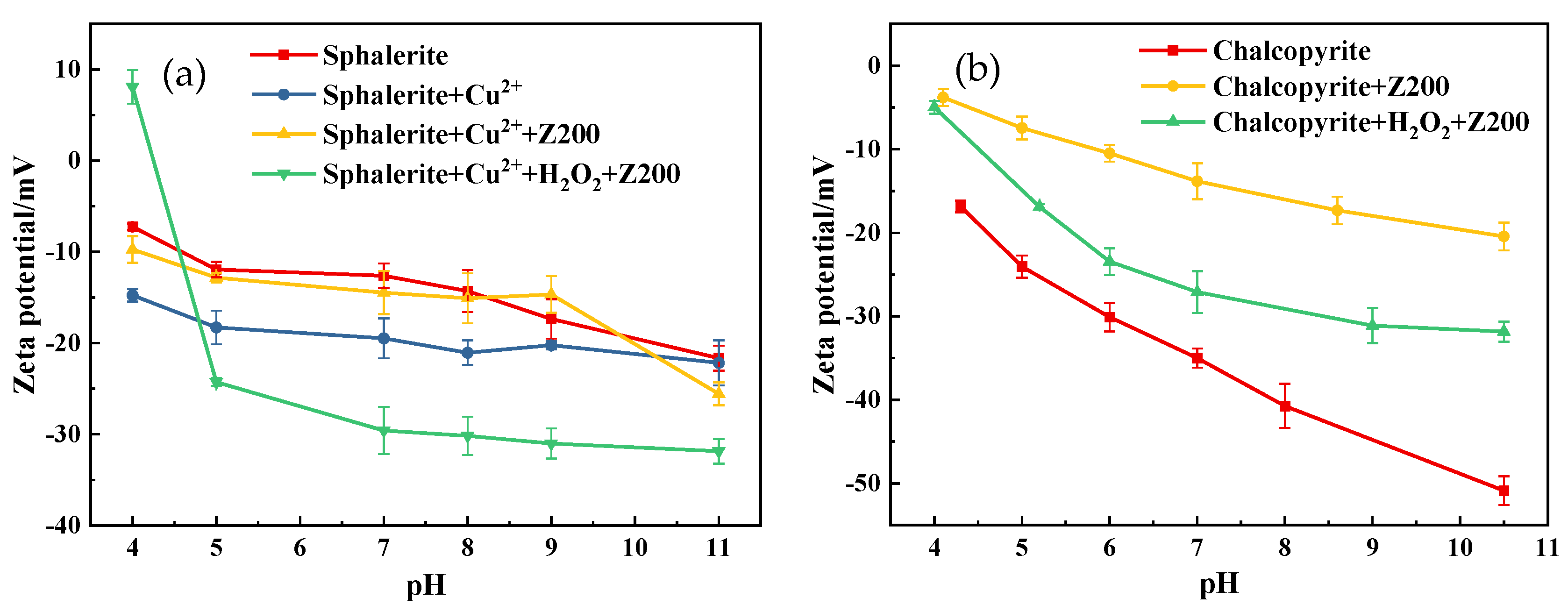
3.4. Zeta Potential Test Results
3.5. Surface Adsorption Analysis
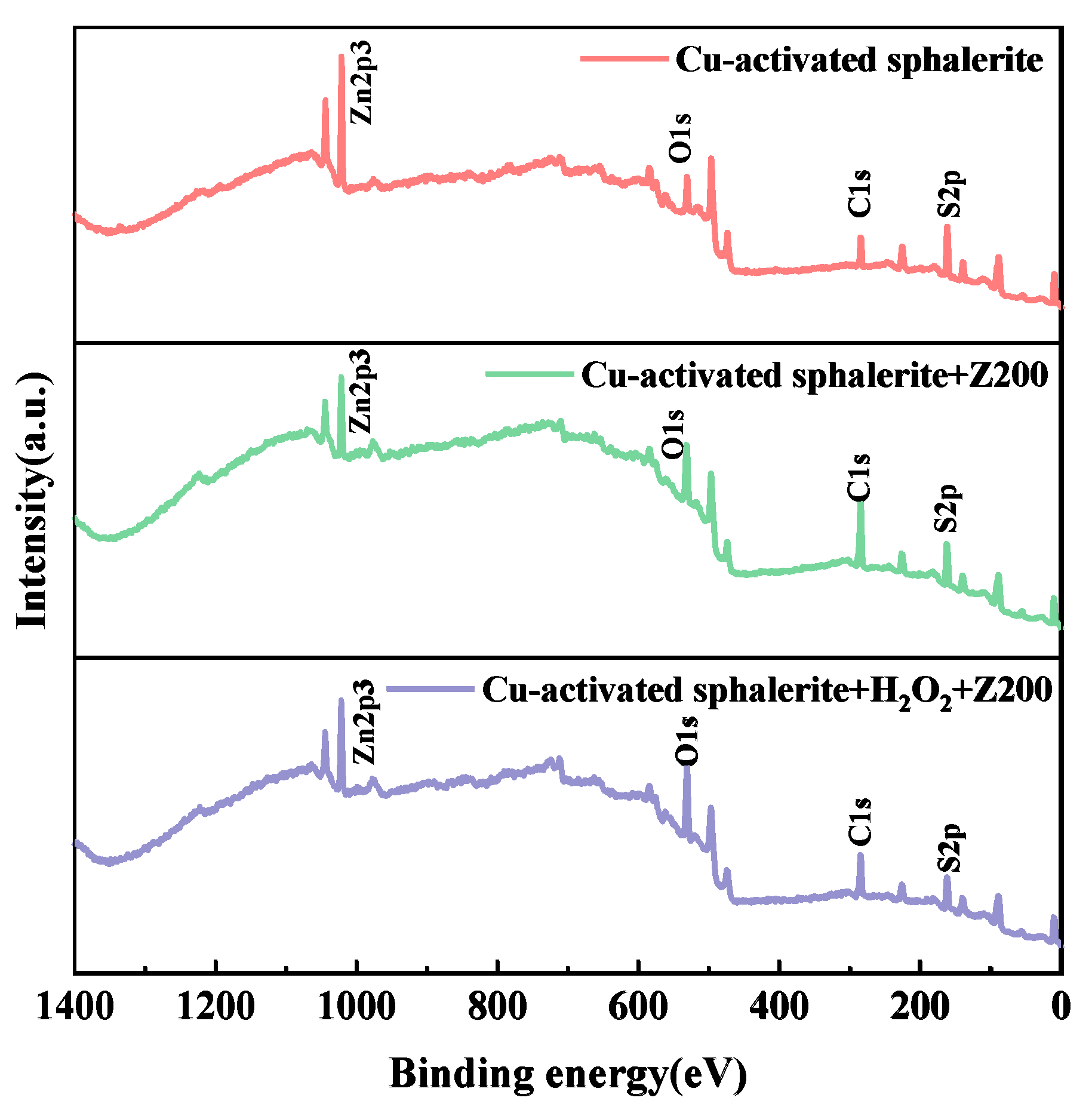
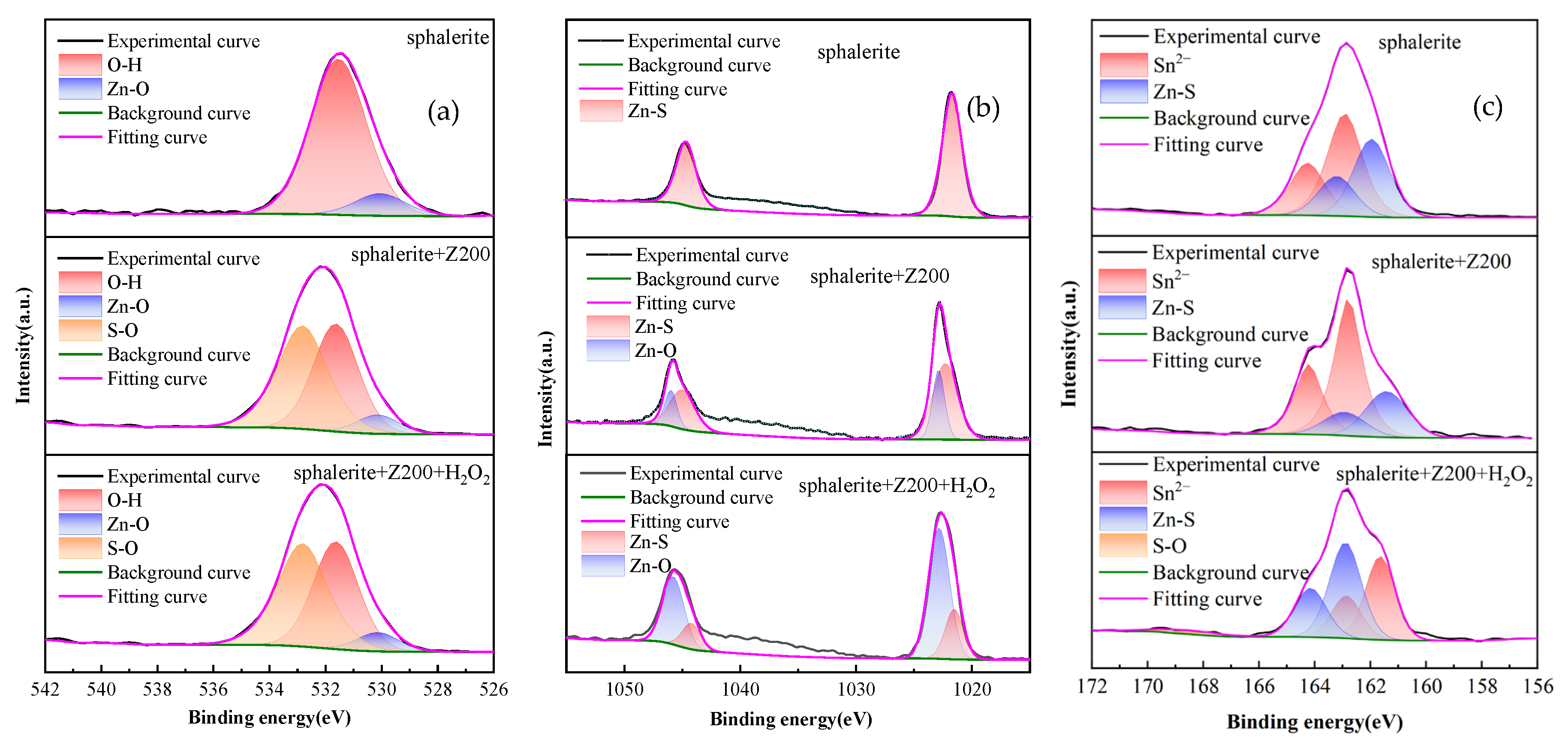
3.6. XPS Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wei, B.H.; Mao, Y.Q.; Wang, L.; Shen, P.L.; Wang, H.; Liu, D.W. Sources, Performance and Mechanisms of Metal Ions in the Flotation Process of Copper, Lead, and Zinc Ores: A Review. Minerals 2024, 14, 1105. [Google Scholar] [CrossRef]
- Piao, Y.C.; He, F.Y.; Pang, Z.R.; Shang, Y.B.; Luo, K.H.; Zhu, Y.G.; Gao, X.Y.; Zhang, X.R. Flotation Mechanism of Sphalerite by Sulfuration–Amine Method. Minerals 2023, 13, 673. [Google Scholar] [CrossRef]
- Bao, L.Y.; Liu, W.A.; Liu, W.B.; Guo, Y.; Chen, Z.X. Experimental and interaction mechanism with novel depressant lysine for the removal of copper ions activation on sphalerite surface. Miner. Eng. 2025, 226, 109247. [Google Scholar] [CrossRef]
- Chandra, A.P.; Gerson, A.R. A review of the fundamental studies of the copper activation mechanisms for selective flotation of the sulfide minerals, sphalerite and pyrite. Adv. Colloid Interface Sci. 2009, 145, 97–110. [Google Scholar] [CrossRef]
- Aikawa, K.; Ito, M.; Orii, N.; Jeon, S.; Park, I.; Haga, K.; Kamiya, T.; Takahashi, T.; Sunada, K.; Sakakibara, T.; et al. Flotation of Copper Ores with High Cu/Zn Ratio: Effects of Pyrite on Cu/Zn Separation and an Efficient Method to Enhance Sphalerite Depression. Minerals 2022, 12, 1103. [Google Scholar] [CrossRef]
- Nooshabadi, A.J.; Rao, K.H. Formation of hydrogen peroxide by sulphide minerals. Hydrometallurgy 2014, 141, 82–88. [Google Scholar] [CrossRef]
- Feng, B.; Guo, Y.T.; Zhang, W.P.; Peng, J.X.; Wang, H.H.; Huang, Z.Q.; Zhou, X.T. Flotation separation behavior of chalcopyrite and sphalerite in the presence of locust bean gum. Miner. Eng. 2019, 143, 105940. [Google Scholar] [CrossRef]
- Wang, C.T.; Liu, R.Q.; Wu, M.R.; Zhai, Q.L.; Luo, Y.J.; Jing, N.W.; Xie, F.F.; Sun, W. Selective separation of chalcopyrite from sphalerite with a novel depressant fenugreek gum: Flotation and adsorption mechanism. Miner. Eng. 2022, 184, 107653. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Yang, B.Q.; Martin, R.; Zhang, H.Q.; He, D.S.; Luo, H.H. Flotation separation of galena from sphalerite using hyaluronic acid (HA) as an environmental-friendly sphalerite depressant. Miner. Eng. 2022, 187, 107771. [Google Scholar] [CrossRef]
- Silvestre, M.O.; Pereira, C.A.; Galery, R.; Peres, A.E.C. Dispersion effect on a lead-zinc sulphide ore flotation. Miner. Eng. 2009, 22, 752–758. [Google Scholar] [CrossRef]
- Zhao, K.; Ma, C.; Gu, G.H.; Gao, Z.Y. Selective Separation of Chalcopyrite from Galena Using a Green Reagent Scheme. Minerals 2021, 11, 796. [Google Scholar] [CrossRef]
- Wei, Q.; Jiao, F.; Dong, L.Y.; Liu, X.D.; Qin, W.Q. Selective depression of copper-activated sphalerite by polyaspartic acid during chalcopyrite flotation. Trans. Nonferrous Met. Soc. China 2021, 31, 1784–1795. [Google Scholar] [CrossRef]
- Huang, P.; Cao, M.; Liu, Q. Selective depression of sphalerite by chitosan in differential Pb-Zn flotation. Int. J. Miner. Process. 2013, 122, 29–35. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, B.; Zhong, C.; Wang, Z. Effect and mechanism of the xanthate/H2O2 addition order on flotation separation of chalcopyrite and sphalerite. Miner. Eng. 2022, 188, 107851. [Google Scholar] [CrossRef]
- Suyantara, G.P.W.; Hirajima, T.; Miki, H.; Sasaki, K.; Yamane, M.; Takida, E.; Kuroiwa, S.; Imaizumi, Y. Effect of Fenton-like oxidation reagent on hydrophobicity and floatability of chalcopyrite and molybdenite. Colloids Surf. A Physicochem. Eng. Asp. 2018, 554, 34–48. [Google Scholar] [CrossRef]
- Xia, L.; Hart, B.R. Correlation between the hydrogen peroxide formed during grinding and the oxidized species present on the surface of sphalerite. Miner. Eng. 2019, 130, 165–170. [Google Scholar] [CrossRef]
- Liu, J.; Feng, B.; Guo, W.; Chen, Y. Utilizing H2O2 for the selective flotation separation of chalcopyrite and galena with their surface pre-adsorbed with Isopropyl Ethyl Thiocarbamate. Sep. Purif. Technol. 2024, 330, 125477. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Ma, Z.; Peng, W.; Huang, Y.; Wang, W.; Cao, Y. Recent advances in oxidation pretreatment and flotation separation of metal sulfide ores. Green Smart Min. Eng. 2025, 2, 142–155. [Google Scholar] [CrossRef]
- Nooshabadi, A.J.; Rao, K.H. Formation of hydrogen peroxide by chalcopyrite and its influence on flotation. Miner. Metall. Process. 2013, 30, 212–219. [Google Scholar] [CrossRef]
- Suyantara, G.P.W.; Berdakh, D.; Miki, H.; Hirajima, T.; Sasaki, K.; Ochi, D.; Aoki, Y. Effect of hydrogen peroxide on selective flotation of chalcocite and enargite. Int. J. Min. Sci. Technol. 2023, 33, 703–716. [Google Scholar] [CrossRef]
- Chen, Z.; Yoon, R.-H. Electrochemistry of copper activation of sphalerite at pH 9.2. Int. J. Miner. Process. 2000, 58, 57–66. [Google Scholar] [CrossRef]
- Flores, D.J.; Graber, T.A.; Angel-Castillo, A.H.; Hernández, P.C.; Taboada, M.E. Use of Hydrogen Peroxide as Oxidizing Agent in Chalcopyrite Leaching: A Review. Metals 2025, 15, 531. [Google Scholar] [CrossRef]
- Cui, Y.F.; Jiao, F.; Qin, W.Q.; Wang, C.; Li, X.C. Flotation separation of sphalerite from galena using eco-friendly and efficient depressant pullulan. Sep. Purif. Technol. 2022, 295, 121013. [Google Scholar] [CrossRef]
- Aikawa, K.; Ito, M.; Segawa, T.; Jeon, S.; Park, I.; Tabelin, C.B.; Hiroyoshi, N. Depression of lead-activated sphalerite by pyrite via galvanic interactions: Implications to the selective flotation of complex sulfide ores. Miner. Eng. 2020, 152, 106367. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, Z.; Wang, Y.; Liu, F.; Liu, S.; Liu, G. Flotation Separation of Chalcopyrite from Sphalerite with 3-amyl-4-amino-1,2,4-triazole-5-thione and Its Underlying Mechanism. Min. Metall. Eng. 2021, 41, 51–56. [Google Scholar]
- Cheng, T.; Wang, T.; Shen, Z.; Ma, S.; Shi, S.; Deng, J.; Deng, J. The depression mechanism of magnesium on the flotation of sphalerite with different iron contents: DFT and experimental studies. Sep. Purif. Technol. 2025, 353, 128379. [Google Scholar] [CrossRef]
- Zhang, C.H.; He, T.S.; Chen, W.; Bu, X.Z.; Wang, S.; Tian, X.Z. The Auxiliary Effect of Copper Ions on the Depressant Effect of Sodium Thioglycolate in Chalcopyrite Flotation. Minerals 2020, 10, 157. [Google Scholar] [CrossRef]
- Rios, L.A.; Barraza, M.J.; Robles, P.A.; Quezada, G.R. Chalcopyrite Flotation, Molecular Design and Smart Industry: A Review. Int. J. Mol. Sci. 2025, 26, 3613. [Google Scholar] [CrossRef]
- Sun, X.S.; Yu, J.W.; Zhang, W.Y.; Li, P.Y.; Li, Y.J.; Han, Y.X. Flotation separation of chalcopyrite from pyrite and sphalerite using a novel collector: An experimental and mechanism investigation. Appl. Surf. Sci. 2024, 661, 160059. [Google Scholar] [CrossRef]
- Ignatkina, V.A.; Bocharov, V.A.; Aksenova, D.D.; Kayumov, A.A. Zeta potential of the surface of ultrafine sulfides and floatability of minerals. Russ. J. Non-Ferr. Met. 2017, 58, 95–100. [Google Scholar] [CrossRef]
- Hong, G.; Choi, J.; Han, Y.; Yoo, K.S.; Kim, K.; Kim, S.B.; Kim, H. Relationship between Surface Characteristics and Floatability in Representative Sulfide Minerals: Role of Surface Oxidation. Mater. Trans. 2017, 58, 1069–1075. [Google Scholar] [CrossRef]
- Gomez-Flores, A.; Heyes, G.W.; Ilyas, S.; Kim, H. Prediction of grade and recovery in flotation from physicochemical and operational aspects using machine learning models. Miner. Eng. 2022, 183, 107627. [Google Scholar] [CrossRef]
- Zhai, Q.L.; Hu, Y.W.; Liu, R.Q.; Sun, W. Investigation of pullulan polysaccharide as a sphalerite depressant for chalcopyrite separation: Flotation behavior and interfacial adsorption mechanism. Adv. Powder Technol. 2024, 35, 104621. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, X.L.; Liu, X.X.; Xu, X.L.; Chen, X.Y.; He, H.Y.; Zhang, Y.; Peng, R.; Shen, P.L.; Liu, D.W. A novel eco-friendly depressant Scutellaria Baicalensis Extract SBE and its performance on flotation separation of chalcopyrite from sphalerite: A combined experimental and mechanism investigation. Chem. Phys. 2025, 591, 112541. [Google Scholar] [CrossRef]
- Kwoka, M.; Kulis-Kapuscinska, A.; Zappa, D.; Comini, E.; Szuber, J. Novel insight on the local surface properties of ZnO nanowires. Nanotechnology 2020, 31, 465705. [Google Scholar] [CrossRef]
- Jnido, G.; Ohms, G.; Viöl, W. Deposition of Zinc Oxide Coatings on Wood Surfaces Using the Solution Precursor Plasma Spraying Process. Coatings 2021, 11, 183. [Google Scholar] [CrossRef]
- Jiang, L.; Feng, B.; Liu, J.; Guo, Y.; Corin, K.C.; Mabounda, J. Influence of surface oxidation rate on the flotation of sphalerite and galena inhibited by gum arabic. Process. Saf. Environ. Prot. 2024, 192, 998–1007. [Google Scholar] [CrossRef]
- Pauly, N.; Yubero, F.; Espinós, J.P.; Tougaard, S. XPS primary excitation spectra of Zn 2p, Fe 2p, and Ce 3d from ZnO, α-Fe2O3, and CeO2. Surf. Interface Anal. 2019, 51, 353–360. [Google Scholar] [CrossRef]
- Li, J.L.; Li, G.L.; Liu, Z.C.; Ning, S.; Liu, R.Z. Interface-coupled PbSO4 dissolution and PbS precipitation and its effect on sulfidization flotation of anglesite. Trans. Nonferrous Met. Soc. China 2023, 33, 3503–3513. [Google Scholar] [CrossRef]
- Cabrera-German, D.; Molar-Velázquez, G.; Gómez-Sosa, G.; de la Cruz, W.; Herrera-Gomez, A. Detailed peak fitting analysis of the Zn 2p photoemission spectrum for metallic films and its initial oxidation stages. Surf. Interface Anal. 2017, 49, 1078–1087. [Google Scholar] [CrossRef]
- Kartio, I.J.; Basilio, C.I.; Yoon, R.-H. An XPS study of sphalerite activation by copper. Langmuir 1998, 14, 5274–5278. [Google Scholar] [CrossRef]
- Pratt, A. Fine Structure in XPS and XANES Spectra Acquired from a Series of Sphalerite Samples. ECS Trans. 2010, 28, 95–103. [Google Scholar] [CrossRef]
- Ejtemaei, M.; Nguyen, A.V. Characterisation of sphalerite and pyrite surfaces activated by copper sulphate. Miner. Eng. 2017, 100, 223–232. [Google Scholar] [CrossRef]

| Content/wt.% | Zn | Cu | Fe | S | Pb | Si | |
|---|---|---|---|---|---|---|---|
| Sample | |||||||
| Sphalerite | 64.01 | <0.005 | 1.49 | 33.75 | 0.031 | 0.34 | |
| Chalcopyrite | 0.024 | 34.32 | 30.32 | 34.12 | 0.068 | 0.27 | |
| Concentration of Depressant H2O2/(mmol·L−1) | Product | Yield/wt.% | Grade/% | Recovery/% | ||
|---|---|---|---|---|---|---|
| Cu | Zn | Cu | Zn | |||
| 0 | Cu concentrate | 94.99 | 16.17 | 29.54 | 95.84 | 91.46 |
| Zn concentrate | 5.01 | 13.31 | 52.33 | 4.16 | 8.54 | |
| Feed | 100.00 | 16.03 | 30.68 | 100.00 | 100.00 | |
| 8 | Cu concentrate | 48.11 | 29.95 | 5.76 | 87.30 | 8.98 |
| Zn concentrate | 51.89 | 4.04 | 54.12 | 12.70 | 91.02 | |
| Feed | 100.00 | 16.51 | 30.85 | 100.00 | 100.00 | |
| Test Condition | C0/mg | C1/mg | V/L | C/% | M/g | |
|---|---|---|---|---|---|---|
| Sphalerite+Cu2++Z-200 | 2.90 | 1.80 | 0.05 | 52.13% | 2 | 0.0528 |
| Sphalerite+Cu2++H2O2+Z-200 | 2.80 | 2.50 | 0.0144 | |||
| Chalcopyrite+Z-200 | 2.90 | 1.00 | 0.0911 | |||
| Chalcopyrite+H2O2+Z-200 | 2.80 | 1.20 | 0.0767 |
| Samples | At.% | |||
|---|---|---|---|---|
| C1s | O1s | Zn2p | S2p | |
| Cu-activated sphalerite | 39.60 | 20.41 | 20.67 | 19.32 |
| Cu-activated sphalerite+Z-200 | 56.66 | 26.02 | 5.47 | 11.84 |
| Cu-activated sphalerite+H2O2+Z-200 | 43.24 | 34.45 | 11.90 | 10.41 |
| Samples | Zn–O | O–H | S–O | |||
|---|---|---|---|---|---|---|
| B.E./eV | At. | B.E./eV | At. | B.E./eV | At. | |
| Cu-activated sphalerite | 530.06 | 11.43 | 531.54 | 88.52 | - | 0.00 |
| Cu-activated sphalerite+Z-200 | 530.50 | 31.25 | 531.78 | 52.74 | 533.00 | 16.01 |
| Cu-activated sphalerite+H2O2+Z-200 | 530.13 | 6.49 | 531.63 | 40.16 | 532.82 | 44.77 |
| Samples | Zn–S | Zn–O | ||
|---|---|---|---|---|
| B.E./eV | At.% | B.E./eV | At.% | |
| Cu-activated sphalerite | 1021.72 | 100.00 | - | 0.00 |
| Cu-activated sphalerite+Z-200 | 1021.31 | 61.54 | 1022.17 | 38.46 |
| Cu-activated sphalerite+H2O2+Z-200 | 1021.53 | 24.91 | 1022.84 | 75.09 |
| Samples | Zn–S | Sn2− | S–O | |||
|---|---|---|---|---|---|---|
| B.E./eV | At.% | B.E./eV | At.% | B.E./eV | At.% | |
| Cu-activated sphalerite | 161.21 | 42.96 | 162.05 | 57.04 | - | 0.00 |
| Cu-activated sphalerite+Z-200 | 160.74 | 31.44 | 161.94 | 68.56 | - | 0.00 |
| Cu-activated sphalerite+H2O2+Z-200 | 161.64 | 43.24 | 162.89 | 53.63 | 168.31 | 3.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, P.; Tong, X.; Xie, X.; Song, Q.; Ma, Y.; Han, B.; Fu, H.; Lu, Z. Selective Oxidation Depression of Copper-Activated Sphalerite by H2O2 During Chalcopyrite Flotation. Metals 2025, 15, 813. https://doi.org/10.3390/met15070813
Fan P, Tong X, Xie X, Song Q, Ma Y, Han B, Fu H, Lu Z. Selective Oxidation Depression of Copper-Activated Sphalerite by H2O2 During Chalcopyrite Flotation. Metals. 2025; 15(7):813. https://doi.org/10.3390/met15070813
Chicago/Turabian StyleFan, Peiqiang, Xiong Tong, Xian Xie, Qiang Song, Yuanlin Ma, Bin Han, Haitao Fu, and Zhiming Lu. 2025. "Selective Oxidation Depression of Copper-Activated Sphalerite by H2O2 During Chalcopyrite Flotation" Metals 15, no. 7: 813. https://doi.org/10.3390/met15070813
APA StyleFan, P., Tong, X., Xie, X., Song, Q., Ma, Y., Han, B., Fu, H., & Lu, Z. (2025). Selective Oxidation Depression of Copper-Activated Sphalerite by H2O2 During Chalcopyrite Flotation. Metals, 15(7), 813. https://doi.org/10.3390/met15070813






