Preparation of Small-Sized and Uniformly Distributed SnO by Ultrasound at Room Temperature
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Experimental Procedure
2.3. Analytical Methods
3. Results and Discussion
3.1. Effect of Experimental Parameters on the Particle Size
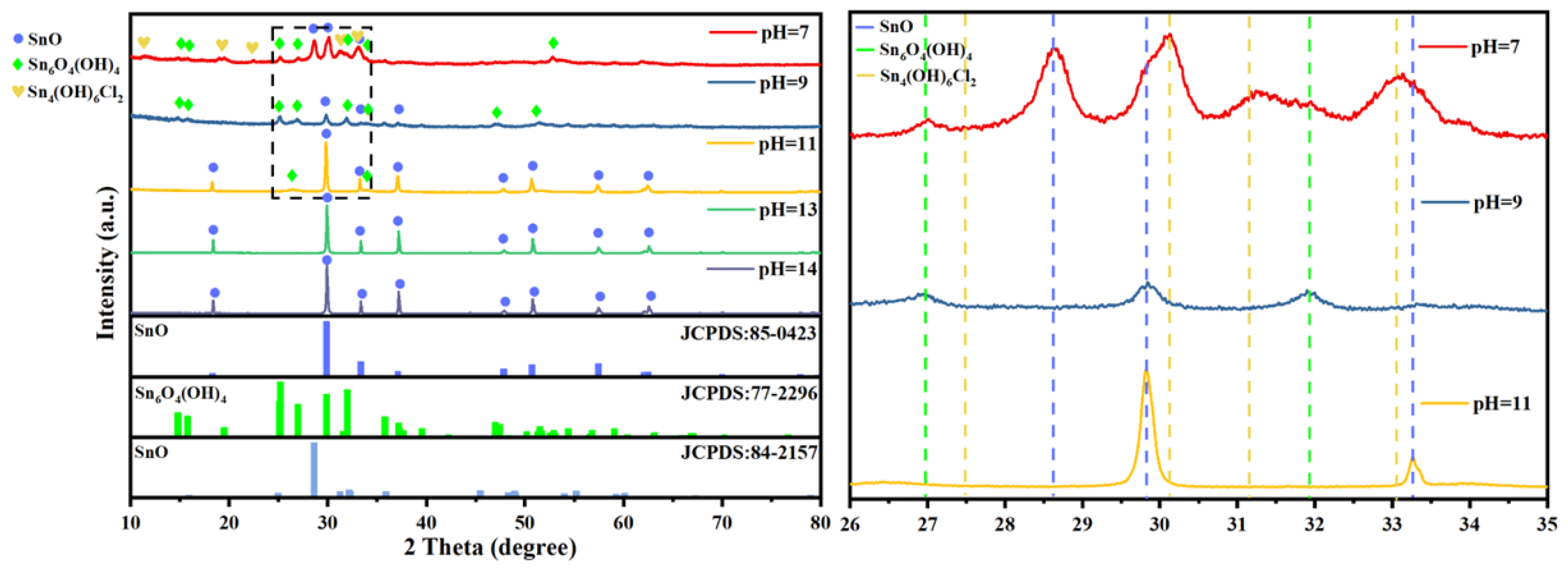
3.1.1. Endpoint pH Value
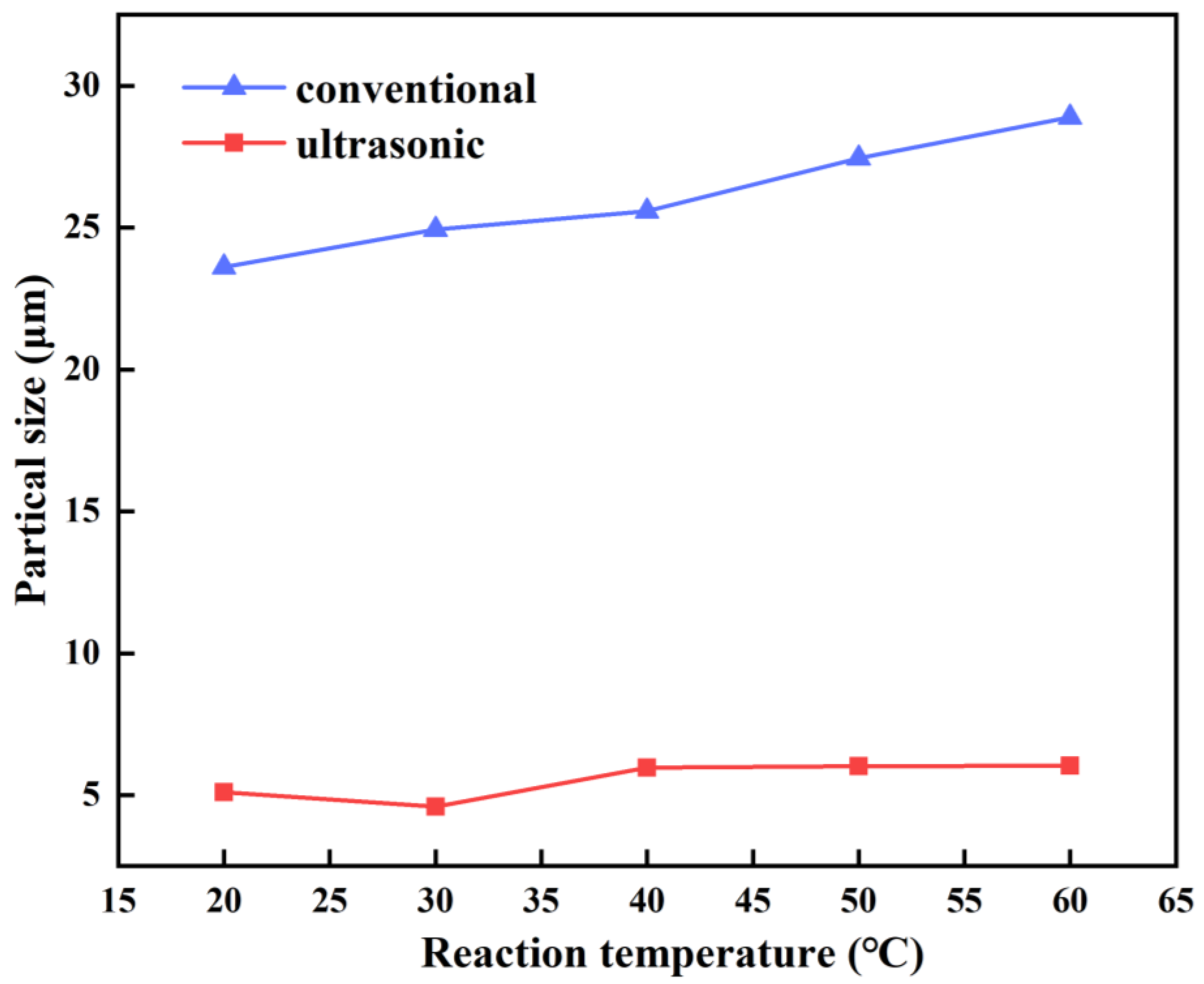
3.1.2. Reaction Temperature
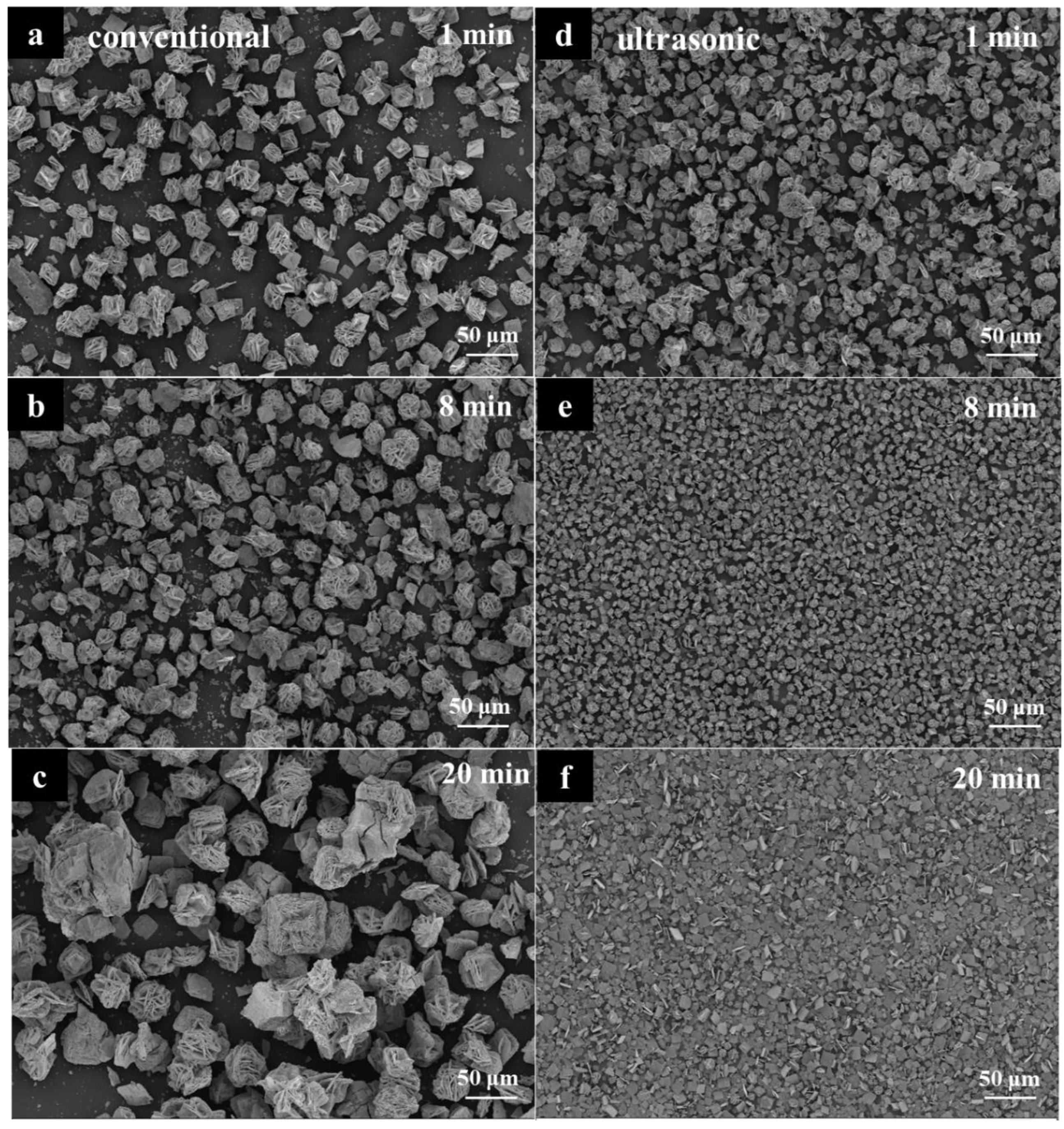
3.1.3. Constant-Temperature Time
3.1.4. Ultrasonic Power
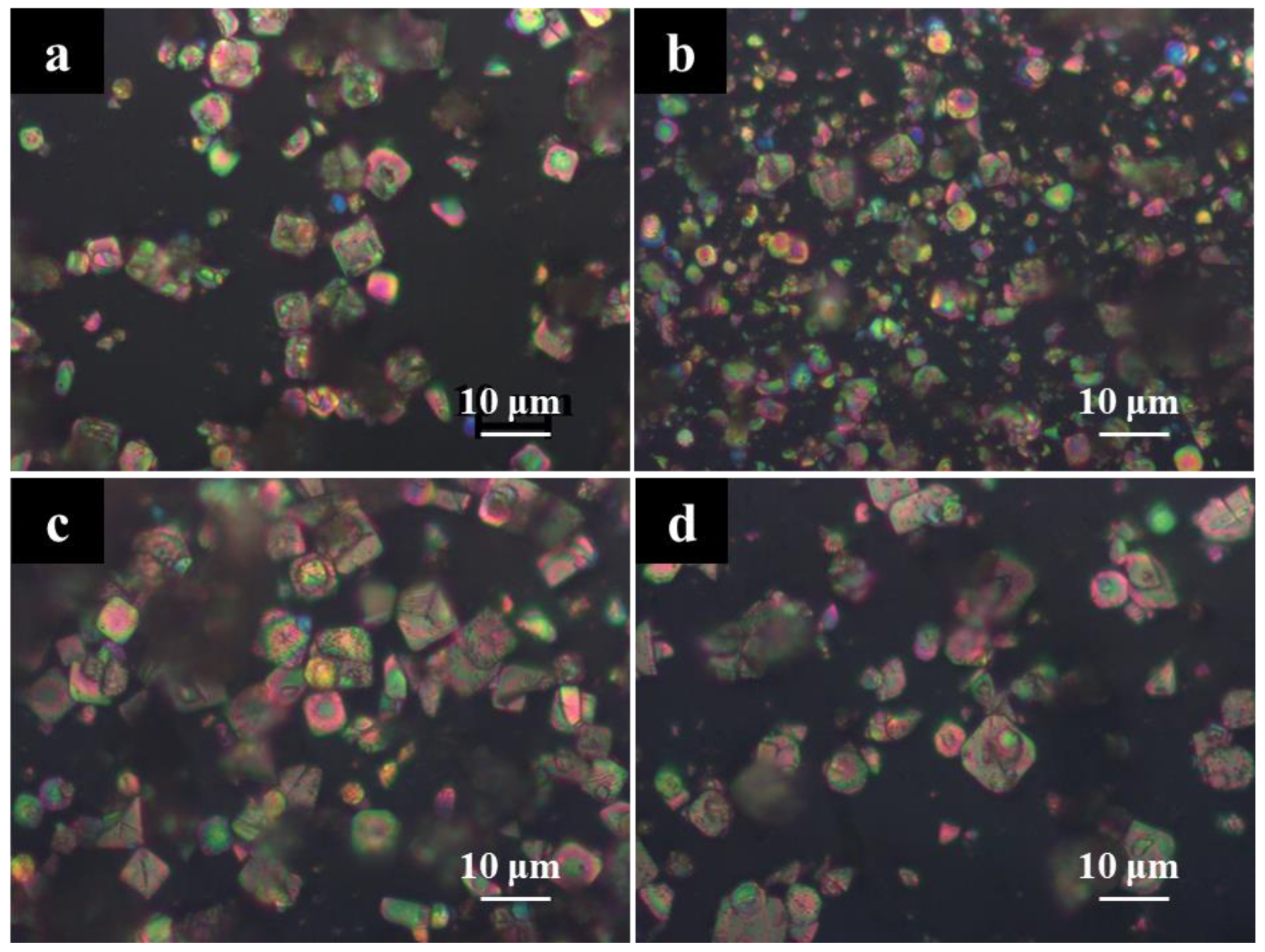
3.2. Characterization of Stannous Oxide Products Obtained by Ultrasonic and Conventional Methods
3.3. Enhanced Mechanism of Ultrasound for Product Size Control
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kelaidis, N.; Bousiadi, S.; Zervos, M.; Chroneos, A.; Lathiotakis, N.N. Electronic Properties of the Sn1−xPbx O Alloy and Band Alignment of the SnO/PbO System: A DFT Study. Sci. Rep. 2020, 10, 16828. [Google Scholar] [CrossRef]
- Panda, S.A.; Barala, S.; Hazra, A.; Gangopadhyay, S. Formation of All Tin Oxide p–n Junctions (SnO–SnO2) during Thermal Oxidation of Thin Sn Films. Phys. Status Solidi A—Appl. Mater. Sci. 2024, 627, 2400698. [Google Scholar] [CrossRef]
- Errico, L.A. Ab Initio FP-LAPW Study of the Semiconductors SnO and SnO2. Phys. B Condens. Matter 2007, 389, 140–144. [Google Scholar] [CrossRef]
- Chen, P.-J.; Jeng, H.-T. Phase Diagram of the Layered Oxide SnO: GW and Electron-Phonon Studies. Sci. Rep. 2015, 5, 16359. [Google Scholar] [CrossRef]
- Shi, H.; Deng, R.; Jiang, D.; Li, Y. Enhancing Photo-Response Performance through Ultrathin Al2O3 Modification at the p-SnO2: Al/n-GaN Heterojunction Interface. Opt. Mater. 2024, 156, 115950. [Google Scholar] [CrossRef]
- Sowmya, B.; John, A.; Panda, P.K. A Review on Metal-Oxide Based p-n and n-n Heterostructured Nano-Materials for Gas Sensing Applications. Sens. Int. 2021, 2, 100085. [Google Scholar] [CrossRef]
- Zhang, X.; Li, D.; Wang, L.; Yang, F.; Wang, Y. Induced Electron Transfer by Oxygen Vacancy Gradient on SnO2 Conductive Glass for Electrocatalytic Reduction. Sci. China Mater. 2021, 64, 2081–2085. [Google Scholar] [CrossRef]
- Han, Y.; Wu, X.; Shen, G.; Dierre, B.; Gong, L.; Qu, F.; Bando, Y.; Sekiguchi, T.; Filippo, F.; Golberg, D. Solution Growth and Cathodoluminescence of Novel SnO2 Core-Shell Homogeneous Microspheres. J. Phys. Chem. C 2010, 114, 8235–8240. [Google Scholar] [CrossRef]
- Zhang, R. Morphology Controlled Synthesis of SnO and TiO2/SnO Heterojunctions with Enhanced Photocatalytic Properties. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2021. [Google Scholar]
- Jain, R.; Lakhnot, A.S.; Bhimani, K.; Sharma, S.; Mahajani, V.; Panchal, R.A.; Kamble, M.; Han, F.; Wang, C.; Koratkar, N. Nanostructuring versus Microstructuring in Battery Electrodes. Nat. Rev. Mater. 2022, 7, 736–746. [Google Scholar] [CrossRef]
- Guo, Y.; Hu, Y.; Zhang, Q.; Ruan, G.; Mao, Y.; Zhang, H.; Wang, R.; Zhao, P.; Li, M.; Tang, H.; et al. Fabrication of SnO2 Micron-Rods Embedding in 3D Composite of Graphene oxide@ MWNTs as High-Performance Anode for Lithium-Ion Batteries. J. Energy Storage 2023, 74, 109479. [Google Scholar] [CrossRef]
- Deng, P.; Ning, H.; Xie, W.; Liu, X.; Deng, Y.; Yao, R.; Peng, J. Research progress in stannous oxide thin film transistors. Mater. Eng. 2020, 48, 83–88. [Google Scholar]
- Jagadish, K.; Rai, R.K.; Pandey, M.; Chandni, U.; Ravishankar, N. Mutual Stabilization of Metastable Phases of Tin Oxide: Epitaxial Encapsulation of Tetragonal SnO Microcrystals by Orthorhombic SnO2. J. Phys. Chem. C 2022, 126, 15001–15010. [Google Scholar] [CrossRef]
- Kılıç, Ç.; Zunger, A. Origins of Coexistence of Conductivity and Transparency in SnO2. Phys. Rev. Lett. 2002, 88, 095501. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Wang, F.; Rafiuddin; Javed, Q.; Rafique, M.Y.; Li, Y.; Li, P. Preparation, Characterization and Optical Properties of Tin Monoxide Micro-Nano Structure via Hydrothermal Synthesis. Mater. Lett. 2012, 68, 409–412. [Google Scholar] [CrossRef]
- Qin, B.; Zhang, H.; Diemant, T.; Geiger, D.; Raccichini, R.; Behm, R.J.; Kaiser, U.; Varzi, A.; Passerini, S. Ultrafast Ionic Liquid-Assisted Microwave Synthesis of SnO Microflowers and Their Superior Sodium-Ion Storage Performance. ACS Appl. Mater. Interfaces 2017, 9, 26797–26804. [Google Scholar] [CrossRef]
- Uchiyama, H.; Nakanishi, S.; Kozuka, H. Hydrothermal Synthesis of Nanostructured SnO Particles through Crystal Growth in the Presence of Gelatin. J. Solid State Chem. 2014, 217, 87–91. [Google Scholar] [CrossRef]
- Jordens, J.; Canini, E.; Gielen, B.; Van Gerven, T.; Braeken, L. Ultrasound Assisted Particle Size Control by Continuous Seed Generation and Batch Growth. Crystals 2017, 7, 195. [Google Scholar] [CrossRef]
- Ashokkumar, M. The Characterization of Acoustic Cavitation Bubbles—An Overview. Ultrason. Sonochem. 2011, 18, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Gielen, B.; Claes, T.; Janssens, J.; Jordens, J.; Thomassen, L.C.J.; Gerven, T.V.; Braeken, L. Particle Size Control during Ultrasonic Cooling Crystallization of Paracetamol. Chem. Eng. Technol. 2017, 40, 1300–1308. [Google Scholar] [CrossRef]
- Furusawa, A.; Hine, K.; Hayashi, Y.; Takizawa, H. Formation of Particle of Bismuth-Indium Alloys and Particle Diameter by Ultrasonic Cavitation. Ultrason. Sonochem. 2019, 50, 322–330. [Google Scholar] [CrossRef]
- Belca, L.M.; Ručigaj, A.; Teslič, D.; Krajnc, M. The Use of Ultrasound in the Crystallization Process of an Active Pharmaceutical Ingredient. Ultrason. Sonochem. 2019, 58, 104642. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hao, J.; Niu, Q.; Du, Q.; Zheng, X.; Liu, H.; Yang, H. Influence of Ultrasonic Assistance on the Microstructure and Friction Properties of Laser Cladded Ni60/WC Composite Coatings. J. Alloys Compd. 2025, 1010, 177149. [Google Scholar] [CrossRef]
- Zhou, N.; Zhang, C.; Zeng, X.; Yuan, J.; Zhou, L. A Molecular Dynamics Study of the Growth Rate of SiC Crystal and Its Dependence on the Temperature. Int. J. Mod. Phys. B 2016, 30, 1650152. [Google Scholar] [CrossRef]
- Ji, S.; Ji, X.; Song, Y.; Wei, Y.; Duan, T. Effect of Sintering Temperature on Crystal Growth and Evolution of CaZrTi2O7–CaNdTiNbO7 Ceramics: Minor Actinides Immobilization Host Materials. Nucl. Eng. Des. 2024, 429, 113643. [Google Scholar] [CrossRef]
- Wang, D.; Zhong, J.; Sun, B.; Bao, X.; Zhang, J. Tin Oxide Nanoparticles with Controlled Morphology and Particle Size by Hydrothermal Method. J. Inorg. Chem. 2008, 24, 892–896. [Google Scholar]
- Al-Attri, R.; Halladj, R.; Askari, S. Green Route of Flexible Al-MOF Synthesis with Superior Properties at Low Energy Consumption Assisted by Ultrasound Waves. Solid State Sci. 2022, 123, 106782. [Google Scholar] [CrossRef]
- Ruecroft, G.; Hipkiss, D.; Ly, T.; Maxted, N.; Cains, P.W. Sonocrystallization: The Use of Ultrasound for Improved Industrial Crystallization. Org. Process Res. Dev. 2005, 9, 923–932. [Google Scholar] [CrossRef]
- Wu, M.; Jiang, B.; Huang, P.; Wu, W. Ultrasound-Assisted Synthesis of PDA Nanoparticles with Controllable Size Regulation. Mater. Lett. 2023, 351, 135073. [Google Scholar] [CrossRef]
- Guo, Y.; Bao, H.; Fu, H.; Zhang, H.; Li, W.; Cai, W. Ultrasonic Emulsification Preparation of Metallic Rubidium Sol and Its Ignition Performance. J. Met. 2022, 58, 792–798. [Google Scholar]
- Yang, G.; Lin, W.; Lai, H.; Tong, J.; Lei, J.; Yuan, M.; Zhang, Y.; Cui, C. Understanding the Relationship between Particle Size and Ultrasonic Treatment during the Synthesis of Metal Nanoparticles. Ultrason. Sonochem. 2021, 73, 105497. [Google Scholar] [CrossRef]
- Aprile, C.; Teruel, L.; Alvaro, M.; Garcia, H. Structured Mesoporous Tin Oxide with Electrical Conductivity. Application in Electroluminescence. J. Am. Chem. Soc. 2009, 131, 1342–1343. [Google Scholar] [CrossRef]
- Grabowy, M.; Wojteczko, A.; Komarek, S.; Wilk, A.; Pędzich, Z. ATZ Composites with Enhanced Mechanical Reliability and Lifetime. J. Eur. Ceram. Soc. 2024, 44, 5319–5325. [Google Scholar] [CrossRef]
- Mahdavi, R.; Ashraf Talesh, S.S. The Effect of Ultrasonic Irradiation on the Structure, Morphology and Photocatalytic Performance of ZnO Nanoparticles by Sol-Gel Method. Ultrason. Sonochem. 2017, 39, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Karmaoui, M.; Jorge, A.B.; McMillan, P.F.; Aliev, A.E.; Pullar, R.C.; Labrincha, J.A.; Tobaldi, D.M. One-Step Synthesis, Structure, and Band Gap Properties of SnO2 Nanoparticles Made by a Low Temperature Nonaqueous Sol–Gel Technique. ACS Omega 2018, 3, 13227–13238. [Google Scholar] [CrossRef]
- Sharif, M.S.; Aqeel, M.; Haider, A.; Naz, S.; Ikram, M.; Ul-Hamid, A.; Haider, J.; Aslam, I.; Nazir, A.; Butt, A.R. Photocatalytic, Bactericidal and Molecular Docking Analysis of Annealed Tin Oxide Nanostructures. Nanoscale Res. Lett. 2021, 16, 33. [Google Scholar] [CrossRef]
- Ibarguen, C.A.; Mosquera, A.; Parra, R.; Castro, M.S.; Rodríguez-Páez, J.E. Synthesis of SnO2 Nanoparticles through the Controlled Precipitation Route. Mater. Chem. Phys. 2007, 101, 433–440. [Google Scholar] [CrossRef]
- Ong, C.; Lee, W.; Lim, L.; Chong, W.; Chew, J.; Hayashi, Y.; Nishikawa, T.; Öhberg, P.; Ahmad, H.; Yap, Y. Dispersion Stability of Tin(IV) Oxide Nanoparticles in Polar Solvents and Water Aided by Ultrasonication. Jpn. J. Appl. Phys. 2023, 62, 035002. [Google Scholar] [CrossRef]
- Xiang, L.; Fu, M.; Wang, T.; Wang, D.; Xv, H.; Miao, W.; Le, T.; Zhang, L.; Hu, J. Application and Development of Ultrasound in Industrial Crystallization. Ultrason. Sonochem. 2024, 111, 107062. [Google Scholar] [CrossRef]





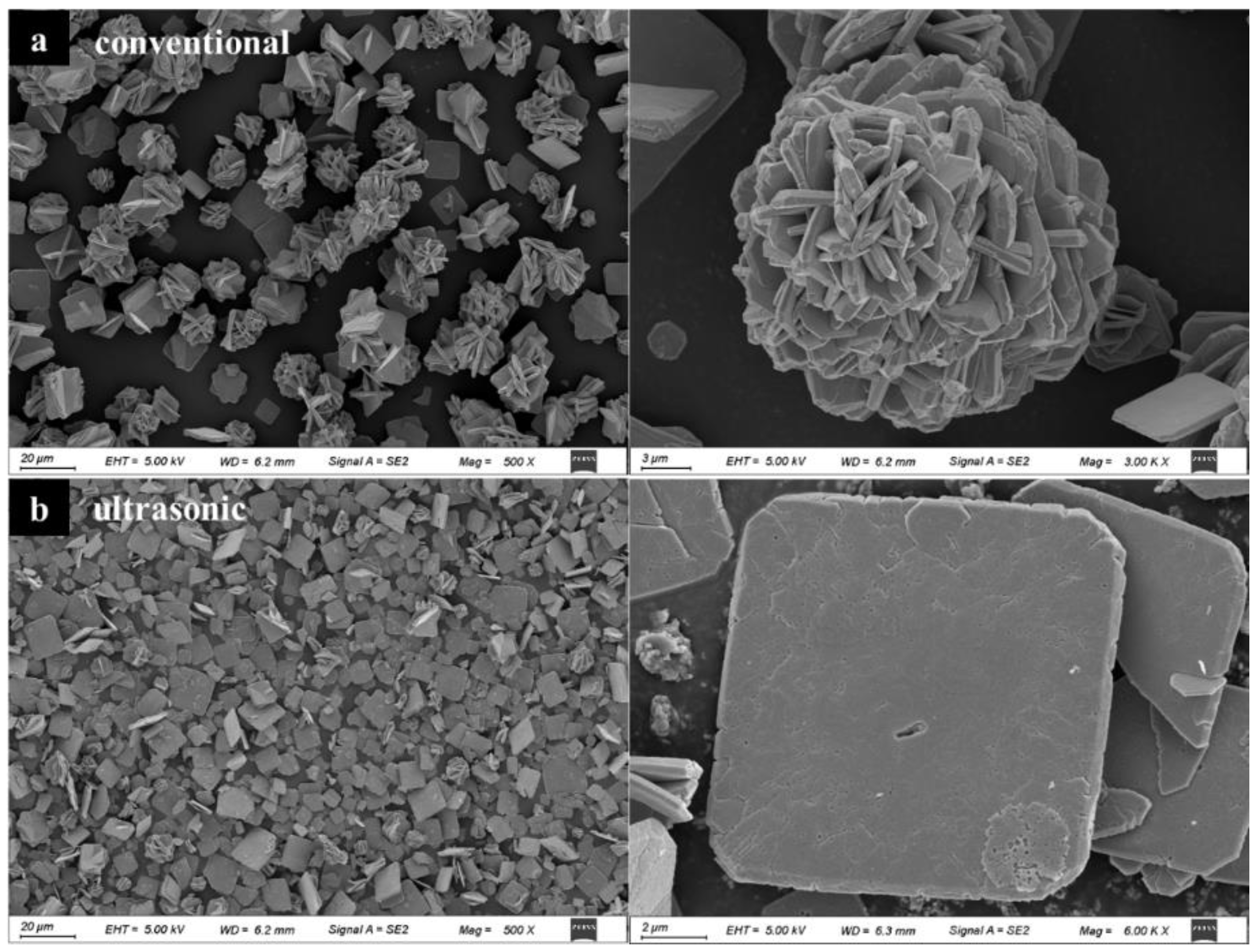
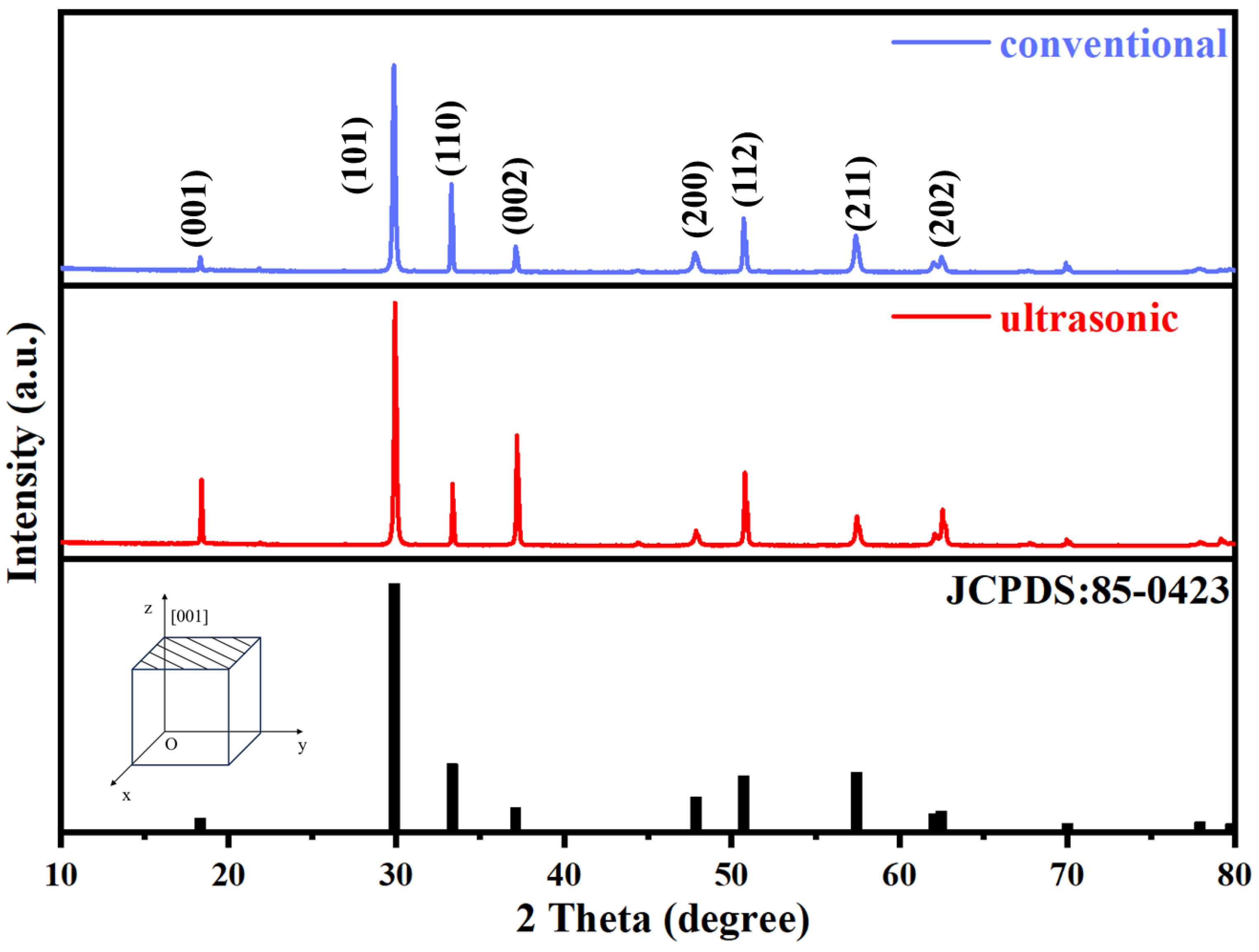
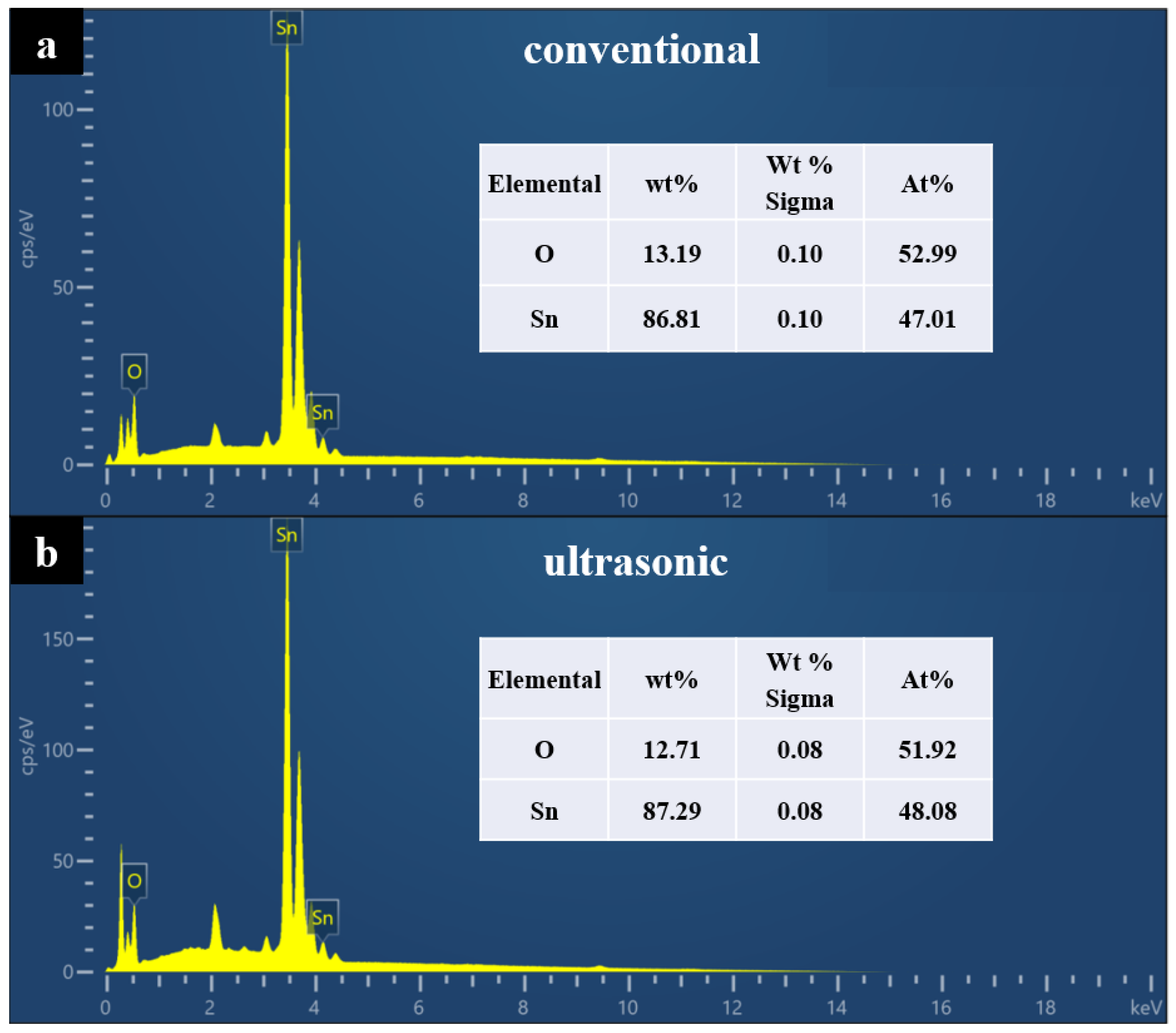
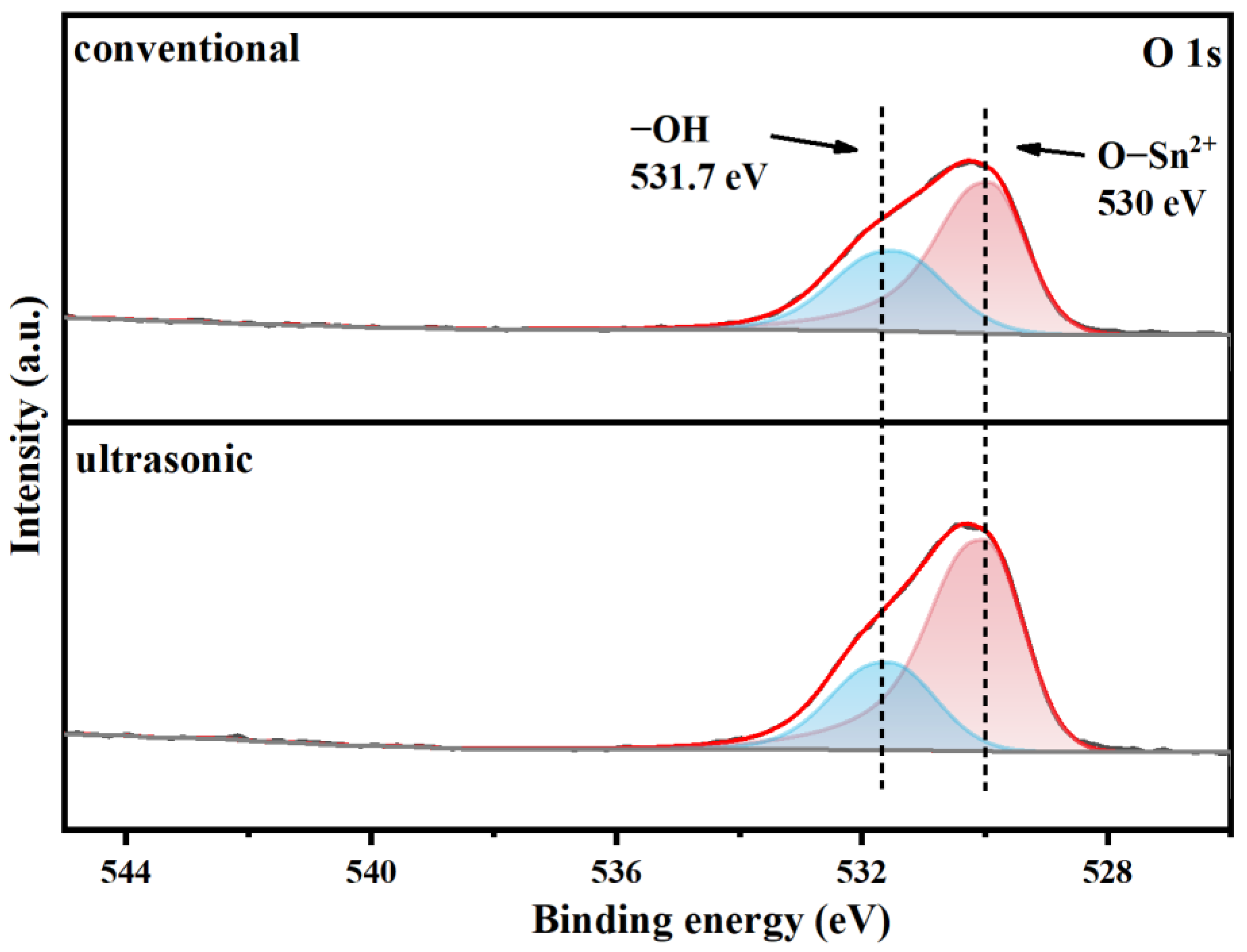
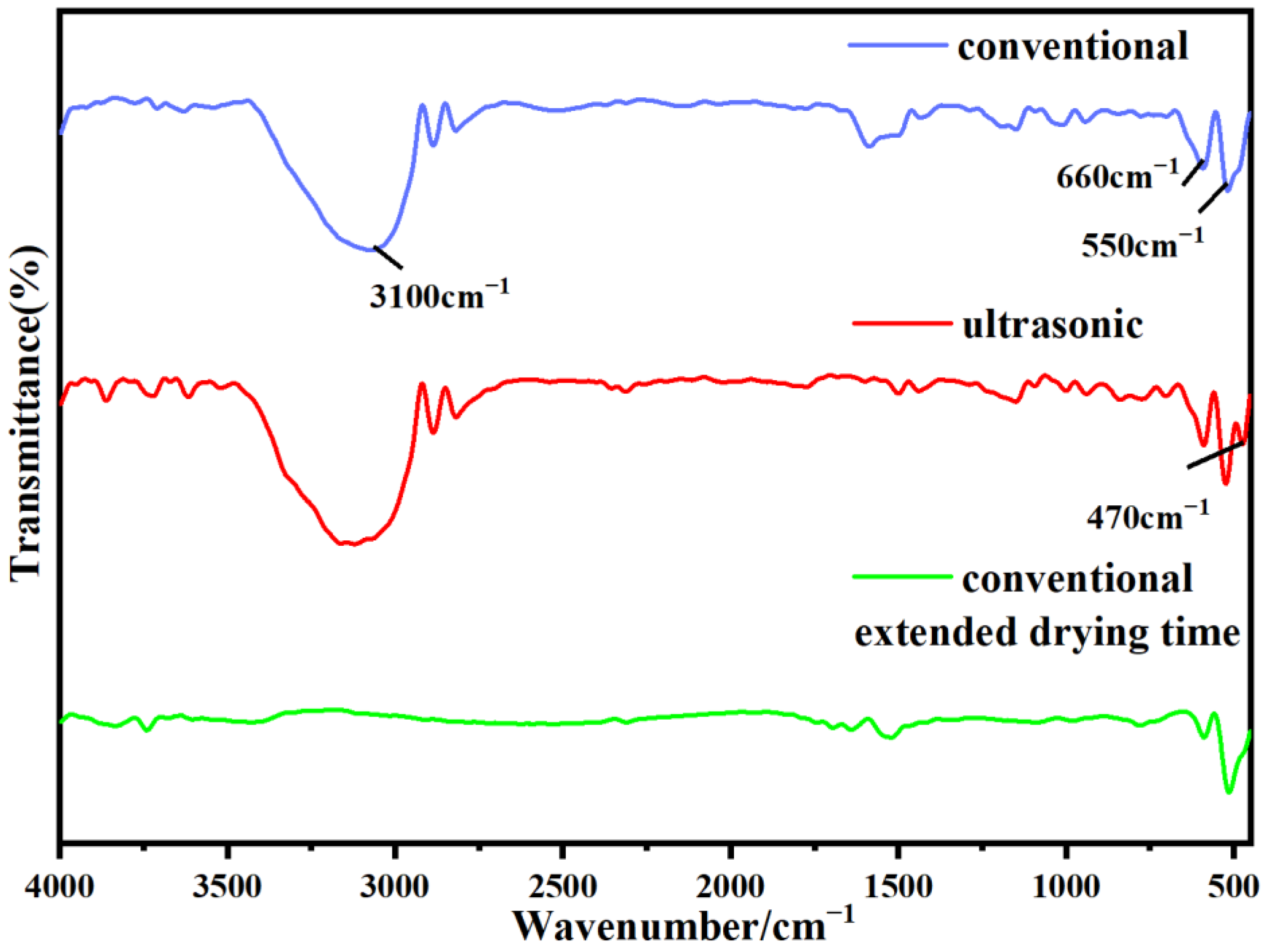
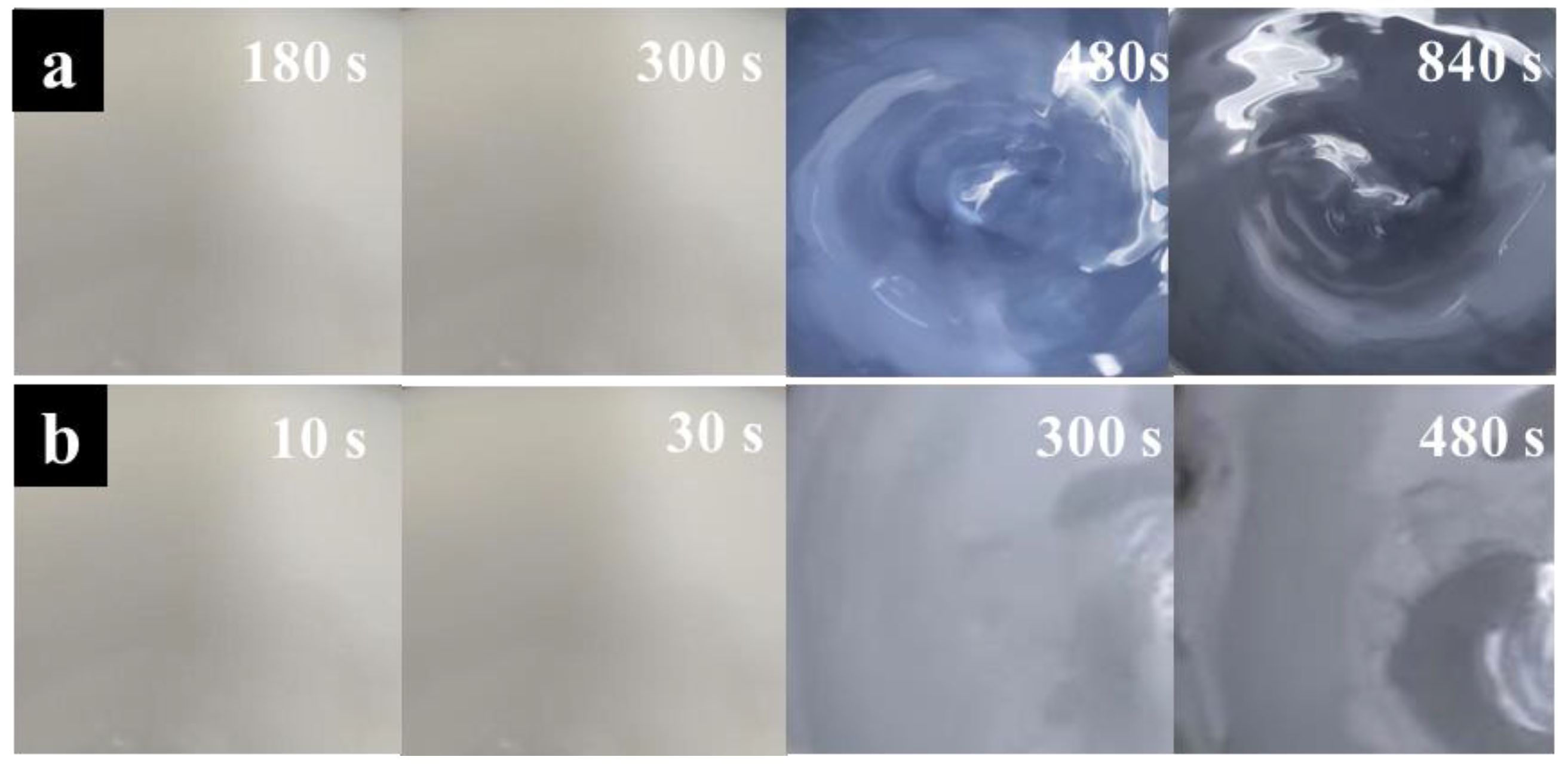
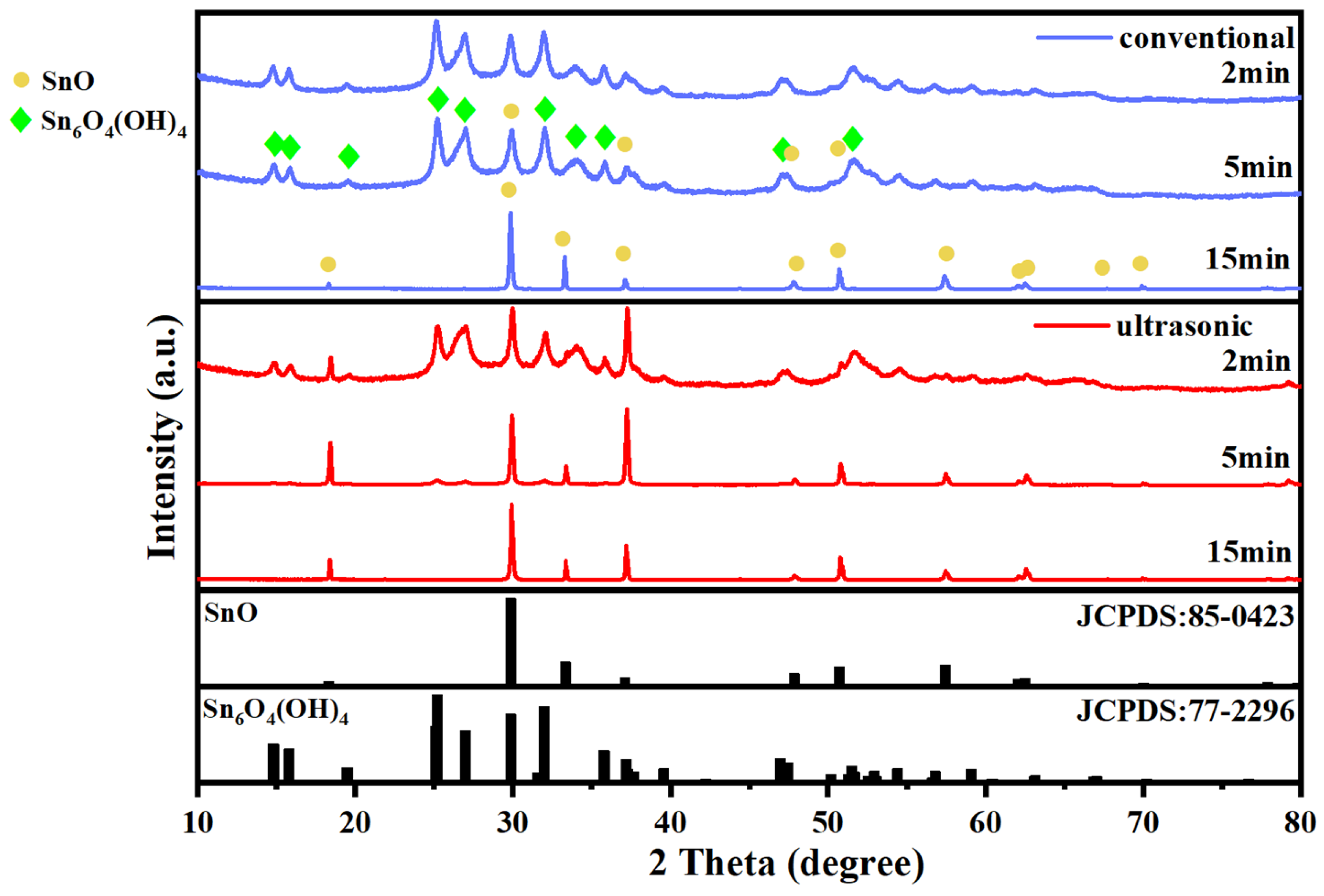
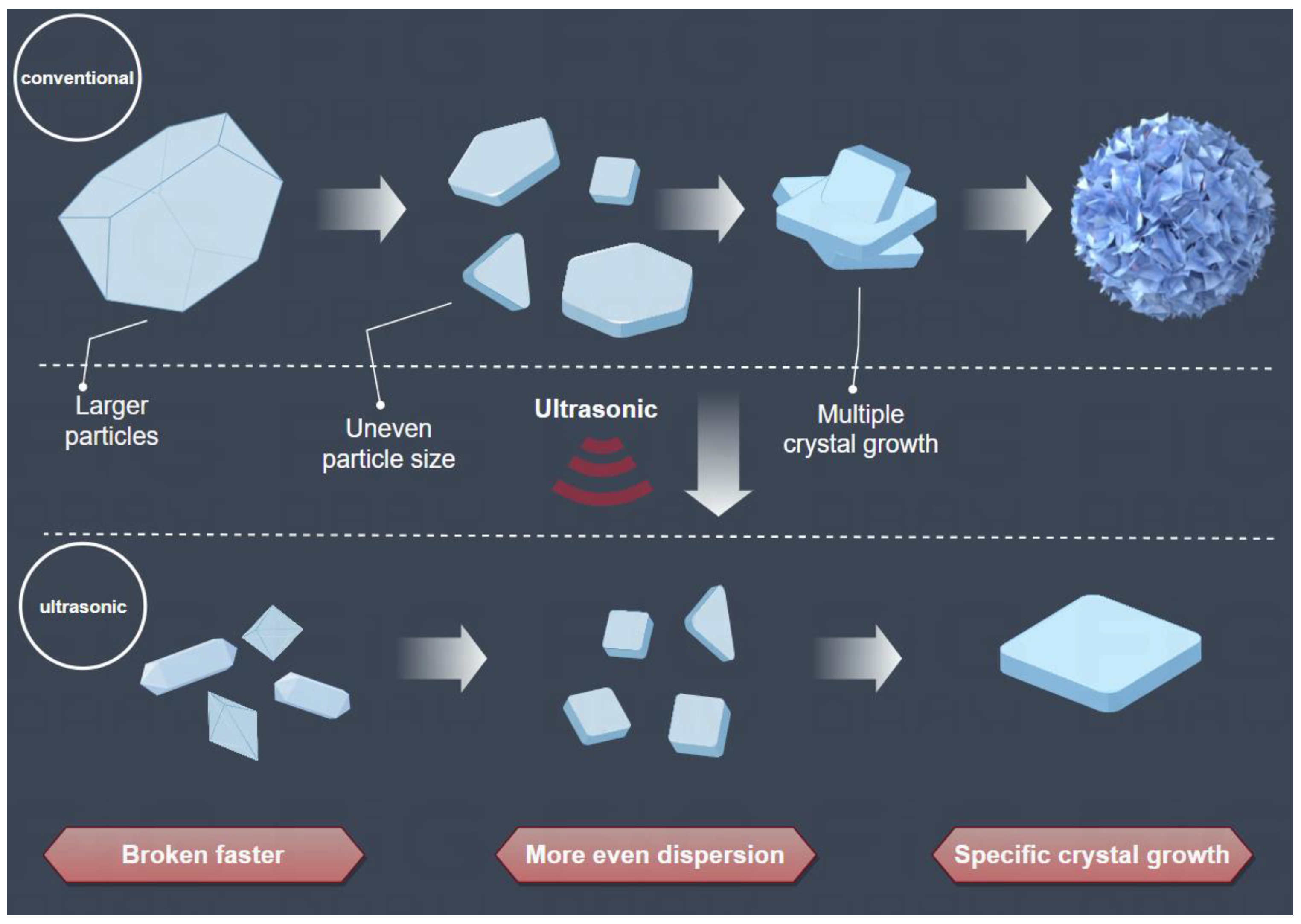
| Samples | Sn% | SnO Content% |
|---|---|---|
| Conventional | 85.48 | 97 |
| Ultrasonic | 86.99 | 98.7 |
| Sample | Specific Surface Area (m2/g) | Pore Volume (m3/g) | Average Pore Size (nm) |
|---|---|---|---|
| Conventional | 1.099 | 0.005 | 3.417 |
| Ultrasonic | 2.877 | 0.006 | 3.407 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, M.; Xiang, L.; Zhang, Q.; Xv, T.; Le, T.; Zhang, L. Preparation of Small-Sized and Uniformly Distributed SnO by Ultrasound at Room Temperature. Metals 2025, 15, 643. https://doi.org/10.3390/met15060643
Fu M, Xiang L, Zhang Q, Xv T, Le T, Zhang L. Preparation of Small-Sized and Uniformly Distributed SnO by Ultrasound at Room Temperature. Metals. 2025; 15(6):643. https://doi.org/10.3390/met15060643
Chicago/Turabian StyleFu, Mingge, Liuxin Xiang, Qian Zhang, Tao Xv, Thiquynhxuan Le, and Libo Zhang. 2025. "Preparation of Small-Sized and Uniformly Distributed SnO by Ultrasound at Room Temperature" Metals 15, no. 6: 643. https://doi.org/10.3390/met15060643
APA StyleFu, M., Xiang, L., Zhang, Q., Xv, T., Le, T., & Zhang, L. (2025). Preparation of Small-Sized and Uniformly Distributed SnO by Ultrasound at Room Temperature. Metals, 15(6), 643. https://doi.org/10.3390/met15060643






