Crystallization of Calcium Sulfate for Mining Wastewater Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
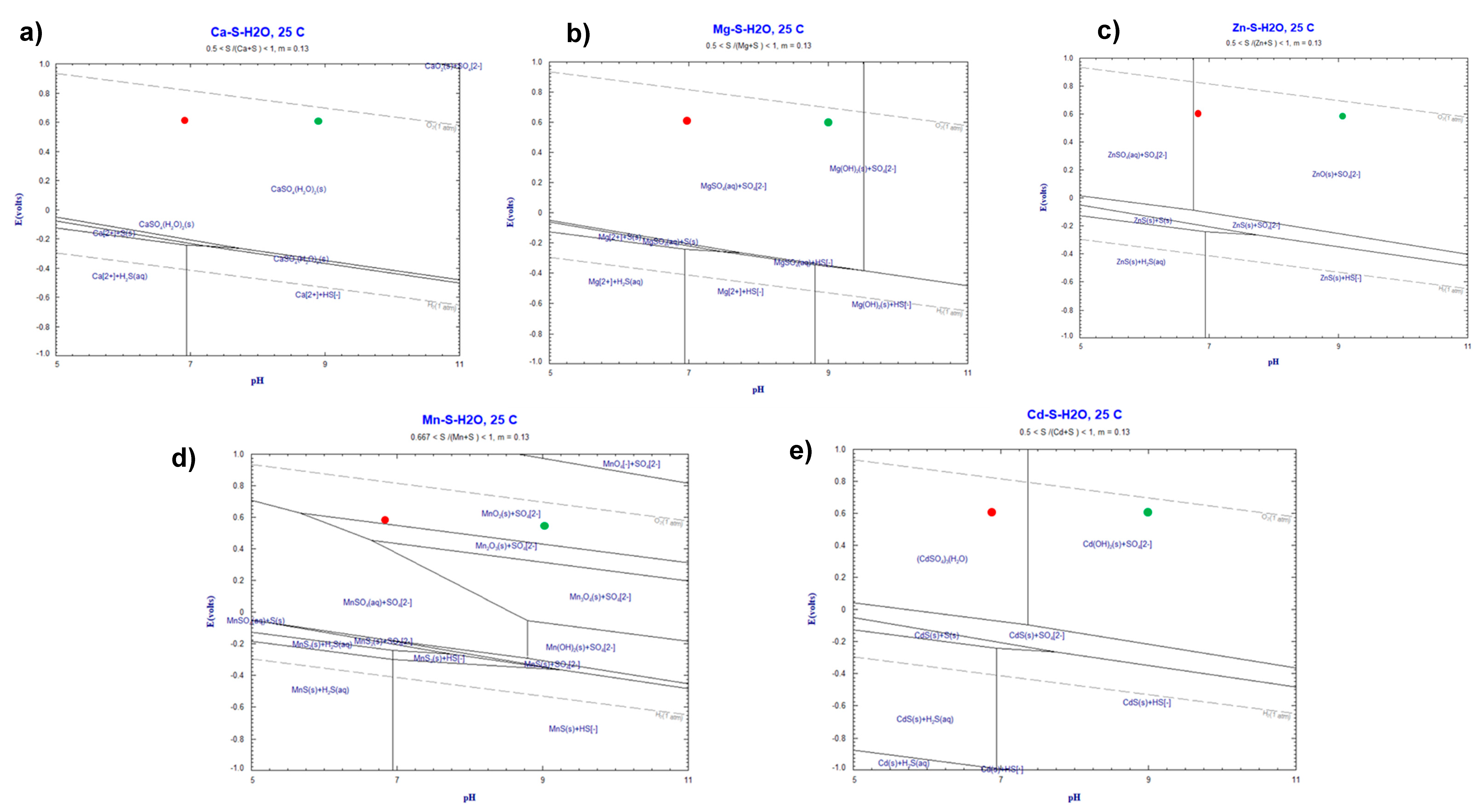
2.2.1. Thermodynamic Simulations
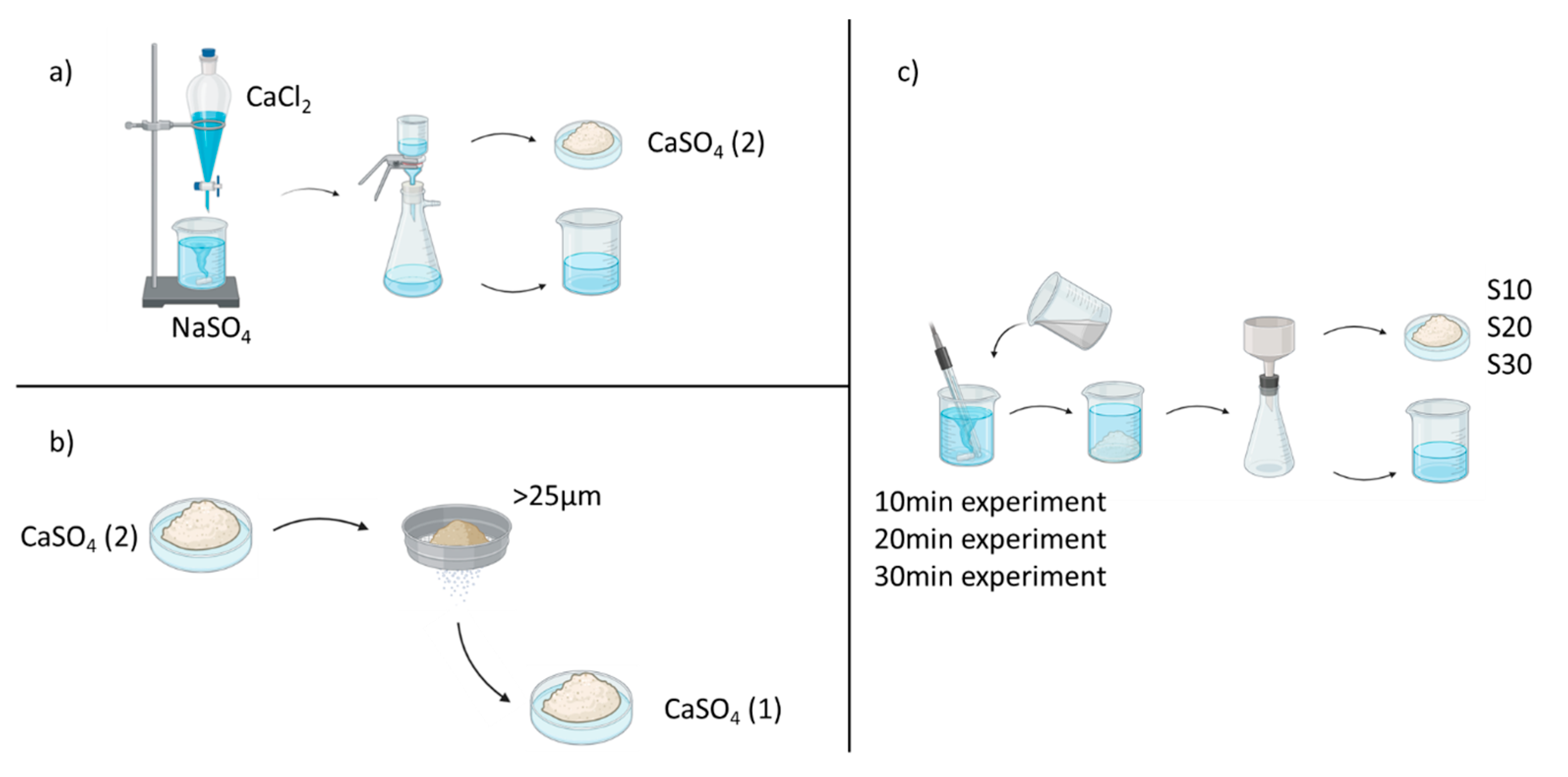
2.2.2. Precipitation Experiments
2.2.3. Decantation Experiments
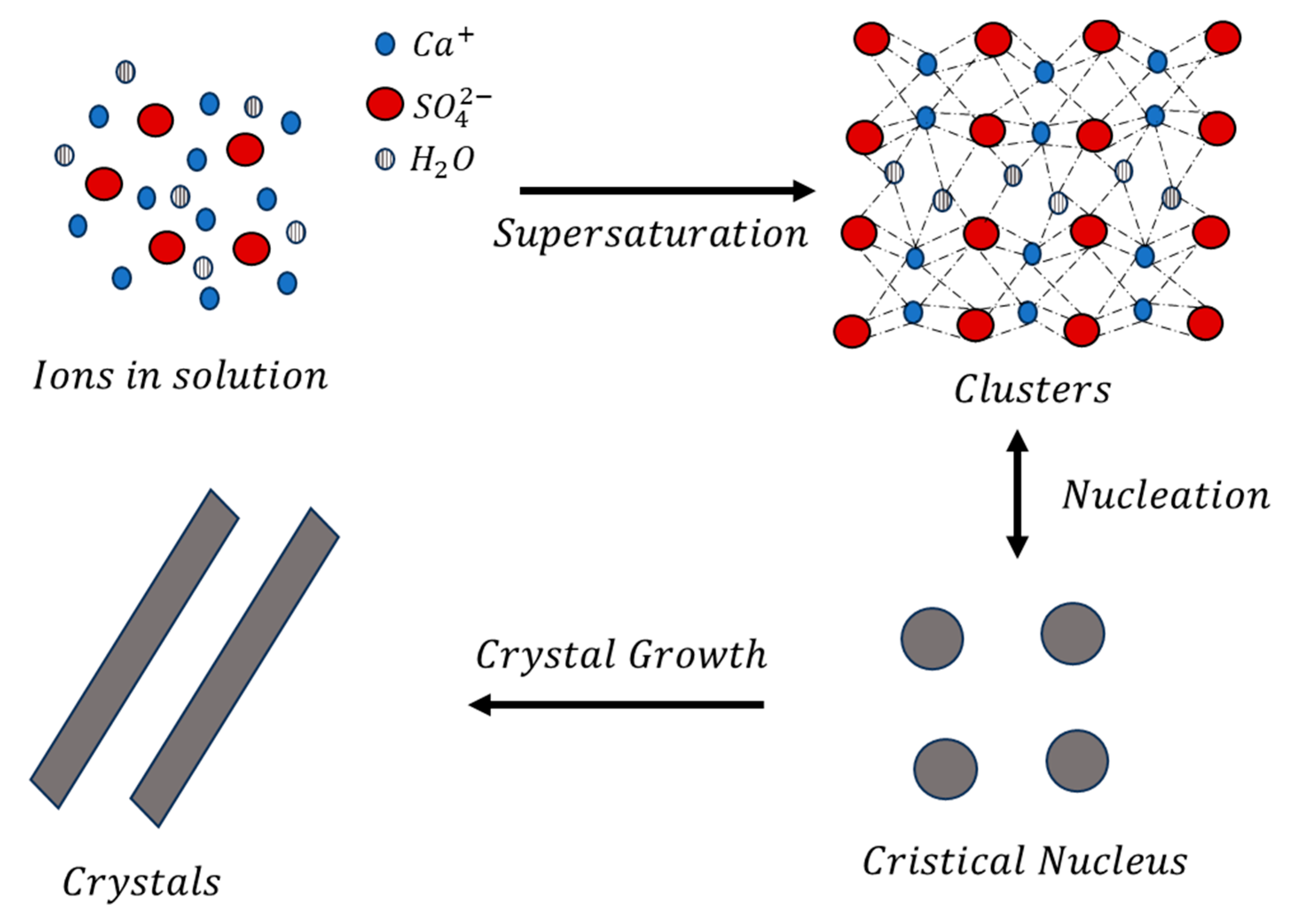
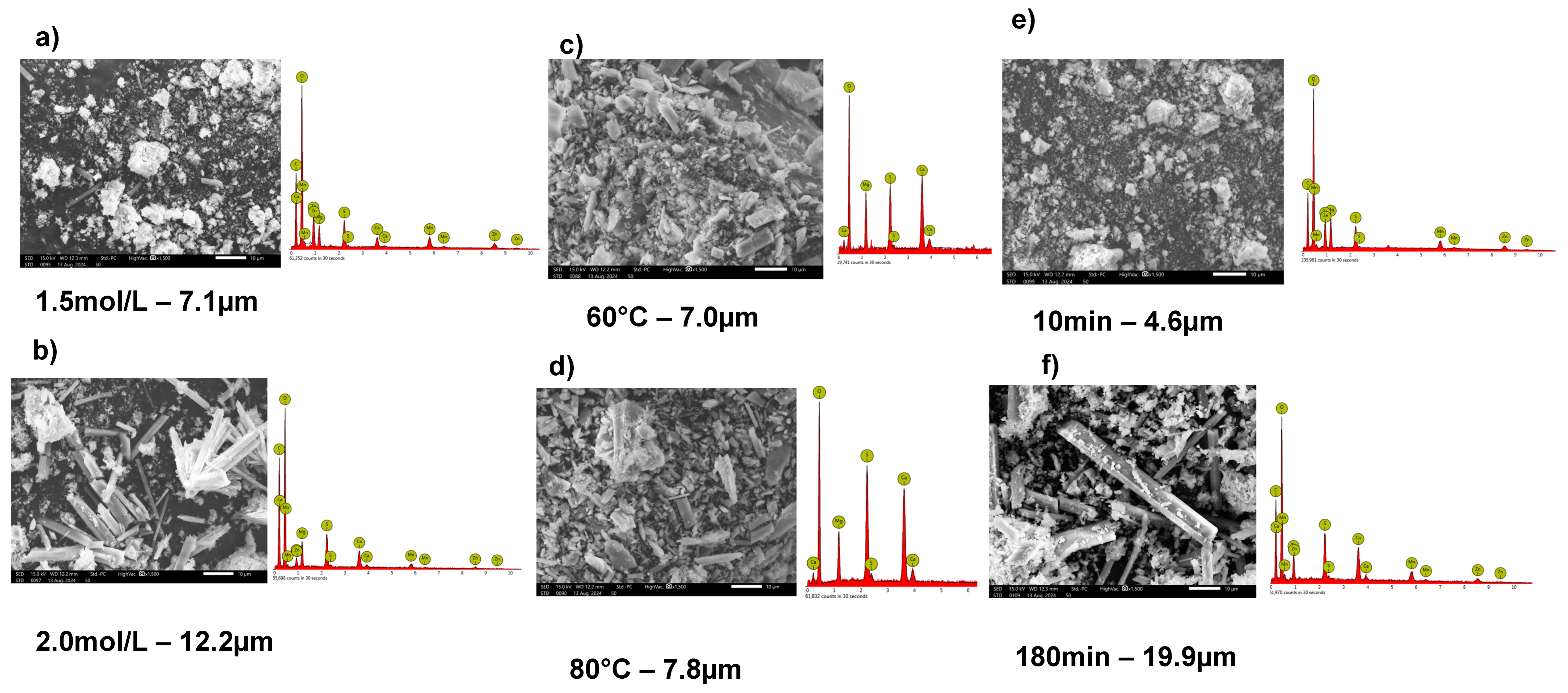
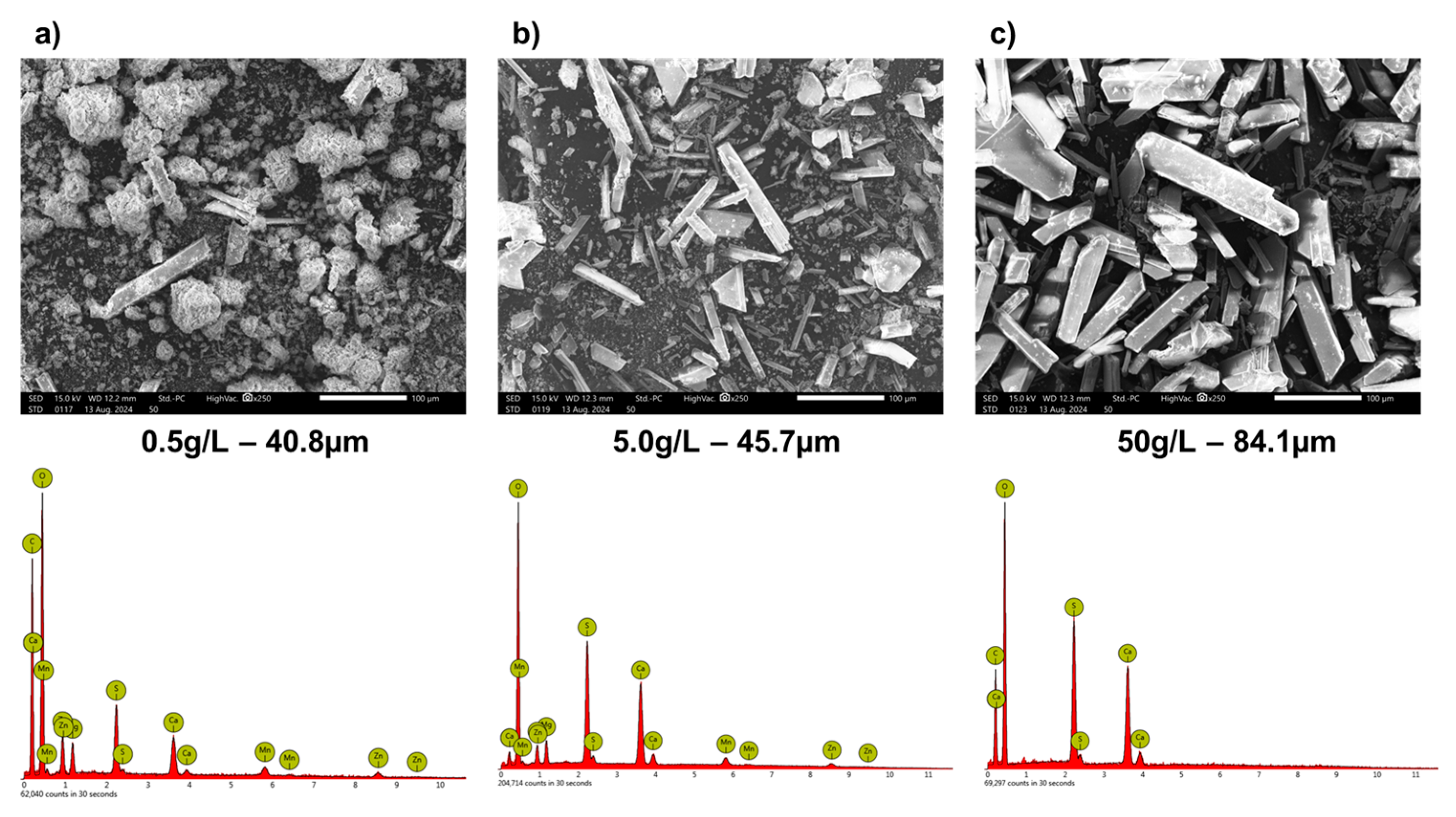
3. Results and Discussion
3.1. Solution Characterization and Thermodynamic Simulations
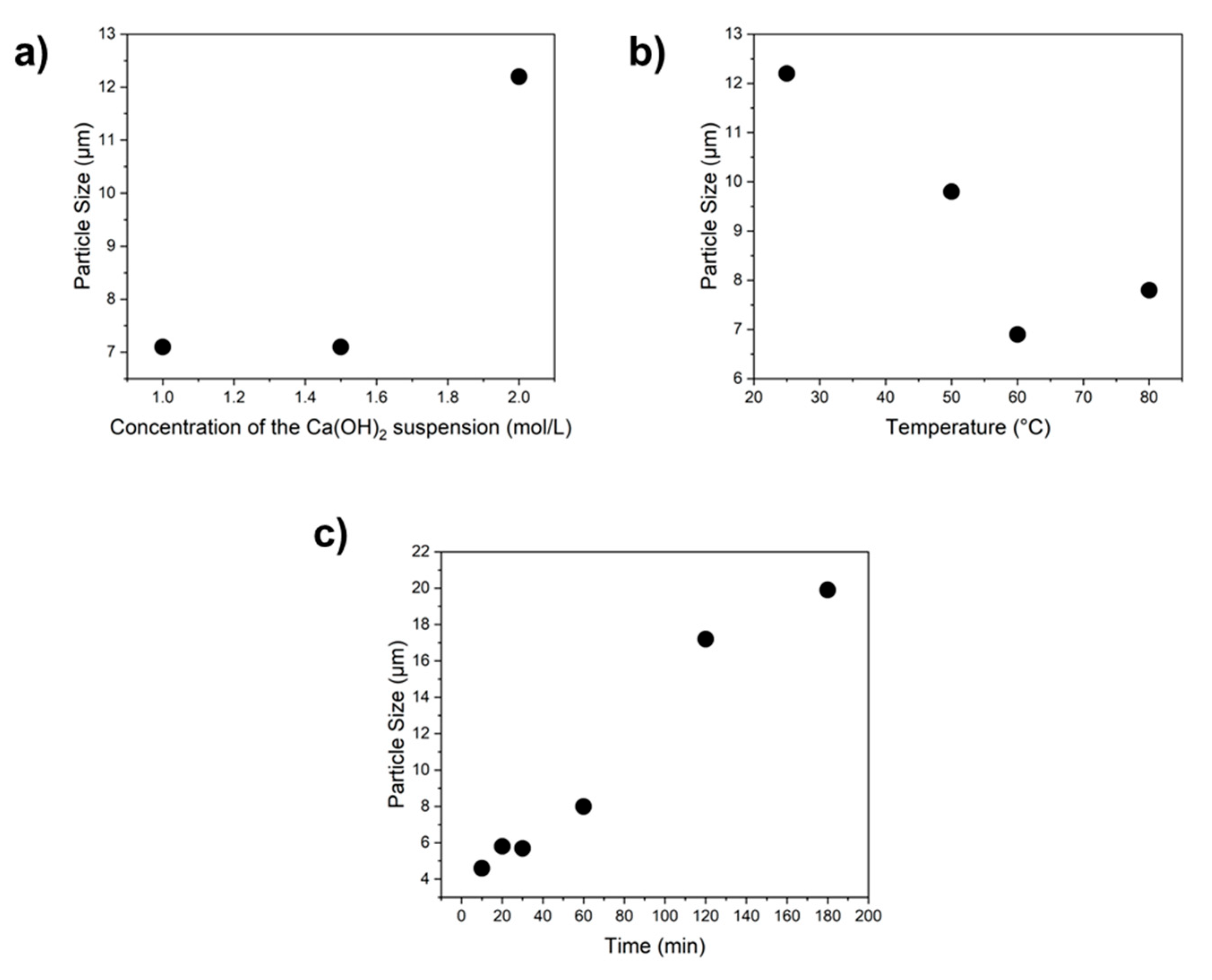
3.2. Precipitation Experiments Without Seeds
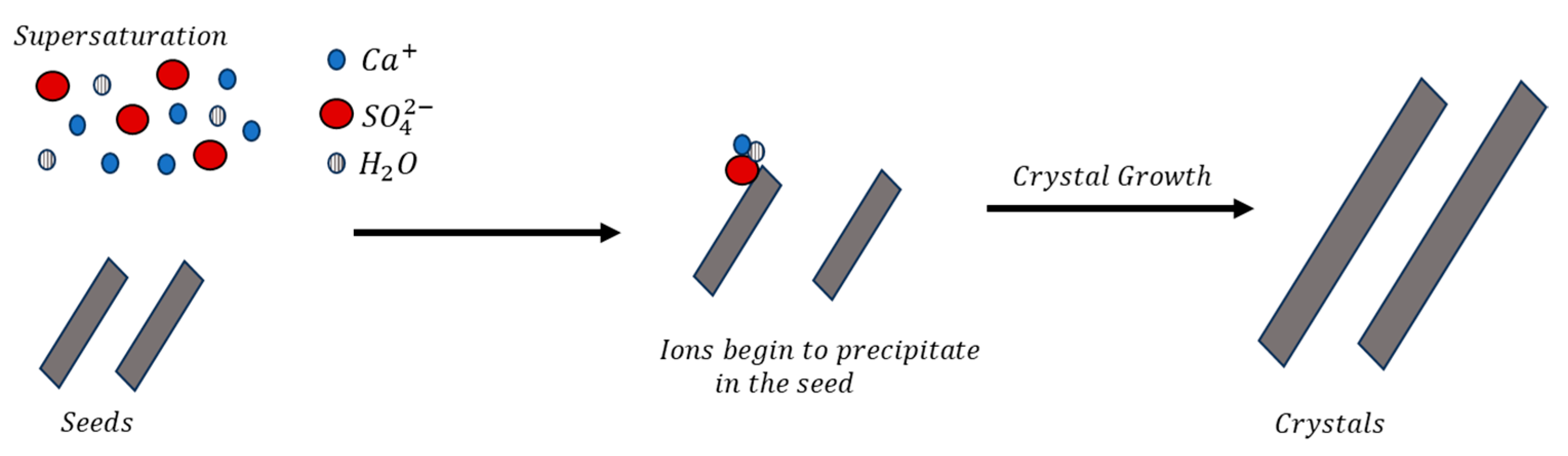
3.3. Precipitation Experiments with Seeds
3.4. Decantation Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lewis, A.; Seckler, M.; Kramer, H.; van Rosmalen, G. Industrial Crystallization: Fundamentals and Applications, 1st ed.; Cambridge University Press: Cambridge, UK, 2015; pp. 4–19, 71–128. [Google Scholar]
- Mullin, J.W. Crystallization, 4th ed.; ButterWorth Heinemann: Oxford, UK, 2001; pp. 181–214. [Google Scholar]
- He, Y.; Gao, Z.; Zhang, T.; Sun, J.; Ma, Y.; Tian, N.; Gong, J. Seeding Techniques and Optimization of Solution Crystallization Processes. Org. Process Res. Dev. 2020, 20, 1839–1849. [Google Scholar] [CrossRef]
- Shang, Y.; Wang, Y.; Huang, J.; Shi, J.; Xu, L.; Li, K.; Jin, P.; Jin, X. A novel strategy for simultaneous removal of hardness, suspended solids and organics from fracturing wastewater: Efficiency, mechanism and application. Sep. Purif. Technol. 2025, 366, 132735. [Google Scholar] [CrossRef]
- Reichl, C.; Schatz, M. World Mining Data 2025; International Organizing Committee for the World Mining Congress: Viena, Austria, 2025. [Google Scholar]
- Talebi, M.; Rezaei, A.; Rafiei, Y. Application of sodium carbonate and sodium sulfate for removal of lithium and strontium from oilfield produced water. Sci. Rep. 2025, 15, 18895. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Shang, Y.; Xu, Y.; Shen, L.; Wang, Y.; Liu, Y.; Zhang, G.; Mei, A.; Liu, H.; Jin, P. Study on the nucleation crystallization pelleting process for manganese ion recovery from hydrometallurgical tailings water. Front. Environ. Sci. Eng. 2025, 19, 98. [Google Scholar] [CrossRef]
- Yang, J.; Chen, L.; Yang, L.; Jiang, X.; Deng, L.; Tang, X.; Wang, W. Organic matter removal mitigates crystallization inhibition to enhance co-recovery of potassium and phosphorus from wastewater. Enrivon. Res. 2025, 279, 121758. [Google Scholar] [CrossRef] [PubMed]
- Jorge, N.; Amor, C.; Teixeira, A.R.; Marchão, L.; Lucas, M.S.; Peres, J.A. Combination of Coagulation-Flocculation-Decantation with Sulfate Radicals for Agro-Industrial Wastewater Treatment. Eng. Proc. 2022, 19, 19. [Google Scholar]
- Alimi, F.; Elfil, H.; Gadri, A. Kinetics of the precipitation of calcium sulfate dihydrate in a desalination unit. Desalination 2003, 158, 9–16. [Google Scholar] [CrossRef]
- Magomedzapir, S.; Ali, K.; Artigat, B.; Zarima, G.; Tamila, M. The disinfecting properties of Penox-1 solutions for sanitation of objects of veterinary supervision. E3S Web Conf. 2020, 175, 03012. [Google Scholar]
- Chagwedera, T.M.; Chivavava, J.; Lewis, A.E. Gypsum Seeding to Prevent Scaling. Crystals 2022, 12, 342. [Google Scholar] [CrossRef]
- Brough, D.; Jouhara, H. The aluminium industry: A review on state-of-the-art technologies, environmental impacts and possibilities for waste heat recovery. Int. J. Thermofluids 2020, 1–2, 100007. [Google Scholar] [CrossRef]
- Chaves, A.P. Teoria e Prática do Tratamento de Minérios: Desaguamento, Espessamento e Filtragem, 3rd ed.; Signus: Rio de Janeiro, Brasil, 2010; pp. 50–85. [Google Scholar]
- Mondillo, N.; Accardo, M.; Boni, M.; Boyce, A.; Herrington, R.; Rumsey, M.; Wilkinson, C. New insights into the genesis of willemite (Zn2SiO4) from zinc nonsulfide deposits, through trace elements and oxygen isotope geochemistry. Ore Geol. Rev. 2020, 118, 103307. [Google Scholar] [CrossRef]
- Xu, H.; Qian, Y.; Zhou, Q.; Wei, C.; Wang, Q.; Zhao, W.; Zhu, B.; Tong, F.; Ren, F.; Zhang, M.; et al. Leaching of Willemite Concentrate in Sulfuric Acid Solution at High Temperature. Sustainability 2023, 15, 161. [Google Scholar] [CrossRef]
- Olivo, G.R.; Monteiro, L.V.; Baia, F.; Slezak, P.; Carvalho, I.; Fernandes, N.A.; Oliveira, G.D.; Botura Neto, B.; McGladrey, A.; Silva, A.M.; et al. The Proterozoic Vazante hypogene zinc silicate district, Minas Gerais, Brazil: A review of the ore system applied to mineral exploration. Minerals 2018, 8, 22. [Google Scholar] [CrossRef]
- Abdel-Aal, E.A.; Rashad, M.M.; El-Shall, H. Crystallization of calcium sulfate dihydrate at different supersaturation ratios and different free sulfate concentrations. Cryst. Res. Technol. 2004, 39, 313–321. [Google Scholar] [CrossRef]
- Kumar, S.; Naiya, T.K.; Kumar, T. Developments in oilfield scale handling towards green technology-A review. J. Pet. Sci. Eng. 2018, 169, 428–444. [Google Scholar] [CrossRef]
- Bahrig, L.; Hickey, S.G.; Eychmüller, A. Mesocrystalline materials and the involvement of oriented attachment—A review. CrystEngComm 2014, 16, 9408–9424. [Google Scholar] [CrossRef]
- Luo, K.; Li, C.; Xiang, L.; Li, H.; Ning, P. Influence of temperature and solution composition on the formation of calcium sulfates. Particuology 2010, 8, 240–244. [Google Scholar] [CrossRef]
- Lochhead, R.Y. Basic Physical Sciences for the Formulation of Cosmetic Products. In Cosmetic Science and Technology: Theoretical Principles and Applications, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 39–76. [Google Scholar]
- Sugimoto, T. Recrystallization. In Monodispersed Particles, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 167–179. [Google Scholar]
- Moran, S. Fluid mechanics. In An Applied Guide to Water and Effluent Treatment Plant Design, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 53–58. [Google Scholar]
- Bratby, J. Coagulation and Flocculation in Water and Wastewater Treatment, 3rd ed.; IWA Publishing: London, UK, 2016. [Google Scholar]

| Reagent (Commercial Name) | Characteristic | Proportion |
|---|---|---|
| F1 (Floticor AD17797) | Polymer coagulant | 0.05% m/m |
| F2 (Floticor AD17097) | Polymer coagulant | 0.05% m/m |
| F3 (Floticor AD18011) | Mineral coagulant | 0.05% m/m |
| F4 (Floticor FL7130) | Anionic flocculant | 0.01% m/m |
| SO42− | Ca | Cd | Mg | Mn | Zn |
|---|---|---|---|---|---|
| 21.8 g/L | 526 mg/L | 2.9 mg/L | 4.7 g/L | 332 mg/L | 206 mg/L |
| Species/Compounds | Concentration (mg/L) |
|---|---|
| CaSO4 | 947 |
| Ca2+ | 263 |
| Cd(SO4)22− | 3.7 |
| CdSO4 | 1.6 |
| Cd2+ | 0.7 |
| MgSO4 | 11,291 |
| Mg2+ | 2584 |
| MnSO4 | 452 |
| Mn2+ | 178 |
| SO42− | 12,536 |
| Zn2+ | 206 |
| Compound | Supersaturation |
|---|---|
| CaSO4 | −0.03 |
| CaSO4·2H2O | 0.14 |
| CdO | −6.99 |
| Cd(OH)2 | −5.75 |
| MgO | −9.26 |
| Mg(OH)2 | −4.78 |
| MnO | −7.09 |
| Mn(OH)2 | −4.49 |
| Seed | Seed Mean Size | Particle Size Achieved |
|---|---|---|
| S10 | 4.6 µm | 8.5 µm |
| S20 | 5.8 µm | 9.0 µm |
| S30 | 5.7 µm | 8.0 µm |
| CaSO4 (1) | 5.6 µm | 23.4 µm |
| CaSO4 (2) | 39.0 µm | 40.8 µm |
| Reagent | Average Speed | Increase in Average Speed % |
|---|---|---|
| - | 50 mL/min | - |
| Seeds 10 g/L | 110 mL/min | 120% |
| F1 0.05% m/m | 120 mL/min | 140% |
| F2 0.05% m/m | 140 mL/min | 180% |
| F3 0.05% m/m | 160 mL/min | 220% |
| F4 0.01% m/m | 166 mL/min | 232% |
| Seeds 10 g/L and F4 0.01% m/m | 177 mL/min | 254% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamengo, F.G.G.; Botelho Junior, A.B.; Seckler, M.M.; Espinosa, D.C.R.; Tenório, J.A.S. Crystallization of Calcium Sulfate for Mining Wastewater Treatment. Metals 2025, 15, 710. https://doi.org/10.3390/met15070710
Zamengo FGG, Botelho Junior AB, Seckler MM, Espinosa DCR, Tenório JAS. Crystallization of Calcium Sulfate for Mining Wastewater Treatment. Metals. 2025; 15(7):710. https://doi.org/10.3390/met15070710
Chicago/Turabian StyleZamengo, Fernanda Gusman Garreta, Amilton Barbosa Botelho Junior, Marcelo Martins Seckler, Denise Crocce Romano Espinosa, and Jorge Alberto Soares Tenório. 2025. "Crystallization of Calcium Sulfate for Mining Wastewater Treatment" Metals 15, no. 7: 710. https://doi.org/10.3390/met15070710
APA StyleZamengo, F. G. G., Botelho Junior, A. B., Seckler, M. M., Espinosa, D. C. R., & Tenório, J. A. S. (2025). Crystallization of Calcium Sulfate for Mining Wastewater Treatment. Metals, 15(7), 710. https://doi.org/10.3390/met15070710









