Abstract
Parts fabricated by laser powder bed fusion (LPBF) contain oxide inclusions, which can be detrimental to fatigue resistance. Under typical LPBF conditions, the atmosphere contains enough oxygen to oxidize reactive elements such as aluminum and titanium, forming oxides in the parts. In this work, mechanisms of oxide formation and oxide alteration were studied by laser-remelting the surfaces of bulk specimens of IN718 and AlSi10Mg, without the addition of metal powder. Calculations based on the mass transfer of oxygen to the melt pool surface indicated that direct oxidation of the melt pool did not play a major role. Rather, both the oxidation of hot spatter and reworking of the pre-existing oxide affected the concentration and morphology of oxides on the metal surface.
1. Introduction
Defects in components produced by laser powder bed fusion (LPBF) include pores and oxide inclusions. Most previous work has focused on pores [1,2,3,4] and some on oxide inclusions [5,6]. This work focuses on oxide inclusions in AlSi10Mg and IN718 LPBF components, studying the mechanism of oxide formation during remelting conditions that were chosen to be the same (in terms of power, laser speed, material, and chamber atmosphere) as for LPBF. Although the concentration of aluminum in IN718 is only 0.2–0.8 mass%, aluminum is the most readily oxidized element in this alloy, forming alumina during LPBF. Oxide inclusions were previously identified as fatigue initiation sites at the surfaces of AlSi10Mg specimens produced by LPBF [5], but the origin of the oxides was not explicitly identified.
One mechanism is the oxidation of spatter (hot, molten droplets ejected from the melt pool). Simonelli et al. reported selective oxidation on the surface of spatter particles ejected from a melt pool for AlSi10Mg and AISI 316L stainless steel [7].
To distinguish powder particles from spatter particles which were ejected from a melt pool during a laser scan of metal powder, in this study, we melted planar IN718 and AlSi10Mg specimens without metal powder and subsequently analyzed the surfaces of the laser-scanned beads by optical microscopy and scanning electron microscopy (SEM). The experimental approach used here has some similarities to laser polishing [8]; however, previous work on laser polishing focused on changes in surface roughness and hardness, and did not give detail on changes in surface oxide (though microanalyses reported in one paper did show the presence of oxides on laser-polished AlSi10Mg [9]).
Instead, the aims of this work were to characterize the oxides on the surfaces of laser-remelted samples, to evaluate the contribution of direct oxidation of the melt pool to oxide formation, and to assess whether pre-existing oxide films are changed (reworked) during the melting of the surface. For comparison with the surface oxides, inclusions within IN718 samples produced by LPBF were imaged. The work indicates that spatter oxidation is the main oxidation mechanism, and that large oxides can form on the melt pool surface from the reworking of the pre-existing oxide.
2. Materials and Methods
2.1. Plate Melting
2.1.1. Materials
In this work, bulk materials were used in melting tests to eliminate the effects of the metal powder. A rolled sheet was used for the Inconel 718 specimens (their composition is in Table 1; supplied by McMaster-Carr, Cleveland, OH, USA). AlSi10Mg samples were cut from a cylinder previously fabricated by LPBF (stress-relieved at 300 °C after building); the oxygen content was approximately 400 ppm (parts per million by mass). The samples were cut by EDM to the dimensions 19.5 × 19.5 × 6.35 mm3. The Inconel 718 samples were wet-ground (with SiC paper) to 800 grit and the AlSi10Mg samples by a diamond suspension to 3 µm. The polished specimens were ultrasonically cleaned in ethanol and dried with compressed air.

Table 1.
IN718 plate composition.
To study the effect of the existing oxide film, a set of AlSi10Mg specimens were anodized after polishing. The electrolyte contained 15% H2SO4 and 85% H2O by volume, the applied current density was 1.2 A/dm2, and the anodization time was 3 s, giving an expected additional oxide film thickness of 13 nm (if expressed as the equivalent thickness of a dense oxide film) [10]. However, the anodized film was porous, as shown in Figure 1, and the actual additional thickness of the oxide film was larger than 13 nm. The anodized sample was subsequently ultrasonically cleaned with ethanol and dried with compressed air.
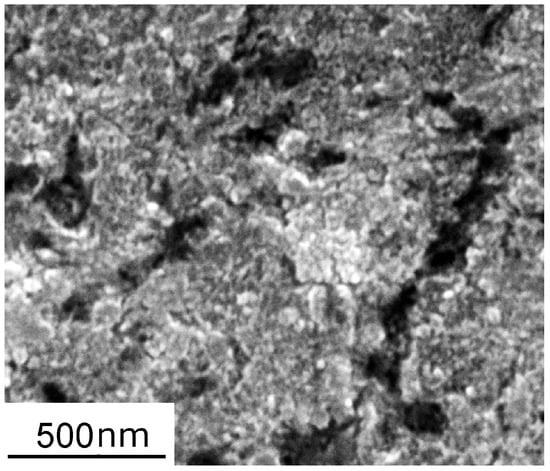
Figure 1.
Secondary electron image of anodized AlSi10Mg surface.
2.1.2. Melting
The specimens were melted with an EOS M290 selective laser melting (SLM) unit using four different combinations of laser power and speed; the melting conditions are summarized in Table 2 and Table 3. The unit uses a continuous Yb fiber laser (wavelength 1.06–1.1 µm) with a spot diameter of approximately 100 µm. The standard condition (“STD” in Table 3) is as recommended by EOS. The hatch spacings were chosen so that adjacent melt pools would overlap, based on melt pool widths estimated with the Rosenthal equation [11]. In one case (IN718, lower power), the area was scanned twice to have the same total melting time as for the low power–low speed case. In all other tests, the area was melted only once. Figure 2 shows schematics of the single and multi-pass scanning patterns.

Table 2.
Basic parameters of SLM process used in this work.

Table 3.
Beam power, beam speed, and scan times for each condition.
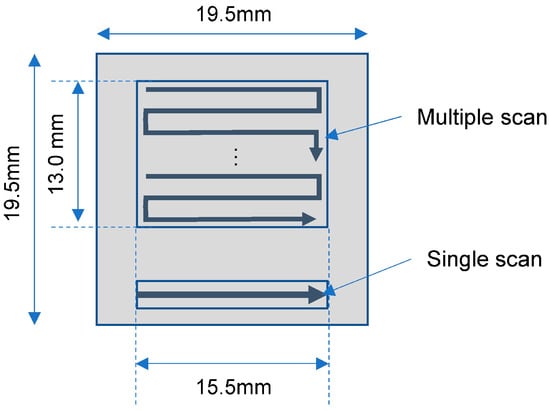
Figure 2.
Laser scan patterns.
2.2. Characterization
The surface morphology of the melted regions and melt pool cross-sections was studied by optical microscopy. Inclusions and oxides were analyzed using scanning electron microscopy with energy-dispersive X-ray spectroscopy (SEM/EDX). To estimate the thickness of the oxides, NIST DTSA-II [12] was used to simulate the detected peaks for various oxide thicknesses on the bulk metal. The parameters used in the DTSA-II simulation are shown in Table 4.

Table 4.
Parameters used for simulation of EDX spectra.
Inclusions were examined in IN718 parts produced by laser powder bed fusion (EOS M290, standard conditions). The specimens were etched electrolytically to remove the metallic matrix, leaving the inclusions on the specimen surface. The electrolyte consisted of 60 mL of anhydrous methanol, 2.0 g of tetramethylammonium chloride, and 4.85 mL of acetylacetone; the cathode was a graphite rod (6 mm diameter), the applied voltage was 10 V, and the etching time was 15 min. The etched specimens were ultrasonically cleaned in methanol and dried in a stream of compressed air. While all the major elements in IN718 are readily chelated by acetylacetone [13], silicon is not [14], the result being that the matrix of the AlSi10Mg samples was not fully dissolved. Rather, the Al-rich phase dissolved, leaving behind the cellular structure of silicon—see Figure 3. This is similar to the structure produced by the electrochemical polishing of AlSi10Mg [15].
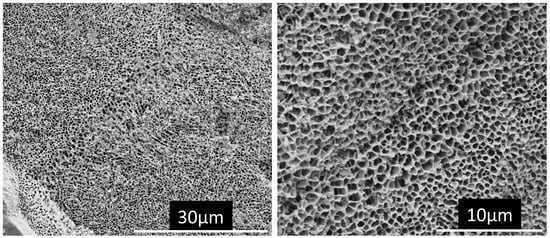
Figure 3.
Secondary electron micrographs of the deep-etched AlSi10Mg LBPF sample.
3. Results
3.1. Inclusion Observation: Deep-Etched Sample
Figure 4 shows typical inclusions in the IN718 LBPF samples. Chemically, most inclusions were alumina; the shapes were spherical or plate-like, with observed lengths of up to about 30 microns (for plate-like inclusions).
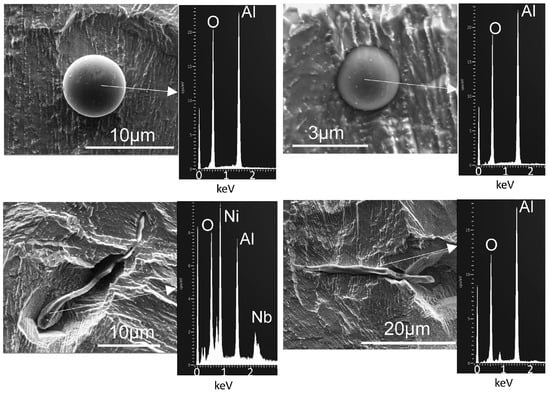
Figure 4.
Secondary electron micrographs of inclusions in deep-etched IN718 LPBF sample, with EDX spectra showing prominent Al and O peaks.
3.2. Melt Bead Analysis of Laser-Melted Plate Samples
3.2.1. IN718
The cross-sections and measured sizes of the single-bead melt pools for the four conditions are shown in Figure 5 (note that the magnifications differ) and Table 5. As expected, the keyholing condition resulted in a deeper melt pool. However, even for the standard and low P–low V conditions, the ratio of the melt pool depth to width was larger than 0.5. A D/W ratio larger than 0.5 indicates that some extent of keyholing has occurred, even under these melting conditions. For alloys with relatively low thermal conductivity —like IN718—the melt pool width is approximately proportional to (P/V)0.5 [16], resulting in a significantly smaller melt pool for the low-power case (P/V = 177 J/m) than for the low P–low V (P/V = 340 J/m) and standard (P/V = 297 J/m) cases.

Figure 5.
Cross-sections of IN718 single beads. Melting conditions: (a) STD, (b) Key, (c) Low P, and (d) Low P–low V.

Table 5.
IN718 single beads: melt-pool dimensions and ratio of melt pool depth to width.
Optical microscopy (Figure 6) shows many dark dots, apparently spherical particles, and elongated streaks on the laser-melted surface. The darker dots also appear dark in the backscattered electron images (Figure 7 and Figure 8), showing that these consist of lighter elements than the metal, and EDX (Figure 8) confirmed that these are aluminum oxide. Oxide shapes include clusters of small spheres, larger spheres, and linear streaks (Figure 8).
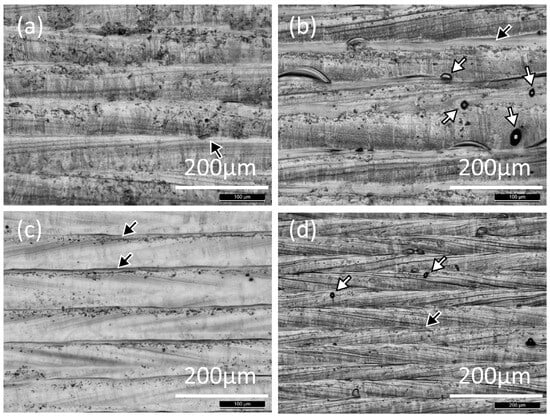
Figure 6.
Optical micrographs of the surface of overlapping laser scans on an IN718 plate, for different melting conditions: (a) STD, (b) Key, (c) Low P, and (d) Low P–low V. The black arrows indicate continuous streaks and the white arrows indicate spherical particles.
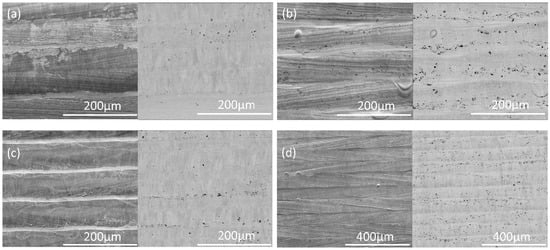
Figure 7.
Scanning electron micrographs of the IN718 plate surfaces melted with different laser conditions: (a) STD, (b) Key, (c) Low P, and (d) Low P–low V. In each pair, a secondary electron image is given on the left and a backscattered electron image on the right.
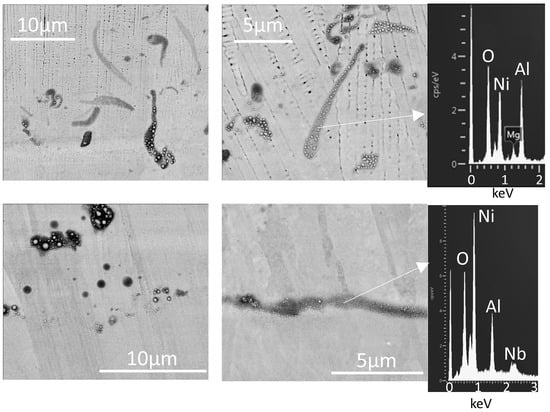
Figure 8.
Backscattered electron micrographs showing dark areas on laser-remelted IN718 at higher magnification, with EDX spectra of these areas.
Figure 9 summarizes the particle sizes. The sizes were measured (using ImageJ version 1.52u [17]) for three areas (each approximately 300 µm × 600 µm) in the BSE images for each melting condition; sizes are presented as equivalent circle diameters. The sizes (and size distributions) were similar for all conditions, but with significantly more particles per unit area for the keyholing condition. Surfaces remelted with a lower scanning speed (Key and Low P–low V) had a larger area fraction of oxides; the larger area fraction for keyholing conditions is consistent with more oxide production by spatter, as discussed later. The large surface area fraction of oxides of the Low P–low V case may be related to the large melt pool (Table 5), significantly larger than for the standard and low-power cases; the larger melt pool would have remained liquid for longer.
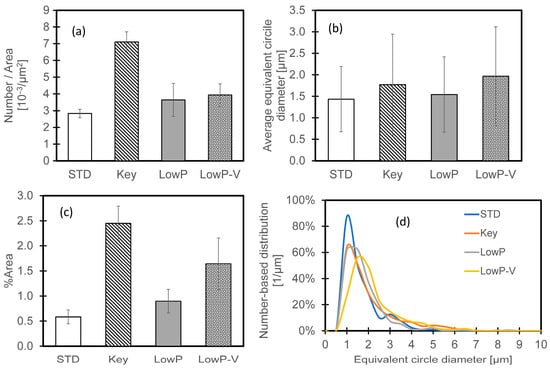
Figure 9.
Particle sizes and numbers on laser-remelted IN718 specimens. (a) Oxide particle numbers per unit area. (b) Average particle sizes. (c) Area fraction of oxide particles. (d) Number-based size distribution. Error bars indicate one standard deviation.
3.2.2. AlSi10Mg
Cross-sections of the single beads (Figure 10 and Table 6) confirmed the deep melt pool for the keyholing condition and nearly semi-circular melt pools for the other cases. The relative difference in melt pool size between the low P and low P–low V cases is not as large for AlSi10Mg (Table 6) as it is for IN718 (Table 5); for metals with high thermal conductivity (like AlSi10Mg), the dependence of melt pool width on (P/V) is weaker than for metals with lower thermal conductivity [16].

Figure 10.
Cross-sections of AlSi10Mg single beads. Process conditions: (a) Std, (b) Key, (c) Low P, and (d) Low P–low V. Broken lines indicate melt pool boundaries.

Table 6.
AlSi10Mg single beads: melt-pool dimensions and ratio of melt pool depth to width.
Similar to IN718, spherical particles and streaks are evident in the optical micrographs of the surface of the laser-remelted AlSi10Mg (Figure 11). However, bands of oxides at the borders of the melt pools are more evident on AlSi10Mg, especially for single beads (Figure 12). Although the oxide bands at the melt pool edges are evident in the optical micrographs, the bands are quite thin, since these regions do not appear darker in the backscattered electron images (Figure 13), except for narrow regions at the edges of the oxide bands (see also the higher-magnification images in Figure 14, with a representative EDX spectrum, confirming the dark particles to be aluminum oxide).

Figure 11.
Optical micrographs of the surface of the AlSi10Mg samples remelted with different laser conditions: (a) STD, (b) Key, and (c) Low P–low V. The black arrows indicate oxide streaks and the white arrows indicate spherical particles.

Figure 12.
Optical micrographs of the surfaces of the single-scan bead surface of the AlSi10Mg samples remelted under different conditions: (a) STD, (b) Key, (c) Low P, and (d) Low P–low V.
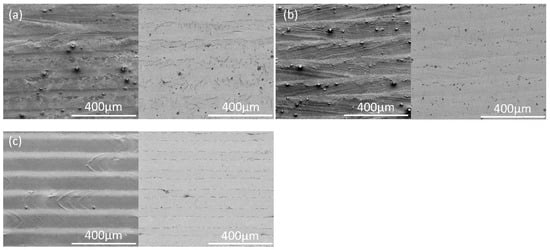
Figure 13.
Scanning electron micrographs of the surfaces of AlSi10Mg parts remelted with different overlapping laser scans. (a) STD, (b) Key, and (c) Low P–low V. In each pair, a secondary electron image is given on the left and a backscattered electron image on the right.
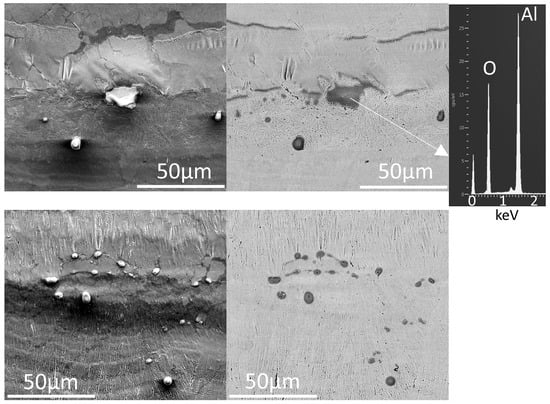
Figure 14.
Higher-magnification scanning electron micrographs of oxides on single bead surfaces for the AlSi10Mg samples, melted under standard conditions (upper pair) and keyholing conditions (lower pair). In each pair, a secondary electron image is given on the left and a backscattered electron image on the right.
The ratio of the oxygen peak to the aluminum peak in the EDX spectra was used to estimate the oxide thickness in the different regions. Based on this ratio (Figure 15), four regions were distinguished (for single-bead regions) based on oxide thickness: (i) the original (unmelted) region, where a weak O peak is detected; (ii) an oxide band at the melt pool edge (the O peak is slightly higher than the unmelted metal), (iii) a thick oxide at the inner edge of the oxide band, and (iv) an exposed metal region along the center of the melt pool (with an O peak is hardly detected). For the keyholing condition, in region (iii) (thicker oxide), more spherical (less elongated) oxides were observed, and these oxides were more discontinuous than for the other conditions. These regions appear to arise from flow on the melt pool surface—that tends to move oxide to the melt pool edge—as well as the tendency for the oxide to agglomerate under the effect of surface and interfacial tension. These proposed steps are consistent with the exposure of metal at the melt pool center (with a very thin oxide layer, estimated below) and accumulation of oxide at the melt pool edges. For keyholing conditions, the melt pool is larger (shown in Figure 10b) and remains molten for longer, allowing for more reworking of the initially thin oxide at the melt pool edge into more spherical particles (see Figure 15b for examples).
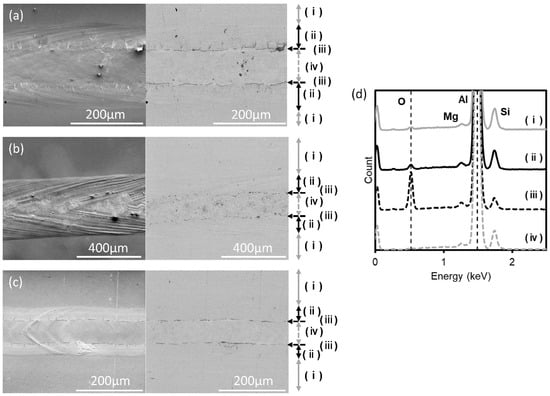
Figure 15.
Scanning micrographs of single beads on AlSi10Mg surfaces (secondary electron images on the left and backscattered electron micrographs on the right), for different melting conditions: (a) STD, (b) keyholing, and (c) Low P–low V conditions. The arrows on the right of the images indicate different regions: (i) unmelted, (ii) oxide band at melt pool edge, (iii) thicker oxides at the edge of the oxide band, and (iv) metal exposed along the center of the melt pool. (d) Representative EDX spectra of the four regions (15 kV acceleration voltage).
Particle sizes (Figure 16) were measured using the same approach as for IN718, measuring four areas (100 µm × 240 µm) for each condition. Compared with the standard condition, the keyholing condition resulted in more, but smaller, particles—consistent with more breaking up of the oxide streaks at the edge of the keyholing melt pool. The lower-speed condition resulted in fewer particles, smaller than for the standard condition and covering much less of the surface.
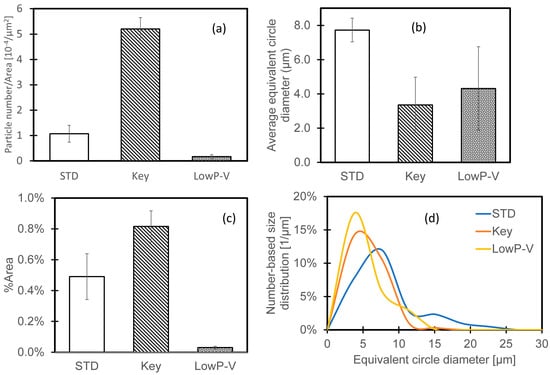
Figure 16.
Oxide particle size analysis results on the surface of laser-remelted AlSi10Mg samples: (a) particle number; (b) average particle; (c) area fraction; (d) number-based particle size distribution. Error bars indicate one standard deviation.
Similar regions were seen on the surfaces of the anodized samples after remelting, but the films along the melt pool edges are more evident in the optical micrographs (Figure 17) of the anodized samples. The widths of the melt pool and of the exposed metal region along the melt pool centerline were similar for the polished and anodized samples for all melting conditions (Figure 18).
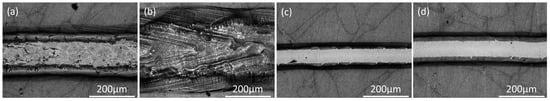
Figure 17.
Optical micrographs of single beads on AlSi10Mg samples that were anodized before laser melting under different conditions: (a) STD, (b) Key, (c) Low P, and (d) Low P–low V.
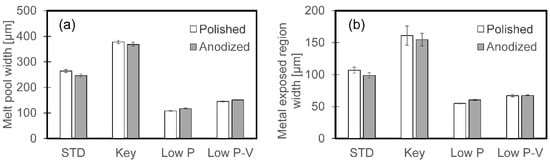
Figure 18.
The widths of (a) the melt pool and (b) metal exposed at the center of the melt pool, for single beads on polished and anodized AlSi10Mg. The error bars indicate one standard deviation.
Scanning electron micrographs (Figure 19) show that the thicker oxides were more continuous on the anodized sample, but with very similar EDX spectra, and with larger oxides for the keyholing condition. The higher-magnification images of the thick oxide regions shown in Figure 20 show that larger spherical oxides occurred at the higher heat input (higher P/V ratio; keyholing condition).
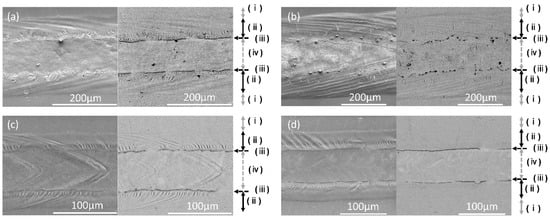
Figure 19.
Scanning electron micrograph images of single beads on the anodized AlSi10Mg samples (secondary electron images on the left and backscattered electron micrographs on the right), for different melting conditions: (a) STD, (b) Key, (c) Low P, and (d) Low P–low V. Arrows to the right of the images indicate the extent of different regions: (i) unmelted region, (ii) oxide band at melt pool edge, (iii) thicker oxides at the edge of the oxide band, and (iv) metal exposed along the center of the melt pool.
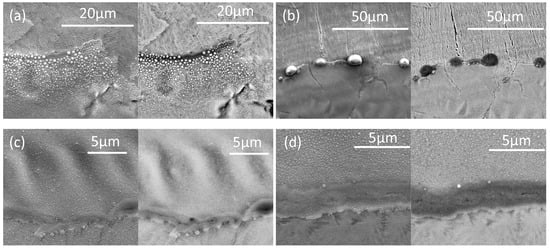
Figure 20.
Higher-magnification scanning micrographs in the thick-oxide regions of single beads on the anodized AlSi10Mg samples, for different melting conditions: (a) STD, (b) Key, (c) Low P, and (d) Low P–low V. In each pair, a secondary electron image is given on the left and a backscattered electron image on the right.
Lower-voltage EDX analyses (at 5 kV, with a shallower interaction volume) were used to estimate the oxide thickness more accurately, with better sensitivity for light elements [18]. Figure 21a,b show simulated EDX spectra of different thicknesses of alumina on bulk AlSi10Mg, simulated with DTSA-II [12]. Compared with 15 kV, the 5 kV spectrum shows a larger oxygen peak relative to aluminum, which allowed more accurate estimation of the thickness of thin alumina layers. The relationship between the O/Al peak ratio and oxide thickness, shown in Figure 21c, was used to estimate the oxide thicknesses.
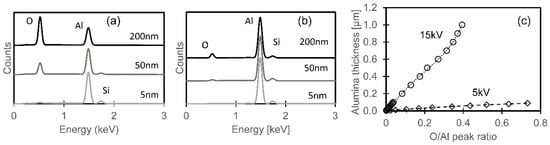
Figure 21.
Simulated EDX spectra for different alumina film thicknesses on bulk AlSi10Mg, at acceleration voltages of (a) 5 kV and (b) 15kV. (c) Relationship between film thickness and simulated O/Al peak count ratio.
The estimated oxide thicknesses for each region (for both polished and anodized AlSi10Mg samples) are shown in Figure 22. For the unmelted regions, the estimated difference in oxide thickness between the polished and anodized specimens was 9.4 nm, similar to but less than the estimated oxide growth by anodization (16 nm); this difference likely reflects the difference between the actual porous nature of the anodized film and the dense oxide assumed when calculating the relationship in Figure 21c. The metal exposed along the melt pool centers had similar oxide thicknesses for each condition and for the polished and anodized specimens; the average thickness (3.7 nm) is close to the thickness of the passive oxide film naturally formed on aluminum [19]. The band of oxide along the melt pool edge was thicker on the anodized samples (than on the polished surfaces); the difference in the average oxide thickness (12.3 nm) is similar to the estimated additional anodized oxide film thickness (13 nm). The oxide particles along the inner edge of the oxide band were much thicker for the keyholing condition (an average thickness of 780 nm).
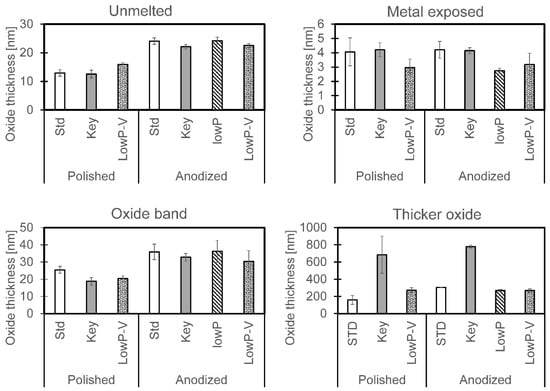
Figure 22.
Estimated oxide thickness for each region and melting condition, for polished and anodized AlSi10Mg. Error bars indicate one standard deviation.
Since the AlSi10Mg sample had a significant internal oxygen content (around 400 ppm), it is of interest whether some of this internal oxide collected on the melt pool surface during remelting. To this end, the volumes of the oxides were calculated based on the estimated oxide thickness and the width of each region (shown in Figure 18). The areas of discontinuous thick oxide regions were measured from the BSE images using ImageJ (version 1.52u). The volume calculation assumed that the oxide thicknesses were uniform in each region. The measured thickness, areas, and calculated oxide amount (expressed as an average thickness) are shown in Figure 23. After the laser scan, the oxide amount increased for all conditions. (Note that the calculated increase in oxide thickness for the Low P–low V condition applied to the polished sample was small because the original oxide thickness of this sample—estimated from its EDX spectrum—was anomalously large. If the oxide thickness of the polished sample remelted under the Low P–low V condition is taken to be the same as for the other conditions [12 nm], the oxide thickness increment after melting would be 3 nm.) The increase in oxide thickness was larger for the standard and keyholing conditions, for both polished and anodized samples (but slightly less for the anodized specimens). However, these increases in thickness are small compared with the total oxygen in the part. For example, taking the total oxygen content of the AlSi10Mg sample to be 400 ppm before melting, and using the melt pool dimensions for standard conditions given in Table 6, the increase in oxide thickness would be approximately 50 nm if all of the internal oxygen were transferred to the surface as Al2O3. This increase in oxide thickness due to the transfer of oxide from the interior of the melt pool was estimated as follows:
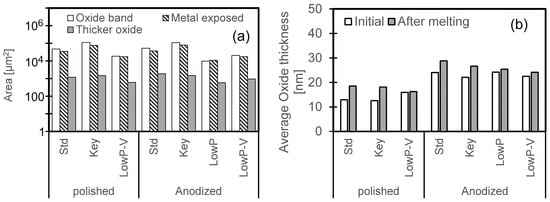
Figure 23.
Oxide areas and thicknesses on polished and anodized AlSi10Mg specimens. (a) Calculated areas of each region. (b) Oxide amount on melted region, expressed as an average thickness for unit area.
Approximating the cross-sectional shape of the melt pool as an ellipse, a melt track of length L, width W, and depth D has a volume of
The volume of oxide (Al2O3) within the melt track is given by
where ρM is the density of the alloy (taken to by 2700 kg/m3), ρAl2O3 is the density of the oxide (3990 kg/m3), and foxide is the mass fraction of oxide in the melt track interior. If the oxygen concentration in the melt pool is 400 ppm, all of it present as Al2O3, the mass fraction of oxide is 400 × (51/27) = 756 ppm, where (51/27) is the ratio of the molar mass of AlO1.5 to that of Al. If this oxide were to be transported to the melt pool surface, the resulting oxide thickness (h) would be given by
Using D = 132 µm (Table 6), and the other values as stated, yields h = 53 nm. This is much more than the observed increase in the oxide thickness on the melt pool surface after melting, which is just a few nanometers. This means that, while oxide on the surface of the metal is significantly reworked during melting, there is little transfer of oxide from the interior of the melt pool to its surface.
4. Discussion
Three possible mechanisms by which the alumina forms were considered. The first is direct oxidation of the melt pool, by the oxygen in the chamber gas (the typical oxygen concentration in the chamber is 0.1%, much higher than for equilibrium with aluminum in either alloy). Second, hot, liquid spatter ejected from the melt pool could oxidize as it travels through the furnace atmosphere, forming spherical particles (or disks if spread out on the part surface). The last is the reworking of oxide that existed before melting, such as the oxide film on the powder or—in this work—that on the part surface. Even though the pre-existing oxide on the powder or plate samples is thin, the melt pool temperature may be sufficiently high to agglomerate these oxides into thicker particles. Each of these mechanisms is considered in turn.
4.1. Melt Pool Oxidation
The combination of temperature, super-equilibrium oxygen concentration, and mobility of aluminum in the liquid alloy allow the oxidation of aluminum at the melt pool surface. However, the solidification time of the melt pool is short in LPBF (10−3–10−4 s) compared with welding (order of 10−1 s) [20], which would limit the extent of oxidation.
To test the possibility of direct melt pool oxidation, the thickness of the oxide which can form on the melt pool surface before solidification was estimated, based on the limiting case of rate control by the mass transfer of oxygen in the gas phase to the melt pool surface. The gas mass transfer coefficient was estimated with the expression of Fyrillas and Kontoghiorghes [21], assuming the melt pool surface to be approximately elliptical, with the gas flow at a constant speed () normal to the scan direction. For this case, the average Sherwood number () can be expressed by the following equation [21]:
where is the Peclet number, given by . W is the width of the melt pool, and is the diffusivity of oxygen in the gas.
The mass transfer coefficient (m) for the transfer of molecular oxygen to the melt pool surface is given by the following equation [21]:
The flux of molecular oxygen to the melt pool surface () is expressed by
where is the molar concentration of oxygen, is the partial pressure of oxygen in the chamber, R is the ideal gas constant, and T is the absolute temperature.
Assuming that all oxygen transferring to the melt pool surface reacts with only Al to form Al2O3, the oxide formation rate per unit area () is described by
If the oxidation time is taken to be where is the beam speed and L is the melt pool length, the thickness of the Al2O3 formed by the oxidation of the melt pool surface () is given by
The values used in this calculation are listed in Table 7. The gas speed is based on the work of Bidare et al. [22]. The film temperature was taken as the average temperature of the chamber temperature (approximately equal to the room temperature, 298 K) and the liquidus temperature.

Table 7.
Input parameters used for estimation of oxide thickness.
Table 8 shows the melt pool sizes calculated by using the Rosenthal equation [11], using an absorptivity of 0.35. The Rosenthal melt pool lengths (L) were used for the oxidation estimate; the comparison with the measured melt pool widths (Table 5 and Table 6) indicates that the Rosenthal values are reasonable.

Table 8.
Beam conditions and estimated melt pool size and oxide thickness.
The estimated oxide thickness resulting from direct melt pool oxidation (Table 8) is around 1 nm for IN718 and 0.1–0.3 nm for AlSi10Mg. This is small compared with the average oxide thickness for AlSi10Mg (Figure 23). For IN718, the typical area coverage by oxides (around 1%, see Figure 9) and typical oxide thickness (around 0.5 µm [23]) give an estimated average thickness of the deposited oxide of approximately 5 nm, which is also larger than the calculated contribution of melt pool oxidation. Therefore, it is concluded that direct melt pool oxidation is not the main cause of oxide inclusions in LPBF parts. This conclusion contrasts with the work of Chia et al. [24,25], who focused on oxidation within the metal vapor plume and oxygen transfer to the melt pool immediately below the plume; as discussed in the next section, the oxidation of spatter—and the incorporation of oxidized spatter in the build—appears to be of greater importance.
4.2. Spatter Oxidation
Previous research showed that oxides cover the surface of spatter [7,26]; the ejection of spatter from the melt pool has been widely reported and studied [7,25,26,27,28,29]. An example of spatter ejection observed in this work (without powder) is shown in Figure 24.
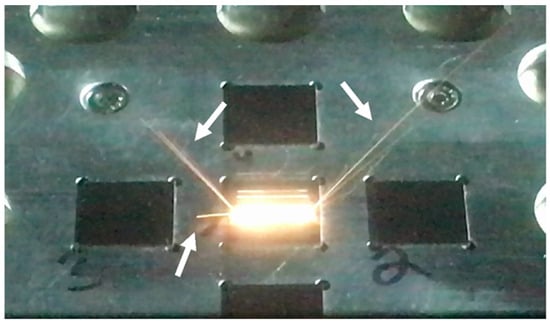
Figure 24.
Image of beam scan of bulk IN718 specimen (standard condition). Arrows indicate spatter ejected from melt pool. Square regions are samples with 20 mm side lengths.
For spatter to oxidize, both the reaction rate and the temperature need to be high. Previous calculations have indicated that the oxidation of aluminum from molten IN718 spatter is limited by oxygen mass transfer to the spatter, that the reaction is rapid for droplets in the size range of microns to tens of microns, and that the exothermicity of oxidation would exceed radiative cooling for the oxidation of droplets smaller than approximately 20 µm in diameter [23] (larger particles would tend to cool upon ejection, and not oxidize to the same extent). This calculated size effect is supported by the observation that oxide particles observed in built parts and on top of the beads were less than 10 µm in size for the IN718 specimens, even though the size range of spatter particles is 1–270 µm [26].
Previous work indicated that spatter production increases as the power density increases [29,30,31,32]. The results from this work did show significantly more deposited particles (in number and area fraction) for keyholing conditions, and for the AlSi10Mg samples, the samples melted at the lower power and lower speed had far fewer deposited particles, even though the P/V ratio was the same as for the standard conditions. For the IN718 specimens, the samples melted under the low-power condition and the low-power and low-speed condition showed similar oxide deposition (number of particles) to the standard condition (all of these are about half that of the keyholing case). However, for the IN718 tests at the low power, the melted area was scanned twice, giving approximately the same total melting time as the low-power–low-speed case, and twice that of the standard conditions—indicating about half the rate of deposition of oxides at the lower power than for standard conditions.
It is concluded that spatter oxidation is a major contribution to the oxides observed on remelted samples and in LPBF parts.
4.3. Oxide Reworking
The metal powders used in LPBF are generally covered with thin oxide films. Even for aluminum alloy atomized in air, the thickness of the oxide film is generally less than 10 nm [33], which is much less than the typical size of oxide inclusions in additively manufactured parts. When the metal powder is melted, the oxygen in the oxide film will not dissolve into the alloy since the solubility of oxygen in aluminum is low (approximately 0.2 ppb by mass) [34]. Therefore, the oxides which cover metal powder can remain in or on the melt pool and agglomerate, growing to form large oxides. In this work, the possibility of such agglomeration leading to larger oxides (“reworking”) was studied by melting the surface of plate samples.
The single-bead scans on AlSi10Mg showed three regions on the bead surface after melting: a central “metal” region covered by a few nanometers of oxide, a film of oxide at the melt pool edge, and thicker oxides between the metal and film regions. The average thickness of the oxide in these regions was similar to (and slightly thicker than) the original oxide thickness before melting (Figure 23). These results indicate that oxides on the melt surface were pushed towards the melt pool edge, by the flow of molten metal on the melt pool surface outwards from the heat source (under the influence of the surface tension gradient) [35,36]. This is the Marangoni flow mechanism that was also discussed in the context of laser polishing of AlSi10Mg [9]; in related work, Gautam et al. reported using the motion of oxide particles on a melt pool in ferrochromium to track Marangoni flow [37]. The outward flow opened up the central “metal” region. In addition, oxide thickening occurred at the boundary between the metal and filmed regions. For anodized samples, with a higher oxide load on the melt surface, agglomeration formed spherical oxides instead of lines of thicker oxides. Especially for keyholing melting, relatively large oxide spheres (approximately 10 µm of diameter) formed in the thick oxide region. For the lower-power conditions (with less intensive stirring and generally faster solidification), the consolidated oxide in the thick oxide region formed lines (streaks) along the beam scan direction. Both the rate of surface flow and the duration of the melt pool are expected to affect the extent of oxide reworking—consistent with the larger oxides found under keyholing conditions (with a larger melt pool that remains liquid for longer).
The formation of these large oxides by the reworking of the thin surface oxide appears to be a new observation, and is another mechanism—in addition to spatter oxidation—that can form micron-sized, approximately round oxides. One related observation in the literature is that the addition of laser heating to wire-arc directed energy deposition of aluminum alloy 7075 changed the distribution of oxides on the metal deposit [38]. In the current work, the location of the surface oxides—in lines parallel to and near the edge of the melt pool—is distinct from the apparently random distribution of spatter oxides. While reworking of the surface oxide was not as evident in the case of the IN718 samples, in the keyholing case (Figure 7b), some oxides appeared to be aligned with the melt pool edges, and the surfaces of samples built with powder showed some streaks of oxides close to and parallel with the melt pool edges [23]. It appears likely that both oxide on powder particles and oxide deposited by spatter can be reworked by melting—these need not be entirely distinct mechanisms.
These results do not address whether either of these two alloys is more susceptible to oxidation under LPBF conditions. If the proposed mechanism is correct, the tendency of an alloy to develop internal oxides would depend on spatter formation, as well as the fraction of the deposited spatter that is retained within the build. In this context, it has been shown that changing the build conditions to increase surface roughness (by using a larger hatch spacing, with other build conditions the same) increases the internal oxide concentration in IN718 [39], consistent with the retention of a larger proportion of the deposited oxide. The results in [39] also support the conclusion that the direct oxidation of the melt pool contributes little to the total oxide: if it did, coupons built with a smaller hatch spacing (increasing the total time of exposure of molten metal to the build atmosphere) would have had a higher internal oxide concentration—but this is the opposite of what was actually observed [39].
5. Conclusions
This work studied mechanisms of oxide inclusion formation by analyzing laser-melted surfaces of plate specimens. The main findings are summarized as follows:
- Direct oxidation of the melt pool surface is not a major contributor to oxide formation.
- Spatter oxidation is a feasible mechanism, consistent with the larger concentration of oxides on the surface of samples that were remelted under keyholing conditions.
- Reworking changes the morphology and distribution of pre-existing oxides, and can form apparently spherical oxides that are several microns in diameter (from an oxide film that is 10–20 nm thick).
- For the conditions tested in this work, the reworking of oxides was much more evident on the AlSi10Mg samples than on IN718.
- A more complete understanding of the size and concentration of oxides would require the quantification of spatter oxidation, oxide reworking, and oxide incorporation within the build.
Author Contributions
Conceptualization, T.O. and P.C.P.; methodology, T.O. and P.C.P.; investigation, T.O.; resources, P.C.P.; data curation, T.O.; writing—original draft preparation, T.O. and P.C.P.; writing—review and editing, P.C.P.; visualization, T.O.; supervision, P.C.P.; project administration, P.C.P.; funding acquisition, P.C.P. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to acknowledge access to the facilities of the Next Manufacturing Institute as well as the Materials Characterization Facility at Carnegie Mellon University under grant # MCF-677785.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Poulin, J.-R.; Kreitcberg, A.; Terriault, P.; Brailovski, V. Long Fatigue Crack Propagation Behavior of Laser Powder Bed-Fused Inconel 625 with Intentionally-Seeded Porosity. Int. J. Fatigue 2019, 127, 144–156. [Google Scholar] [CrossRef]
- Rao, S.; Cunningham, R.; Ozturk, T.; Rollett, A.D. Measurement and Analysis of Porosity in Al-10Si-1Mg Components Additively Manufactured by Selective Laser Melting. Mater. Perform. Charact. 2016, 5, 701–716. [Google Scholar] [CrossRef]
- Wycisk, E.; Solbach, A.; Siddique, S.; Herzog, D.; Walther, F.; Emmelmann, C. Effects of Defects in Laser Additive Manufactured Ti-6Al-4V on Fatigue Properties. Phys. Procedia 2014, 56, 371–378. [Google Scholar] [CrossRef]
- Gong, H.; Rafi, K.; Gu, H.; Starr, T.; Stucker, B. Analysis of Defect Generation in Ti–6Al–4V Parts Made Using Powder Bed Fusion Additive Manufacturing Processes. Addit. Manuf. 2014, 1–4, 87–98. [Google Scholar] [CrossRef]
- Tang, M.; Pistorius, P.C. Oxides, Porosity and Fatigue Performance of AlSi10Mg Parts Produced by Selective Laser Melting. Int. J. Fatigue 2017, 94, 192–201. [Google Scholar] [CrossRef]
- Louvis, E.; Fox, P.; Sutcliffe, C.J. Selective Laser Melting of Aluminium Components. J. Mater. Process. Technol. 2011, 211, 275–284. [Google Scholar] [CrossRef]
- Simonelli, M.; Tuck, C.; Aboulkhair, N.T.; Maskery, I.; Ashcroft, I.; Wildman, R.D.; Hague, R. A Study on the Laser Spatter and the Oxidation Reactions During Selective Laser Melting of 316L Stainless Steel, Al-Si10-Mg, and Ti-6Al-4V. Met. Mater Trans A 2015, 46, 3842–3851. [Google Scholar] [CrossRef]
- Gisario, A.; Barletta, M.; Veniali, F. Laser Polishing: A Review of a Constantly Growing Technology in the Surface Finishing of Components Made by Additive Manufacturing. Int. J. Adv. Manuf. Technol. 2022, 120, 1433–1472. [Google Scholar] [CrossRef]
- Zhou, J.; Han, X.; Li, H.; Liu, S.; Shen, S.; Zhou, X.; Zhang, D. In-Situ Laser Polishing Additive Manufactured AlSi10Mg: Effect of Laser Polishing Strategy on Surface Morphology, Roughness and Microhardness. Materials 2021, 14, 393. [Google Scholar] [CrossRef]
- Stevenson, M.F. Anodizing. In Surface Engineering; Cotell, C.M., Sprague, J.A., Smidt, F.A., Eds.; ASM International: Metals Park, OH, USA, 1994; pp. 482–493. [Google Scholar]
- Rosenthal, D. The Theory of Moving Sources of Heat and Its Application to Metal Treatments. J. Fluids Eng. 1946, 68, 849–865. [Google Scholar] [CrossRef]
- Ritchie, N.W.M. Spectrum Simulation in DTSA-II. Microsc. Microanal. 2009, 15, 454–468. [Google Scholar] [CrossRef] [PubMed]
- Stary, J.; Liljenzin, J.O. Critical Evaluation of Equilibrium Constants Involving Acetylacetone and Its Metal Chelates. Pure Appl. Chem. 1982, 54, 2557–2592. [Google Scholar] [CrossRef]
- Sato, K.; Kammori, O. Studies of the Direct Dissolution of Metal in a β-Diketone Reagent. Bull. Chem. Soc. Jpn. 1969, 42, 2778–2790. [Google Scholar] [CrossRef]
- Liu, H.; Ye, M.; Shen, X.; Ye, Z.; Wang, L.; Wang, G.; Xu, P.; Wang, C. Improving Surface Electrochemical Polishing Quality of Additive Manufactured AlSi10Mg by Reconstituting the Si Phase. Surf. Coat. Technol. 2024, 479, 130549. [Google Scholar] [CrossRef]
- Tang, M.; Pistorius, P.C.; Beuth, J.L. Prediction of Lack-of-Fusion Porosity for Powder Bed Fusion. Addit. Manuf. 2017, 14, 39–48. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ, U.S. National Institutes of Health, Bethesda, Maryland, USA. 1997–2018. Available online: https://imagej.net/ij/ (accessed on 1 March 2020).
- Goldstein, J.I.; Newbury, D.E.; Michael, J.R.; Ritchie, N.W.; Scott, J.H.J.; Joy, D.C. Scanning Electron Microscopy and X-Ray Microanalysis, 4th ed.; Springer: New York, NY, USA, 2018. [Google Scholar]
- McCafferty, E.; Natishan, P.M. (Invited) Localized Breakdown of the Natural Oxide Film on Aluminum by Chloride Ions and the Formation of Oxide Blisters. ECS Trans. 2011, 33, 47–56. [Google Scholar] [CrossRef]
- Huang, C.; Kou, S. Partially Melted Zone in Aluminum Welds—Liquation Mechanism and Directional Solidification. Weld. J. 2000, 79, 113-s–120-s. [Google Scholar]
- Fyrillas, M.M.; Kontoghiorghes, E.J. Numerical Calculation of Mass Transfer from Elliptical Pools in Uniform Flow Using the Boundary Element Method. Transp. Porous Media 2004, 55, 91–102. [Google Scholar] [CrossRef]
- Bidare, P.; Bitharas, I.; Ward, R.M.; Attallah, M.M.; Moore, A.J. Fluid and Particle Dynamics in Laser Powder Bed Fusion. Acta Mater. 2018, 142, 107–120. [Google Scholar] [CrossRef]
- Ohtsuki, T.; Smith, L.; Tang, M.; Pistorius, P.C. Origin of Oxides and Oxide-Related Pores in Laser Powder Bed Fusion Parts. In Structural Integrity of Additive Manufactured Materials & Parts; Shamsaei, N., Seifi, M., Eds.; ASTM International: West Conshohocken, PA, USA, 2020; pp. 45–60. [Google Scholar]
- Chia, H.Y.; Wang, L.; Yan, W. Influence of Oxygen Content on Melt Pool Dynamics in Metal Additive Manufacturing: High-Fidelity Modeling with Experimental Validation. Acta Mater. 2023, 249, 118824. [Google Scholar] [CrossRef]
- Chia, H.Y.; Zhang, Y.; Wang, L.; Yan, W. Unveiling Gas–Liquid Metal Reactions in Metal Additive Manufacturing: High-Fidelity Modeling Validated with Experiments. Acta Mater. 2024, 275, 120029. [Google Scholar] [CrossRef]
- Gasper, A.N.D.; Szost, B.; Wang, X.; Johns, D.; Sharma, S.; Clare, A.T.; Ashcroft, I.A. Spatter and Oxide Formation in Laser Powder Bed Fusion of Inconel 718. Addit. Manuf. 2018, 24, 446–456. [Google Scholar] [CrossRef]
- Khairallah, S.A.; Anderson, A.T.; Rubenchik, A.; King, W.E. Laser Powder-Bed Fusion Additive Manufacturing: Physics of Complex Melt Flow and Formation Mechanisms of Pores, Spatter, and Denudation Zones. Acta Mater. 2016, 108, 36–45. [Google Scholar] [CrossRef]
- Taheri Andani, M.; Dehghani, R.; Karamooz-Ravari, M.R.; Mirzaeifar, R.; Ni, J. A Study on the Effect of Energy Input on Spatter Particles Creation during Selective Laser Melting Process. Addit. Manuf. 2018, 20, 33–43. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Mai, S.; Wang, D.; Song, C. Investigation into Spatter Behavior during Selective Laser Melting of AISI 316L Stainless Steel Powder. Mater. Des. 2015, 87, 797–806. [Google Scholar] [CrossRef]
- Ye, D.; Zhu, K.; Fuh, J.Y.H.; Zhang, Y.; Soon, H.G. The Investigation of Plume and Spatter Signatures on Melted States in Selective Laser Melting. Opt. Laser Technol. 2019, 111, 395–406. [Google Scholar] [CrossRef]
- Gunenthiram, V.; Peyre, P.; Schneider, M.; Dal, M.; Coste, F.; Koutiri, I.; Fabbro, R. Experimental Analysis of Spatter Generation and Melt-Pool Behavior during the Powder Bed Laser Beam Melting Process. J. Mater. Process. Technol. 2018, 251, 376–386. [Google Scholar] [CrossRef]
- Yin, J.; Yang, L.; Yang, X.; Zhu, H.; Wang, D.; Ke, L.; Wang, Z.; Wang, G.; Zeng, X. High-Power Laser-Matter Interaction during Laser Powder Bed Fusion. Addit. Manuf. 2019, 29, 100778. [Google Scholar] [CrossRef]
- Joys, J. Production of Aluminum and Aluminum-Alloy Powder. In Powder Metallurgy; Samal, P., Newkirk, J., Eds.; ASM International: Metals Park, OH, USA, 2015; pp. 569–580. [Google Scholar]
- Wriedt, H.A. The Al-O (Aluminum-Oxygen) System. Bull. Alloy Phase Diagr. 1985, 6, 548–553. [Google Scholar] [CrossRef]
- Mukherjee, T.; Wei, H.L.; De, A.; DebRoy, T. Heat and Fluid Flow in Additive Manufacturing—Part I: Modeling of Powder Bed Fusion. Comput. Mater. Sci. 2018, 150, 304–313. [Google Scholar] [CrossRef]
- Raghavan, A.; Wei, H.L.; Palmer, T.A.; DebRoy, T. Heat Transfer and Fluid Flow in Additive Manufacturing. J. Laser Appl. 2013, 25, 052006. [Google Scholar] [CrossRef]
- Gautam, P.; Biswal, H.J.; Lucon, J.; Stefanescu, C.; LaDouceur, R.; Lucon, P. Particle Tracking in a Simulated Melt Pool of Laser Powder Bed Fusion. J. Laser Appl. 2023, 35, 042021. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Huang, X.; Pan, Y.; Zhang, C. Regulating Oxide Behavior and Forming Stability of Arc-Directed Energy Deposition in 7075 Aluminum Alloy via Beam Oscillation. Addit. Manuf. 2025, 98, 104650. [Google Scholar] [CrossRef]
- Smith, L.A.; Pistorius, P.C. Build Surface Roughness and Internal Oxide Concentration for Laser Powder Bed Fusion of IN718. JMMP 2022, 6, 25. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).