Selective Recovery and Enrichment of Cobalt from Cobalt-Containing Slag by Carbothermal Reduction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Reduction Roasting Experiments and Analysis Method
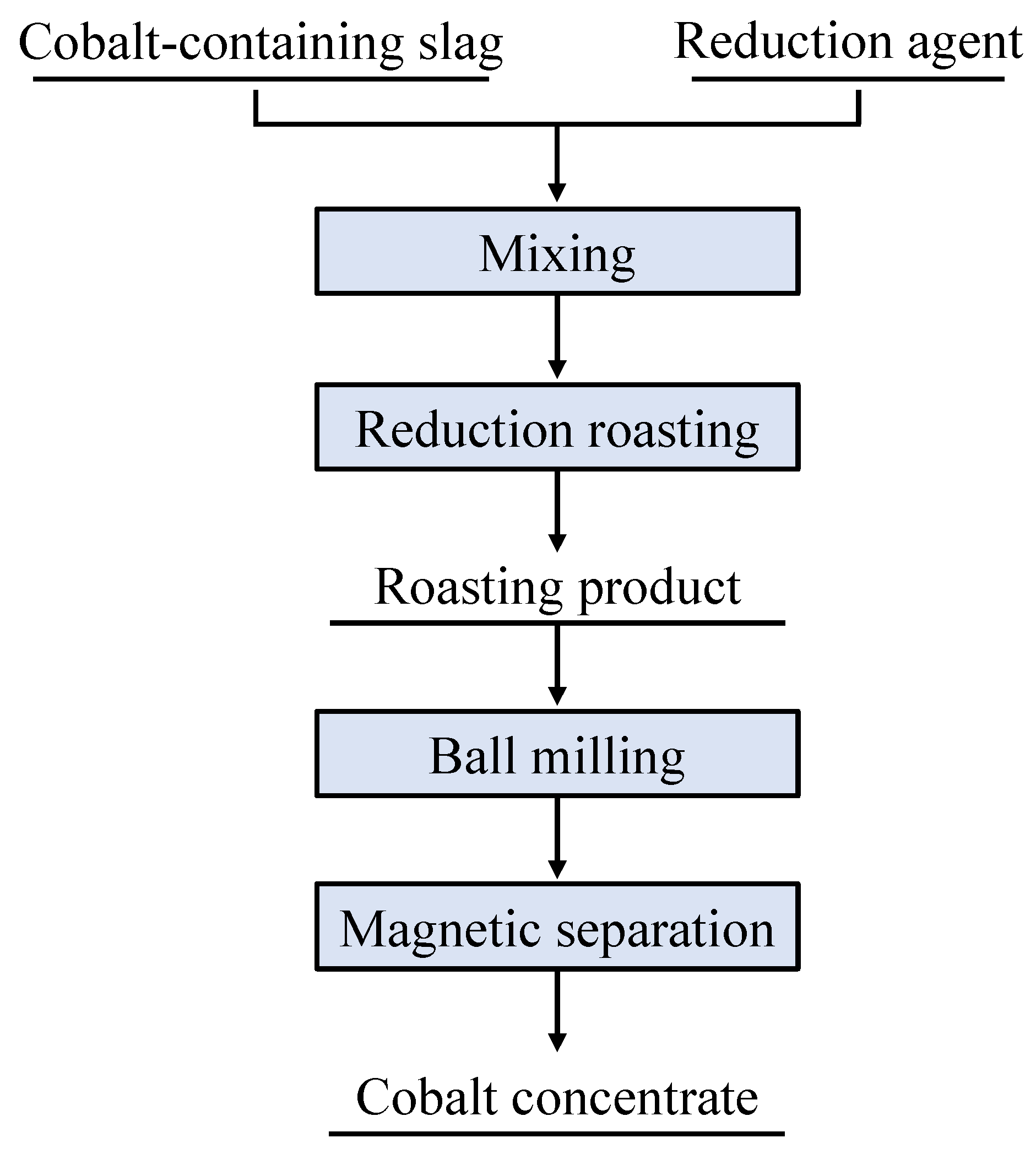
2.2.1. Pretreatment of Cobalt-Containing Slag
2.2.2. Reduction Roasting Experiments
2.2.3. Analytical Methods
3. Results and Discussion
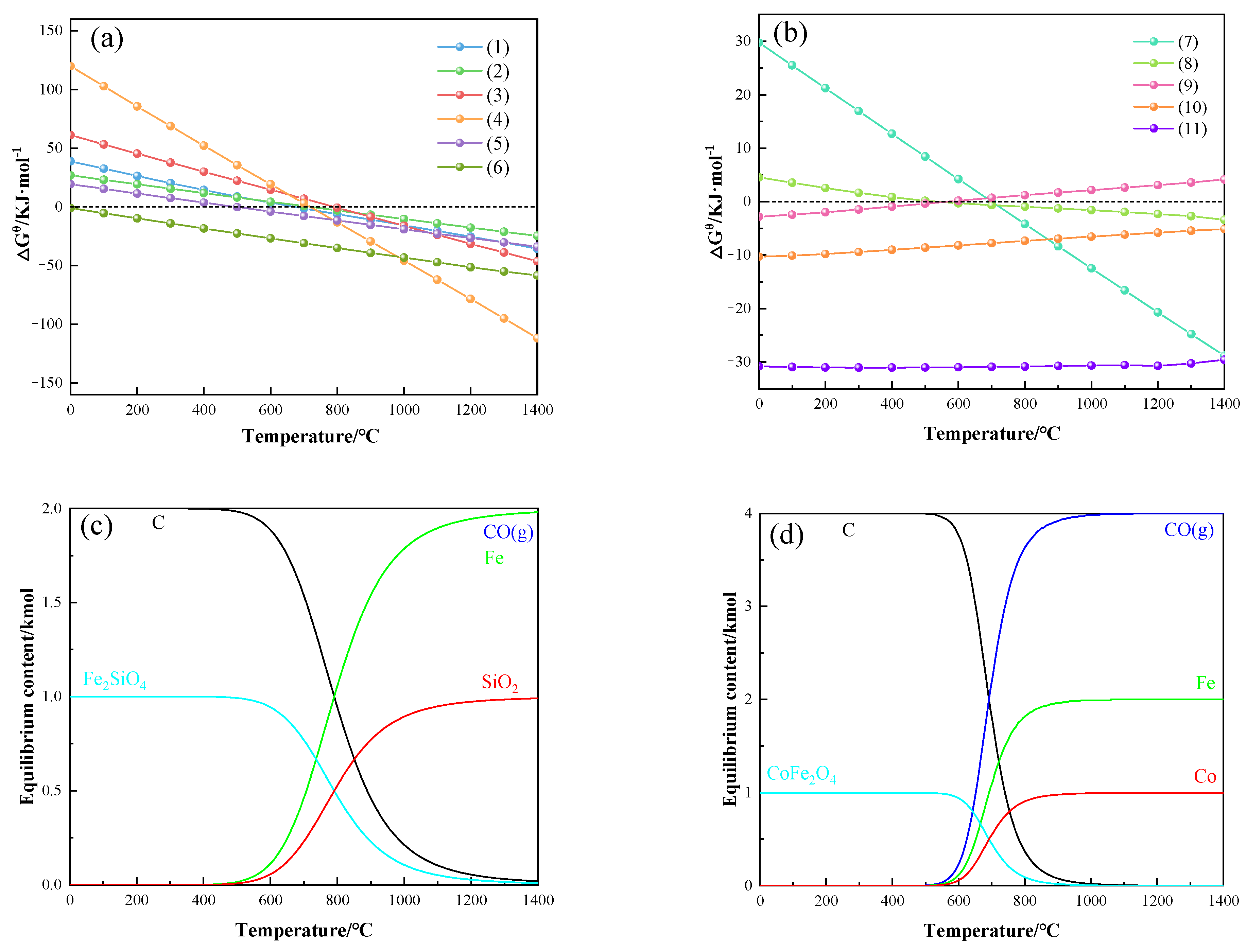
3.1. Thermodynamic Analysis
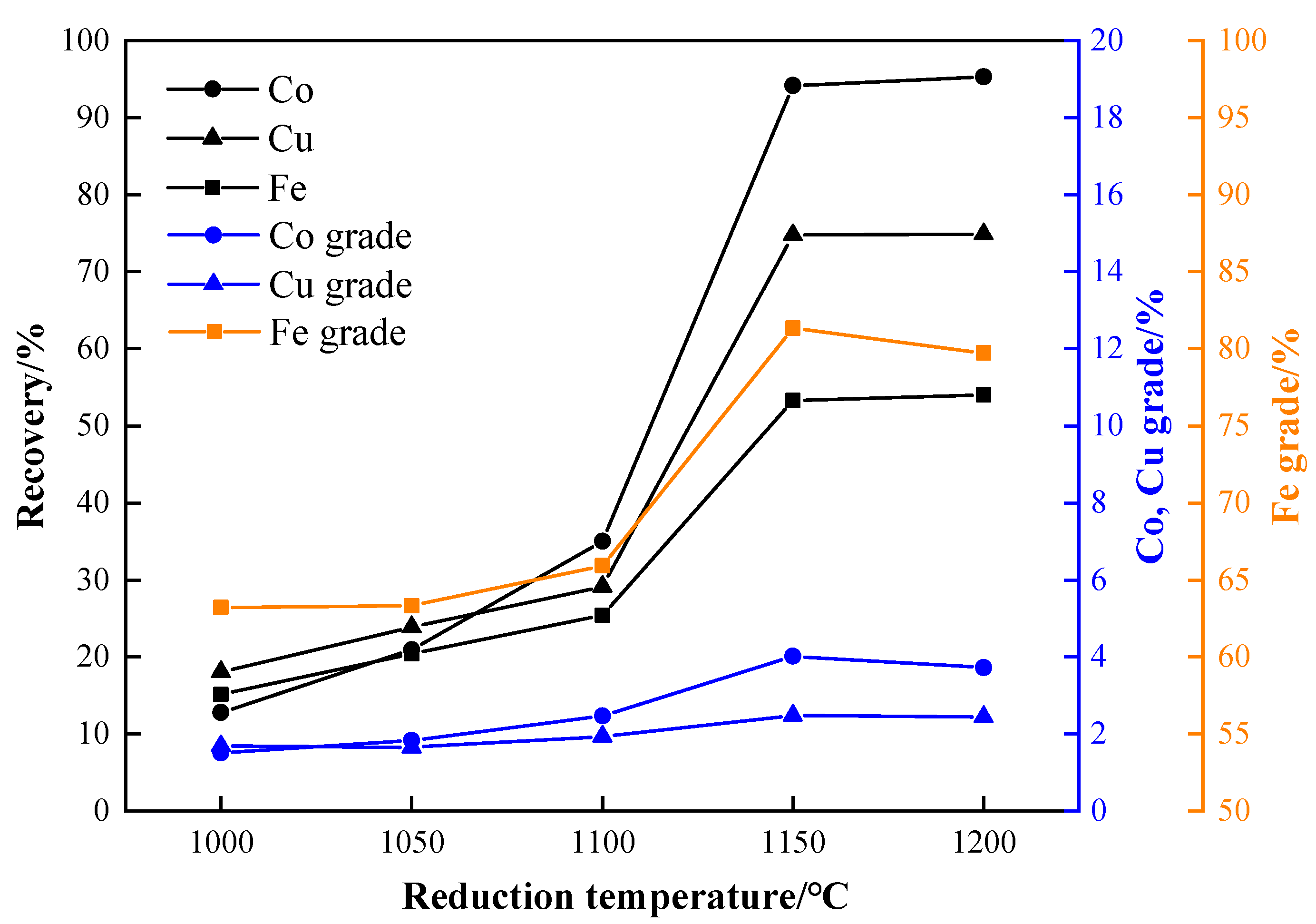
3.2. Effect of Reduction Temperature
3.3. Effect of Reduction Agent Ratio
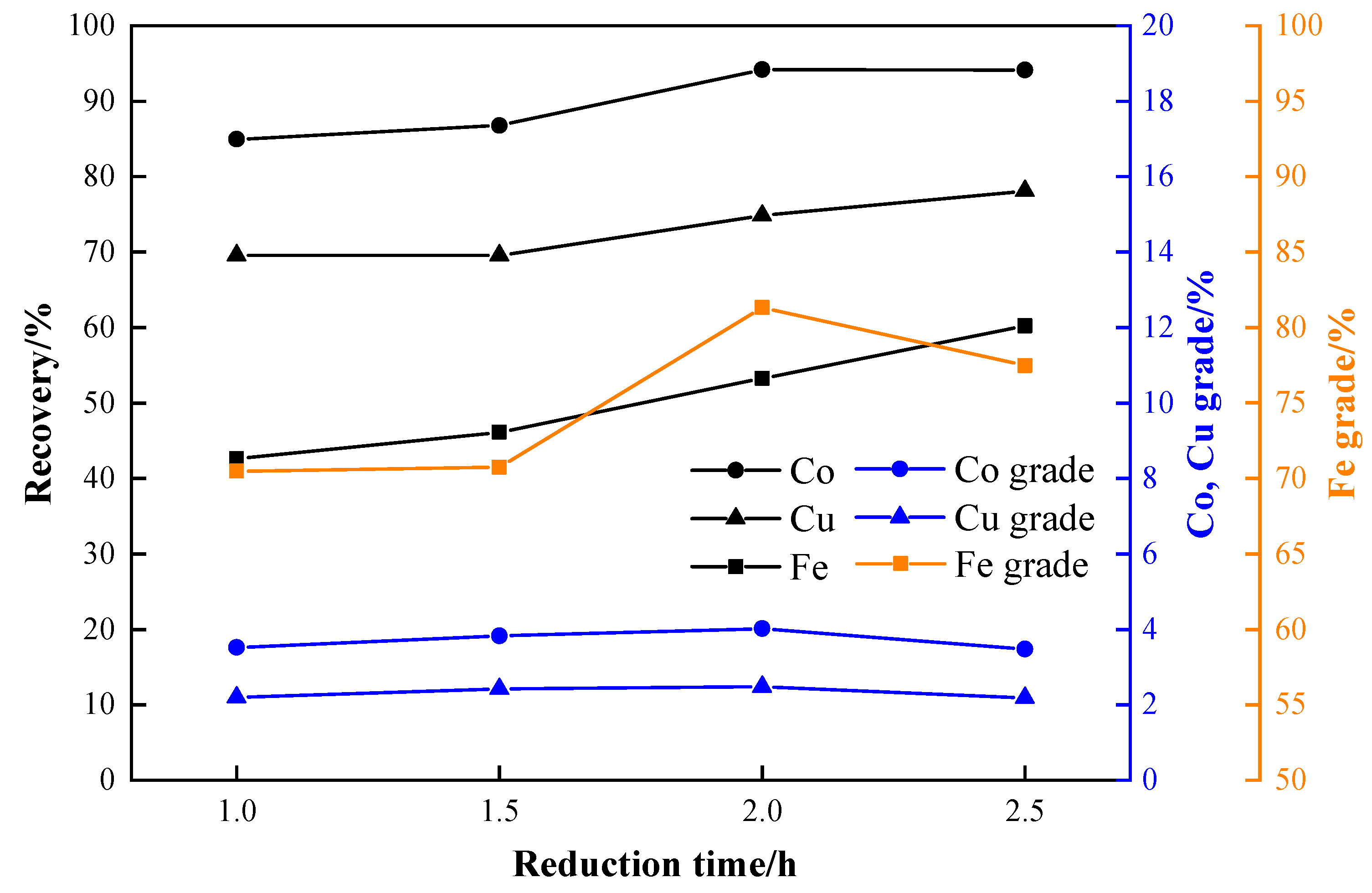
3.4. Effects of Reduction Time
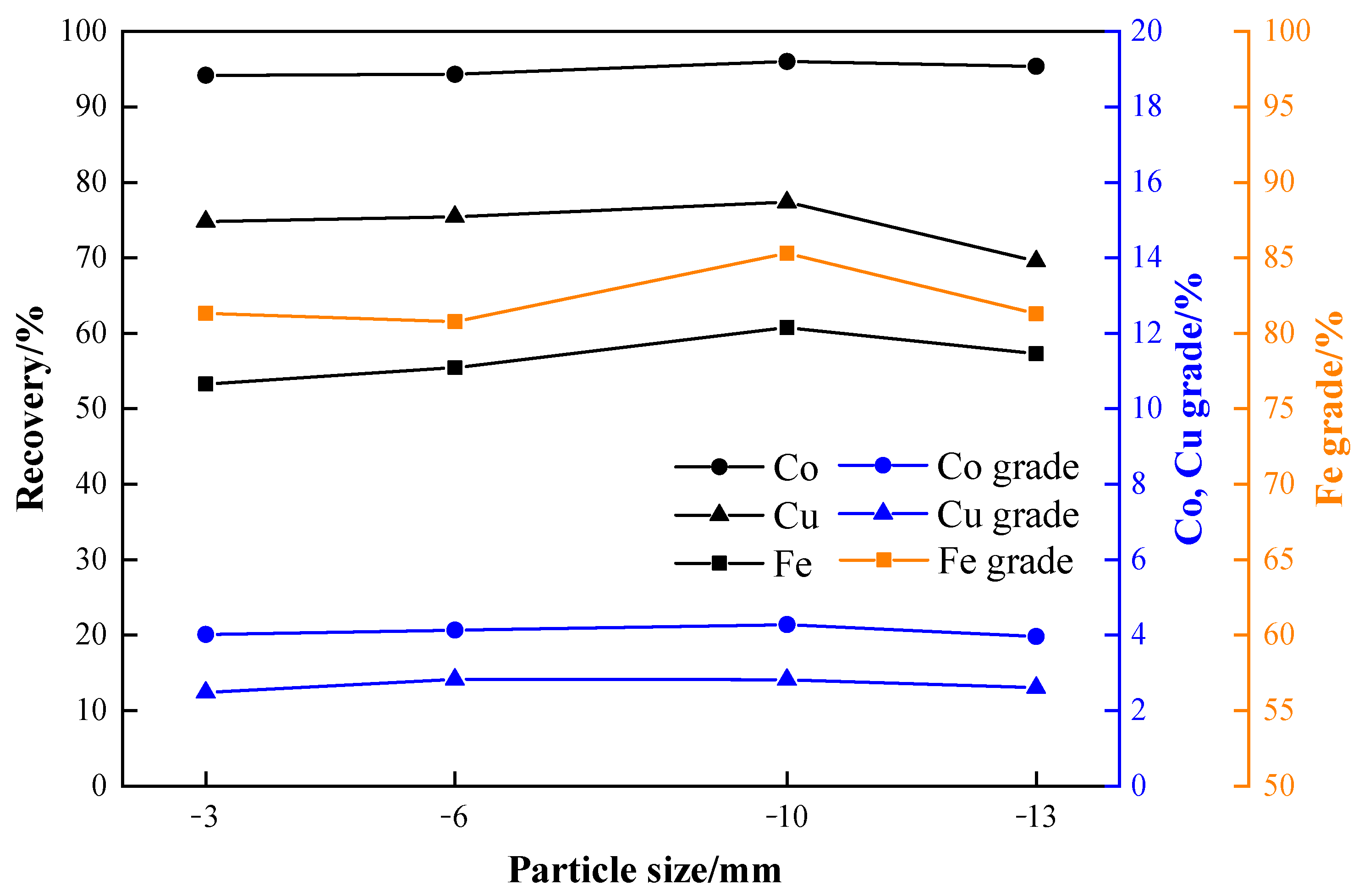
3.5. Effects of Particle Size
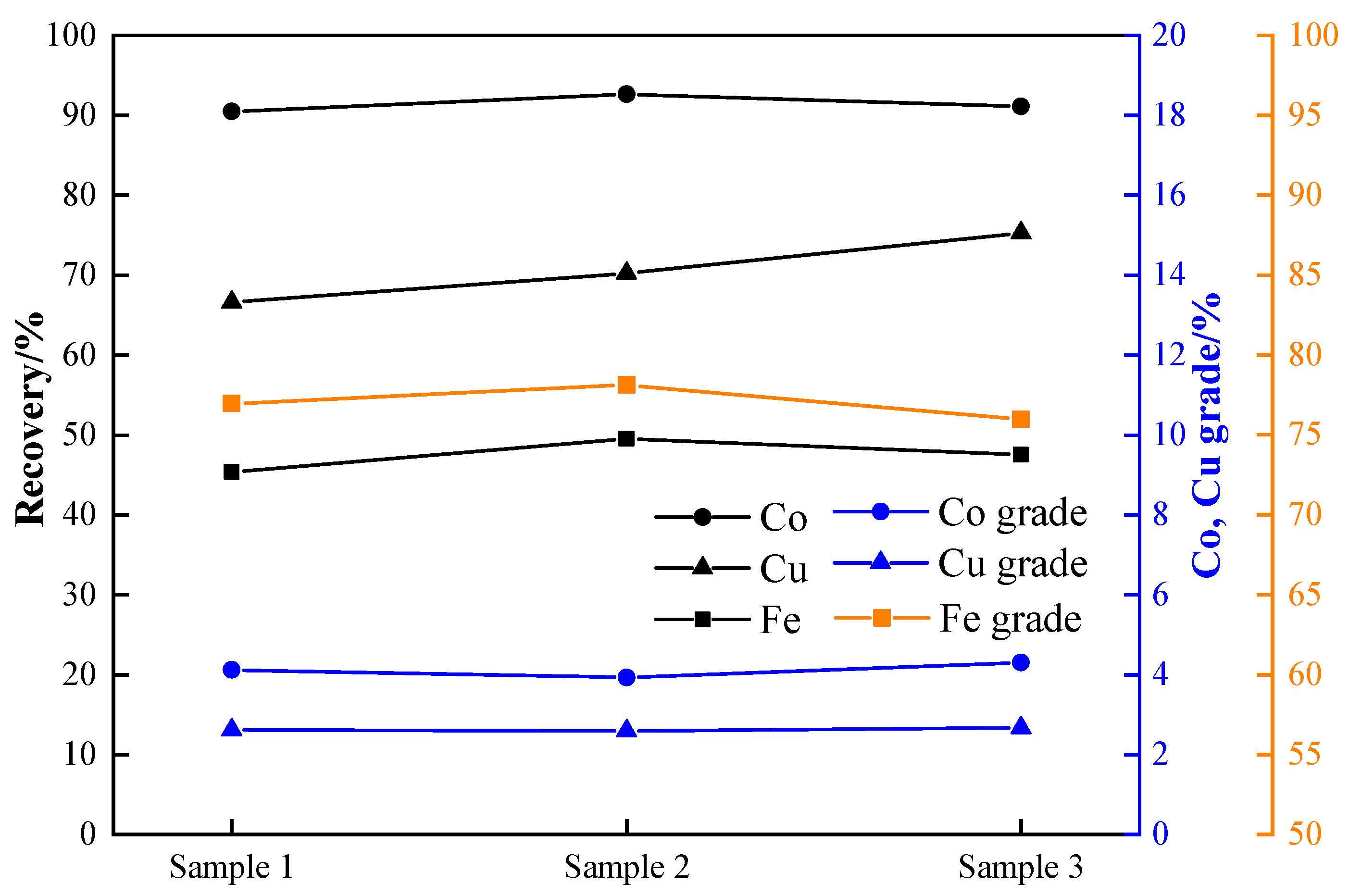
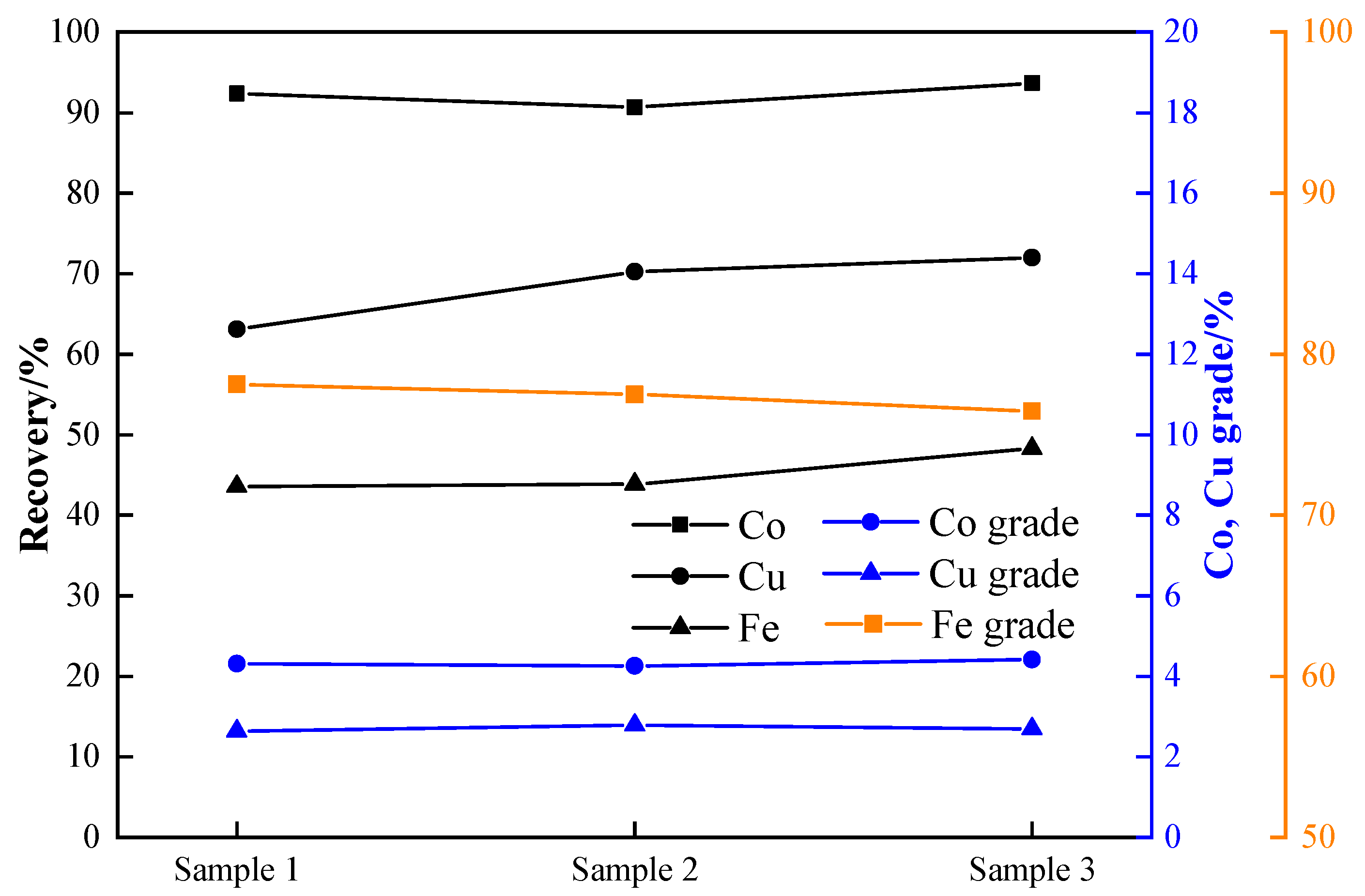
3.6. Reduction Verification Experiment
3.7. Migration of Cobalt
4. Conclusions
- (1)
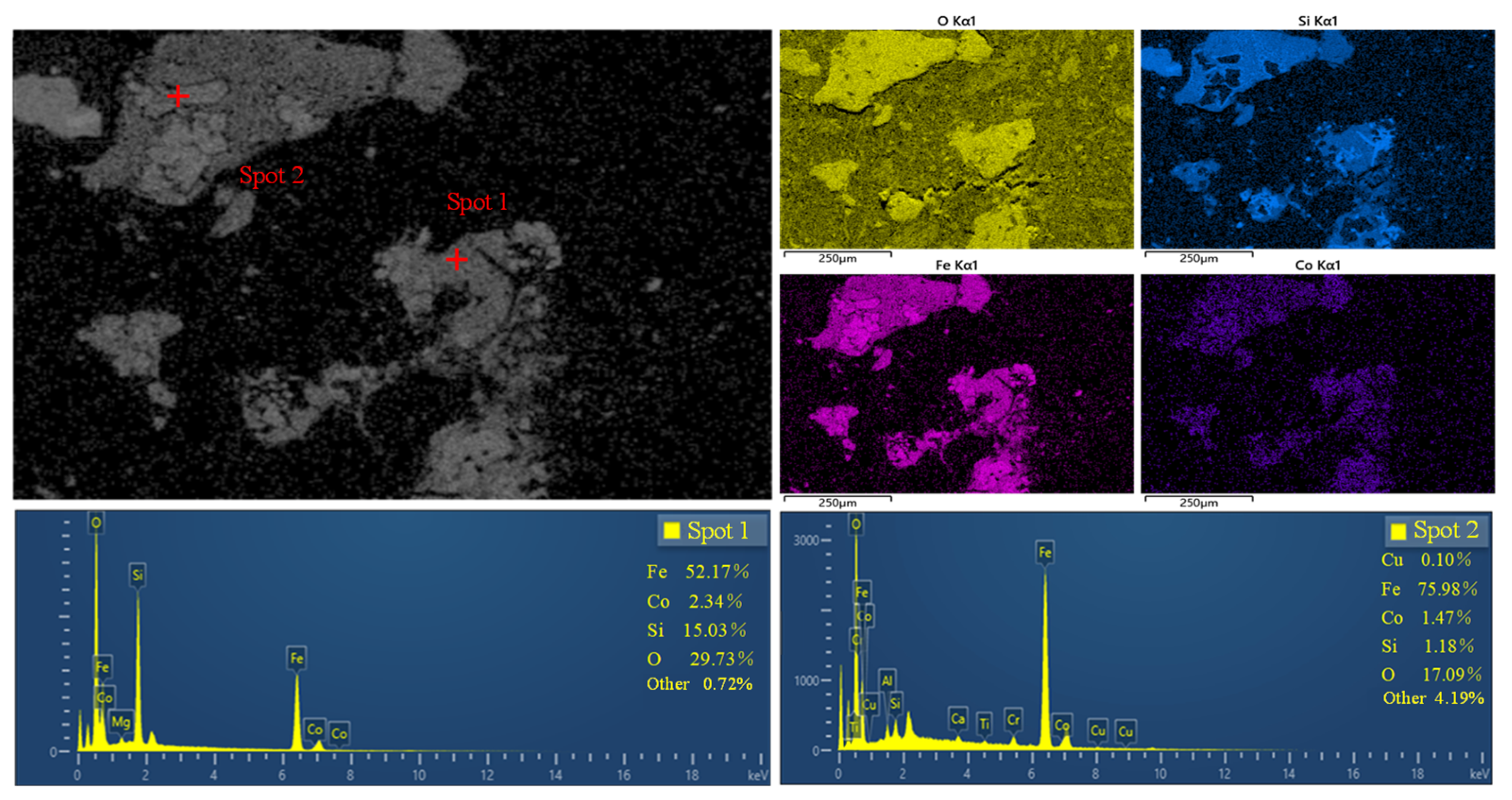
- The primary minerals in cobalt-containing slag include iron silicate minerals, glassy minerals, magnetite, and copper sulfide minerals, with smaller amounts of cobalt-nickel sulfide minerals, metallic copper, and pyrrhotite. The main cobalt-bearing minerals are cobalt–nickel sulfide minerals, pyrrhotite, iron silicate minerals, and magnetite. Cobalt is predominantly hosted in the iron silicate minerals.
- (2)
- The optimal process conditions for the carbothermic reduction of cobalt-containing slag, based on experimental results, are as follows: a reduction temperature of 1150 °C, a reductant ratio of 40%, a reduction time of 2 h, a particle size of −3 mm, a grinding fineness of −0.075 mm with an 80% passing rate, and a magnetic field strength of 95.54 kA/m. The results show that the grades of cobalt, copper, and iron in the concentrate after magnetic separation are 4.02%, 2.48%, and 81.33%, respectively, and the recoveries are 94.17%, 74.80%, and 53.27%.
- (3)
- The static reduction in a muffle furnace and the dynamic reduction in a rotary kiln were validated under the optimal conditions, resulting in stable cobalt grade and recovery rates, which are suitable for industrial applications. The cobalt grade is between 3.93 and 4.30%, and the recovery rate is between 90.49% and 92.66% according to the static reduction experiment. The cobalt grade and cobalt recovery rate reached 4.26–4.41% and 90.67–93.67%, respectively, with the rotary kiln dynamic reduction experiment.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Song, Y.; Zhang, Z.; Cheng, J.; Zeng, A.; Zhang, Y. Effects of technical advance on cobalt supply and its supply trends: A perspective of the whole industrial chains. Resour. Conserv. Recycl. 2024, 209, 107760. [Google Scholar] [CrossRef]
- Chandra, M.; Yu, D.; Tian, Q.; Guo, X. Recovery of Cobalt from Secondary Resources: A Comprehensive Review. Miner. Process. Extr. Metall. Rev. 2021, 43, 679–700. [Google Scholar] [CrossRef]
- Santoro, L.; Tshipeng, S.; Pirard, E.; Bouzahzah, H.; Kaniki, A.; Herrington, R. Review of Economic, Technical and Environmental Aspects of Electric Vehicles. Miner. Eng. 2019, 137, 277–289. [Google Scholar] [CrossRef]
- Ettler, V.; Mihaljevič, M.; Drahota, P.; Kříbek, B.; Nyambe, I.; Vaněk, A.; Penížek, V.; Sracek, O.; Natherová, V. Cobalt-bearing copper slags from Luanshya (Zambian Copperbelt): Mineralogy, geochemistry, and potential recovery of critical metals. J. Geochem. Explor. 2022, 237, 106987. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, Y.; Liu, Y.; Zhou, T.; Ou, B. Distribution and enrichment processes of cobalt in the Longqiao iron skarn deposit in Eastern China. Ore Geol. Rev. 2024, 174, 106277. [Google Scholar] [CrossRef]
- Horn, S.; Gunn, A.G.; Petavratzi, E.; Shaw, R.A.; Eilu, P.; Törmänen, T.; Bjerkgård, T.; Sandstad, J.S.; Jonsson, E.; Kountourelis, S.; et al. Cobalt resources in Europe and the potential for new discoveries. Ore Geol. Rev. 2021, 130, 103915. [Google Scholar] [CrossRef]
- Santoro, L.; Tshipeng, S.; Pirard, E.; Bouzahzah, H.; Kaniki, A.; Herrington, R. Mineralogical reconciliation of cobalt recovery from the acid leaching of oxide ores from five deposits in Katanga (DRC). Miner. Eng. 2019, 137, 277–289. [Google Scholar] [CrossRef]
- Dehaine, Q.; Tijsseling, L.T.; Glass, H.J.; Törmänen, T.; Butcher, A.R. Geometallurgy of cobalt ores: A review. Miner. Eng. 2021, 160, 106656. [Google Scholar] [CrossRef]
- Shannak, S.D.; Cochrane, L.; Bobarykina, D. Strategic analysis of metal dependency in the transition to low-carbon energy: A critical examination of nickel, cobalt, lithium, graphite, and copper scarcity using IEA future scenarios. Energy Res. Soc. Sci. 2024, 118, 013773. [Google Scholar] [CrossRef]
- Savinova, E.; Evans, C.; Lèbre, É.; Stringer, M.; Azadi, M.; Valenta, R.K. Will global cobalt supply meet demand? The geological, mineral processing, production and geographic risk profile of cobalt. Resour. Conserv. Recycl. 2023, 190, 106855. [Google Scholar] [CrossRef]
- Wesselkämper, J.; Dahrendorf, L.; Mauler, L.; Lux, S.; von Delft, S. Towards circular battery supply chains: Strategies to reduce material demand and the impact on mining and recycling. Resour. Policy 2024, 95, 105160. [Google Scholar] [CrossRef]
- Tisserant, A.; Pauliuk, S. Matching global cobalt demand under different scenarios for co-production and mining attractiveness. J. Econ. Struct. 2016, 5, 4. [Google Scholar] [CrossRef]
- Liu, Y.; Xia, Q.; Duan, J.; Dai, J.; Wu, S.; Zhao, Z. Geochemical anomalies of critical metals in the Eastern Kunlun Orogenic Belt, China: Implications for nickel and cobalt mineral exploration. Ore Geol. Rev. 2024, 171, 106168. [Google Scholar] [CrossRef]
- Seck, G.S.; Hache, E.; Barnet, C. Potential bottleneck in the energy transition: The case of cobalt in an accelerating electro-mobility world. Resour. Policy 2022, 75, 102516. [Google Scholar] [CrossRef]
- Piçarra, A.; Annesley, I.R.; Otsuki, A.; de Waard, R. Market assessment of cobalt: Identification and evaluation of supply risk patterns. Resour. Policy 2021, 73, 102206. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, L.; Xu, Z. Tracking and quantifying the cobalt flows in mainland China during 1994–2016: Insights into use, trade and prospective demand. Sci. Total Environ. 2019, 672, 752–762. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, Y.; Ma, X.; Shuai, C.; Zhao, Y. How critical mineral supply security affects China NEVs industry? Based on a prediction for chromium and cobalt in 2030. Resour. Policy 2023, 85, 103861. [Google Scholar] [CrossRef]
- Liu, W.; Li, X.; Liu, C.; Wang, M.; Liu, L. Resilience assessment of the cobalt supply chain in China under the impact of electric vehicles and geopolitical supply risks. Resour. Policy 2023, 80, 103183. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.A.; Wang, L.; Qin, W.; Han, J. An unconventional approach for the efficient recovery of iron, cobalt, copper and silicon from copper slag. J. Hazard. Mater. 2024, 476, 135168. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Ning, Y.; Wei, G.; Qu, J.; Yu, Z.; Liu, X. Process and mechanism of Zn and Co leaching from cobalt xanthate slag enhanced by oxidative roasting. Process Saf. Environ. Prot. 2024, 192, 1249–1259. [Google Scholar] [CrossRef]
- Godirilwe, L.L.; Haga, K.; Altansukh, B.; Jeon, S.; Danha, G.; Shibayama, A. Establishment of a Hydrometallurgical Scheme for the Recovery of Copper, Nickel, and Cobalt from Smelter Slag and Its Economic Evaluation. Sustainability 2023, 15, 10496. [Google Scholar] [CrossRef]
- Li, L.; Xiao, Y.; Lei, Y.; Xu, J.; Xu, Z. An approach of cobalt recovery from waste copper converter slags using pig iron as capturing agent and simultaneous recovery of copper and tin. Waste Manag. 2023, 165, 1–11. [Google Scholar] [CrossRef]
- Li, H.; Guo, X.-Y.; Shi, Y.; Wang, C.; Yu, D.-W.; Tian, Q.-H. Selective hydrogen reduction of binary iron-cobalt chlorides. J. Cent. South Univ. 2024, 30, 3991–4003. [Google Scholar] [CrossRef]
- Li, Y.; Chang, C.; Jie, Y.; Jin, W.; Chen, Y.; Wan, X.; Tang, C.; Yang, S. Thermodynamic Phase Conversion Mechanism on Copper–Cobalt Slag Cleaning Process Using Gypsum Wastes as Sulfurizing Agent. J. Sustain. Metall. 2021, 7, 1643–1653. [Google Scholar] [CrossRef]
- Huang, Y.; Duan, Z.; Bai, N.; Wang, H.; Cao, Y.; Song, X.; Peng, W.; Zhu, X. Highly selective dissolution and synchronous extraction of zinc from zinc-cobalt slag by an ionic liquid [Hbet][Tf2N]–H2O system: A novel method for separating zinc and cobalt. J. Clean. Prod. 2021, 315, 128301. [Google Scholar] [CrossRef]
- Zhao, H.; Ma, B.; Hong, S.; Huang, H.; Liu, F.; Sohn, H.Y. Recovery of Copper and Cobalt from Converter Slags via Reduction–Sulfurization Smelting Using Spent Pot Lining as the Reductant. ACS Sustain. Chem. Eng. 2021, 9, 4234–4246. [Google Scholar] [CrossRef]
- Alvial-Hein, G.; Mahandra, H.; Ghahreman, A. Separation and recovery of cobalt and nickel from end of life products via solvent extraction technique: A review. J. Clean. Prod. 2021, 297, 126592. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, J.; Zhang, J.; Hu, J.; Li, J.; Zhang, L.; Chen, Y.; Wang, C. Efficient Recovery of Copper and Cobalt from the Matte–Slag Mixture of ISA Furnace by Injection of Coke and Pyrite. Metall. Mater. Trans. B 2018, 49, 3118–3126. [Google Scholar] [CrossRef]
- Phiri, T.C.; Singh, P.; Nikoloski, A.N. Mineralogical Characterisation of Copper Slag and Phase Transformation after Carbocatalytic Reduction for Hydrometallurgical Extraction of Copper and Cobalt. Metals 2024, 14, 1119. [Google Scholar] [CrossRef]
- Rudnik, E.; Burzyńska, L.; Gumowska, W. Hydrometallurgical recovery of copper and cobalt from reduction-roasted copper converter slag. Miner. Eng. 2009, 22, 88–95. [Google Scholar] [CrossRef]
- Zhai, X.-J.; Li, N.-J.; Zhang, X.; Fu, Y.; Jiang, L. Recovery of cobalt from converter slag of Chambishi Copper Smelter using reduction smelting process. Trans. Nonferrous Met. Soc. China 2011, 21, 2117–2121. [Google Scholar] [CrossRef]
- Bhatti, S.A.; Qiao, X.-C. Synergistic effect of carbothermal reduction and sodium salts leaching in the process of iron recovery from copper slag. Process Saf. Environ. Prot. 2025, 193, 170–182. [Google Scholar] [CrossRef]
- GB/T 212-2008; Industrial Analysis of Coal. National Standard of the People’s Republic of China: Beijing, China, 2021.




| Serial Number | Chemical Composition | |||||||
|---|---|---|---|---|---|---|---|---|
| Si | Al | Ca | Mg | S | Cu | Co | Fe | |
| 1 | 13.96 | 0.516 | 0.456 | 0.353 | 0.060 | 1.01 | 1.27 | 47.29 |
| 2 | 14.07 | 0.444 | 0.420 | 0.360 | 0.058 | 1.03 | 1.29 | 46.92 |
| 3 | 14.00 | 0.964 | 0.438 | 0.354 | 0.061 | 1.03 | 1.27 | 47.06 |
| Mineral | Content | Cobalt Distribution Rate |
|---|---|---|
| Cobalt–nickel sulfide | 0.10 | 2.52 |
| Copper sulfide | 1.87 | 0 |
| Magnetite | 5.01 | 0.49 |
| Pyrrhotite | 0.02 | 0.06 |
| Metallic copper | 0.05 | 0 |
| Iron-containing silicate | 77.88 | 96.93 |
| Vitreous mineral | 15.07 | 0 |
| Cobalt–iron alloy | - | - |
| Fixed Carbon/wt. % | Volatile Matter/wt. % | Ash Content/wt. % | Moisture Content/wt. % |
|---|---|---|---|
| 67.21 | 17.76 | 13.48 | 1.55 |
| Particle Size | Weight/g | Distribution Rate/% | Positive Accumulation/% | Negative Accumulation/% |
|---|---|---|---|---|
| +4.00 | 17.67 | 1.39 | 1.39 | 100.00 |
| −4.00 + 3.35 | 40.05 | 3.15 | 4.54 | 98.61 |
| −3.35 + 3.00 | 117.81 | 9.27 | 13.81 | 95.46 |
| −3.00 + 2.00 | 298.58 | 23.50 | 37.31 | 86.19 |
| −2.00 + 1.00 | 260.19 | 20.48 | 57.79 | 62.69 |
| −1.00 + 0.5 | 186.05 | 14.64 | 72.43 | 42.21 |
| −0.5 + 0.15 | 133.12 | 10.48 | 82.91 | 27.57 |
| −0.15 + 0.106 | 77.36 | 6.09 | 89.00 | 17.09 |
| −0.106 + 0.075 | 54.74 | 4.30 | 93.30 | 11.00 |
| −0.075 | 85.02 | 6.70 | 100.00 | 6.70 |
| Total | 1270.59 | 100.00 | — | — |
| Mineral | Content of Cobalt-Containing Slag | Cobalt Distribution Rate | Content of Roasting Product | Cobalt Distribution Rate |
|---|---|---|---|---|
| Cobalt–nickel sulfide | 0.10 | 2.52 | - | - |
| Copper sulfide | 1.87 | 0 | 0.75 | 0.45 |
| Magnetite | 5.01 | 0.49 | 0.83 | 0 |
| Pyrrhotite | 0.02 | 0.06 | - | - |
| Metallic copper | 0.05 | 0 | 0.08 | 0.02 |
| Iron-containing silicate | 77.88 | 96.93 | 51.13 | 15.79 |
| Vitreous mineral | 15.07 | 0 | 28.87 | 1.64 |
| Cobalt–iron alloy | - | - | 18.34 | 82.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, J.; Pan, J.; Zhao, J.; Zhang, Q.; Hao, G.; Liu, Y.; Yu, H. Selective Recovery and Enrichment of Cobalt from Cobalt-Containing Slag by Carbothermal Reduction. Metals 2025, 15, 622. https://doi.org/10.3390/met15060622
Gong J, Pan J, Zhao J, Zhang Q, Hao G, Liu Y, Yu H. Selective Recovery and Enrichment of Cobalt from Cobalt-Containing Slag by Carbothermal Reduction. Metals. 2025; 15(6):622. https://doi.org/10.3390/met15060622
Chicago/Turabian StyleGong, Jiachen, Jian Pan, Jingfu Zhao, Qian Zhang, Guansheng Hao, Yan Liu, and Helei Yu. 2025. "Selective Recovery and Enrichment of Cobalt from Cobalt-Containing Slag by Carbothermal Reduction" Metals 15, no. 6: 622. https://doi.org/10.3390/met15060622
APA StyleGong, J., Pan, J., Zhao, J., Zhang, Q., Hao, G., Liu, Y., & Yu, H. (2025). Selective Recovery and Enrichment of Cobalt from Cobalt-Containing Slag by Carbothermal Reduction. Metals, 15(6), 622. https://doi.org/10.3390/met15060622







