1. Introduction
The ultrasonic spray pyrolysis (USP) technique is a droplet generation phenomenon induced by ultrasonic waves with several interesting properties, including its simplicity, cost-effectiveness, continuous operation, high deposition rate, and ability to deposit on broad surface areas [
1,
2]. The size of the obtained droplets is less than 20 μm in average for a low in-flight speed. This can impede the removal of droplets from the gas phase due to their collision with the reactor walls and collisions between the droplets themselves and the consequent merging.
The spray pyrolysis technique has several advantages, including the higher stability of the obtained coatings as compared to coatings deposited in vacuum, the diversity of its solution precursors, its cost-effectiveness, and its ease of use.
The spray pyrolysis technique is an adaptable processing method for preparing single- and multi-layered films as dense or porous ceramic coatings and various material powders.
Ultrasonic atomization operates based on an electromechanical device vibrating at high frequencies. Only Newtonian fluids with low viscosities can be atomized when being passed over the vibrating surface, and the vibration causes the solution to be atomized into droplets.
As compared to the other techniques for depositing thin films, the spray pyrolysis technique has several advantages, including its open-atmosphere process, its open reaction chamber, its adjustability during deposition, and the ability to observe the deposition procedure [
3].
Due to the easily controllable morphologies of the powders obtained using the USP method as well as the availability of its inexpensive precursors, it is a versatile and powerful tool for synthesizing particles of controlled as well as uniform particle sizes. This technique has great potential for synthesizing nanoscale metallic and oxidic particles [
4,
5,
6]. Zhao et al. performed the synthesis of core–shell particles using the ultrasonic spray pyrolysis synthesis of Ag/TiO
2 Nanocomposite Photocatalysts for simultaneous H
2 production and CO
2 reduction [
7]. Because of its excellent properties, titanium oxide was used as a carrier for catalytic active silver particles.
In the USP method, the starting solution precursor is atomized to form an aerosol [
8,
9,
10,
11]. Droplets of the aerosol are carried by a carrier gas, usually a reducing gas, into a hot chamber, where the droplets are dried, contracted, precipitated, thermolized, and sintered, and spherical particles will be formed. Very short residence times, typically of a few seconds, are usually sufficient in order to guarantee the formation of spherical nanoparticles [
12,
13,
14].
The size of the obtained nanoparticles is related to the droplet diameter and the initial concentration of the precursor solution [
15]. Increasing the ultrasonic frequency reduces the droplet diameter of the produced aerosol and also increases the ratio of smaller nanoparticles in the final product. The formation of droplets by ultrasound was first described by Wood and Loomis in 1927. Lang [
16], in 1962, and Peskin and Raco [
17], in 1963, formulated an equation to describe the connection between the ultrasonic frequency and the mean droplet diameter. A theoretical equation for the prediction of the sizes of the obtained nanoparticles was formulated in previous work, combining Kelvin’s equation and an equation based on the capillary theory.
Dmat is the final particle diameter; Csol, ρsol, and Msol are the solute concentration, density, and molar mass; Mmat and ρmat are the molar mass and density of the desired material; σ is the surface tension; and f is the frequency of the ultrasonic atomizer.
Stopić et al. [
18] synthesized nanosized TiO
2 particles from C
16H
36O
4Ti using ultrasonic spray pyrolysis (USP) in single- and multi-step processes with horizontal and vertical reactors. Spherical submicron TiO
2 core–RuO
2 shell particles were obtained via multi-step USP using two ultrasonic atomizers (0.8 MHz and 2.5 MHz) fed with separate RuCl
4 and C
16H
36O
4Ti solutions at 850 °C in oxidizing conditions. Particle characterization was performed using XRD, SEM, and TEM, with the core–shell structure confirmed through a comparison with a hard sphere model. It was shown that the aerosol mixing of Ru and TiO
2 streams significantly affected core–shell formation.
In a follow-up study [
19], RuO
2/TiO
2 particles synthesized by USP from a mixed solution were deposited on titanium substrates in a second reactor under a high-voltage electrostatic field at 500 °C. The coatings’ electrochemical behavior was studied, establishing structure–activity/stability correlations for the TiO
2@RuO
2 microspheres.
To avoid the use of carbon-based precursors [
20,
21], Kostić et al. [
22] investigated the thermal decomposition of titanium oxysulfate via USP between 700 and 1000 °C. The effects of the solution concentration and H
2/Ar flow rates were examined. The resulting powders exhibited a spherical morphology and varied in size based on the process conditions. However, wet powder collection led to material loss, prompting the current study’s use of electrostatic precipitation for improved recovery.
Other studies have confirmed USP’s efficacy in producing oxide nanoparticles like alumina and ZnO [
23,
24,
25]. Janacković et al. [
26] synthesized spherical alumina particles via USP from 0.5 M aqueous solutions of aluminum nitrate and chloride at 800–1000 °C. Yet, these studies focused on pure precursors and did not implement advanced powder collection.
This study presents a novel application of USP for synthesizing TiO
2 and Al
2O
3 nanopowders from industrial waste-derived precursors such as red mud (bauxite residue), a byproduct of the Bayer process [
27,
28]. With ~180 million tons generated annually and over 4 billion tons accumulated [
29,
30], red mud poses a serious environmental challenge. By incorporating electrostatic precipitation, this work improved the collection efficiency and purity while promoting resource recovery and environmental sustainability.
2. Materials and Methods
Ultrasonic spray pyrolysis (USP) was employed for the synthesis of aluminum oxide (Al
2O
3) and titanium dioxide (TiO
2) nanopowders, with hydrogen reduction applied exclusively in the production of the titanium-based powders. For aluminum oxide synthesis, aluminum nitrate was prepared by dissolving aluminum hydroxide oxide (AlOOH) in hydrochloric acid to form aluminum chloride, followed by the addition of nitric acid to convert it into aluminum nitrate. In contrast, titanium oxysulfate was used as the precursor for TiO
2 synthesis under a hydrogen reduction atmosphere. This study introduces the novel application of titanium oxysulfate (TiOSO
4) and aluminum nitrate (Al(NO
3)
3) solutions as precursors for the production of nanosized TiO
2 and Al
2O
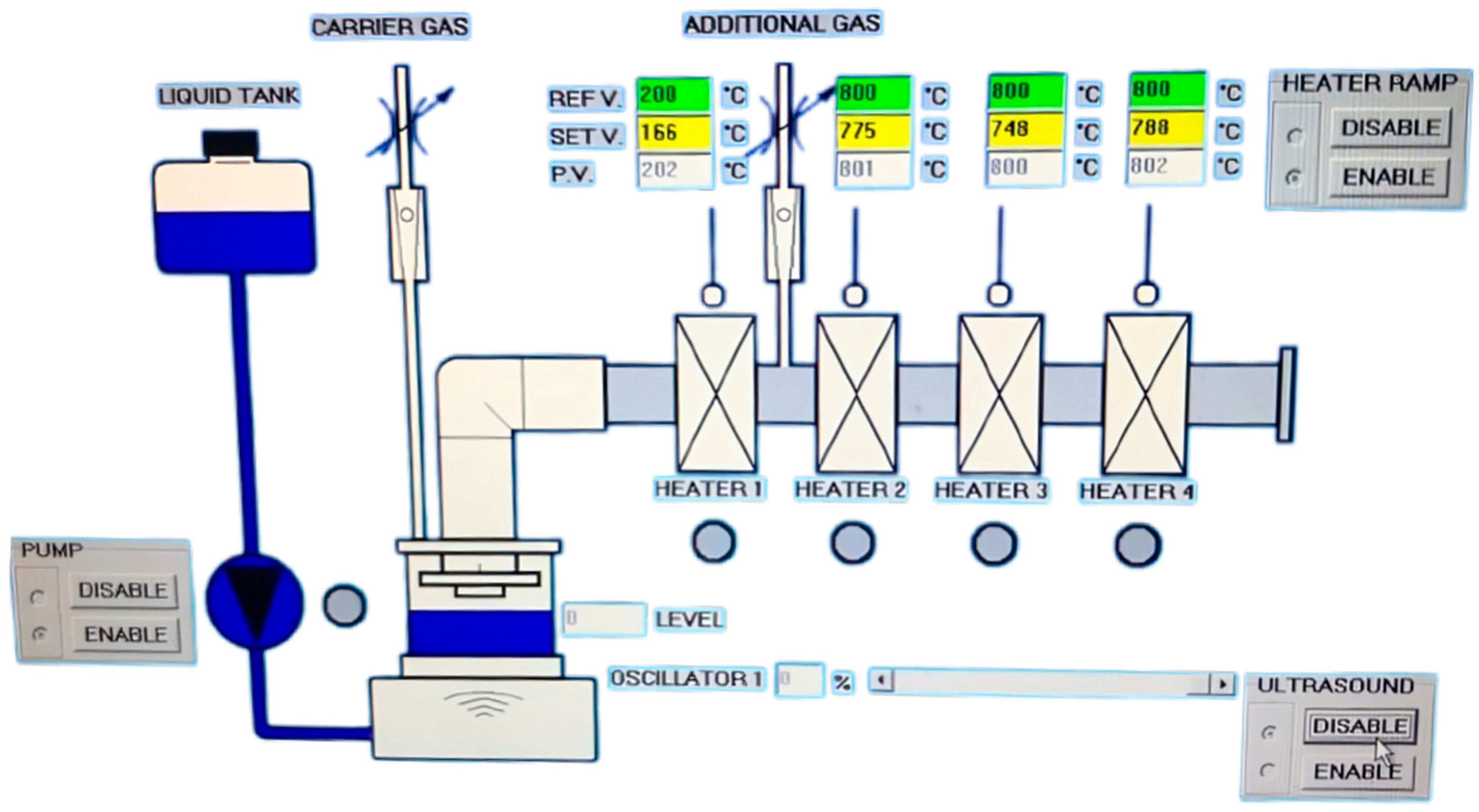
3 powders, addressing a significant gap in the literature. Both precursors, each with a 1 M molar concentration, were processed using an ultrasonic generator (PRIZNANO, Kragujevac, Serbia) to produce aerosols, highlighting the scalability and versatility of the USP method for oxide nanopowder synthesis. This furnace had 4 different heaters with a liquid tank with a pump for the continuous transport of the produced aerosols, as shown at
Figure 1.
The temperature in the furnace was controlled and regulated during the experimental work. The reaction tube was made from stainless steel, and a ceramic tube was used for the second titanium dioxide nanopowder experiments. Both tubes had a diameter of 100 mm. The level of liquid fluid was regulated using a special sensor. The frequency of the ultrasound was 1.7 MHz. The production of the aerosols was 1 L/h. The transport of the produced aerosols was performed using air. The collection of the powder was performed using one electrostatic precipitator (voltage: 7–12 KV), as shown in
Figure 2.
This image shows an advanced setup for ultrasonic spray pyrolysis (USP), highlighting its essential components for nanoparticle synthesis. On the left, the ultrasonic generator was used to atomize precursor solutions into a fine aerosol using ultrasonic waves. This step ensured the production of uniform droplets, which were then transported into the central unit, a high-temperature tubular furnace. The tubular furnace served as the reaction zone where the aerosol underwent thermal decomposition in a controlled environment, resulting in the formation of oxide powders. The furnace allowed for precise adjustments of the temperature and atmosphere, such as the reducing or inert gases, to achieve the desired material properties. On the right, the electrostatic precipitator efficiently captured the synthesized nanopowders. Employing an electrostatic field ensured the collection of the fine particles produced during pyrolysis, minimizing the material loss and ensuring environmental safety. The maximal temperature in the electrostatic precipitator was 200 °C in order to prevent condensation in the system, enabling the production of dried powder. This integrated setup produced by PRIZMA, Kragujevac, Serbia, demonstrates the efficiency and precision of USP as a scalable method for producing high-purity oxide nanopowders like titanium dioxide (TiO2) and aluminum oxide (Al2O3).
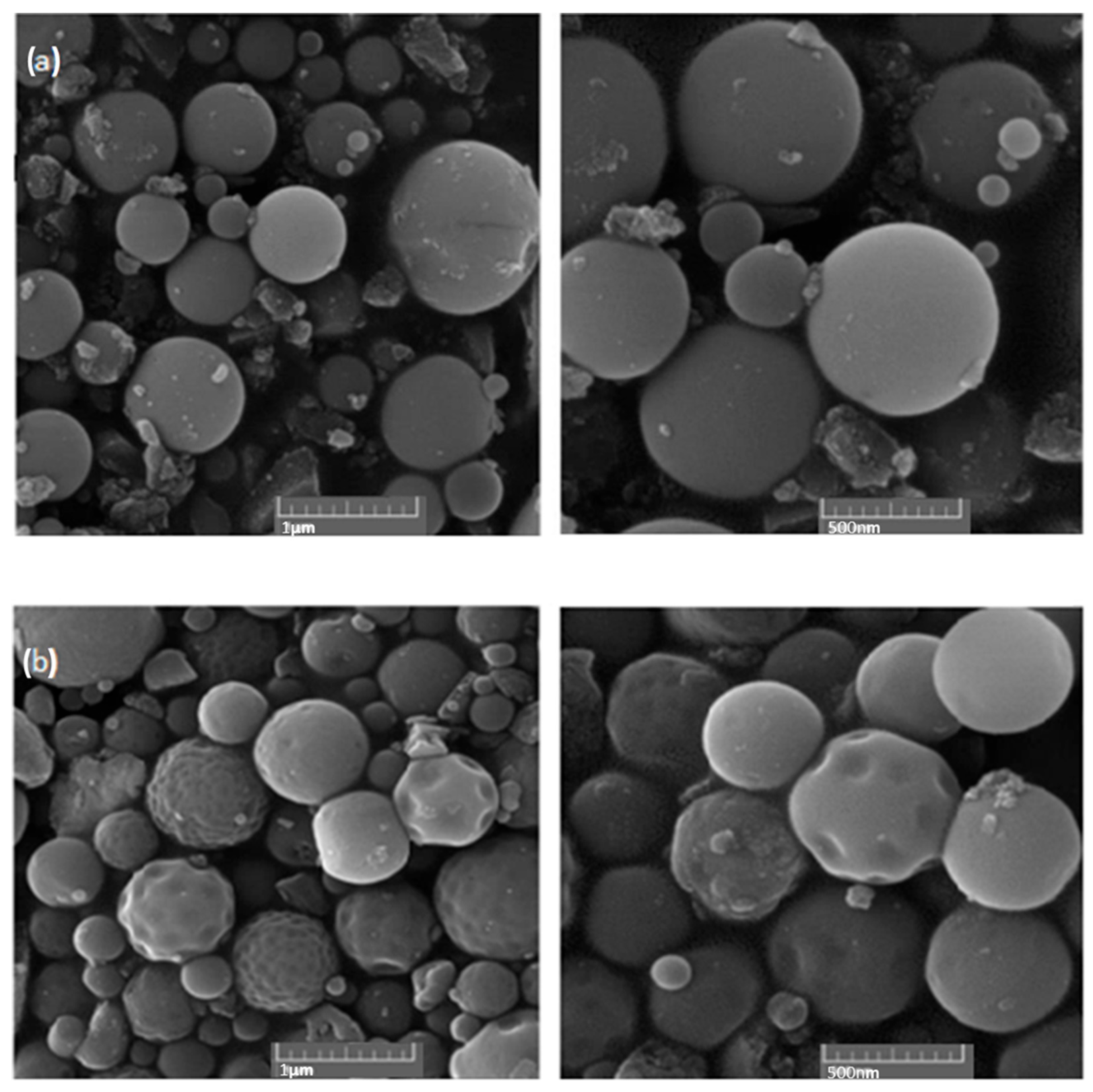
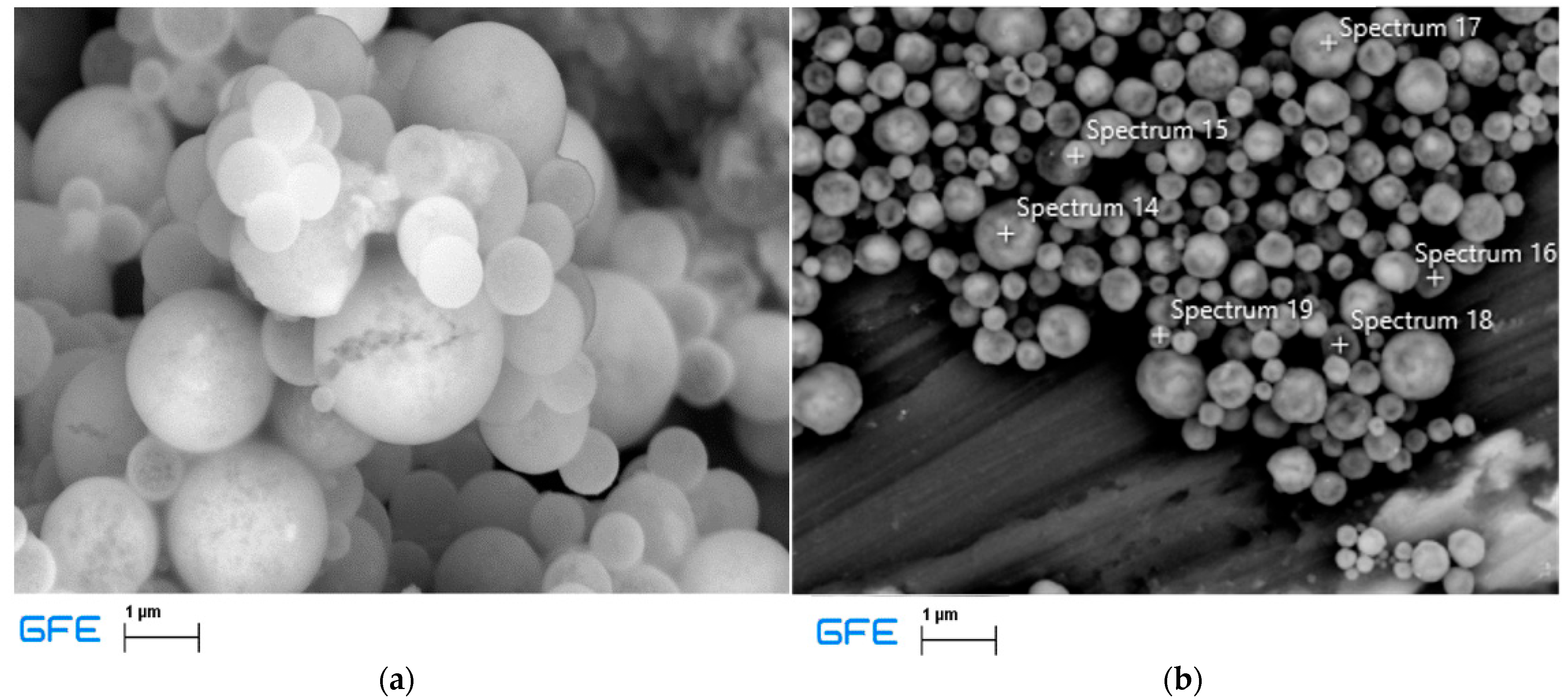
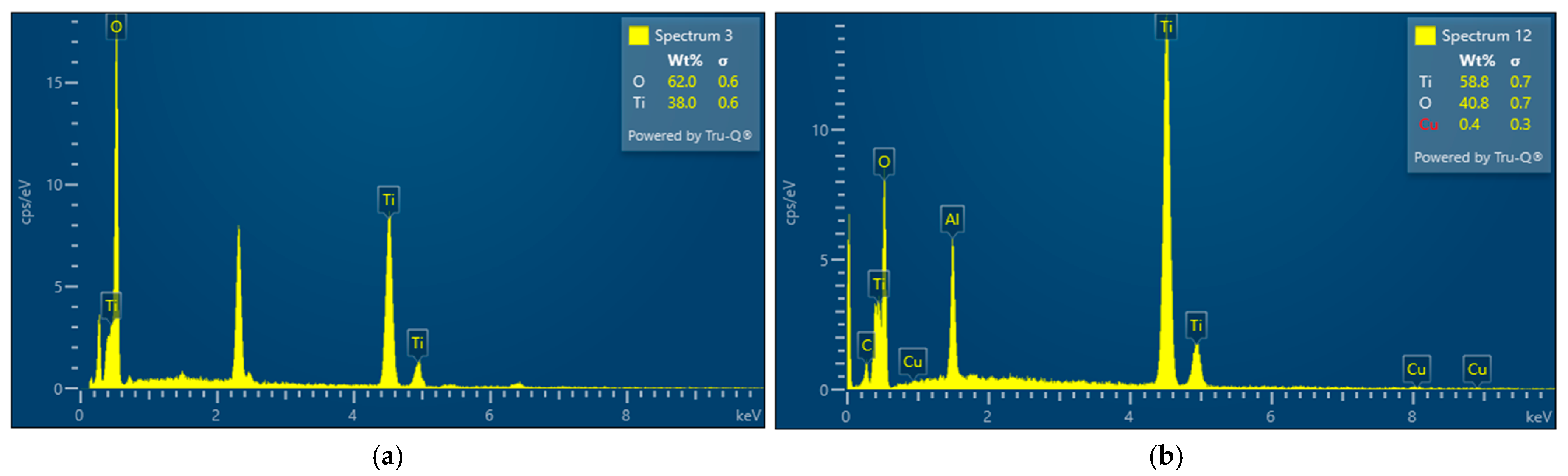
All the experiments were conducted using a PRIZNano generator. Various methods were used for sample characterization, which included XRD analysis, EDX analysis, particle size distribution measurements, and SEM analysis. SEM analysis was performed using the JSM 7000F by JEOL (manufactured in 2006, JEOL Ltd., Tokyo, Japan) coupled with EDX analysis using the Octane Plus-A by Ametek-EDAX (manufactured in 2015, AMETEK Inc., Berwyn, PA, USA), with the equipment operated using Genesis V 6.53 software by Ametek-EDAX. These analyses revealed the irregular structure of the precursor, as depicted in
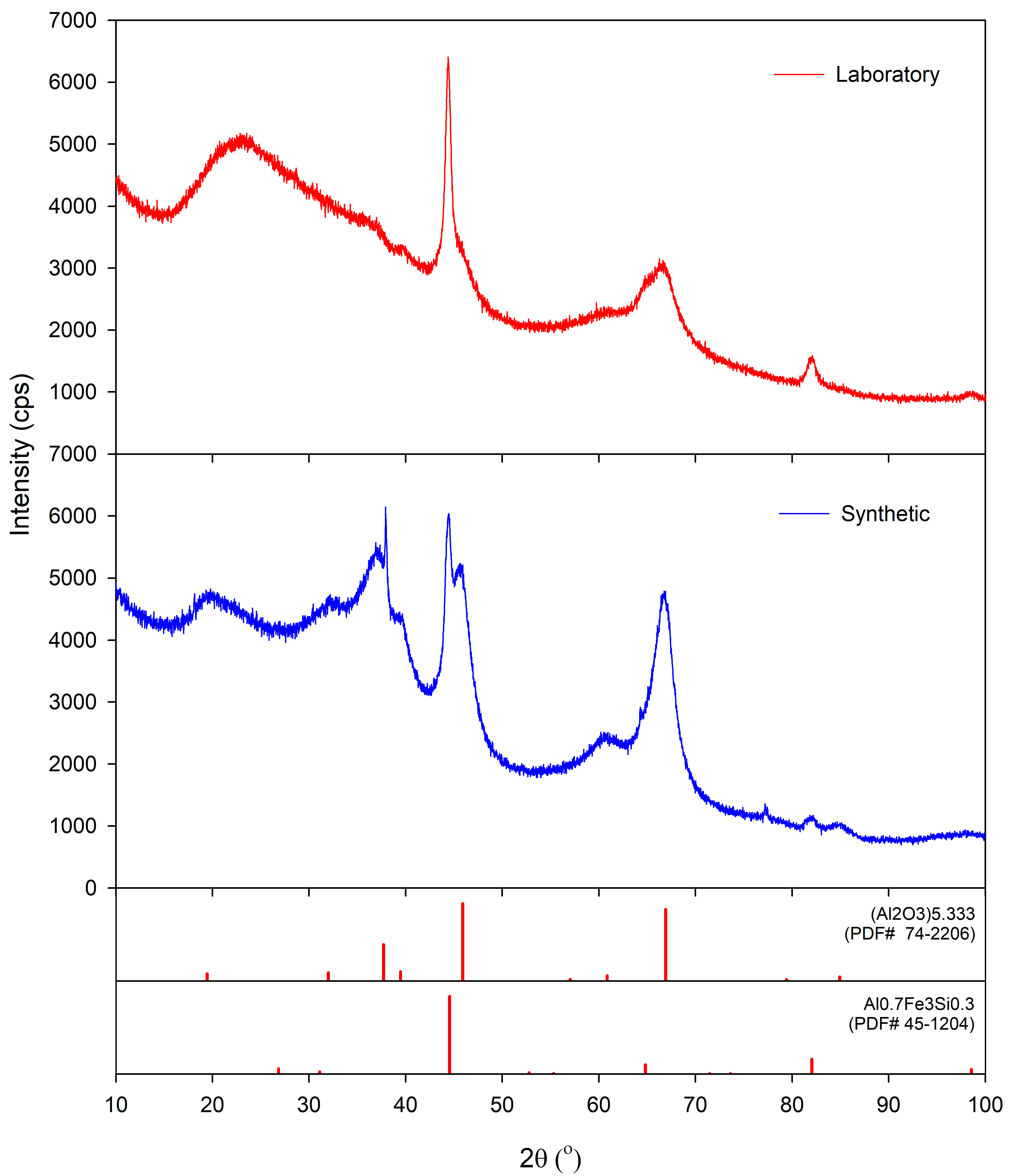
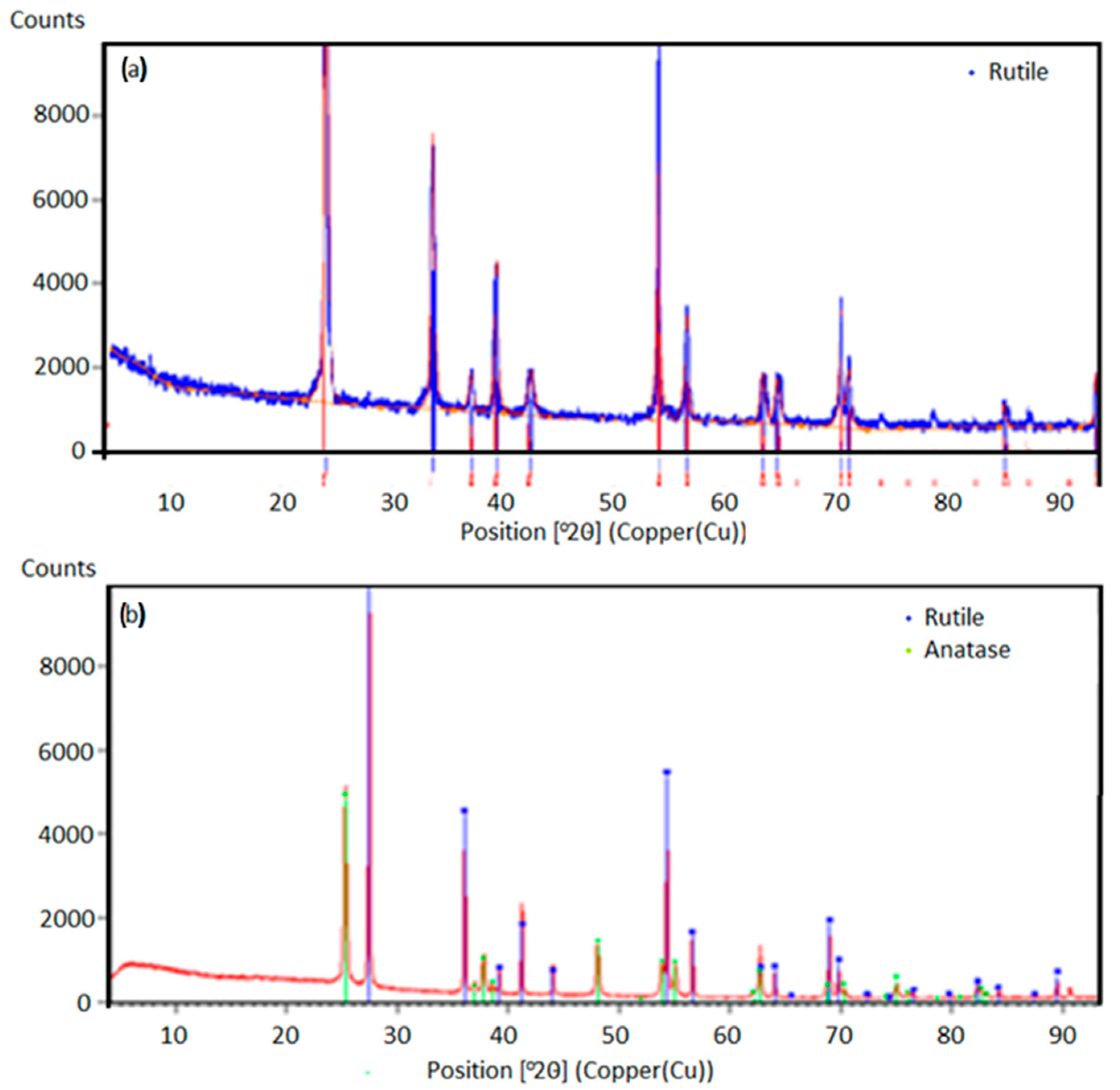
Figure 2. The XRD analysis of the obtained powders was carried out on a Bruker D8 Advance equipped with a LynxEye detector (Bruker AXS, Karlsruhe, Germany). The X-ray diffraction patterns were obtained using a Bruker-AXS D4 Endeavor diffractometer (Bruker AXS, Karlsruhe, Germany) in a Bragg–Brentano geometry. The system featured a copper tube and a primary nickel filter providing Cu Kα1,2 radiation (λ = 1.54187 Å). HighScore software, version 5.0, in conjunction with the COD database, was used to analyze the diffraction data.
Author Contributions
Conceptualization, S.S., R.F. and B.F.; methodology, S.S., D.K., M.P. and R.F.; software, V.D. and N.N.; validation, S.S. and V.D., formal analysis, D.K. and V.D.; investigation, D.K., R.F. and N.N.; resources, M.P., N.N. and B.F.; data curation, V.D. and M.P.; writing—original draft preparation, S.S., D.K. and V.D.; writing—review and editing, N.N.; visualization, M.P.; supervision, M.P., R.F. and B.F.; project administration, S.S., R.F. and B.F.; funding acquisition, B.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Commission, grant number 101135077 (EURO-TITAN).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Authors Vladimir Damjanović and Radislav Filipović were employed by Alumina d.o.o. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Majeric, P.; Rudolf, R. Advances in Ultrasonic Spray Pyrolysis Processing of Noble Metal Nanoparticles—Review. Materials 2020, 13, 3485. [Google Scholar] [CrossRef] [PubMed]
- Rahemi Ardekani, S.; Sabour Rouh Aghdam, A.; Nazari, M.; Bayat, A.; Yazdani, E.; Saievar-Iranizad, E. A Comprehensive Review on Ultrasonic Spray Pyrolysis Technique: Mechanism, Main Parameters and Applications in Condensed Matter. J. Anal. Appl. Pyrolysis 2019, 141, 104631. [Google Scholar] [CrossRef]
- Tsai, S.C.; Song, Y.L.; Tsai, C.S.; Yang, C.C.; Chiu, W.Y.; Lin, H.M. Ultrasonic Spray Pyrolysis for Nanoparticles Synthesis. J. Mater. Sci. 2004, 39, 3647–3657. [Google Scholar] [CrossRef]
- Tiyyagura, H.R.; Majerič, P.; Anžel, I.; Rudolf, R. Low-Cost Synthesis of AuNPs through Ultrasonic Spray Pyrolysis. Mater. Res. Express 2020, 7, 055017. [Google Scholar] [CrossRef]
- Mädler, L. Liquid-Fed Aerosol Reactors for One-Step Synthesis of Nano-Structured Particles. KONA Powder Part. J. 2004, 22, 107–120. [Google Scholar] [CrossRef]
- Emil, E.; Alkan, G.; Gurmen, S.; Rudolf, R.; Jenko, D.; Friedrich, B. Tuning the Morphology of ZnO Nanostructures with the Ultrasonic Spray Pyrolysis Process. Metals 2018, 8, 569. [Google Scholar] [CrossRef]
- Zhao, C.; Krall, A.; Zhao, H.; Zhang, Q.; Li, Y. Ultrasonic Spray Pyrolysis Synthesis of Ag/TiO2 Nanocomposite Photocatalysts for Simultaneous H2 Production and CO2 Reduction. Int. J. Hydrogen Energy 2012, 37, 9967–9976. [Google Scholar] [CrossRef]
- Miloševic, O.; Jordovic, B.; Uskokovic, D. Preparation of Fine Spherical ZnO Powders by an Ultrasonic Spray Pyrolysis Method. Mater. Lett. 1994, 19, 165–170. [Google Scholar] [CrossRef]
- Choi, W.J.; Kim, J.H.; Lee, H.; Park, C.W.; Lee, Y.I.; Byun, J. Hydrogen Reduction Behavior of W/Y2O3 Powder Synthesized by Ultrasonic Spray Pyrolysis. Int. J. Refract. Met. Hard Mater. 2021, 95, 105450. [Google Scholar] [CrossRef]
- Ebin, B.; Gurmen, S. Synthesis and Characterization of Nickel Particles by Hydrogen Reduction Assisted Ultrasonic Spray Pyrolysis(USP-HR) Method. KONA Powder Part. J. 2011, 29, 134–140. [Google Scholar] [CrossRef]
- Shatrova, N.; Yudin, A.; Levina, V.; Dzidziguri, E.; Kuznetsov, D.; Perov, N.; Issi, J.P. Elaboration, Characterization and Magnetic Properties of Cobalt Nanoparticles Synthesized by Ultrasonic Spray Pyrolysis Followed by Hydrogen Reduction. Mater. Res. Bull. 2017, 86, 80–87. [Google Scholar] [CrossRef]
- Li, M.; Zhao, L.; Guo, L. Preparation and Photoelectrochemical Study of BiVO4 Thin Films Deposited by Ultrasonic Spray Pyrolysis. Int. J. Hydrogen Energy 2010, 35, 7127–7133. [Google Scholar] [CrossRef]
- Küçükelyas, B.; Safaltln, S.; Sam, E.D.; Gurmen, S. Synthesis, Structural and Magnetic Characterization of Spherical High Entropy Alloy CoCuFeNi Particles by Hydrogen Reduction Assisted Ultrasonic Spray Pyrolysis. Int. J. Mater. Res. 2022, 113, 306–315. [Google Scholar] [CrossRef]
- Koo, Y.; Oh, S.; Im, K.; Kim, J. Ultrasonic Spray Pyrolysis Synthesis of Nano-Cluster Ruthenium on Molybdenum Dioxide for Hydrogen Evolution Reaction. Appl. Surf. Sci. 2023, 611, 155774. [Google Scholar] [CrossRef]
- Rajan, R.; Pandit, A. Correlation to predict Droplet Size in Ultrasonic Atomisation. Ultrasonics 2001, 39, 235–255. [Google Scholar] [CrossRef]
- Lang, R. Ultrasonic atomization of liquids. J. Acoust. Soc. Am. 1962, 34, 6–8. [Google Scholar] [CrossRef]
- Peskin, R.L.; Raco, R.J. Ultrasonic Atomization of Liquids. J. Acoust. Soc. Am. 1963, 35, 1378–1381. [Google Scholar] [CrossRef]
- Stopic, S.; Schroeder, M.; Weirich, T.; Friedrich, B. Synthesis of TiO2 Core/RuO2 Shell Particles using Multistep Ultrasonic Spray Pyrolysis. Mater. Res. Bull. 2013, 48, 3633–3635. [Google Scholar] [CrossRef]
- Košević, M.; Stopic, S.; Bulan, A.; Kintrup, J.; Weber, R.; Stevanović, J.; Panic, V.; Friedrich, B. A continuous process for the ultrasonic spray pyrolysis synthesis of RuO2-TiO2 particles and their application as an active coating of activated titanium anode. Adv. Powder Technol. 2017, 28, 43–49. [Google Scholar] [CrossRef]
- Ahonen, P.; Kauppinen, E.; Joubert, J.; Deschanvres, J.; Van Tendeloo, G. Preparation of Nanocrystalline Titania Powder via Aerosol Pyrolysis of Titanium tetrabutooxide. J. Mater. Res. 1999, 14, 3938–3948. [Google Scholar] [CrossRef]
- Messing, G.; Zhang, S.; Jayanthi, G. Ceramic powder synthesis by spray pyrolysis. J. Am. Ceram. Soc. 1993, 76, 2707–2726. [Google Scholar] [CrossRef]
- Kostic, D.; Stopic, S.; Keutmann, M.; Emil-Kaya, E.; Volkov-Husovic, T.; Perušic, M.; Friedrich, B. Synthesis of Titanium-Based Powders from Titanium Oxy-Sulfate Using Ultrasonic Spray Pyrolysis. Materials 2024, 17, 4779. [Google Scholar] [CrossRef]
- Das, R.; Pachfule, P.; Banerjee, R.; Poddar, P. Metal and metal oxide nanoparticle synthesis from metal organic frameworks (MOFs): Finding the border of metal and metal oxides. Nanoscale 2012, 4, 591–599. [Google Scholar] [CrossRef]
- Bang, H.; Suslick, K. Application of Ultrasound to the Synthesis of nanostructured Materials. Adv. Mater. 2010, 22, 1039–1059. [Google Scholar] [CrossRef] [PubMed]
- Perendis, D. Thin Film Deposition by Spray Pyrolysis and the Application in Solid Oxide Fuel Cells. Ph.D. Thesis, ETH Zurich, Zürich, Switzerland, 2003. [Google Scholar]
- Jokanović, V.; Janaćković, D.; Spasić, A.; Uskoković, D. Synthesis and formation mechanism of ultrafine spherical Al203 powders by ultrasonic spray pyrolysis method, Materials Transactions. JIM 1996, 37, 627–635. [Google Scholar]
- Tang, W.; Khavarian, M.; Yousefi, A. Red Mud. In Sustainable Concrete Made with Ashes and Dust from Different Sources: Materials, Properties and Applications; Woodhead Publishing: Sawston, UK, 2022; pp. 577–606. [Google Scholar]
- Verma, A.S.; Suri, N.M.; Kant, S. Applications of Bauxite Residue: A Mini-Review. Waste Manag. Res. 2017, 35, 999–1012. [Google Scholar] [CrossRef]
- Sutar, H.; Mishra, S.C.; Sahoo, S.K.; Chakraverty, A.P.; Maharana, H.S. Progress of Red Mud Utilization: An Overview. Chem. Sci. Int. J. 2014, 4, 255–279. [Google Scholar] [CrossRef]
- Morsali, S.; Yildirim, F. Environmental Impact Assessment of Red Mud Utilization in Concrete Production: A Life Cycle Assessment Study. Environ. Dev. Sustain. 2024, 26, 12219–12238. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).