Effect of Micro-Arc Oxidation Voltage on the Surface Morphology and Properties of Ceramic Coatings on 7075 Aluminum Alloy
Abstract
1. Introduction
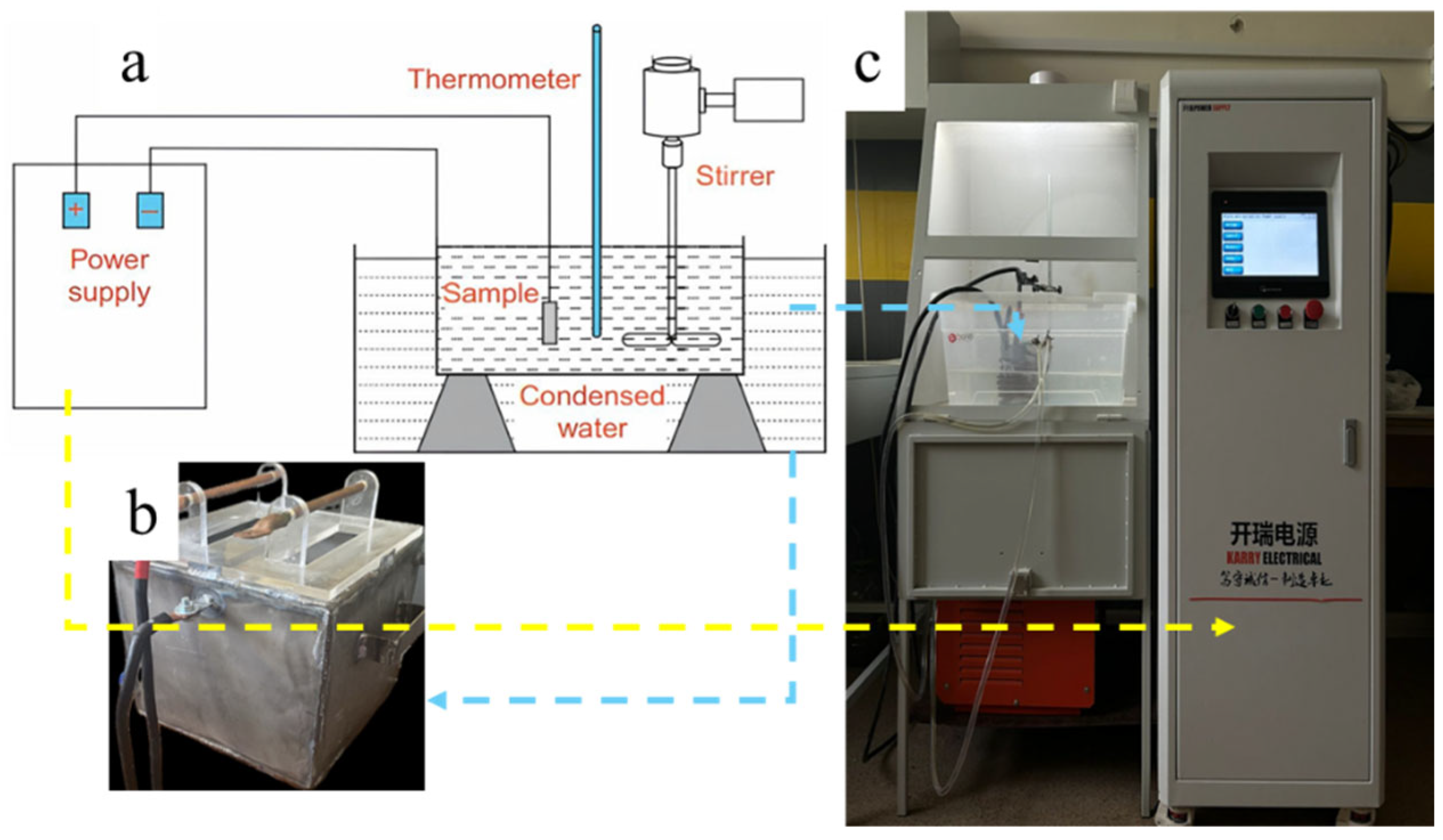
2. Materials and Methods
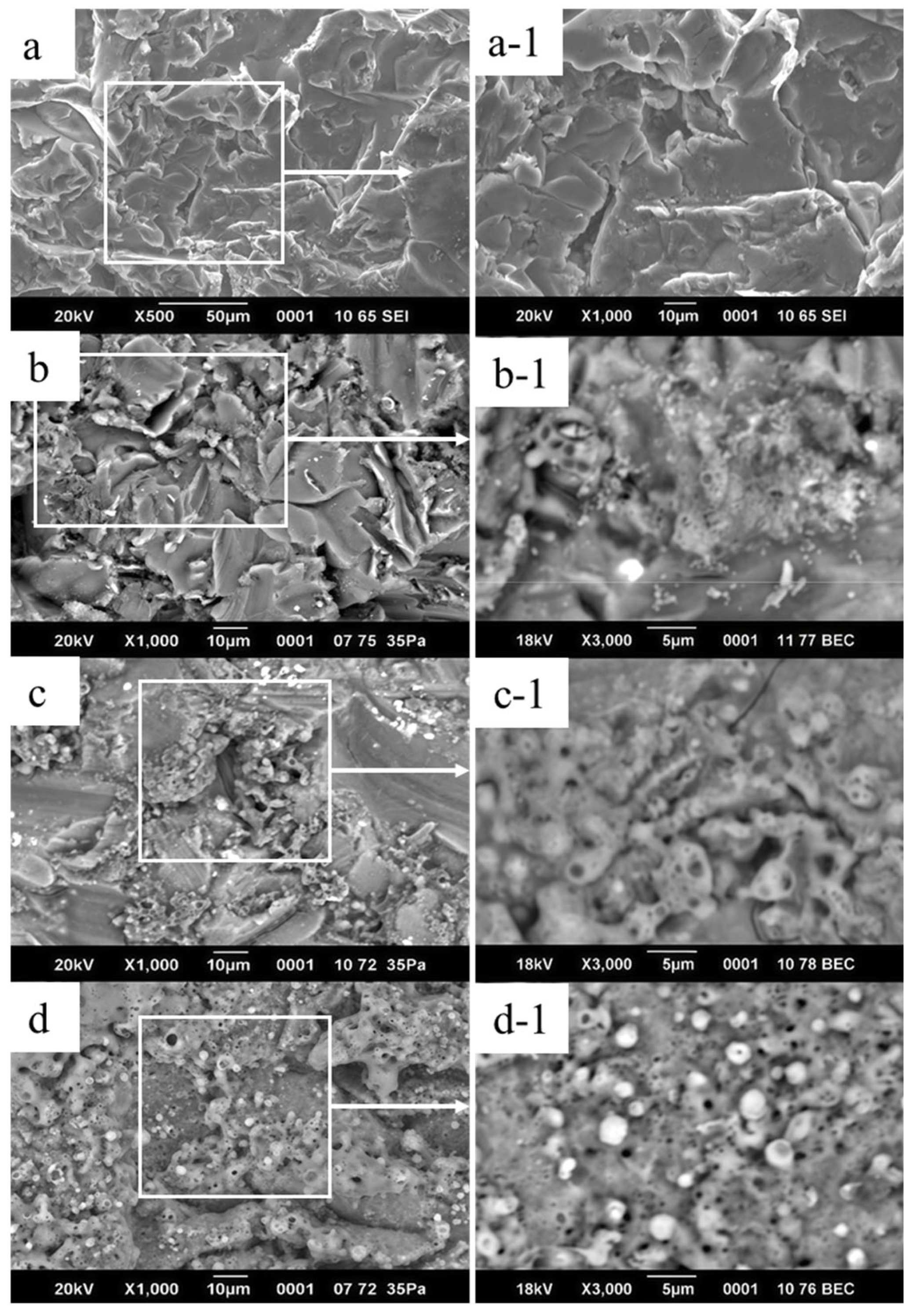
3. Results and Discussion
4. Conclusions
- -
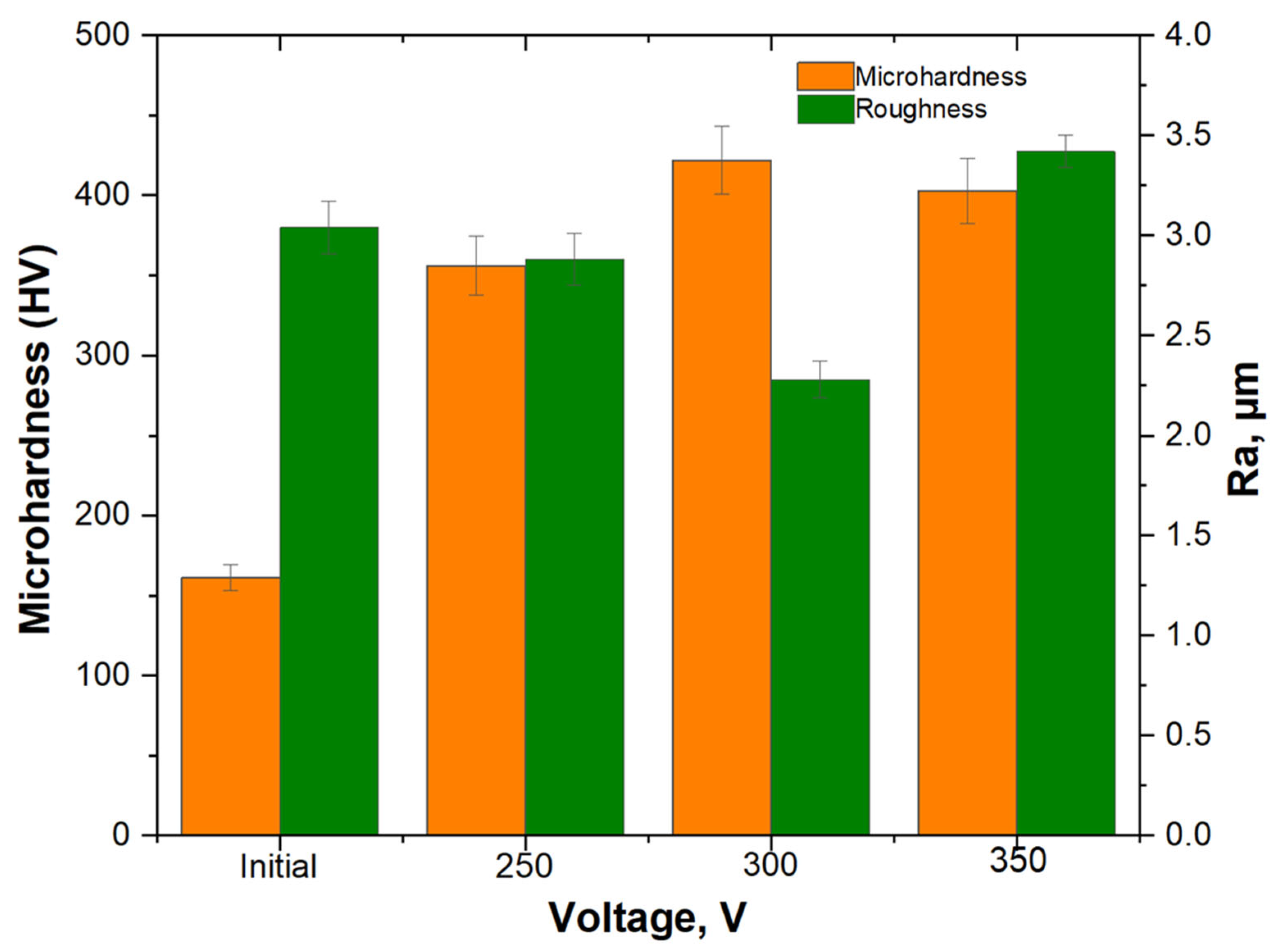
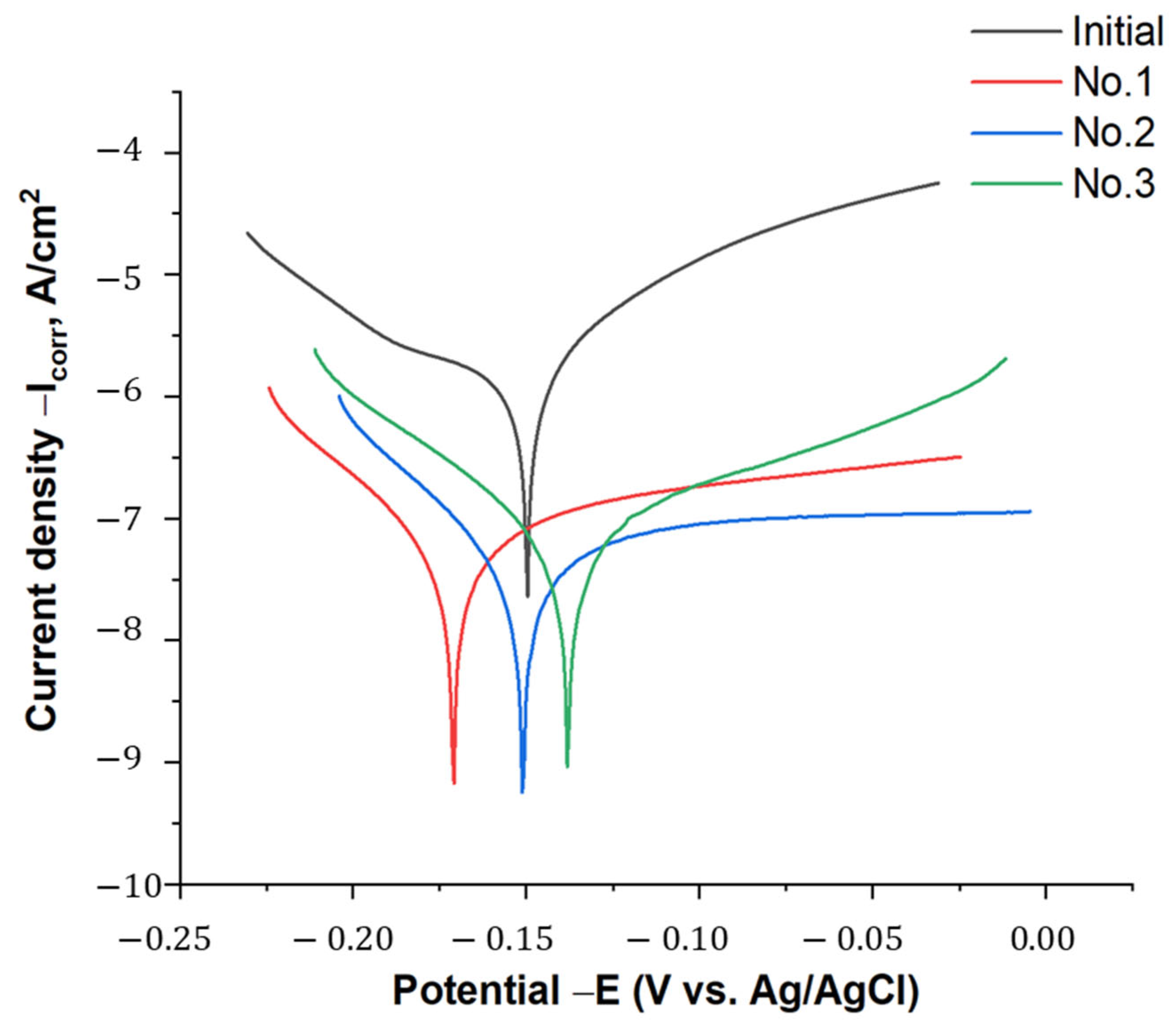
- The coating obtained at 300 V showed the most balanced characteristics, including moderate porosity, microhardness (422 ± 21.2 HV), low surface roughness (2.28 ± 0.09 μm), and excellent corrosion resistance. Although sample No. 3 exhibited a slightly lower corrosion current density, its higher porosity and less favorable Ecorr made sample No. 2 more suitable for long-term protection.
- -
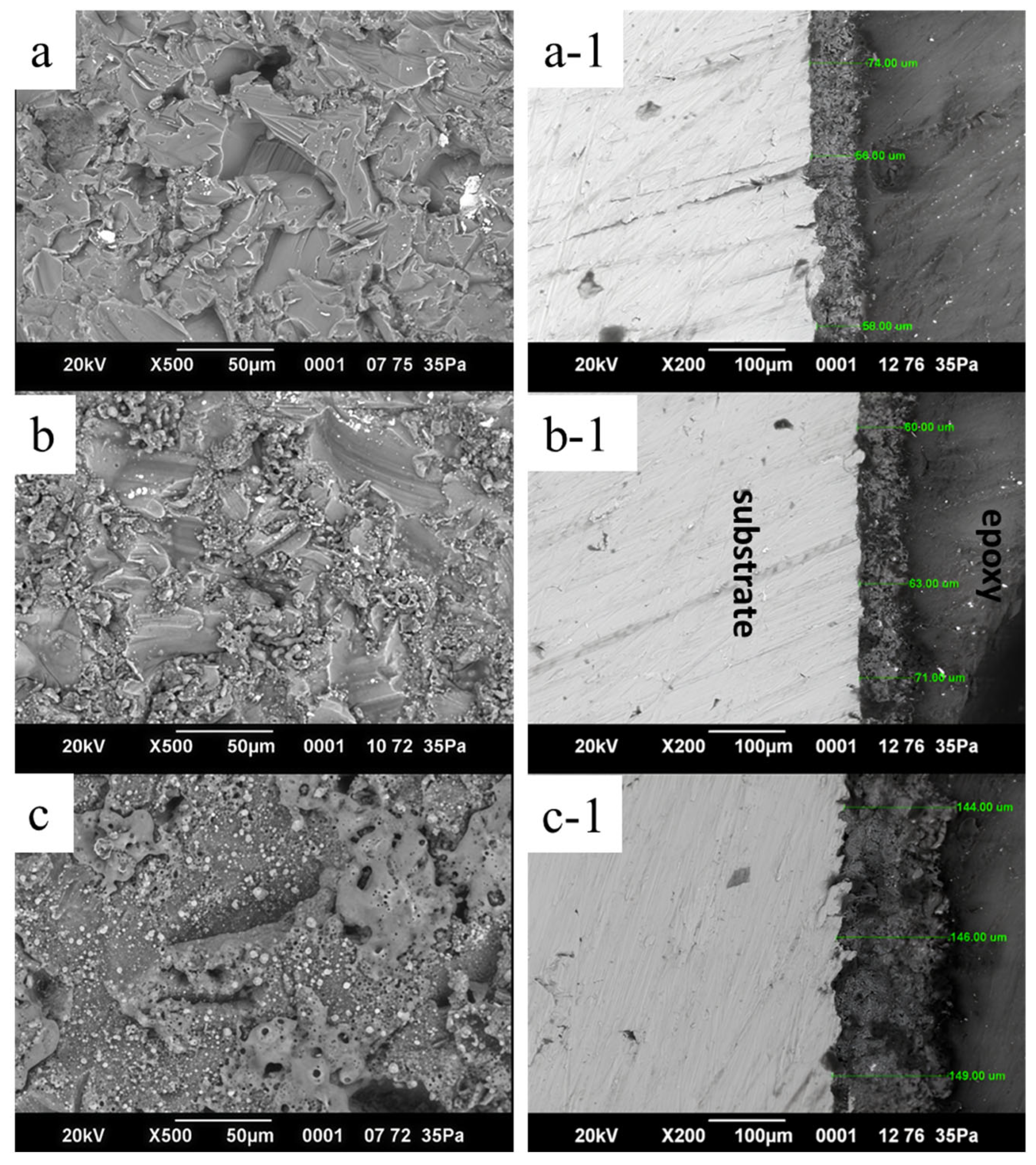
- The coating thickness increased with the voltage, reaching from 56 to 149 μm. However, excessive voltage led to structural defects and high porosity, which negatively affected the protective properties.
- -
- The X-ray diffraction analysis revealed the presence of both γ- and α-Al2O3 phases in all coatings, with increased intensity and crystallinity of the α-phase at higher voltages.
- -
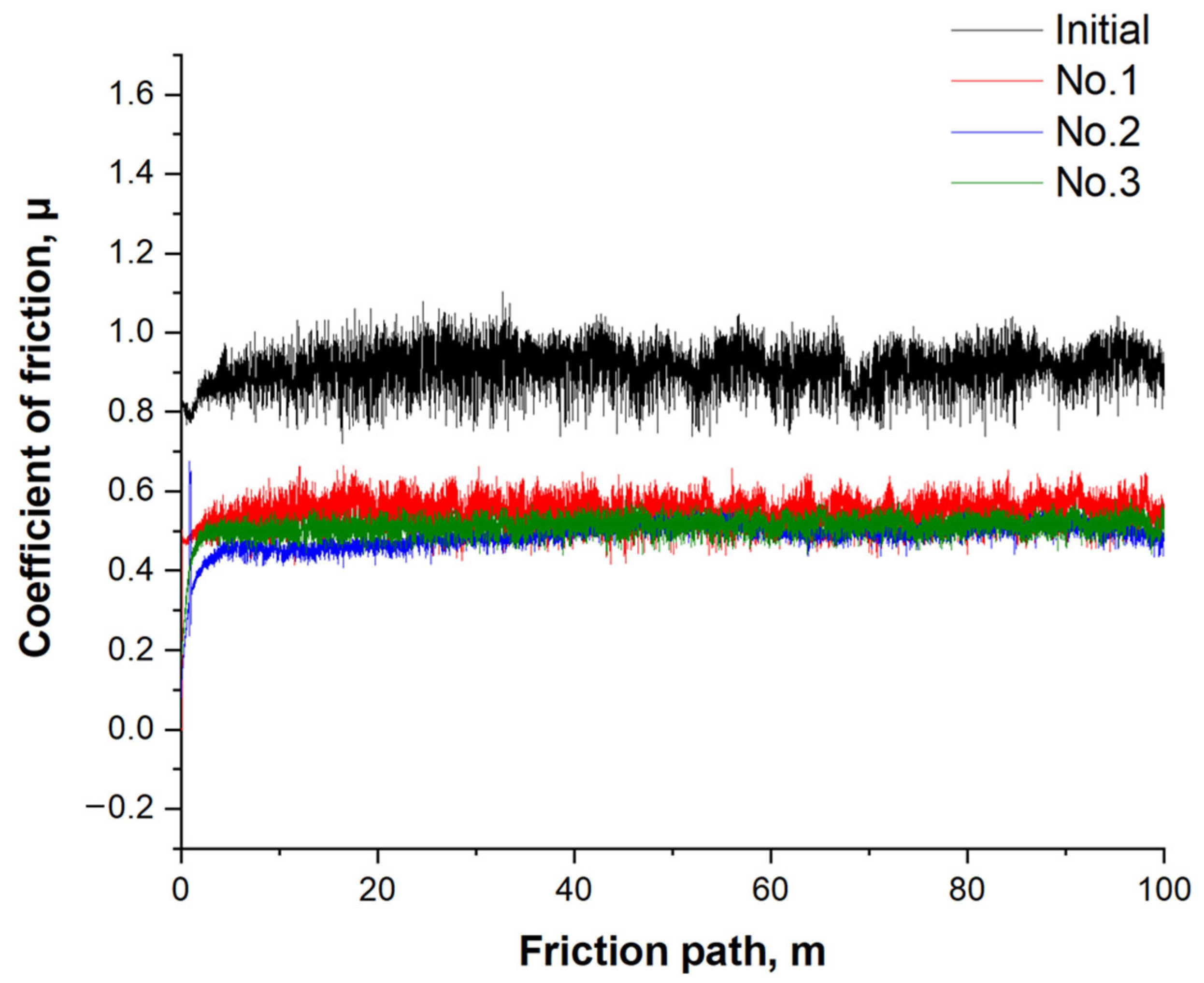
- Sample No. 2 demonstrated the lowest and most stable friction coefficient (0.489), confirming the excellent wear resistance and adhesion of the coating under sliding conditions.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wahid, M.A.; Siddiquee, A.N.; Khan, Z.A. Aluminum alloys in marine construction: Characteristics, application, and problems from a fabrication viewpoint. Mar. Syst. Ocean Technol. 2020, 15, 70–80. [Google Scholar] [CrossRef]
- Yadav, R.; Dwivedi, V.K.; Dwivedi, S.P. Analysis of Mechanical Properties and Manufacturing Process of Aluminum Series. J. Inst. Eng. (India) Ser. D 2024, 1–13. [Google Scholar] [CrossRef]
- Khalid, M.Y.; Umer, R.; Khan, K.A. Review of recent trends and developments in aluminium 7075 alloy and its metal matrix composites (MMCs) for aircraft applications. Results Eng. 2023, 20, 101372. [Google Scholar] [CrossRef]
- Qi, X.; Li, J.; He, Y.; Liu, Y.; Liu, R.; Song, R. Study on the wear resistance and corrosion behaviour of self-sealed MAO/ZrO2 coatings prepared on 7075 aluminium alloy. J. Alloys Compd. 2023, 969, 172436. [Google Scholar] [CrossRef]
- Imran, M.; Khan, A.R.A. Characterization of Al-7075 metal matrix composites: A review. J. Mater. Res. Technol. 2019, 8, 3347–3356. [Google Scholar] [CrossRef]
- Rao, Y.; Wang, Q.; Oka, D.; Ramachandran, C.S. On the PEO treatment of cold sprayed 7075 aluminum alloy and its effects on mechanical, corrosion and dry sliding wear performances thereof. Surf. Coat. Technol. 2020, 383, 125271. [Google Scholar] [CrossRef]
- Jayappa, K.; Anil, K.C.; Khan, Z.A. Enhancing wear resistance in Al-7075 composites through conventional mixing and casting techniques. J. Mater. Res. Technol. 2023, 27, 7935–7945. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, X.; Yu, B.; Cai, M.; Yu, Q.; Zhou, F. Research progress on the wear and corrosion resistant plasma electrolytic oxidation composite coatings on magnesium and its alloys. Coatings 2023, 13, 1189. [Google Scholar] [CrossRef]
- Sikdar, S.; Menezes, P.V.; Maccione, R.; Jacob, T.; Menezes, P.L. Plasma electrolytic oxidation (PEO) process—Processing, properties, and applications. Nanomaterials 2021, 11, 1375. [Google Scholar] [CrossRef]
- Fernández-López, P.; Alves, S.A.; San-Jose, J.T.; Gutierrez-Berasategui, E.; Bayón, R. Plasma electrolytic oxidation (PEO) as a promising technology for the development of high-performance coatings on cast Al-Si alloys: A review. Coatings 2024, 14, 217. [Google Scholar] [CrossRef]
- Wierzbicka, E.; Vaghefinazari, B.; Mohedano, M.; Visser, P.; Posner, R.; Blawert, C.; Zheludkevich, M.; Lamaka, S.; Matykina, E.; Arrabal, R. Chromate-free corrosion protection strategies for magnesium alloys—A review: Part II—PEO and anodizing. Materials 2022, 15, 8515. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Cai, R.; Zhao, C.; Sun, J.; Zhang, J.; Matthews, D.T. Advancement of plasma electrolytic oxidation towards non-valve metals. Surf. Coat. Technol. 2022, 442, 128403. [Google Scholar] [CrossRef]
- Jadhav, P.; Bongale, A.; Kumar, S. A review of process characteristics of plasma electrolytic oxidation of aluminium alloy. J. Phys. Conf. Ser. 2021, 1854, 012030. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Fardosi, A.; Karbasi, M.; Kaseem, M. Advancements in enhancing corrosion protection of Mg alloys: A comprehensive review on the synergistic effects of combining inhibitors with PEO coating. J. Magnes. Alloys 2024, 12, 465–489. [Google Scholar] [CrossRef]
- Varghese, J.; Vieth, P.; Xie, X.; Grundmeier, G. Enhanced corrosion resistance of epoxy-films on ultra-thin SiOx PECVD film coated laser surface melted Al-alloys. SN Appl. Sci. 2023, 5, 29. [Google Scholar] [CrossRef]
- Jiang, B.; Wen, Z.; Wang, P.; Huang, X.; Yang, X.; Yuan, M.; Xi, J. Study on the Optimization of the Preparation Process of ZM5 Magnesium Alloy Micro-Arc Oxidation Hard Ceramic Coatings and Coatings Properties. Metals 2024, 14, 594. [Google Scholar] [CrossRef]
- Li, G.; Ma, F.; Liu, P.; Qi, S.; Li, W.; Zhang, K.; Chen, X. Review of micro-arc oxidation of titanium alloys: Mechanism, properties and applications. J. Alloys Compd. 2023, 948, 169773. [Google Scholar] [CrossRef]
- Rakhadilov, B.; Zhassulan, A.; Baizhan, D.; Shynarbek, A.; Ormanbekov, K.; Aldabergenova, T. The effect of the electrolyte composition on the microstructure and properties of coatings formed on a titanium substrate by microarc oxidation. AIMS Mater. Sci. 2024, 11, 547–564. [Google Scholar] [CrossRef]
- Rakhadilov, B.; Zhassulan, A.; Ormanbekov, K.; Shynarbek, A.; Baizhan, D.; Aldabergenova, T. Surface Modification and Tribological Performance of Calcium Phosphate Coatings with TiO2 Nanoparticles on VT1-0 Titanium by Micro-Arc Oxidation. Crystals 2024, 14, 945. [Google Scholar] [CrossRef]
- Bayatanova, L.B.; Zhassulankyzy, A.Z.; Magazov, N.M.; Rakhadilov, B.K.; Muktanova, N.; Uazyrkhanova, G.K. Effect of plasma-electrolytic oxidation on mechanical properties of titanium coatings. Bull. Karaganda Univ. Phys. Ser. 2023, 111, 65–74. [Google Scholar] [CrossRef]
- Sobolev, A.; Peretz, T.; Borodianskiy, K. Micro-Arc Oxidation Coating of 7075 Aluminum Alloy in Molten Salt. Coatings 2020, 10, 993. [Google Scholar] [CrossRef]
- Chen, X.; Tang, S.; Zhang, D.; Yan, J.; Xie, W.; Ran, Q. Influence of graphene additions on the corrosion resistance of micro-arc oxidation coatings formed on 7075 aluminum alloy. Int. J. Appl. Ceram. Technol. 2024, 21, 3516–3525. [Google Scholar] [CrossRef]
- Barati, N.; Jiang, J.; Meletis, E. Microstructural Evolution of Ceramic Nanocomposites on 7075 Al Alloy by Plasma Electrolytic Oxidation. Adv. Eng. Mater. 2021, 437, 128345. [Google Scholar] [CrossRef]
- Sun, H.O.; Dela Pena, E.M.B. Effects of pulse unipolar voltage on microstructure and corrosion behavior of MAO coatings on AA7075. Coatings 2024, 14, 1498. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhao, Y.; Li, B.Y. Corrosion Resistance of Al2O3-ZrO2 Composite Coatings on Aluminum Alloy by MAO. Adv. Mater. Res. 2011, 189, 672–675. [Google Scholar] [CrossRef]
- Thunaipragasam, S.; Kolekar, A.B.; Chandrashekhar, K.G.; Pandey, R.; Shahid, M.; Rajesh, K.; Singh, B. Investigation of mechanical behavior and surface characteristics of cold spray metallized B4C/AA7075 composites coated by AZ64 alloy through plasma electrolytic oxidation. J. Nanomater. 2023, 2023, 7267093. [Google Scholar] [CrossRef]
- Su, K.; Zhang, J.; Wu, S.; Guan, J.; Li, H.; Ji, D.; Xie, H. Corrosion and corrosion-fatigue properties of composite ceramic coated aluminum alloy formed by combining surface nanocrystallization pre-treatment, micro-arc oxidation, and sealing post-treatment. Int. J. Fatigue 2025, 190, 108661. [Google Scholar] [CrossRef]
- Wu, X.; Li, H.; Lu, J.; Li, Y.; Yang, C.; Cen, Y.; Song, R. MoS2 additive to the MAO Al2O3 composite coatings with enhanced mechanical performances. Mater. Res. Express 2019, 6, 016543. [Google Scholar] [CrossRef]
- Krishtal, M.M.; Ivashin, P.V.; Yasnikov, I.S.; Polunin, A.V. Effect of nanosize SiO2 particles added into electrolyte on the composition and morphology of oxide layers formed in alloy AK6M2 under microarc oxidizing. Met. Sci. Heat Treat. 2015, 57, 428–435. [Google Scholar] [CrossRef]
- Pesode, P.; Barve, S.; Dayane, S. Antibacterial coating on magnesium alloys by MAO for biomedical applications. Res. Biomed. Eng. 2024, 40, 409–433. [Google Scholar] [CrossRef]
- Hashemzadeh, M.; Raeissi, K.; Ashrafizadeh, F.; Hakimizad, A.; Santamaria, M. Incorporation mechanism of colloidal TiO2 nanoparticles and their effect on properties of coatings grown on 7075 Al alloy from silicate-based solution using plasma electrolytic oxidation. Trans. Nonferrous Met. Soc. China 2024, 31, 3659–3676. [Google Scholar] [CrossRef]
- Zhu, M.; Song, Y.; Liu, Z.; Xu, D.; Dong, K.; Han, E.H. Optimization of thermal control and corrosion resistance of PEO coatings on 7075 aluminum alloy by frequency alteration. Surf. Coat. Technol. 2022, 446, 128797. [Google Scholar] [CrossRef]
- Muhaffel, F.; Baydogan, M.; Cimenoglu, H. A study to enhance the mechanical durability of the MAO coating fabricated on the 7075 Al alloy for wear-related high temperature applications. Surf. Coat. Technol. 2021, 409, 126843. [Google Scholar] [CrossRef]
- Shew-E Steel. Aluminum Alloy 7075. SHEW-E Steel Pipe Co., Ltd. Available online: https://ru.shew-esteelpipe.com/aluminum-alloys/aluminum-alloy-7075.html (accessed on 9 May 2025).
- ASTM G5-13; Standard Reference Test Method for Making Potentiostatic and Potentiodynamic Anodic Polarization Measurements. ASTM International: West Conshohocken, PA, USA, 2013.
- Cao, G.P.; Song, R.G. Microstructure and properties of ceramic coatings prepared by micro-arc oxidation on 7075 aluminum alloy. Mater. Res. Express 2018, 5, 026407. [Google Scholar] [CrossRef]
- Kushwaha, A.K.; Maccione, R.; John, M.; Lanka, S.; Misra, M.; Menezes, P.L. Influence of cryomilling on crystallite size of aluminum powder and spark plasma sintered component. Nanomaterials 2022, 12, 551. [Google Scholar] [CrossRef]
- Rodriguez, L.; Paris, J.Y.; Denape, J.; Delbé, K. Micro-arcs oxidation layer formation on aluminium and coatings tribological properties—A review. Coatings 2023, 13, 373. [Google Scholar] [CrossRef]
- Szkodo, M.; Stanisławska, A.; Komarov, A.; Bolewski, Ł. Effect of MAO coatings on cavitation erosion and tribological properties of 5056 and 7075 aluminum alloys. Wear 2021, 474, 203709. [Google Scholar] [CrossRef]
- Wang, C.; Chen, J.; He, J.; Jiang, J.; Zhang, Q. Effect of electrolyte concentration on the tribological performance of MAO coatings on aluminum alloys. Front. Chem. Sci. Eng. 2020, 14, 1065–1071. [Google Scholar] [CrossRef]
- Wang, S.; Gu, Y.; Geng, Y.; Liang, J.; Zhao, J.; Kang, J. Investigating local corrosion behavior and mechanism of MAO coated 7075 aluminum alloy. J. Alloys Compd. 2020, 826, 153976. [Google Scholar] [CrossRef]
- Gong, Y.; Geng, J.; Huang, J.; Chen, Z.; Wang, M.; Chen, D.; Wang, H. Self-healing performance and corrosion resistance of novel CeO2-sealed MAO film on aluminum alloy. Surf. Coat. Technol. 2021, 417, 127208. [Google Scholar] [CrossRef]
- Dai, W.; Zhang, C.; Yue, H.; Li, Q.; Guo, C.; Zhang, J.; Yang, X. A review on the fatigue performance of micro-arc oxidation coated Al alloys with micro-defects and residual stress. J. Mater. Res. Technol. 2023, 25, 4554–4581. [Google Scholar] [CrossRef]
- Liu, X.; Wang, C.; Lei, J.; Song, P.; Huang, T.; Zhang, X.; Ji, V. Micro-Arc Oxidation of Aluminum Alloys: Mechanism, Defects, and Corrosion Resistance. Adv. Eng. Mater. 2025, 27, 2402748. [Google Scholar] [CrossRef]
- Paidar, M.; Vignesh, R.V.; Khorram, A.; Ojo, O.O.; Rasoulpouraghdam, A.; Pustokhina, I. Dissimilar modified friction stir clinch- ing of AA2024-AA6061 aluminum alloys: Effects of materials positioning. J. Mater. Res. Technol. 2020, 9, 6037–6047. [Google Scholar] [CrossRef]
- Li, H.; Huang, Y.; Meng, Q.; Wang, X. Effect of electrical parameters on the structure and properties of aluminum foil microarc oxidation film. Metals 2024, 14, 1108. [Google Scholar] [CrossRef]
- Li, H.; Kong, S.; Liu, Z.; Wang, Z.; Geng, Y. Hardness distribution and growth behavior of micro-arc oxide ceramic film with positive and negative pulse coordination. Nanomaterials 2024, 14, 842. [Google Scholar] [CrossRef]
- Wang, J.; Xu, G.; Jia, E.; Zhao, Z.; Wang, Z.; Lu, H. Preparation of wear-resistant ceramic coating by dynamically controlling the resistance during MAO. Int. J. Appl. Ceram. Technol. 2024, 21, 876–887. [Google Scholar] [CrossRef]
- Astuti, N.H.; Wibowo, N.A.; Ayub, M. The porosity calculation of various types of paper using image analysis. J. Pendidik. Fis. Indones. 2018, 14, 46–51. [Google Scholar] [CrossRef]
- Fernández-Hernán, J.P.; Campos, G.G.; García, A.G.; Rodríguez, J.R.; Mendoza, A.A.; Martínez, R.P. Anticorrosion and cyto- compatibility assessment of graphene-doped hybrid silica and plasma electrolytic oxidation coatings for biomedical applications. ACS Biomater. Sci. Eng. 2021, 7, 5861–5877. [Google Scholar] [CrossRef]
- Venugopal, A.; Panda, R.; Manwatkar, S.; Sreekumar, K.; Krishna, L.R.; Sundararajan, G. Effect of micro arc oxidation treatment on localized corrosion behaviour of AA7075 aluminum alloy in 3.5% NaCl solution. Trans. Nonferrous Met. Soc. China 2012, 22, 700–710. [Google Scholar] [CrossRef]
- Zhou, S.; Tong, R.; Li, H.; Tao, X.; Chen, J. Effects of Current Output Modes on Corrosion Resistance of Micro-Arc Oxidation Black Coatings on Aluminum Alloy. Materials 2025, 18, 2949. [Google Scholar] [CrossRef]

| Cr | Cu | Fe | Mg | Mn | Si | Ti | Zn | Other Elements | Al |
|---|---|---|---|---|---|---|---|---|---|
| 0.18–0.28 | 1.2–2.0 | <0.50 | 2.10–2.90 | <0.30 | <0.40 | <0.20 | 5.1–6.1 | <0.15 | balance |
| Sample | Voltage, V | Frequency, Hz | Duty Cycle, % | Time, s | Pulse Duration, µs | Current Density, A/dm2 |
|---|---|---|---|---|---|---|
| No. 1 | 250 | 500 | 30 | 600 | 100 | 9 |
| No. 2 | 300 | 500 | 30 | 600 | 100 | 14 |
| No. 3 | 350 | 500 | 30 | 600 | 100 | 18 |
| Sample | −Ecorr (mV) | Icorr (A/cm2) | Vcorr (mm/a) | Tafel Slopes (mV) | |
|---|---|---|---|---|---|
| ba | bc | ||||
| Initial | 149.79 | 6.495 × 10−6 | 0.055186 | 121.51 | 2704.8 |
| No. 1 | 171.02 | 1.1356 × 10−7 | 0.00096493 | 321.7 | 61.856 |
| No. 2 | 151.02 | 9.186 × 10−0.8 | 0.00078051 | 1435.7 | 59.534 |
| No. 3 | 138.05 | 8.6833 × 10−0.8 | 0.0007378 | 104.27 | 56.784 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satbayeva, Z.; Zhassulan, A.; Rakhadilov, B.; Shynarbek, A.; Ormanbekov, K.; Leonidova, A. Effect of Micro-Arc Oxidation Voltage on the Surface Morphology and Properties of Ceramic Coatings on 7075 Aluminum Alloy. Metals 2025, 15, 746. https://doi.org/10.3390/met15070746
Satbayeva Z, Zhassulan A, Rakhadilov B, Shynarbek A, Ormanbekov K, Leonidova A. Effect of Micro-Arc Oxidation Voltage on the Surface Morphology and Properties of Ceramic Coatings on 7075 Aluminum Alloy. Metals. 2025; 15(7):746. https://doi.org/10.3390/met15070746
Chicago/Turabian StyleSatbayeva, Zarina, Ainur Zhassulan, Bauyrzhan Rakhadilov, Aibek Shynarbek, Kuanysh Ormanbekov, and Aiym Leonidova. 2025. "Effect of Micro-Arc Oxidation Voltage on the Surface Morphology and Properties of Ceramic Coatings on 7075 Aluminum Alloy" Metals 15, no. 7: 746. https://doi.org/10.3390/met15070746
APA StyleSatbayeva, Z., Zhassulan, A., Rakhadilov, B., Shynarbek, A., Ormanbekov, K., & Leonidova, A. (2025). Effect of Micro-Arc Oxidation Voltage on the Surface Morphology and Properties of Ceramic Coatings on 7075 Aluminum Alloy. Metals, 15(7), 746. https://doi.org/10.3390/met15070746






