Abstract
The chemical extraction of rare-earth elements (REEs) from Estonian graptolite-argillite (GA) and phosphate rock (Phosphorite, PH) samples has been conducted and analyzed. For the initial leaching process, HCl and HNO3 with different concentrations were used to extract REEs from GA and PH. Different extraction agents, including ionic liquids, were examined for the extraction of REEs from acidic aqueous solutions in the liquid–liquid extraction step. After leaching and extraction, all samples were characterized using the inductively coupled plasma mass spectrometry method (ICP-MS/MS). The highest REE extraction efficiencies from GA were established with 1-ethyl-3-methyl imidazolium diethyl phosphate (EMImDEPO4) and from PH using bis(2-ethylhexyl) phosphate (D2EHPA).
1. Introduction
Rare-earth elements (REEs) include 15 lanthanide elements (Z = 57 to 71) together with scandium (Z = 21) and yttrium (Z = 39), as stated in the International Union of Pure and Applied Chemistry (IUPAC) [1,2,3,4]. Due to their high reactivity and chemical similarity, REEs are complicated to separate and refine into pure metal. As a result, efforts to develop efficient separation processes began early in the 20th century [5].
Nowadays, REEs have an enormous impact on societies’ daily lives since it is impossible to develop any high-tech technology that would not contain any REEs [1,6,7,8]. Energy storage devices have become indispensable for storing and maintaining a continuous power supply to meet established renewable energy targets. Furthermore, REE-based permanent magnets are extensively used in wind turbines and electric vehicles [9,10,11]. Current supply chains are mainly controlled and fulfilled by the Chinese REE industry [12,13]. China dominates the REE industry by producing more than 90% of rare-earth requirements. Therefore, recycling REEs is currently necessary to alleviate market fluctuations [14].
Black shale formations, known as graptolite-argillite, are found along the northern coast of Estonia and on Vormsi and Hiiumaa islands [15,16]. The thickness of GA reaches up to 7.4 m in Northwest Estonia and decreases towards the east and south [15,16]. Black shales can be divided into classes based on the degree of commodity metal accumulation: black shale (0.01%), metalliferous black shales (0.1%), and hyper-enriched black shales (1%) [17]. The distribution of metals in GA has a complex pattern, but Mo, V, and U are the most characteristic. Moreover, GA contains high variability in the trace metal composition, including heterogeneous REE patterns [17,18,19].
Presumably, the largest unexploited sedimentary phosphate rock reserves in the European Union are also located in Estonia (about 700 million tons of P2O5) [20]. Estonian phosphate rock contains low amounts of Cd (1–5 ppm) and depleted U content compared to other worldwide deposit locations, but the total content of REEs has been identified with an average of 1500–2000 ppm [20].
REE leaching used in primary REE production is integral to hydrometallurgical REE processing [21]. These processes are defined as noncatalytic heterogeneous reactions between liquid and solid phases, ranging from acid leaching (H2SO4, HCl, or HNO3) of primary ores to leaching with NaCl or (NH4)2SO4 of ion-adsorbed clays [22,23]. For industrial design and optimization processes, the kinetics aspect of the leaching is considered to be highly relevant, including various types of diffusion and chemical reaction mechanisms [24]. Therefore, establishing the most optimal pathway would significantly enhance the leaching process’s effectiveness and consider the environmental aspects.
Extraction of cations is an efficient technique for separating rare earths from acid solutions—it is relatively simple, rapid, and applicable in various concentrations, pH, and temperature ranges [25,26]. For example, triphenyl arsine in kerosene can extract yttrium and ytterbium from sulfuric acid medium. Ionic liquids (ILs) are studied as extraction agents, emphasizing the existing technologies and the principles of sustainable and green chemistry [27,28]. Common acidic extractants (organic phosphonic acids and carboxylic acids) require saponification, resulting in large amounts of wastewater [29]. Thus, designing alternative extractants is a matter of great urgency. The non-functional ILs showed better extraction ability and selectivity than conventional organic solvent systems [30].
This paper has two main novel objectives regarding the high amount of black shale formations and large phosphate rock reserves in Estonia. Firstly, develop a leaching process for Estonian graptolite-argillite and phosphorite ore samples. Secondly, to extract REEs (separate HREEs and LREEs) and other distributed rare metal cations (DRM) from prepared aqueous solutions using ionic liquids and other extraction agents. The concentration of REEs and DRM was determined before and after extraction by ICP-MS/MS to calculate the respective extraction efficiencies. The influence of different molar concentrations of acids has been discussed.
2. Experimental Section
2.1. Leaching Step of GA and PH Samples
The raw GA powder samples from Northeast Estonia (samples have been designated as Sillamäe, Sõtke top, Sõtke bottom, and Argillite from the collection) were homogenized using ball milling (stabilized ZrO2 balls) and then treated with aqueous acidic solutions. GA was leached using Aqua Regia, 1 M HCl, 1 M HNO3, 3 M HCl, 3 M HNO3, and 7.5 M HNO3 aqueous solutions in 1:10 solid-to-liquid mass ratios in separate vials. Demineralized water was used to prepare the acid solutions. The mixtures of GA and acid were stirred (300 rpm) at 70 °C for 3 h, followed by a cooling step (to room temperature), centrifugation for 30 min, and decantation (solid residual separation).
PH leaching experiments contained phosphorite ore samples from Northeast Estonia (designated as Ülgase, Toolse, and Iru outcrop) that were processed in 100 mL 7.5 M HNO3, 7.5 M HCl, 3 M HNO3, and 3 M HCl acidic aqueous solutions for 24 h at room temperature (22 ± 1 °C) in 1:10 solid-to-liquid mass ratios. A relatively high acid concentration and leaching time were necessary to dissolve REEs from the PH ore completely. Thereafter, the solution was filtered to remove any undissolved substances.
Estonian concentrated phosphorite ore (Ülgase, Toolse, and Iru from Northern Estonia) and graptolite-argillite (Sillamäe, Sõtke top, Sõtke bottom, and Argillite from the collection) samples were obtained from geological collections at the Institute of Ecology and Earth Sciences, University of Tartu.
Chemicals: HNO3 and HCl (ACS grade) were purchased from Merck (Burlington, MA, USA).
2.2. Details of the Extraction Process
Acidic aqueous feed solution from the leaching step and extractant were added to the separation funnel at a fixed 9:1 (aqueous/organic) ratio in all cases (GA and PH samples) at room temperature. The mixture was shaken carefully in a separation funnel for 1 min and kept still to reach the reaction equilibration of the extraction for one hour. The procedure was repeated two more times for the best separation efficiencies.
Extractants: Tributyl phosphate (TBP, ≥99.0%) from Merck; 1-ethyl-3-methyl imidazolium diethyl phosphate (EMImDEPO4, ≥97%) and tricaprylylmethylammonium chloride (Aliquat 336, ≥97.0%) from Sigma-Aldrich; bis(2-ethylhexyl) phosphate (D2EHPA, ≥95.0%) from Merck.
2.3. Experimental Methods for Determining the Detailed Chemical Composition of GA and PH
Trace element analysis of samples after the leaching step and extraction process was performed using Agilent 8800 QQQ ICP-MS/MS (Agilent Technologies, Santa Clara, CA, USA). All untreated extraction solutions were diluted to a final dilution factor of 4000 before the analysis using a 2% HNO3 solution. ICP-MS/MS measurements were performed in NoGas mode, and collision mode was used for masses that exhibited polyatomic spectral overlaps. Calibration standard solutions were used.
3. Results and Discussion
3.1. GA Raw Deposit Analysis After Leaching with Modified Aqua Regia Using ICP-MS/MS Method
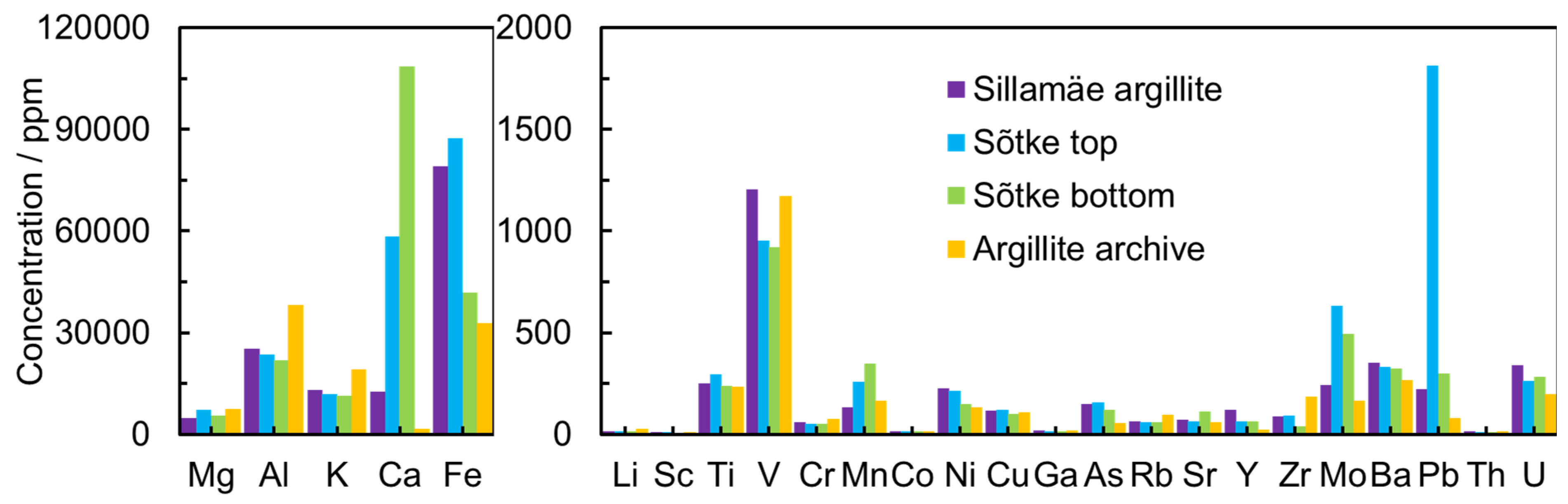
The GA is an argillaceous rock enriched with organic matter that contains high concentrations of several elements (U, V, Mo, Pb, Zn, Cd, Cu, As) and very low concentrations of REEs [31]. The current study of geochemical generalizations of Estonian GA shows that the content of elements in the solution obtained from leaching is heterogeneous and highly dependent on the location and origin (Figure 1). The concentration of metal cations in GA obtained after the leaching process with modified Aqua Regia is in good agreement with the data by Soesoo et al. [15,32,33].
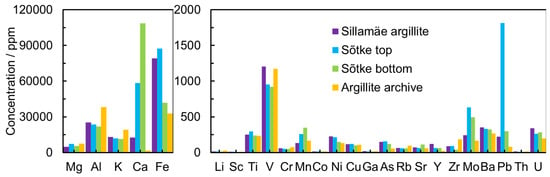
Figure 1.
The concentration of elements after the leaching process in modified Aqua Regia aqueous solution from leaching for different GA deposits collected from Northern Estonia: Sõtke top, Sõtke bottom, Sillamäe, and Argillite.
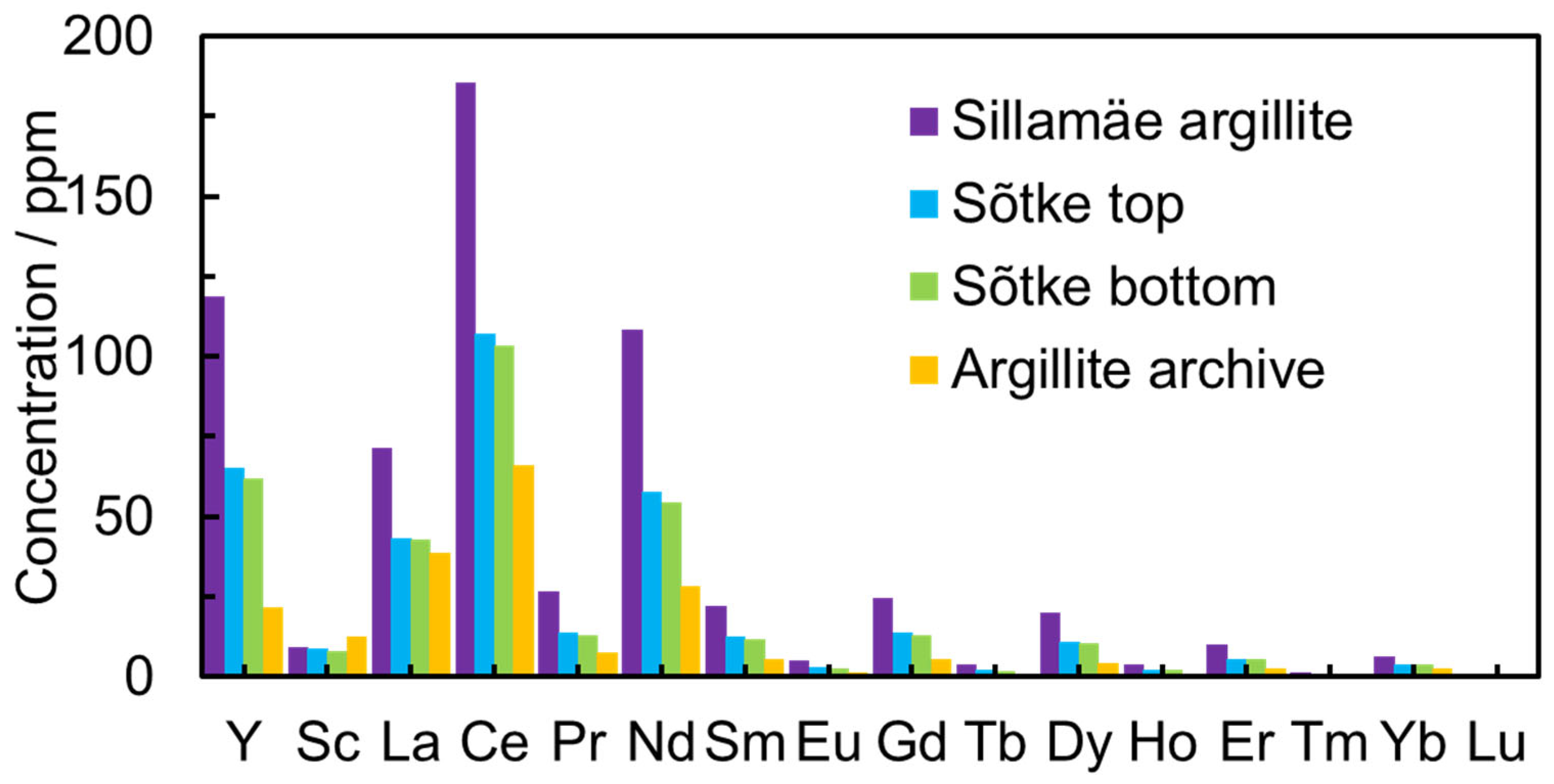
The concentrations of U (198–280 ppm) and Th (10–13 ppm) were moderate in all GA samples. The REEs’ total concentration (in the solution after the leaching process) in the Sillamäe sample reached 600 ppm but stayed between 200 and 350 ppm in other samples (Figure 2).
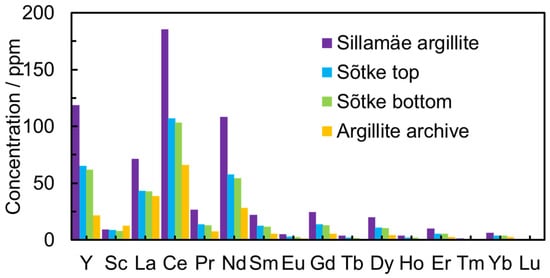
Figure 2.
The concentration of REEs after the leaching process in modified Aqua Regia aqueous solution from leaching for different GA deposits from Northern Estonia, including Sõtke top, Sõtke bottom, Sillamäe, and Argillite.
3.2. GA and PH Composition Analysis After Leaching with HCl and HNO3 Aqueous Solutions Using the ICP-MS/MS Method
For metal extraction, various forms of hydrometallurgical routes are used for black shale processing [17]. The leaching process with typical mineral acids (H2SO4, HCl) with higher concentrations increases the leachability of metals at atmospheric pressure [34]. In this study, H2SO4 was excluded due to earlier findings that most of the REEs transfer to the gypsum (CaSO4·2H2O) during the sulfuric acid leaching [35]. Therefore, to analyze the influence of the acid concentration on the extraction efficiency (E %), HCl and HNO3 with different concentrations were used for the GA and PH treatment processes.
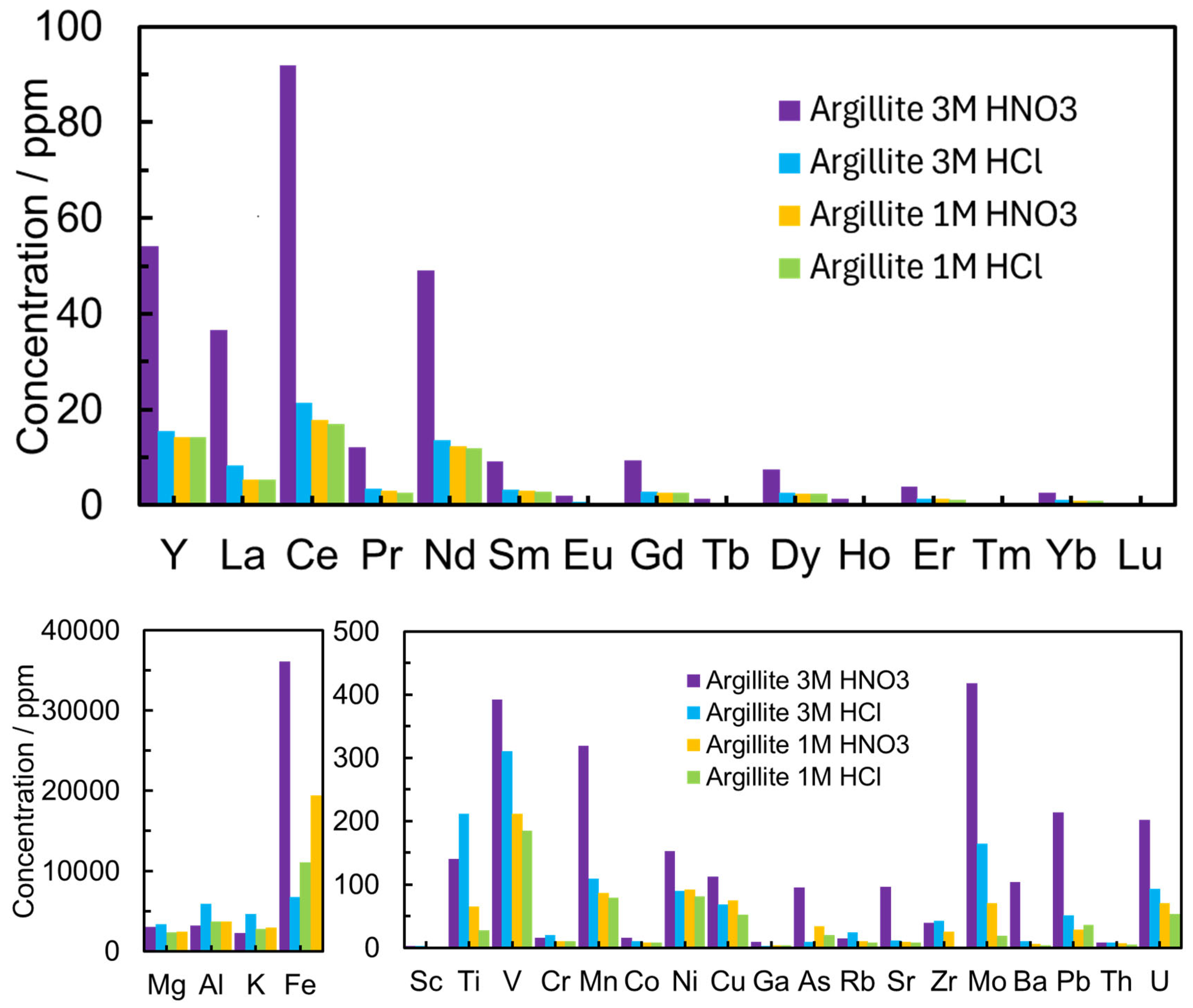
The leaching process results of GA using acids with different concentrations show that the concentration of REEs decreases with the dilution of acid from 3 M to 1 M (with increasing pH of acid solution) based on the ICP-MS/MS measurement results (Figure 3). The lower leaching efficiency of REEs in more dilute acid solution can be explained by the lack of protons.
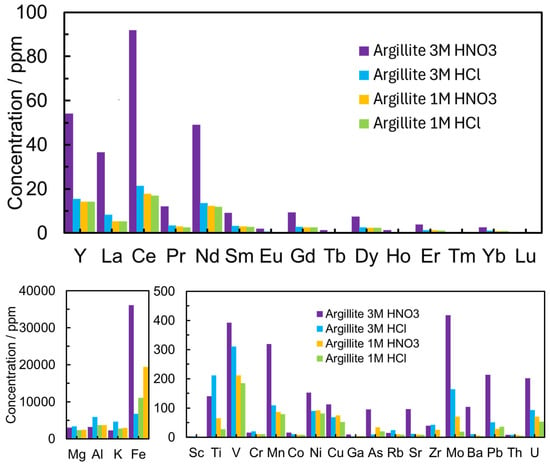
Figure 3.
The concentration of elements in 1 M HCl, 3 M HCl, 1 M HNO3, and 3 M HNO3 aqueous solutions for different GA deposits from Argillite from the collection of the Institute of Geology UT.
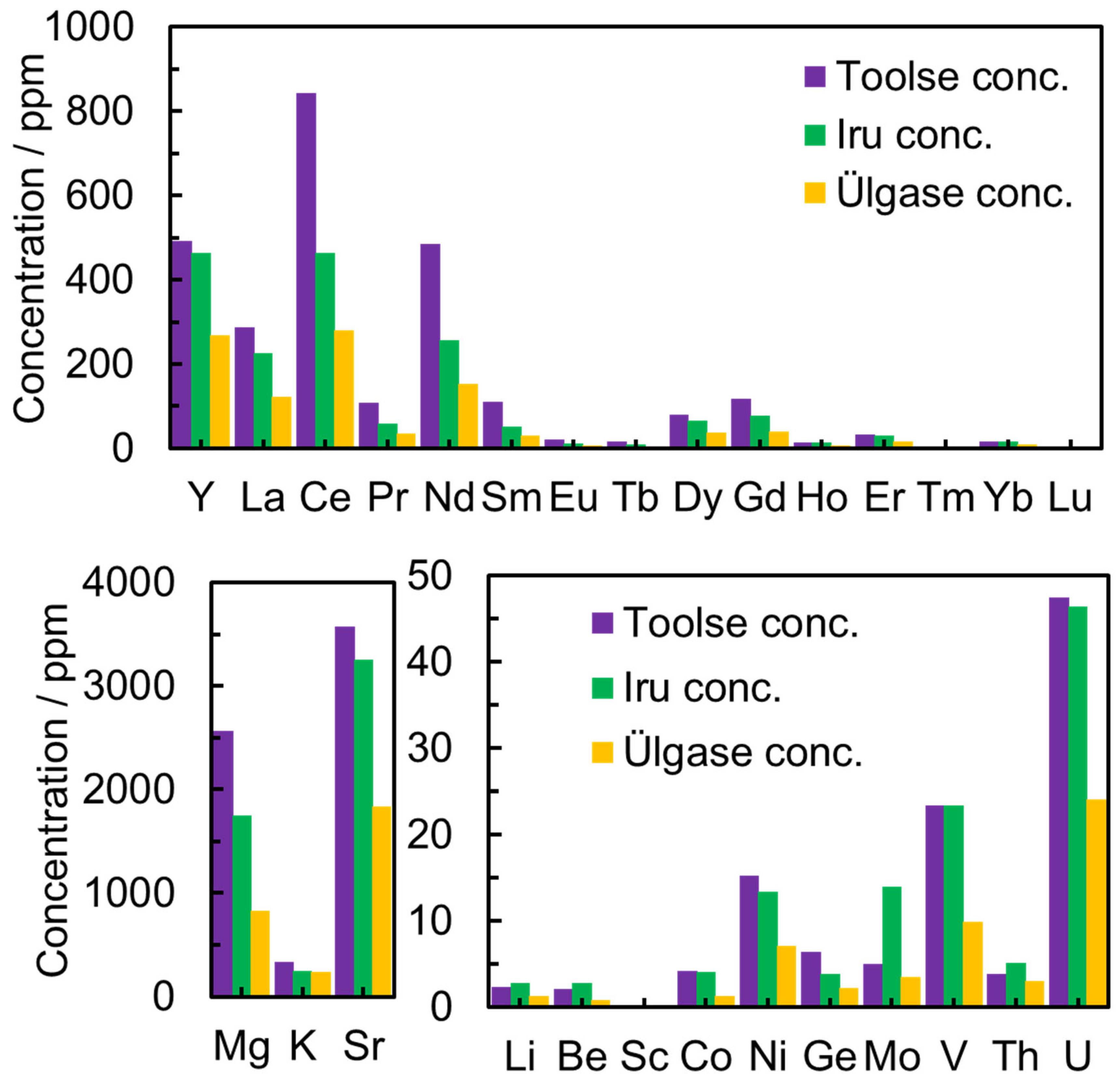
In the PH leaching experiments, the phosphorite ore samples from the Ülgase outcrop were leached in 7.5 M HNO3, and the leaching efficiencies for different deposits are presented in Figure 4. High concentrations of La and Ce might be due to mineral grains of monazite (a mineral that contains rare-earth elements) in the sample [36].
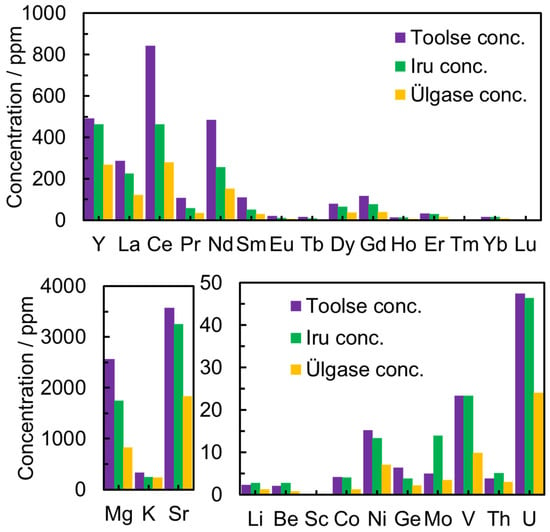
Figure 4.
The concentration of elements in 7.5 M HNO3 aqueous solution for different deposits collected from Toolse, Iru, and Ülgase outcrops.
A more systematic ICP-MS/MS data analysis (Figure 3 and Figure 4) indicates that HNO3 gives more concentrated REE solutions in both ore samples (GA and PH) since (NO3─) is a slightly harder Pearson base than Cl─. Kinetically, hard acids are electrophiles that react more quickly with hard bases (nucleophiles), while soft acids react more rapidly with soft bases. This principle also allows for the prediction of displacement reaction kinetics. Thus, the softness of ions can be used to rationalize the relative stability of the cation–anion interaction in water, which can influence the stability of ion pairs and metal–ligand complexes in water relevant to hydrometallurgical processes [37].
Previous studies have also demonstrated that nitric acid is considered a mineral structure weakener of black shales [38]. Zhu et al. demonstrated that the Mo concentration can be increased with a higher HCl concentration in shales [39]. At the same time, the presence of HNO3 resulted in 74% of the total shale Mo being leached and caused a preferential release of heavy Mo isotopes.
3.3. Extraction Process of GA and PH Using Different Extractants
Based on the low waste materials production and natural resources conception accepted by the EU (recycling industry conditions), all REEs occurring in Estonian GA and PH should be extracted and separated [40].
All extraction agents applied were carefully chosen based on the literature data and our previous results [41,42,43]. Quaternary ammonium-based ionic liquid, Aliquat 336, modified with KNO3 solution, has shown good extraction efficiency for La and Ce. The extraction efficiency with Aliquat 336 (NO3) increased with an increase in the solution pH [44,45]. Therefore, the Aliquat 336(NO3) extraction efficiency was also tested in the Estonian GA and PH systems. Phosphate-based extractants were applied to treat GA solutions, including TBP, EMImDEPO4, and Aliquat 336 (NO3). Meanwhile, D2EHPA was used to extract REEs from PH aqueous acidic solutions in different concentrations, as it has been used in the literature before [46]. It is important to note that all REE concentrations (ppm) in this work depend on the sample’s geological and mineralogical properties (mining pit) in addition to the experimental conditions of the leaching and extraction processes.
Extraction efficiency values were calculated using the previously introduced equation [43]:
Table 1 demonstrates the extraction efficiencies of REEs from GA aqueous solutions. The REE extraction efficiencies of a common extractant, such as TBP, in Estonian GA systems are nonexistent compared to the 3 M HNO3 solution before the extraction process. The calculated extraction efficiency values for ionic liquid, EMImDEPO4, are excellent, higher than 98% for all REEs. It suggests that DEPO4─ strongly interacts with REEs in the acidic aqueous solution of GA. Based on previous results, imidazolium, ammonium, bifunctional, and other ionic liquids have high potential as extraction agents for REEs with high extraction efficiencies, replacing conventional extractants due to sustainability requirements [47,48]. Surprisingly, Aliquat 336(NO3) did not give the expected REE extraction results, with the extraction efficiency values being extremely low in GA solutions, except for La (~80%) and Ce (~24%).

Table 1.
Extraction efficiencies (E %) of REEs from graptolite-argillite using different extraction agents (marked in Table and text) from 3 M HNO3 aqueous solutions.
The extraction efficiencies calculated for the PH from the ICP-MS/MS results indicate that the concentration of HCl significantly influences the extraction profile using D2EHPA as an extraction agent (Table 2). The extraction efficiencies for Aliquat 336(NO3) were low, and the results are not included in the analysis of this paper. Experimental data for D2EHPA show that in more concentrated HCl solutions (5 M and 3 M), HREE and Sc can be extracted selectively. In contrast, LREEs (La-Sm), Y, Eu, Gd, and Tb are not extracted under these conditions. This is in good agreement with previous observations that D2EHPA has better selectivity of HREE over LREE, which can be attributed to the higher charge density of HREE [49]. The LREEs, Y, Eu, Gd, Tb, and Dy can be extracted more effectively using lower HCl (2 M and 1 M) concentrations (Table 2). It is important to note that the extraction efficiency of Y increases significantly at lower concentrations, but the extraction efficiency of Sc is higher in a more acidic environment. We propose that for the extraction of LREE (La-Sm), the concentration of HCl needs to be lower than 1 M. Still, so far, our results indicate that leaching at less acidic concentrations becomes problematic. Therefore, leaching in a more acidic environment, followed by partial neutralization of leachate, might be an option. It can be concluded that extraction in HCl media using different acid concentrations shows promising results for separating HREE and Sc from LREE.

Table 2.
Extraction efficiencies of REEs from phosphorite ore (Ülgase) at different hydrochloric acid molar concentrations using D2EHPA as an extractant.
The extraction results of the PH (Iru) acidic solutions with D2EHPA are highly dependent on the concentration of HNO3 (Table 3), similar to the HCl results. High extraction efficiency values were calculated for all REEs from dilute nitric acid solution with D2EHPA. However, the extraction efficiency values from the concentrated nitric acid solution are better for HREE and negligible for LREE.

Table 3.
Extraction efficiencies of phosphorite ore (Iru) at different nitric acid molar concentrations using D2EHPA as an extractant.
It is essential to point out that the extraction efficiency of Sc is not influenced by the acidic concentration in nitric acid media and remains higher than 90%, which is different from hydrochloric acid media. Thus, for the selective extraction of HREE with D2EHPA, more concentrated HCl and HNO3 should be used. All the results presented in this paper are promising, mainly if ionic liquid extraction has been conducted. However, additional systematic extraction and separation should be conducted to selectively separate REE and DRM from Estonian GA and PH ore samples.
4. Conclusions
The leaching process of Estonian GA and PH was thoroughly studied using HCl and HNO3 with different concentrations, including Aqua Regia. A higher concentration of REEs in GA and PH was achieved in more concentrated acid solutions, especially in HNO3. A variation in phosphate-based extractants and ILs was explored for the extraction process of REEs. The extraction efficiencies in GA complex systems are highest when EMImDEPO4 is used as an extraction agent. In PH systems, more concentrated HCl and HNO3 solutions gave better results for the selective extraction of HREEs from LREEs.
Continuous and systematic analysis of different leaching and extraction agents should be conducted to extract and separate REEs from Estonian GA and PH successfully.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/met15060608/s1.
Author Contributions
Conceptualization: S.J., L.S. and O.O.; methodology: S.J. and L.S.; software: C.S. and O.O.; validation: S.J. and C.S.; formal analysis: L.S.; investigation; S.J. and C.S.; resources: E.L.; data curation: L.S. and O.O.; writing—original draft: C.S. and S.J.; preparation: L.S. and O.O.; writing—review and editing: E.L., L.S., O.O. and S.J.; visualization: O.O.; supervision: E.L.; project administration, E.L.; funding acquisition, E.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Estonian Research Council Grant PRG676 and PRG2677; the Centre of Nanomaterials Technologies and Research project TT13 (NAMUR+); Estonian Ministry of Education and Research project TK210 “Centre of Excellence in Sustainable Green Hydrogen and Energy Technologies” (01.01.2024–31.12.2030); and by the projects “Increasing the knowledge intensity of Ida-Viru entrepreneurship” (ÕÜF12, ÕÜF13) co-funded by the European Union.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
Päärn Paiste and Liis Vitsut for contribution to this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dushyantha, N.; Batapola, N.; Ilankoon, I.M.S.K.; Rohitha, S.; Premasiri, R.; Abeysinghe, B.; Ratnayake, N.; Dissanayake, K. The story of rare earth elements (REEs): Occurrences, global distribution, genesis, geology, mineralogy and global production. Ore Geol. Rev. 2020, 122, 103521. [Google Scholar] [CrossRef]
- Kanazawa, Y.; Kamitani, M. Rare earth minerals and resources in the world. J. Alloys Compd. 2006, 408–412, 1339–1343. [Google Scholar] [CrossRef]
- McLennan, S.M.; Ross Taylor, S. Geology, Geochemistry and Natural Abundances. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; ISBN 978-1-119-95143-8. [Google Scholar]
- Jordens, A.; Cheng, Y.P.; Waters, K.E. A review of the beneficiation of rare earth element bearing minerals. Miner. Eng. 2013, 41, 97–114. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Chen, J.; Kallem, P.; Banat, F.; Qiu, H. Recent advances in selective separation technologies of rare earth elements: A review. J. Environ. Chem. Eng. 2022, 10, 107104. [Google Scholar] [CrossRef]
- Ilankoon, I.M.S.K.; Dushyantha, N.P.; Mancheri, N.; Edirisinghe, P.M.; Neethling, S.J.; Ratnayake, N.P.; Rohitha, L.P.S.; Dissanayake, D.M.D.O.K.; Premasiri, H.M.R.; Abeysinghe, A.M.K.B.; et al. Constraints to rare earth elements supply diversification: Evidence from an industry survey. J. Clean. Prod. 2022, 331, 129932. [Google Scholar] [CrossRef]
- Zhou, B.; Li, Z.; Chen, C. Global Potential of Rare Earth Resources and Rare Earth Demand from Clean Technologies. Minerals 2017, 7, 203. [Google Scholar] [CrossRef]
- Tukker, A. Rare Earth Elements Supply Restrictions: Market Failures, Not Scarcity, Hamper Their Current Use in High-Tech Applications. Environ. Sci. Technol. 2014, 48, 9973–9974. [Google Scholar] [CrossRef]
- Coey, J.M.D. Perspective and Prospects for Rare Earth Permanent Magnets. Engineering 2020, 6, 119–131. [Google Scholar] [CrossRef]
- Podmiljšak, B.; Saje, B.; Jenuš, P.; Tomše, T.; Kobe, S.; Žužek, K.; Šturm, S. The Future of Permanent-Magnet-Based Electric Motors: How Will Rare Earths Affect Electrification? Materials 2024, 17, 848. [Google Scholar] [CrossRef]
- Bailey, G.; Mancheri, N.; Van Acker, K. Sustainability of Permanent Rare Earth Magnet Motors in (H)EV Industry. J. Sustain. Metall. 2017, 3, 611–626. [Google Scholar] [CrossRef]
- Fernandez, V. Rare-earth elements market: A historical and financial perspective. Resour. Policy 2017, 53, 26–45. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, P.; Chen, W.; Wang, L.; Wang, Q.-C.; Chen, W.-Q. Supply and demand conflicts of critical heavy rare earth element: Lessons from gadolinium. Resour. Conserv. Recycl. 2023, 199, 107254. [Google Scholar] [CrossRef]
- Tokimatsu, K.; Murakami, S.; Adachi, T.; Ii, R.; Yasuoka, R.; Nishio, M. Long-term demand and supply of non-ferrous mineral resources by a mineral balance model. Miner. Econ. 2017, 30, 193–206. [Google Scholar] [CrossRef]
- Voolma, M.; Soesoo, A.; Hade, S.; Hints, R.; Kallaste, T. Geochemical heterogeneity of estonian graptolite argillite. Oil Shale 2013, 30, 377. [Google Scholar] [CrossRef]
- Kaljuvee, T.; Tõnsuaadu, K.; Einard, M.; Mikli, V.; Kivimäe, E.-K.; Kallaste, T.; Trikkel, A. Thermal Behavior of Estonian Graptolite–Argillite from Different Deposits. Processes 2022, 10, 1986. [Google Scholar] [CrossRef]
- Vind, J.; Tamm, K. Review of the extraction of key metallic values from black shales in relation to their geological and mineralogical properties. Miner. Eng. 2021, 174, 107271. [Google Scholar] [CrossRef]
- Vind, J.; Ofili, S.; Mänd, K.; Soesoo, A.; Kirsimäe, K. Redox-sensitive trace metal hyper-enrichment in Tremadocian Alum Shale (graptolite argillite) in northwestern Estonia, Baltic Palaeobasin. Chem. Geol. 2023, 640, 121746. [Google Scholar] [CrossRef]
- Menert, A.; Korb, T.; Orupõld, K.; Teemusk, A.; Sepp, H.; Mander, Ü.; Ilmjärv, T.; Truu, J.; Paiste, P.; Kirsimäe, K.; et al. Methanogenesis and metal leaching on anaerobic decomposition of graptolite argillite. Environ. Technol. Innov. 2023, 31, 103139. [Google Scholar] [CrossRef]
- Yang, X.; Tamm, K.; Piir, I.; Kuusik, R.; Trikkel, A.; Tõnsuaadu, K. Evaluation of Estonian phosphate rock by flotation. Miner. Eng. 2021, 171, 107127. [Google Scholar] [CrossRef]
- Brahim, J.A.; Hak, S.A.; Achiou, B.; Boulif, R.; Beniazza, R.; Benhida, R. Kinetics and mechanisms of leaching of rare earth elements from secondary resources. Miner. Eng. 2022, 177, 107351. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumari, A.; Panda, R.; Kumar, J.R.; Yoo, K.; Lee, J.Y. Review on hydrometallurgical recovery of rare earth metals. Hydrometallurgy 2016, 165, 2–26. [Google Scholar] [CrossRef]
- Peelman, S.; Sun, Z.H.I.; Sietsma, J.; Yang, Y. Hydrometallurgical Extraction of Rare Earth Elements from Low Grade Mine Tailings. In Rare Metal Technology 2016; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 17–29. ISBN 978-1-119-27483-4. [Google Scholar]
- Costis, S.; Mueller, K.K.; Coudert, L.; Neculita, C.M.; Reynier, N.; Blais, J.-F. Recovery potential of rare earth elements from mining and industrial residues: A review and cases studies. J. Geochem. Explor. 2021, 221, 106699. [Google Scholar] [CrossRef]
- El-Nadi, Y.A.; El-Hefny, N.E.; Aly, H.F. Solvent extraction and recovery of Y(III) and Yb(III) from fluorspar mineral. Int. J. Miner. Metall. Mater. 2013, 20, 713–719. [Google Scholar] [CrossRef]
- Liu, T.; Chen, J. Extraction and separation of heavy rare earth elements: A review. Sep. Purif. Technol. 2021, 276, 119263. [Google Scholar] [CrossRef]
- Bandara, H.M.D.; Field, K.D.; Emmert, M.H. Rare earth recovery from end-of-life motors employing green chemistry design principles. Green Chem. 2016, 18, 753–759. [Google Scholar] [CrossRef]
- Arrachart, G.; Couturier, J.; Dourdain, S.; Levard, C.; Pellet-Rostaing, S. Recovery of Rare Earth Elements (REEs) Using Ionic Solvents. Processes 2021, 9, 1202. [Google Scholar] [CrossRef]
- Wang, K.; Adidharma, H.; Radosz, M.; Wan, P.; Xu, X.; Russell, C.K.; Tian, H.; Fan, M.; Yu, J. Recovery of rare earth elements with ionic liquids. Green Chem. 2017, 19, 4469–4493. [Google Scholar] [CrossRef]
- Baba, Y.; Kubota, F.; Kamiya, N.; Goto, M. Kinetics and mechanisms of leaching of rare earth elements from secondary resources. J. Chem. Eng. Jpn. 2011, 44, 679–685. [Google Scholar] [CrossRef]
- Soesoo, A. Main Precambrian and Paleozoic Mineral Resources of Estonia. Asp. Min. Miner. Sci. 2021, 6, 729–732. [Google Scholar] [CrossRef]
- Soesoo, A.; Vind, J.; Hade, S. Uranium and Thorium Resources of Estonia. Minerals 2020, 10, 798. [Google Scholar] [CrossRef]
- Hade, S.; Soesoo, A. Estonian graptolite argillites revisited: A future resource? Oil Shale 2014, 31, 4. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, Y.; Liu, T.; Huang, J.; Xue, N. Vanadium extraction from black shale: Enhanced leaching due to fluoride addition. Hydrometallurgy 2019, 187, 141–148. [Google Scholar] [CrossRef]
- Sevim, F.; Saraç, H.; Kocakerim, M.M.; Yartaşı, A. Dissolution Kinetics of Phosphate Ore in H2SO4 Solutions. Ind. Eng. Chem. Res. 2003, 42, 2052–2057. [Google Scholar] [CrossRef]
- Clavier, N.; Podor, R.; Dacheux, N. Crystal chemistry of the monazite structure. J. Eur. Ceram. Soc. 2011, 31, 941–976. [Google Scholar] [CrossRef]
- Senanayake, G. Gold leaching by copper(II) in ammoniacal thiosulphate solutions in the presence of additives. Part I: A review of the effect of hard–soft and Lewis acid-base properties and interactions of ions. Hydrometallurgy 2012, 115–116, 21–29. [Google Scholar] [CrossRef]
- Watling, H.R. Review of Biohydrometallurgical Metals Extraction from Polymetallic Mineral Resources. Minerals 2014, 5, 1–60. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Z.; Chen, H. Advances in Isotope Geochronology and Isotope Geochemistry: A Preface. J. Earth Sci. 2022, 33, 1–4. [Google Scholar] [CrossRef]
- Waste and Recycling. Available online: https://environment.ec.europa.eu/topics/waste-and-recycling_en (accessed on 18 November 2022).
- Jürjo, S.; Oll, O.; Paiste, P.; Külaviir, M.; Zhao, J.; Lust, E. Electrochemical co-reduction of praseodymium and bismuth from 1-butyl-1-methylpyrrolidinium bis (fluorosulfonyl) imide ionic liquid. Electrochem. Commun. 2022, 138, 107285. [Google Scholar] [CrossRef]
- Jürjo, S.; Oll, O.; Lust, E. Yttrium Separation from Phosphorite Extract Using Liquid Extraction with Room Temperature Ionic Liquids Followed by Electrochemical Reduction. Metals 2024, 14, 927. [Google Scholar] [CrossRef]
- Jürjo, S.; Siinor, L.; Siimenson, C.; Paiste, P.; Lust, E. Two-Step Solvent Extraction of Radioactive Elements and Rare Earths from Estonian Phosphorite Ore Using Nitrated Aliquat 336 and Bis(2-ethylhexyl) Phosphate. Minerals 2021, 11, 388. [Google Scholar] [CrossRef]
- Sepúlveda, R.; Toro, N.; Hernández, P.; Navarro, P.; Vargas, C.; Gálvez, E.; Castillo, J. Solvent Extraction of Metal Ions from Synthetic Copper Leaching Solution Using R4NCy. Metals 2022, 12, 1053. [Google Scholar] [CrossRef]
- Gorzin, H.; Ghaemi, A.; Hemmati, A.; Maleki, A. Studies on effective interaction parameters in extraction of Pr and Nd using Aliquat 336 from NdFeB magnet-leaching solution: Multiple response optimizations by desirability function. J. Mol. Liq. 2021, 324, 115123. [Google Scholar] [CrossRef]
- Cheremisina, O.; Ponomareva, M.; Sergeev, V.; Mashukova, Y.; Balandinsky, D. Extraction of Rare Earth Metals by Solid-Phase Extractants from Phosphoric Acid Solution. Metals 2021, 11, 991. [Google Scholar] [CrossRef]
- Quijada-Maldonado, E.; Romero, J. Solvent extraction of rare-earth elements with ionic liquids: Toward a selective and sustainable extraction of these valuable elements. Curr. Opin. Green Sustain. Chem. 2021, 27, 100428. [Google Scholar] [CrossRef]
- Kaim, V.; Rintala, J.; He, C. Selective recovery of rare earth elements from e-waste via ionic liquid extraction: A review. Sep. Purif. Technol. 2023, 306, 122699. [Google Scholar] [CrossRef]
- Gergoric, M.; Ekberg, C.; Steenari, B.-M.; Retegan, T. Separation of Heavy Rare-Earth Elements from Light Rare-Earth Elements via Solvent Extraction from a Neodymium Magnet Leachate and the Effects of Diluents. J. Sustain. Metall. 2017, 3, 601–610. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).