Effects of Post-Treatment on the Microstructure Evolution and High-Temperature Oxidation Properties of Nickel-Based Superalloys Fabricated by Selective Laser Melting
Abstract
1. Introduction
2. Experimental Procedure
2.1. SLM Process
2.2. Heat Treatment
2.3. Residual Stress Test
2.4. Microstructure Characterization
2.5. High-Temperature Oxidation Test
3. Results
3.1. Residual Stress Analysis
3.2. Microstructural Analysis
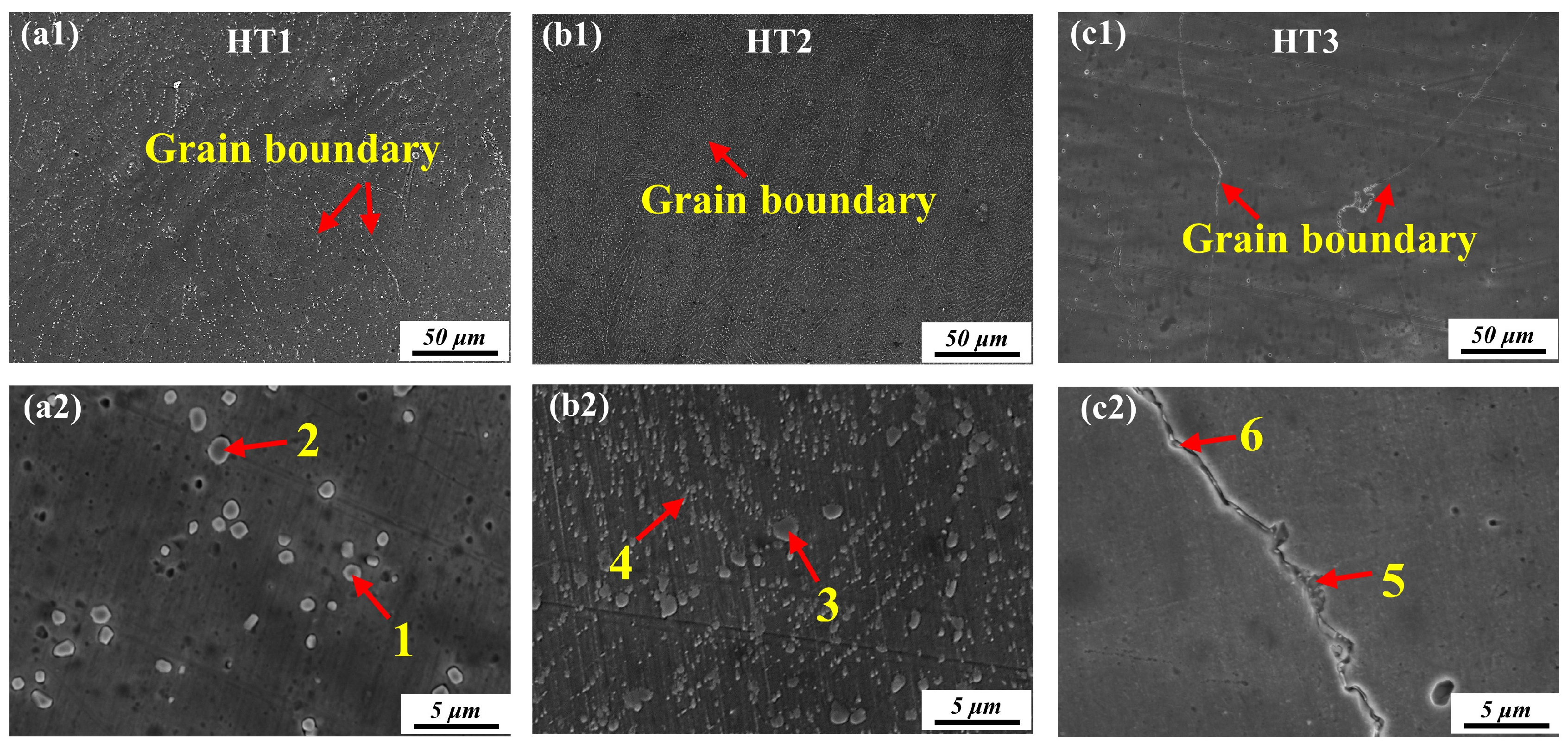
3.2.1. Carbide Precipitation
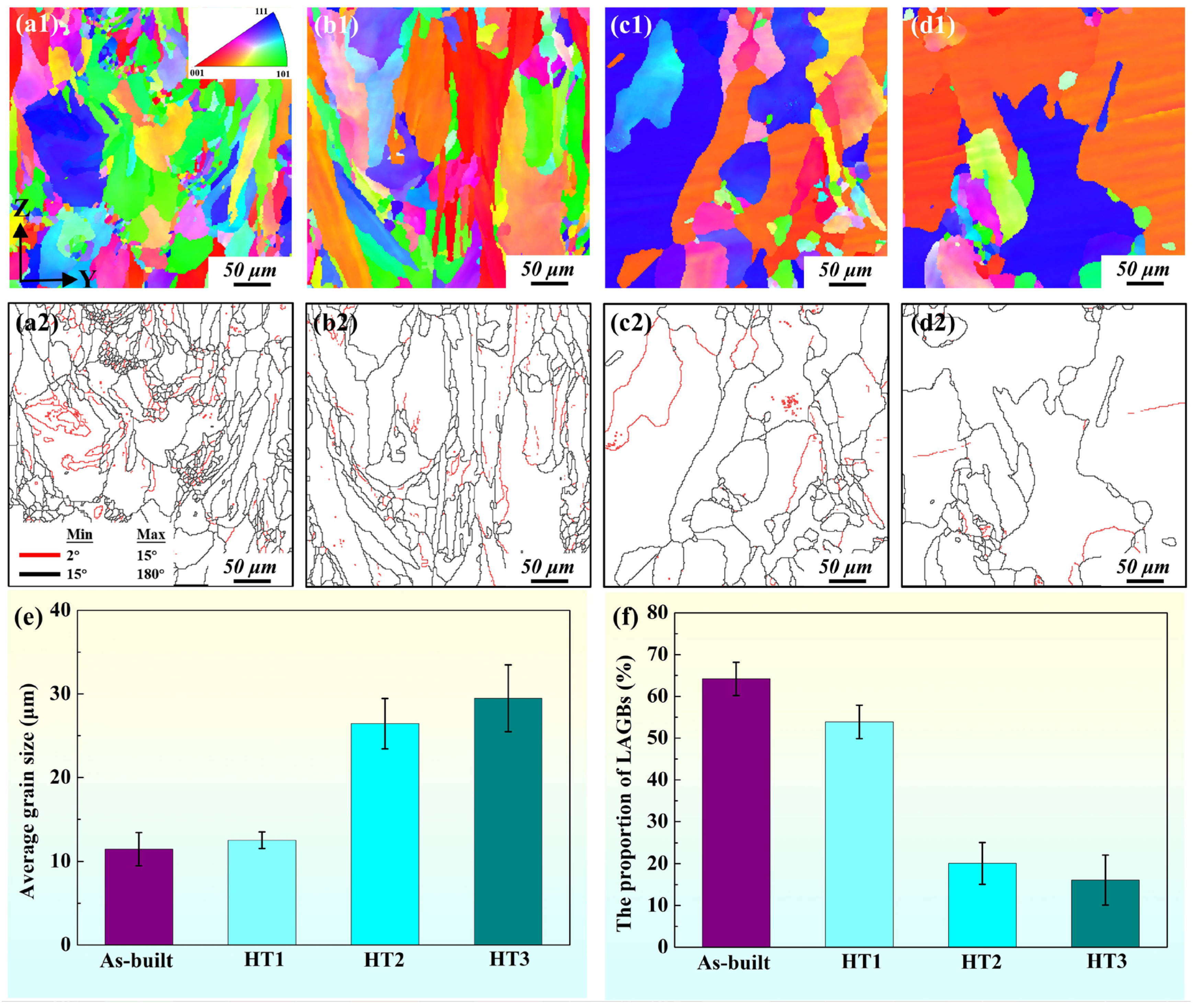
3.2.2. Microstructure
3.3. High-Temperature Oxidation Performance Analysis
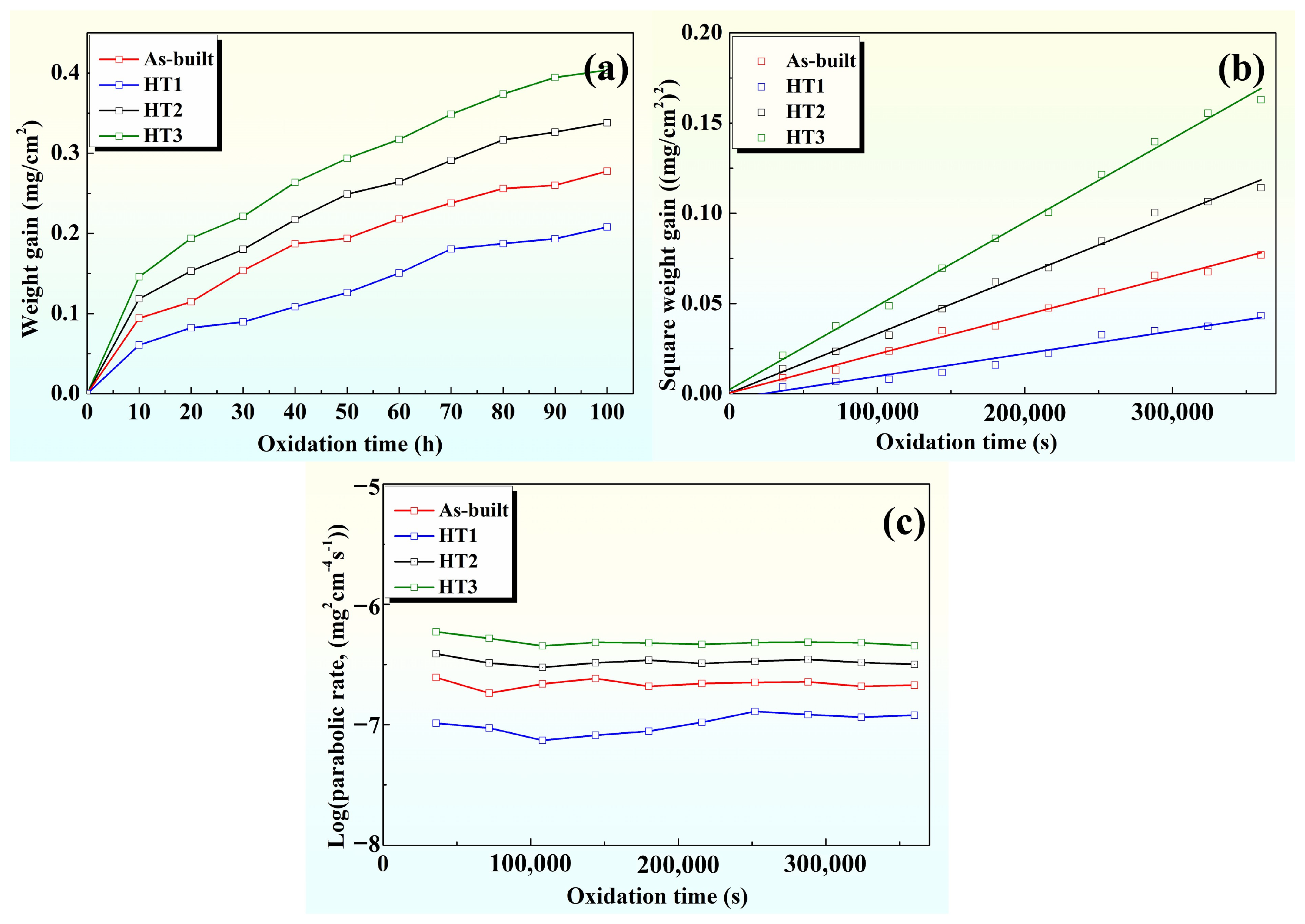
3.3.1. High-Temperature Oxidation Kinetics
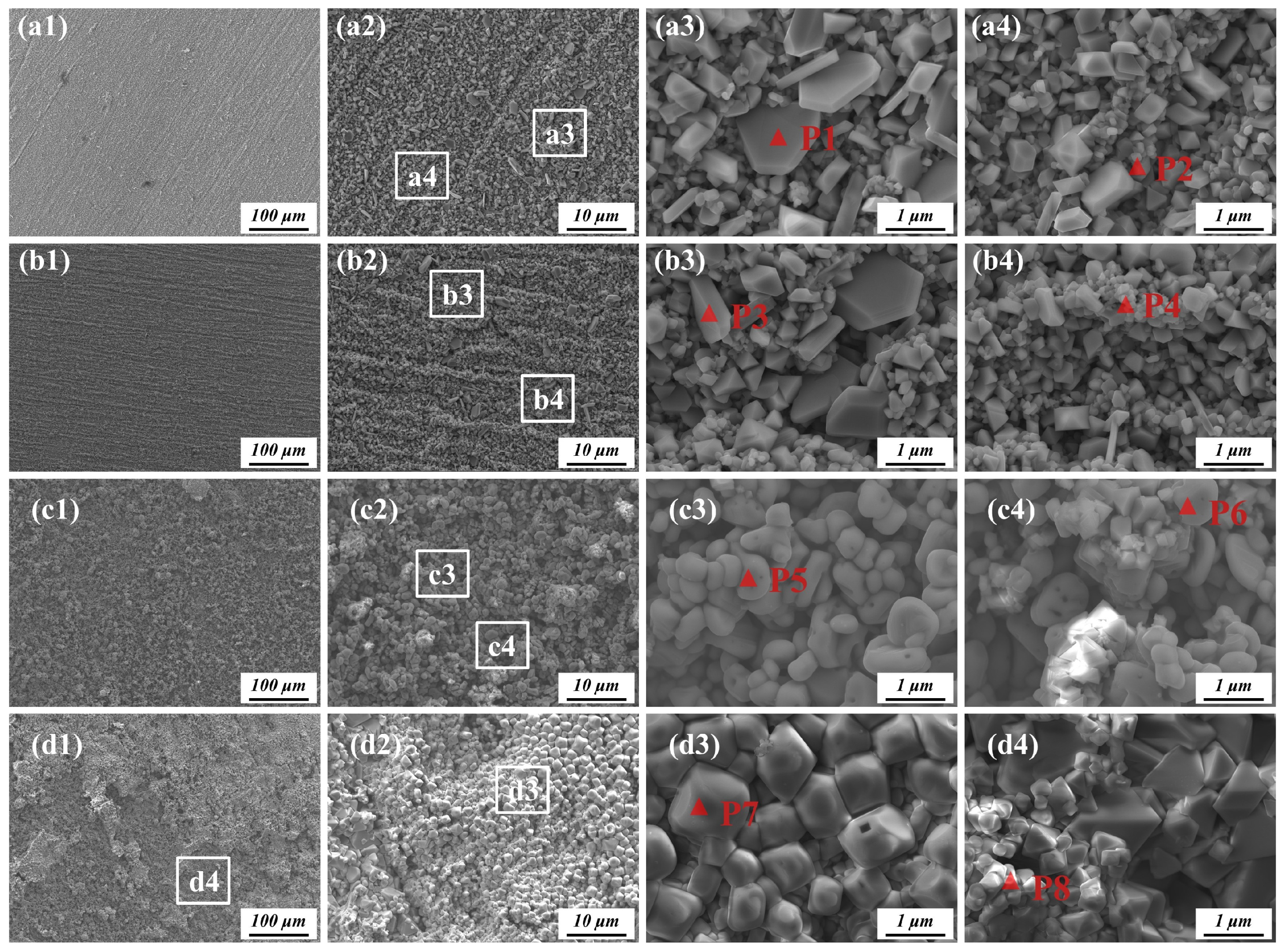
3.3.2. Surface Oxidation Products
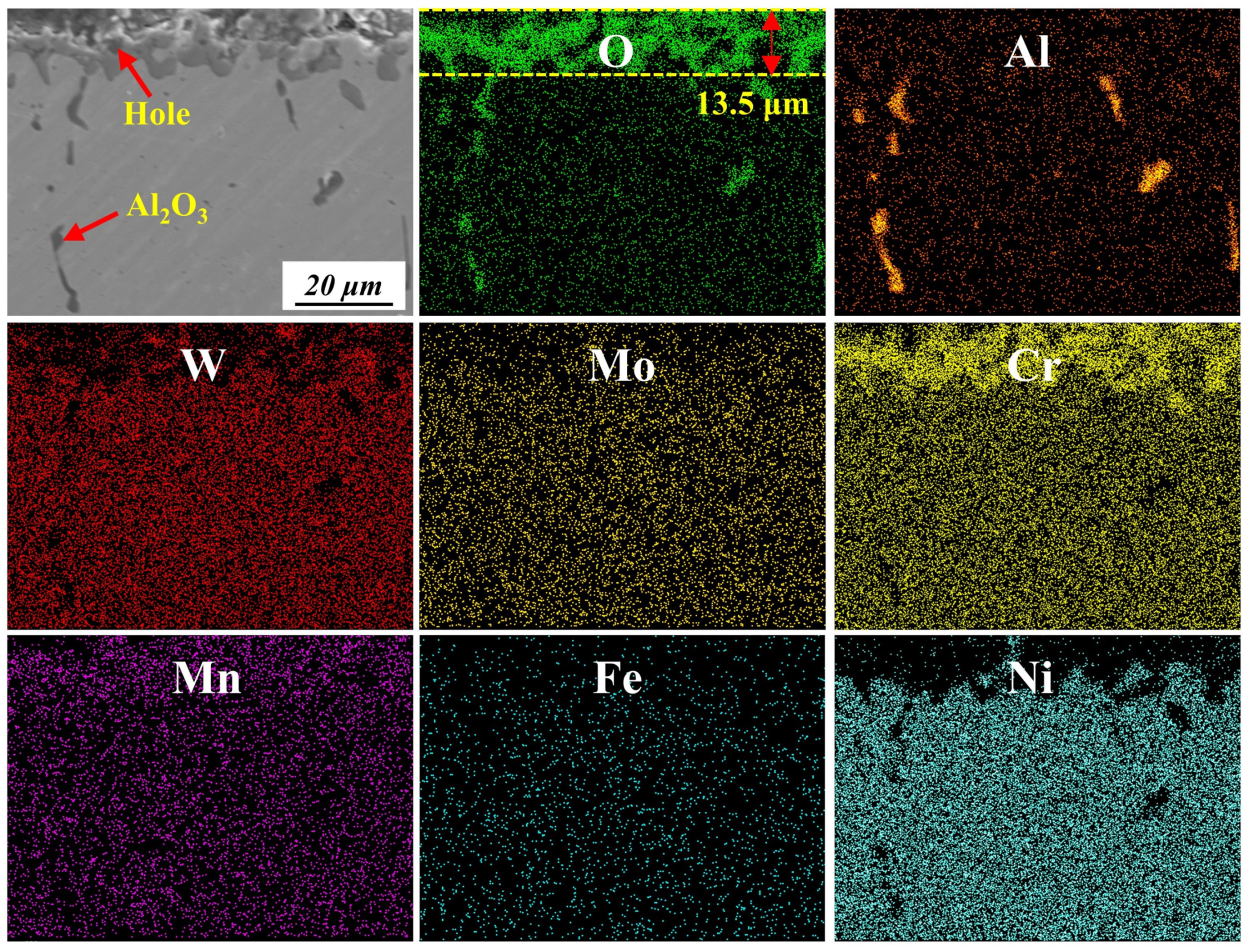
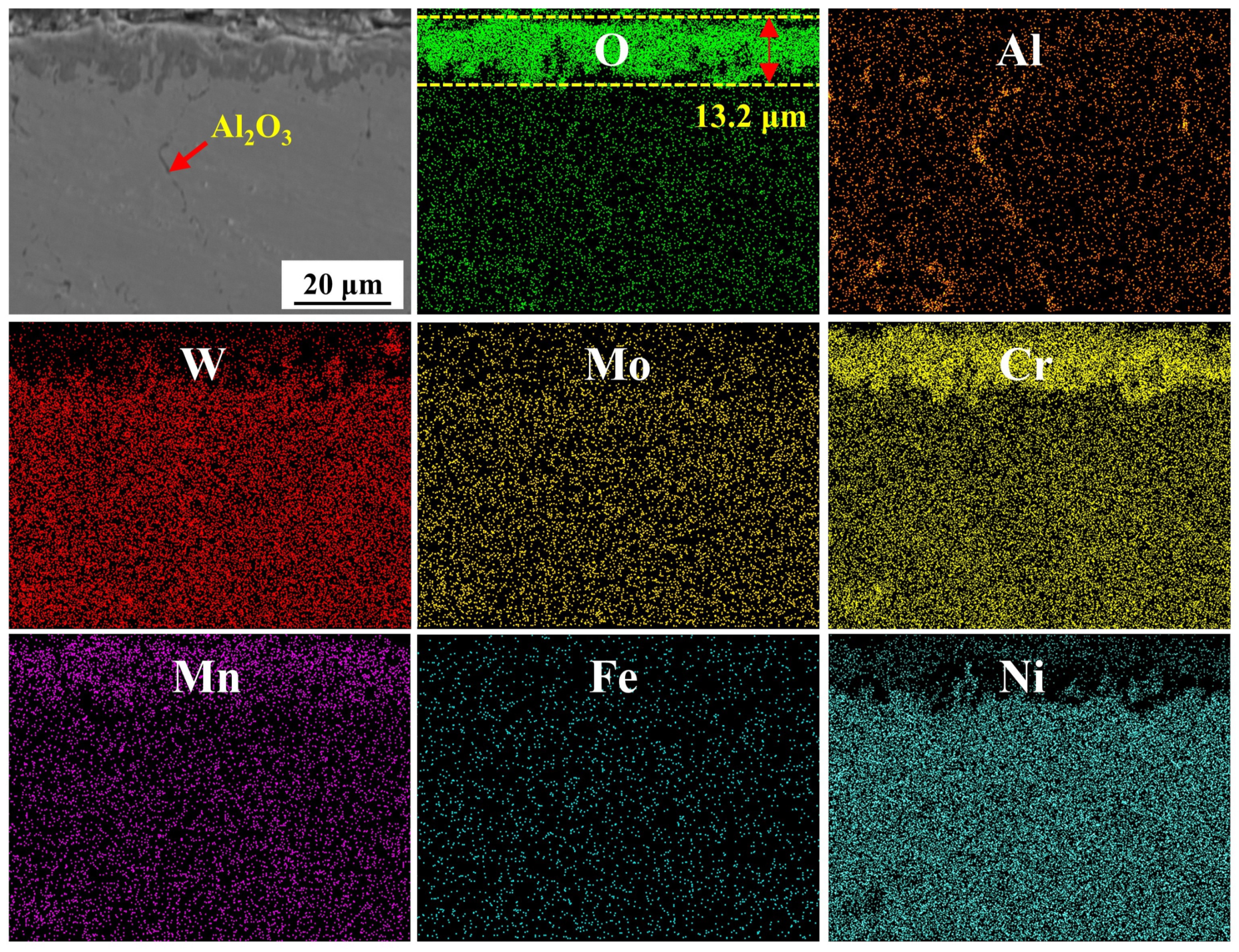
3.3.3. Cross-Sections of Oxidation Products
4. Discussion
4.1. The Influence of Residual Stress and Grain Features on High-Temperature Oxidation
4.2. The Influence of Carbide Precipitation on High-Temperature Oxidation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, N.; Parthasarathy, M.; Patil, S.; Coates, D.; Myers, K.; Zhu, H.; Li, W. Direct additive manufacturing of metal parts for automotive applications. J. Manuf. Syst. 2023, 68, 368–375. [Google Scholar] [CrossRef]
- Kirchhöfer, H.; Schubert, F.; Nickel, H. Precipitation behavior of Ni–Cr–22 Fe–18 Mo (Hastelloy-X) and Ni-Cr-22 Co-12 Mo (Inconel-617) after isothermal aging. Nucl. Technol. 1984, 66, 139–148. [Google Scholar] [CrossRef]
- McNeff, P.S.; Paul, B.K. Electroplasticity effects in Haynes 230. J. Alloy. Compd. 2020, 829, 154438. [Google Scholar] [CrossRef]
- Boehlert, C.J.; Longanbach, S.C. A comparison of the microstructure and creep behavior of cold rolled HAYNES® 230 alloy™ and HAYNES® 282 alloy™. Mater. Sci. Eng. A 2011, 528, 4888–4898. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, R.; Li, J.; Kou, H.; Li, H.; Chang, H.; Fu, H. Characterization of hot deformation behavior of Haynes230 by using processing maps. J. Mater. Process. Technol. 2009, 209, 4020–4026. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, D.; Zhu, X. Study of mechanical properties and fracture mechanism of a new high-temperature alloys. Appl. Mech. Mater. 2012, 271–272, 64–68. [Google Scholar] [CrossRef]
- Rothman, M.F.; Patriarca, C.R. New materials for fabricated gas turbine hot section components. Aircr. Eng. Aerosp. Tec. 1987, 62, 2–7. [Google Scholar] [CrossRef]
- Dutta, B.; Froes, F.H. The additive manufacturing (AM) of titanium alloys. Met. Powder Rep. 2017, 72, 96–106. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.; Yu, T.; Zhang, X.; Ren, X. Effects of ultrasonic shot peening process on the microstructure and mechanical properties of nickel-based superalloys formed by selective laser melting. J. Mater. Process. Technol. 2025, 335, 118667. [Google Scholar] [CrossRef]
- Chen, L.; Xie, J.; Li, Y.; Yu, G.; Zhang, X.; Ren, X. Effect of laser/ultrasonic shock peening on the microstructure and mechanical properties of nickel-based superalloys prepared by Powder Bed Fusion Laser Beam (PBF-LB). J. Mater. Res. Technol. 2024, 33, 7668–7680. [Google Scholar] [CrossRef]
- Chen, L.; Yu, G.; Zhang, X.; Zhu, L.; Li, Y.; Ren, X. Effect of heat treatment on the microstructural evolution and mechanical property of nickel-based superalloy K447A manufactured by Powder Bed Fusion Laser Beam (PBF-LB). Mat. Sci. Eng. A 2024, 910, 146874. [Google Scholar] [CrossRef]
- Idell, Y.; Levine, L.E.; Allen, A.J.; Zhang, F.; Campbell, C.E.; Olson, G.B.; Gong, J.; Snyder, D.R.; Deutchman, H.Z. Unexpected δ-phase formation in additive-manufactured Ni-Based superalloy. JOM 2016, 68, 950–959. [Google Scholar] [CrossRef]
- Jägle, E.A.; Sheng, Z.; Wu, L.; Lu, L.; Risse, J.; Weisheit, A.; Raabe, D. Precipitation reactions in age-hardenable alloys during laser additive manufacturing. JOM 2016, 68, 943–949. [Google Scholar] [CrossRef]
- Deng, D. Additively Manufactured Inconel 718: Microstructures and Mechanical Properties. Ph.D. Thesis, Linkopings Universitet, Linköping, Sweden, 2018. [Google Scholar]
- Zhong, M.; Sun, H.; Liu, W.; Zhu, X.; He, J. Boundary liquation and interface cracking characterization in laser deposition of Inconel 738 on directionally solidified Ni-based superalloy. Scr. Mater. 2005, 53, 159–164. [Google Scholar] [CrossRef]
- Liu, X.; Hu, R.; Luo, X.; Yang, C.; Gao, X. A high-strength Ni–Cr–W based superalloy prepared by laser powder bed fusion: Printability, microstructure and tensile properties. Mater. Sci. Eng. A 2022, 853, 143744. [Google Scholar] [CrossRef]
- Tomus, D.; Rometsch, P.A.; Heilmaier, M.; Wu, X. Effect of minor alloying elements on crack-formation characteristics of Hastelloy-X manufactured by selective laser melting. Addit. Manuf. 2017, 16, 65–72. [Google Scholar] [CrossRef]
- Chen, L.; Sun, Y.; Li, L.; Ren, X. Microstructure evolution; mechanical properties, and strengthening mechanism of TiC reinforced Inconel 625 nanocomposites fabricated by selective laser melting. Mater. Sci. Eng. A 2020, 792, 139655. [Google Scholar] [CrossRef]
- Chen, L.; Sun, Y.; Li, L.; Ren, X. Microstructural evolution and mechanical properties of selective laser melted a nickel-based superalloy after post treatment. Mater. Sci. Eng. A 2020, 792, 139649. [Google Scholar] [CrossRef]
- Amato, K.N.; Gaytan, S.M.; Murr, L.E.; Martinez, E.; Shindo, P.W.; Hernandez, J.; Collins, S.; Medina, F. Microstructures and mechanical behavior of Inconel 718 fabricated by selective laser melting. Acta Mater. 2012, 60, 2229–2239. [Google Scholar] [CrossRef]
- Smith, D.H.; Bicknell, J.; Jorgensen, L.; Patterson, B.M.; Cordes, N.L.; Tsukrov, I.; Knezevic, M. Microstructure and mechanical behavior of direct metal laser sintered Inconel alloy 718. Mater. Charact. 2016, 113, 1–9. [Google Scholar] [CrossRef]
- Shankar, V.; Rao, K.B.S.; Mannan, S.L. Microstructure and mechanical properties of Inconel 625 superalloy. J. Nucl. Mater. 2001, 288, 222–232. [Google Scholar] [CrossRef]
- Han, Q.; Gu, Y.; Setchi, R.; Lacan, F.; Johnston, R.; Evans, S.L.; Yang, S. Additive manufacturing of high-strength crack-free Ni-based Hastelloy X superalloy. Addit. Manuf. 2019, 30, 100919. [Google Scholar] [CrossRef]
- Cheng, X.; Wei, L.; Wang, R.; He, J.; Sha, J.; Ma, Z. Achieving well-balanced strength and ductility in laser powder bed fusion additively manufactured nickel-based superalloys by doping multiple oxides. Mater. Sci. Eng. A 2023, 887, 145773. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Li, Y.; Zhao, D.; Yang, Y.; Li, Q.; Xie, K. The synergy effect of elements on the oxidation resistance of a high-purity directionally solidification nickel-based superalloy. J. Mater. Res. Technol. 2023, 26, 2796–2805. [Google Scholar] [CrossRef]
- Lee, C.M.; Han, Y.-S.; Jeong, J.S.; Jung, W.S.; Song, S.H.; Kim, J.-S. Effect of pre-strain on oxidation behaviour of nickel-based superalloys in air at 700 °C. Corros. Sci. 2022, 200, 110229. [Google Scholar]
- Jiang, W.; Li, P.; Yao, W.-X.; Rui, S.-S.; Shi, H.-J.; Huang, J. The effect of porosity size and oxidation on the HCF property of nickel-based single crystal superalloy at 980 °C. Theor. Appl. Fract. Mec. 2022, 120, 103423. [Google Scholar] [CrossRef]
- Yang, L.; Bo, C.; Junwei, W.; Zhiping, W.; Wensheng, L. Corrosion behavior of Cr, Fe and Ni based superalloy in molten NaCl. Rare Metal Mat. Eng. 2014, 43, 17–23. [Google Scholar] [CrossRef]
- Tung, H.M.; Chen, T.L.; Lan, K.C.; Liu, P.W.; Li, Y.C. The incipient oxidation behaviors of Haynes 282 at high temperatures. Mater. Chem. Phys. 2024, 316, 129109. [Google Scholar] [CrossRef]
- Qin, Y.; Liang, J.; Long, X.; Zhang, N.; Chen, M.; Tang, J.; Liu, W.; Chen, L.; Yan, D.; Li, Q.; et al. Effects of heat treatment on microstructure and high-temperature tensile performance of Ni-based GH3230 superalloy processed by laser powder bed fusion. J. Alloys Compd. 2025, 1021, 179619. [Google Scholar] [CrossRef]
- Zhang, B.; Dembinski, L.; Coddet, C. The study of the laser parameters and environment variables effect on mechanical properties of high compact parts elaborated by selective laser melting 316L powder. Mater. Sci. Eng. A 2013, 584, 21–31. [Google Scholar] [CrossRef]
- Thijs, L.; Sistiaga, M.L.M.; Wauthle, R.; Xie, Q.; Kruth, J.P.; Van Humbeeck, J. Strong morphological and crystallographic texture and resulting yield strength anisotropy in selective laser melted tantalum. Acta Mater. 2013, 61, 4657–4668. [Google Scholar] [CrossRef]
- Liu, F.; Lin, X.; Yu, X.; Huang, C.; Huang, W. Evolution of interface and crystal orientation of laser solid formed GH4169 superalloy during recrystallization. Acta Metall. Sin. Chin. Ed. 2014, 50, 463–470. [Google Scholar]
- Zhang, B.C.; Xiu, M.Z.; Tan, Y.T.; Wei, J.; Wang, P. Pitting corrosion of SLM Inconel 718 sample under surface and heat treatments. Appl. Surf. Sci. 2019, 490, 556–567. [Google Scholar] [CrossRef]
- Keshavarzkermani, A.; Esmaeilizadeh, R.; Enrique, P.D.; Asgari, H.; Zhou, N.Y.; Bonakdar, A.; Toyserkani, E. Static recrystallization impact on grain structure and mechanical properties of heat-treated Hastelloy X produced via laser powder-bed fusion. Mater. Charact. 2021, 173, 110969. [Google Scholar] [CrossRef]
- Jiang, L.; Ye, X.X.; Wang, Z.Q.; Yu, C.; Dong, J.S.; Xie, R.; Li, Z.J.; Yan, L.; Sham, T.K.; Zhou, X.T.; et al. The critical role of Si doping in enhancing the stability of M6C carbides. J. Alloy. Compd. 2017, 728, 917–926. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, L.; Dong, J.; Li, Z.; Zhou, X. The effect of silicon on precipitation and decomposition behaviors of M6C carbide in a Ni–Mo–Cr superalloy. J. Alloy. Compd. 2015, 620, 197–203. [Google Scholar] [CrossRef]
- Fedoseeva, A.; Nikitin, I.; Dudova, N.; Kaibyshev, R. Nucleation of W-rich carbides and Laves phase in a Re-containing 10% Cr steel during creep at 650 °C. Mater. Charact. 2020, 169, 110651. [Google Scholar] [CrossRef]
- Muhammad, R.G.M.; Gradl, P.R.; Shao, S.; Shamsaei, N. Additively manufactured Haynes 230 by laser powder directed energy deposition (LP-DED) effect of heat treatment on microstructure and tensile properties. In Proceedings of the Solid Freeform Fabrication Symposium, Virtual, 2–4 August 2021; pp. 935–947. [Google Scholar]
- Tomus, D.; Tian, Y.; Rometsch, P.A.; Heilmaier, M.; Wu, X. Influence of post heat treatments on anisotropy of mechanical behaviour and microstructure of Hastelloy-X parts produced by selective laser melting. Mater. Sci. Eng. A 2016, 667, 42–53. [Google Scholar] [CrossRef]
- Mahajan, S. Critique of mechanisms of formation of deformation, annealing and growth twins: Face-centered cubic metals and alloys. Scr. Mater. 2013, 68, 95–99. [Google Scholar] [CrossRef]
- Kreitcberg, A.; Brailovski, V.; Turenne, S. Effect of heat treatment and hot isostatic pressing on the microstructure and mechanical properties of Inconel 625 alloy processed by laser powder bed fusion. Mater. Sci. Eng. A 2017, 689, 1–10. [Google Scholar] [CrossRef]
- Zhao, Y.A.; Guo, Q.Y.; Du, Z.F.; Chen, S.H.; Tan, J.; Yang, Z.W.; Ma, Z.Q. Critical role of subgrain orientation on the stability of mechanical properties of selective laser melting manufactured alloys. Mater. Sci. Eng. A 2022, 832, 142505. [Google Scholar] [CrossRef]
- Cheng, X.P.; Du, Z.F.; Chu, S.X.; Wu, J.; Dong, J.; Wang, H.; Ma, Z.Q. The effect of subsequent heating treatment on the microstructure and mechanical properties of additive manufactured Hastelloy X alloy. Mater. Charact. 2022, 186, 111799. [Google Scholar] [CrossRef]
- Chen, L.; Sun, Y.; Li, L.; Ren, X. Improvement of high temperature oxidation resistance of additively manufactured TiC/Inconel 625 nanocomposites by laser shock peening treatment. Addit. Manuf. 2020, 34, 101276. [Google Scholar] [CrossRef]
- Cao, J.; Jiang, B.; Cao, X.; Yuan, Z.; Yao, D.; Huang, J. High temperature oxidation resistance of an amorphous layer induced by ion implantation on the surface of Ni-based superalloy GH202, Appl. Surf. Sci. 2022, 586, 152825. [Google Scholar] [CrossRef]
- Min, S.; Zhang, H.; Liu, H.; Zhang, K.; Huang, A.; Hou, J. Influence of defects on high-temperature oxidation performance of GH3536 superalloys fabricated by laser powder bed fusion. Addit. Manuf. Lett. 2022, 3, 100064. [Google Scholar] [CrossRef]
- Sharifitabar, M.; Khorshahian, S.; Afarani, M.S.; Kumar, P.; Jain, N.K. High-temperature oxidation performance of Inconel 625 superalloy fabricated by wire arc additive manufacturing. Corros. Sci. 2022, 197, 110087. [Google Scholar] [CrossRef]
- Wang, K.; Du, D.; Liu, G.; Pu, Z.; Chang, B.; Ju, J. High-temperature oxidation behaviour of high chromium superalloys additively manufactured by conventional or extreme high-speed laser metal deposition. Corros. Sci. 2020, 176, 108922. [Google Scholar] [CrossRef]
- Prashanth, K.G.; Eckert, J. Formation of metastable cellular microstructures in selective laser melted alloys. J. Alloy. Compd. 2017, 707, 27–34. [Google Scholar] [CrossRef]
- Beckitt, F.R.; Clark, B.R. The shape and mechanism of formation of M23C6 carbide in austenite. Acta Metall. 1967, 15, 113–129. [Google Scholar] [CrossRef]
- Xi, X.; Lin, D.; Song, X.; Luo, X.; Ma, R.; Shi, Z.; Bian, H.; Fu, W.; Dong, Z.; Tan, C. Strength-plasticity transition mechanism after the solution treatment of GH3230 superalloy fabricated via laser powder bed fusion. Mater. Sci. Eng. A 2023, 876, 145124. [Google Scholar] [CrossRef]
- Jo, T.S.; Lim, J.H.; Kim, Y.D. Dissociation of Cr-rich M23C6 carbide in Alloy 617 by severe plastic deformation. J. Nucl. Mater. 2010, 406, 360–364. [Google Scholar] [CrossRef]
- Tan, Q.; Zhu, G.; Zhou, W.; Tian, Y.; Zhang, L.; Dong, A.; Shu, D.; Sun, B. Precipitation; transformation, and coarsening of carbides in a high-carbon Ni-based superalloy during selective laser melting and hot isostatic pressing processes. J. Alloys Compd. 2022, 913, 165196. [Google Scholar] [CrossRef]
- He, X.; Wang, L.; Kong, D.; Li, R.; Zhang, W.; Dai, K.; Ni, X.; He, K.; Dong, C. Recrystallization effect on surface passivation of Hastelloy X alloy fabricated by laser powder bed fusion. J. Mater. Sci. Technol. 2023, 163, 245–258. [Google Scholar] [CrossRef]
- Emam, I.S.; Saber, D.; Waheed, A. High-Temperature Cyclic Oxidation of 800H Superalloy at 750–950 °C in Air. J. Test. Eval. 2020, 48, 1277–1287. [Google Scholar] [CrossRef]
- Bi, Z.; Dong, J.; Zheng, L.; Xie, X. Phenomenon and mechanism of high temperature low plasticity in high-Cr nickel-based superalloy. J. Mater. Sci. Technol. 2013, 29, 187–192. [Google Scholar] [CrossRef]
- Guo, C.; Li, Y.; Li, Y.; Li, G.; Lu, X.; Li, Z.; Chen, X.; Li, X.; Zhou, L.; Zhu, Q.; et al. High-temperature oxidation behaviour of a Co-based superalloy fabricated through laser powder bed fusion. Corros. Sci. 2024, 227, 111703. [Google Scholar] [CrossRef]
- Wang, M.; Cheng, X.; Jiang, W.; Cao, T.; Liu, X.; Lu, J.; Zhang, Y.; Zhang, Z. The effect of amorphous coating on high temperature oxidation resistance of Ni-based single crystal superalloy. Corros. Sci. 2023, 213, 111000. [Google Scholar] [CrossRef]
- Jian, L.; Jian, P.; Bing, H.; Xie, G. Oxidation kinetics of Haynes 230 alloy in air at temperatures between 650 and 850 °C. J. Power Sources 2006, 159, 641–645. [Google Scholar] [CrossRef]
- Jian, P.; Jian, L.; Bing, H.; Xie, G. Oxidation kinetics and phase evolution of a Fe–16Cr alloy in simulated SOFC cathode atmosphere. J. Power Sources 2006, 158, 354–360. [Google Scholar] [CrossRef]
- Yiliti, Y.; Dong, G.; Liu, X.; You, X.; Han, W.; Dong, L.; Zhao, Y.; Yi, L.; Wang, Y. The high temperature oxidation behavior of a superalloy prepared by vacuum induction melting and electron beam smelting: A comparative study. J. Mater. Res. Technol. 2023, 25, 6977–6991. [Google Scholar] [CrossRef]
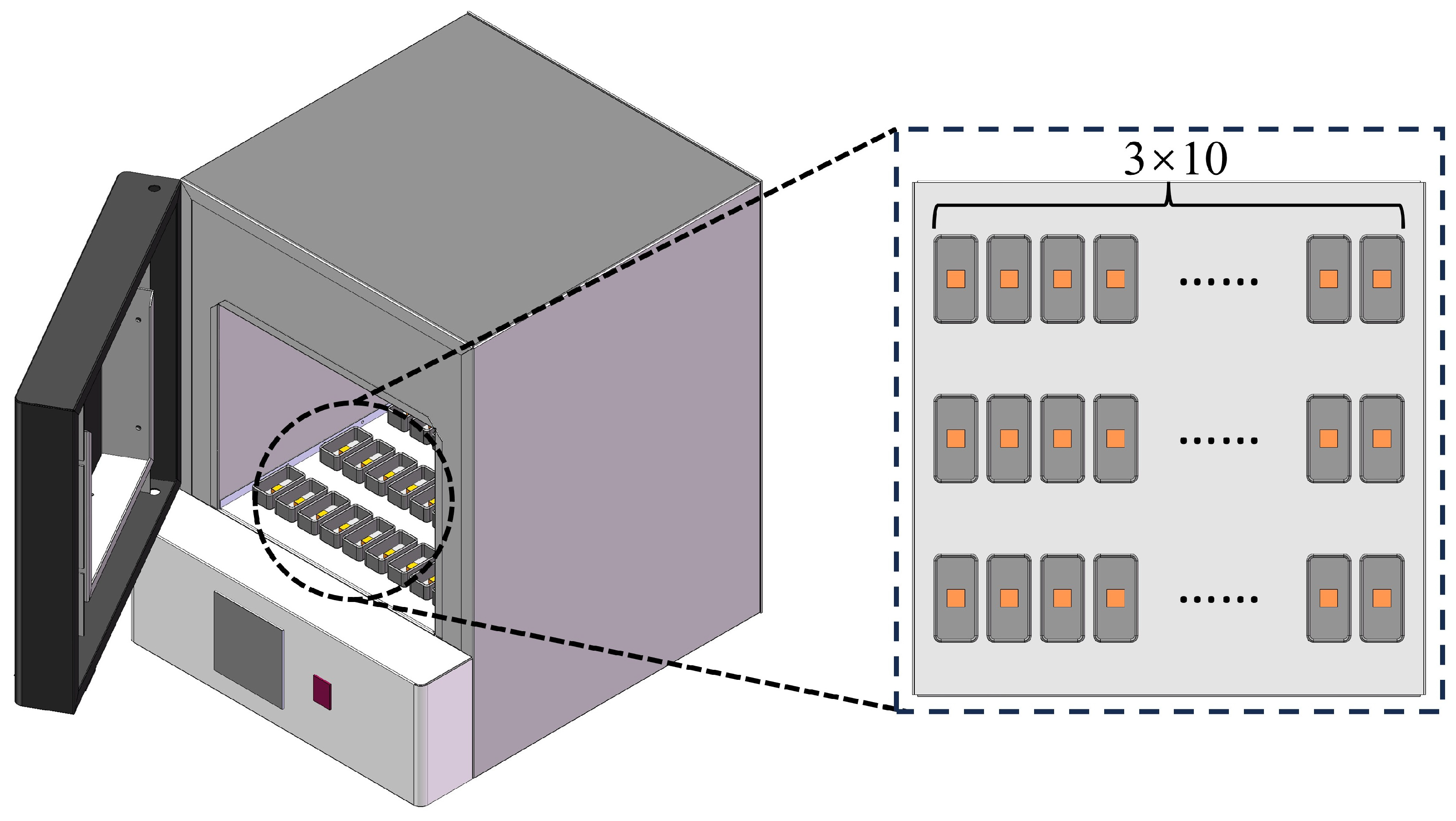
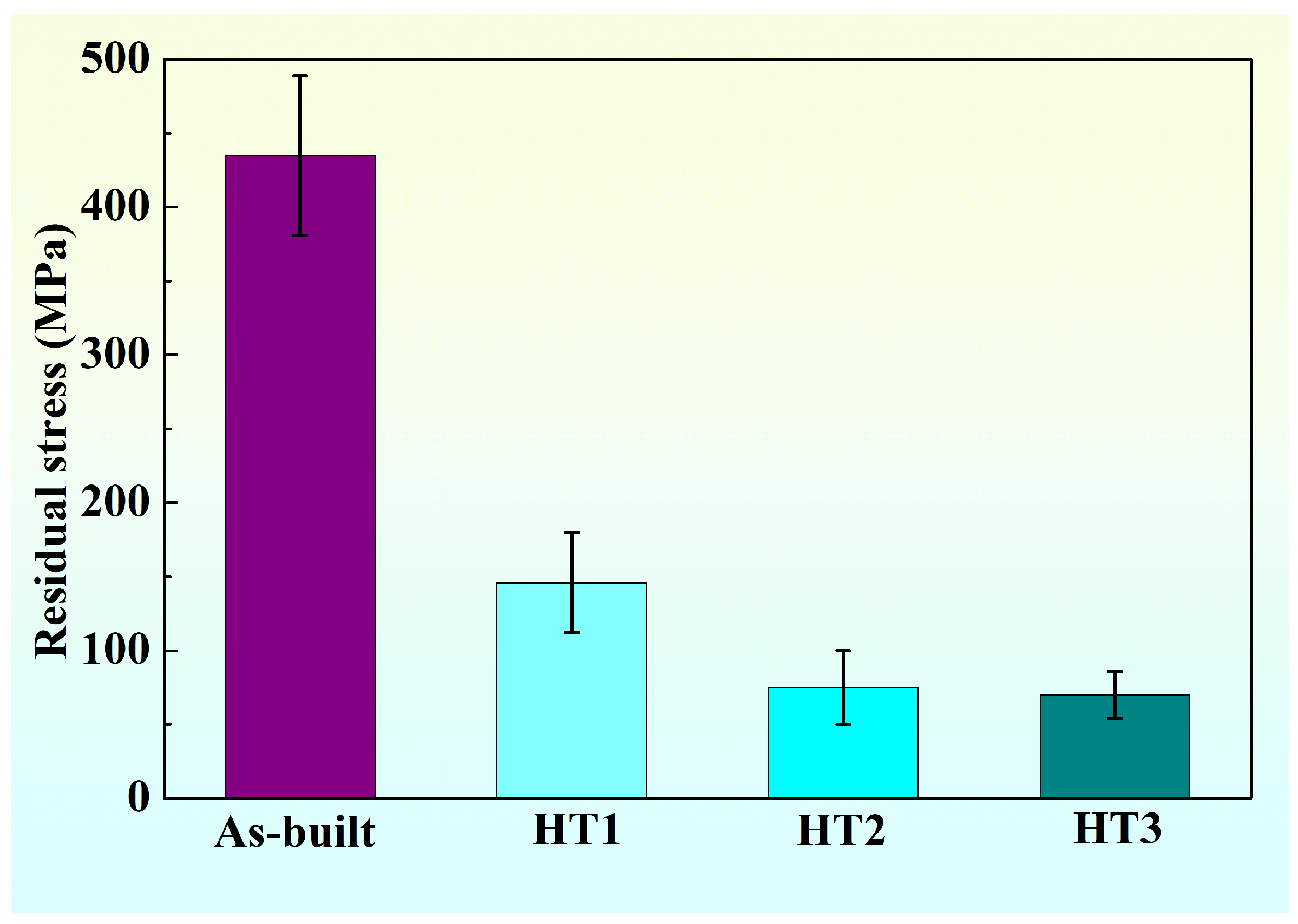
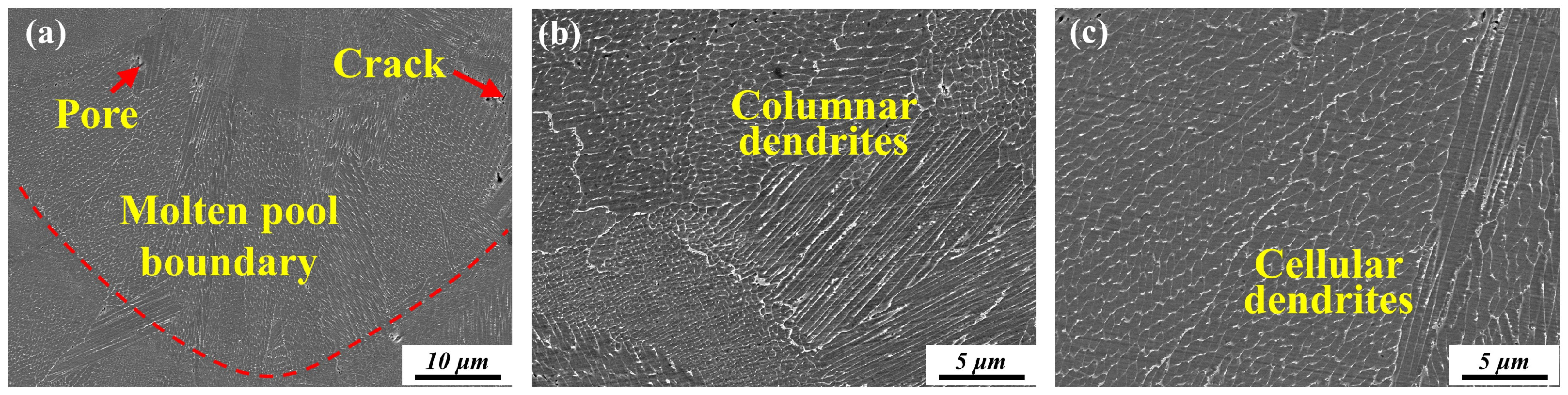
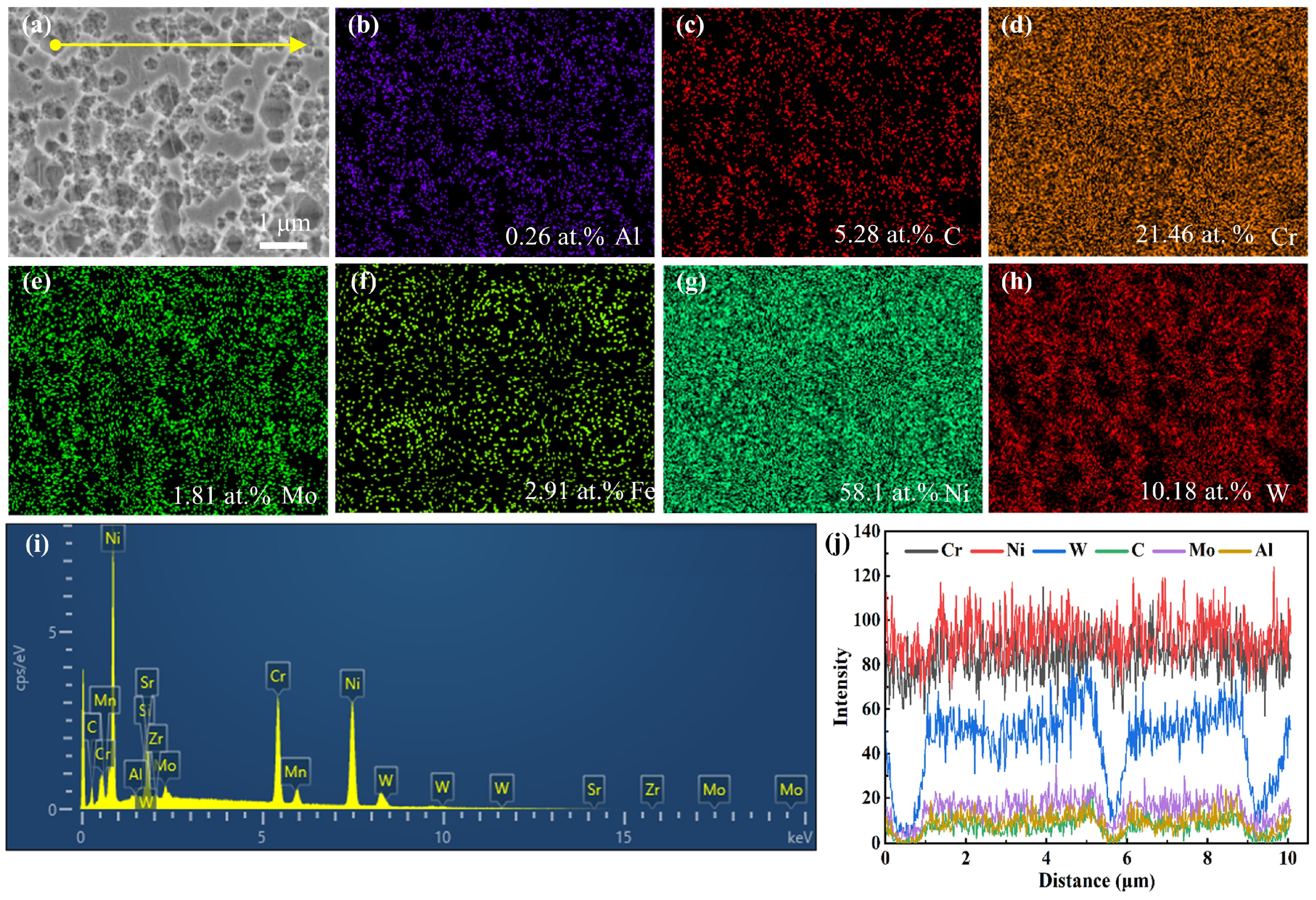





| Temperature (°C) | Time (Min) | Cooling Method | |
|---|---|---|---|
| HT1 | 1100 | 30 | Water Cooling |
| HT2 | 1230 | 30 | Water Cooling |
| HT3 | 1320 | 30 | Water Cooling |
| Point | Elements Composition (at. %) | |||||
|---|---|---|---|---|---|---|
| Ni | W | Cr | Mo | C | Fe | |
| 1 | 25.23 | 28.32 | 13.03 | 11.12 | 15.52 | 6.78 |
| 2 | 26.12 | 26.12 | 18.56 | 14.48 | 12.45 | 2.27 |
| 3 | 28.45 | 31.43 | 11.93 | 9.77 | 14.86 | 3.56 |
| 4 | 33.25 | 29.14 | 10.23 | 10.26 | 15.69 | 1.43 |
| 5 | 25.85 | 32.73 | 12.67 | 9.67 | 16.67 | 2.41 |
| 6 | 29.65 | 30.12 | 13.23 | 9.45 | 15.64 | 1.91 |
| Point | Elements Composition (at.%) | |||||||
|---|---|---|---|---|---|---|---|---|
| O | Al | W | Mo | Cr | Mn | Fe | Ni | |
| P1 | 33.10 | 0.00 | 0.04 | 0.13 | 65.15 | 0.07 | 0.10 | 1.40 |
| P2 | 34.92 | 0.54 | 2.90 | 0.44 | 42.82 | 10.20 | 0.02 | 8.16 |
| P3 | 30.00 | 0.60 | 0.85 | 0.18 | 66.49 | 0.32 | 0.03 | 1.53 |
| P4 | 30.25 | 0.00 | 0.16 | 0.02 | 47.53 | 19.68 | 0.01 | 2.35 |
| P5 | 14.97 | 0.00 | 0.00 | 0.00 | 71.25 | 3.90 | 0.17 | 9.70 |
| P6 | 34.39 | 0.26 | 1.47 | 0.13 | 46.29 | 6.96 | 0.17 | 10.33 |
| P7 | 30.04 | 0.26 | 0.49 | 0.10 | 47.88 | 3.90 | 0.11 | 17.22 |
| P8 | 32.14 | 0.28 | 0.43 | 0.10 | 47.71 | 2.73 | 0.05 | 16.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, R.; Yao, Y.; Han, D.; Fang, J.; Chen, C. Effects of Post-Treatment on the Microstructure Evolution and High-Temperature Oxidation Properties of Nickel-Based Superalloys Fabricated by Selective Laser Melting. Metals 2025, 15, 708. https://doi.org/10.3390/met15070708
Ren R, Yao Y, Han D, Fang J, Chen C. Effects of Post-Treatment on the Microstructure Evolution and High-Temperature Oxidation Properties of Nickel-Based Superalloys Fabricated by Selective Laser Melting. Metals. 2025; 15(7):708. https://doi.org/10.3390/met15070708
Chicago/Turabian StyleRen, Rui, Yunxia Yao, Dongsheng Han, Jun Fang, and Cai Chen. 2025. "Effects of Post-Treatment on the Microstructure Evolution and High-Temperature Oxidation Properties of Nickel-Based Superalloys Fabricated by Selective Laser Melting" Metals 15, no. 7: 708. https://doi.org/10.3390/met15070708
APA StyleRen, R., Yao, Y., Han, D., Fang, J., & Chen, C. (2025). Effects of Post-Treatment on the Microstructure Evolution and High-Temperature Oxidation Properties of Nickel-Based Superalloys Fabricated by Selective Laser Melting. Metals, 15(7), 708. https://doi.org/10.3390/met15070708







