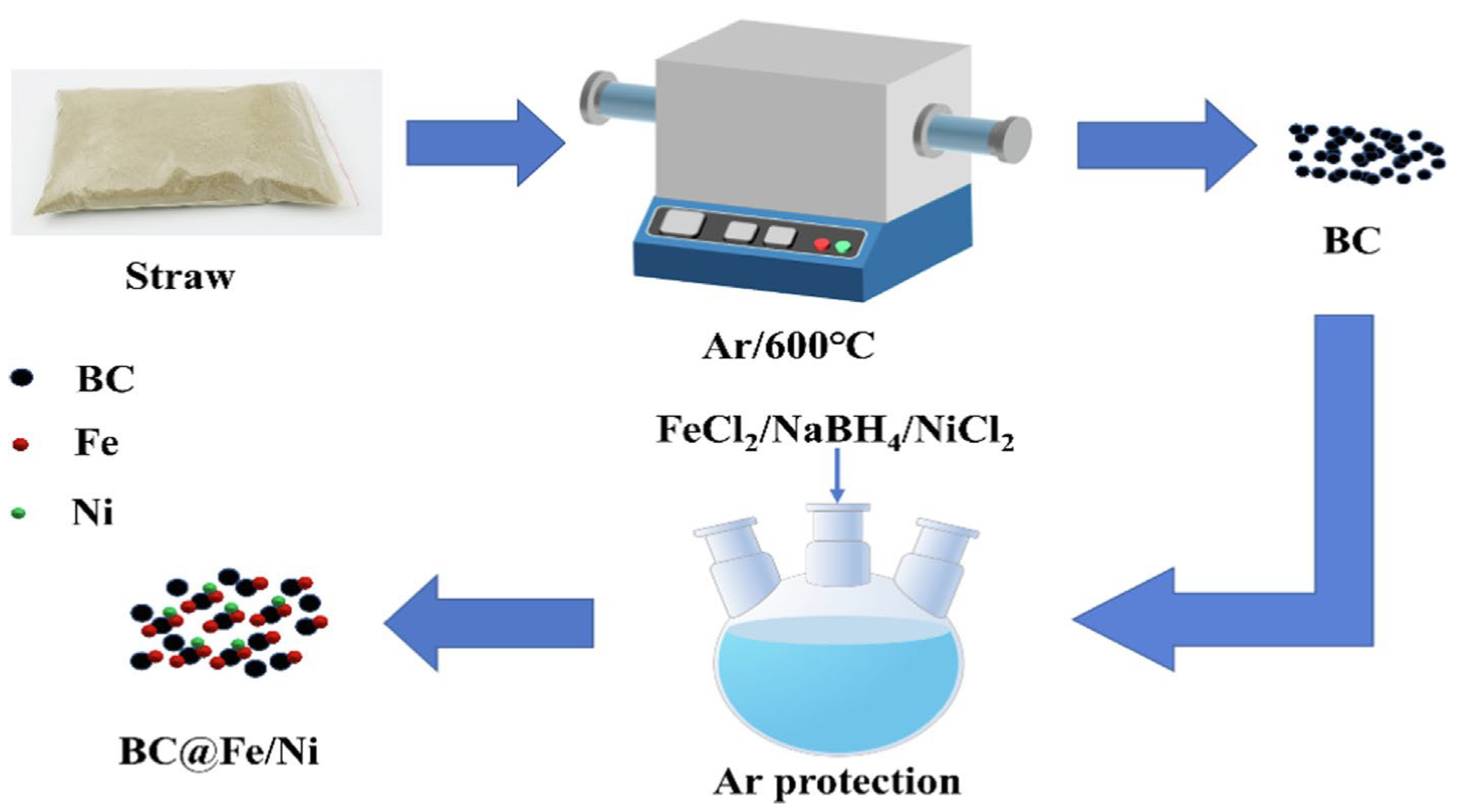
Synthesis of Iron-Based and Aluminum-Based Bimetals: A Systematic Review
Abstract
1. Introduction
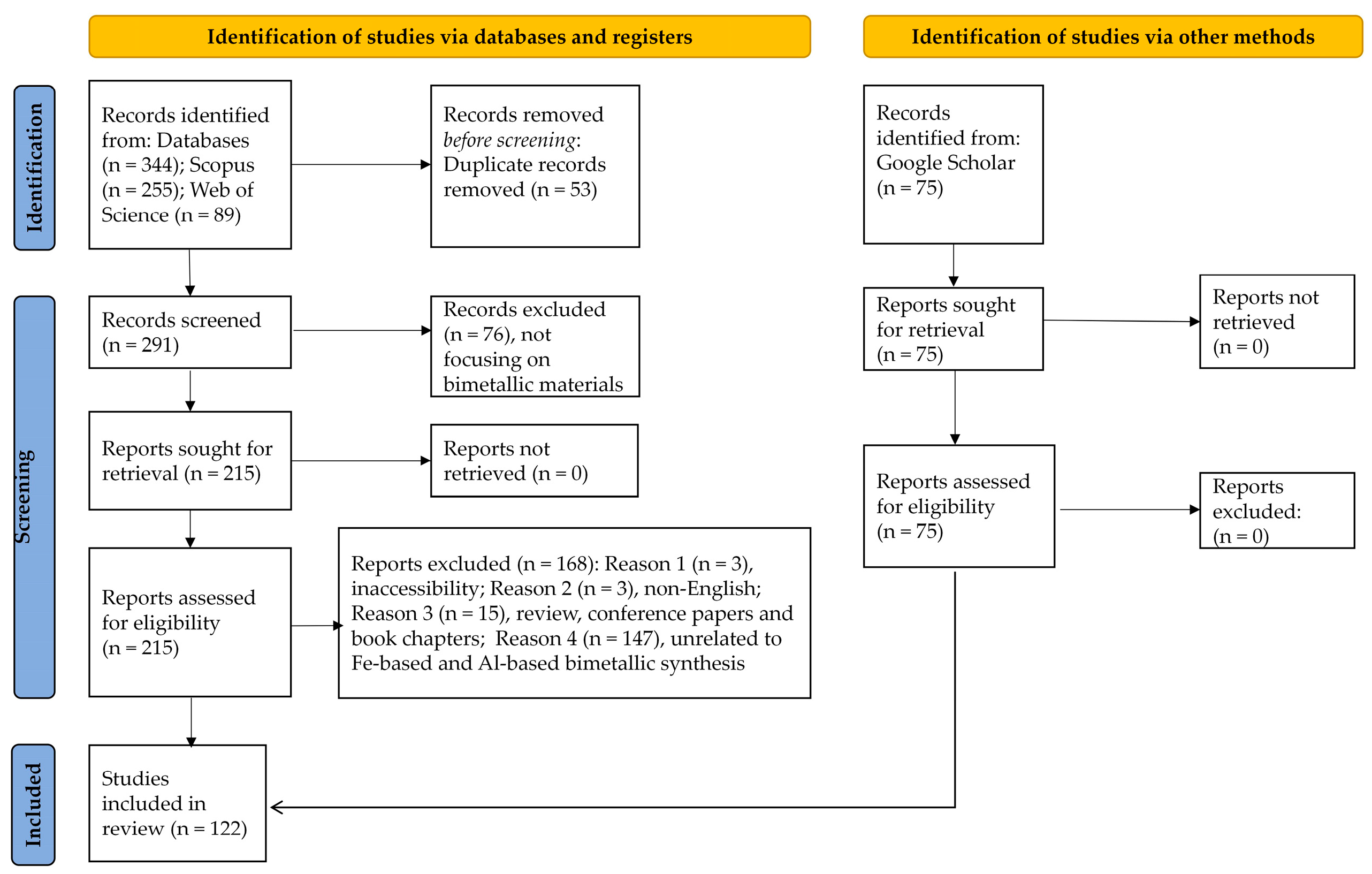
2. Materials and Methods
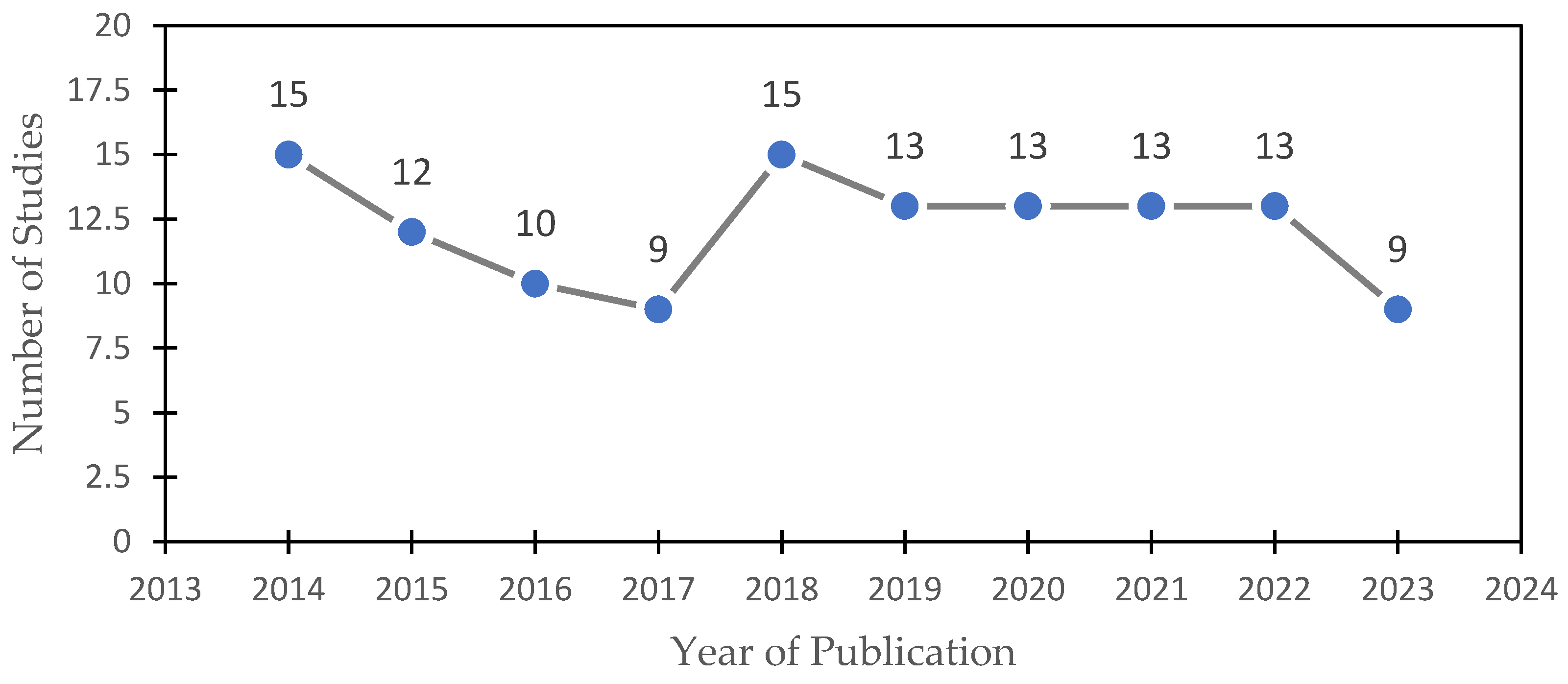
2.1. Bimetal Research Publication Trends
2.2. Active Countries
2.3. Active Journals
2.4. Active Institutions
2.5. Active Authors
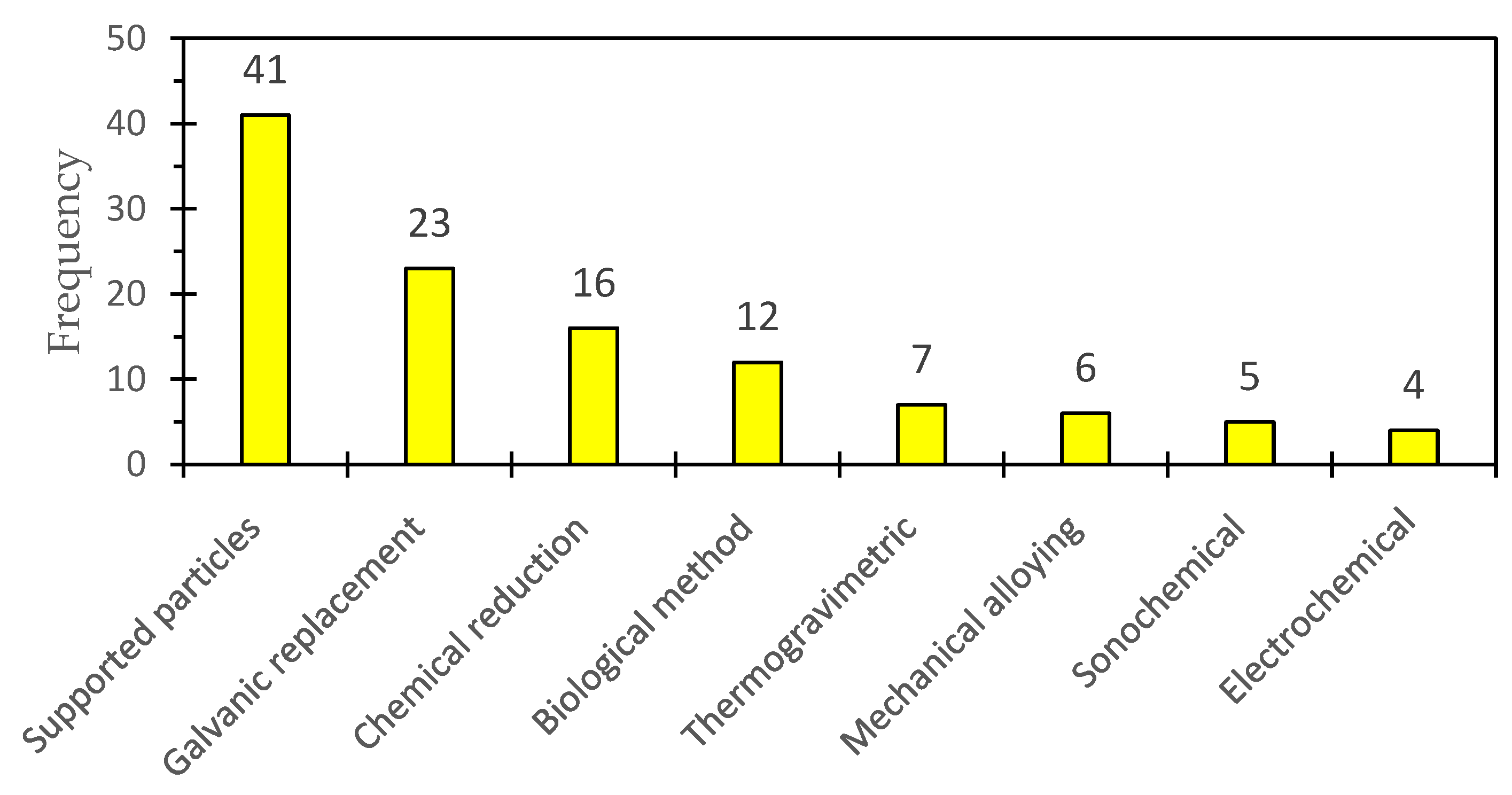
2.6. Most-Used Synthesis Methods
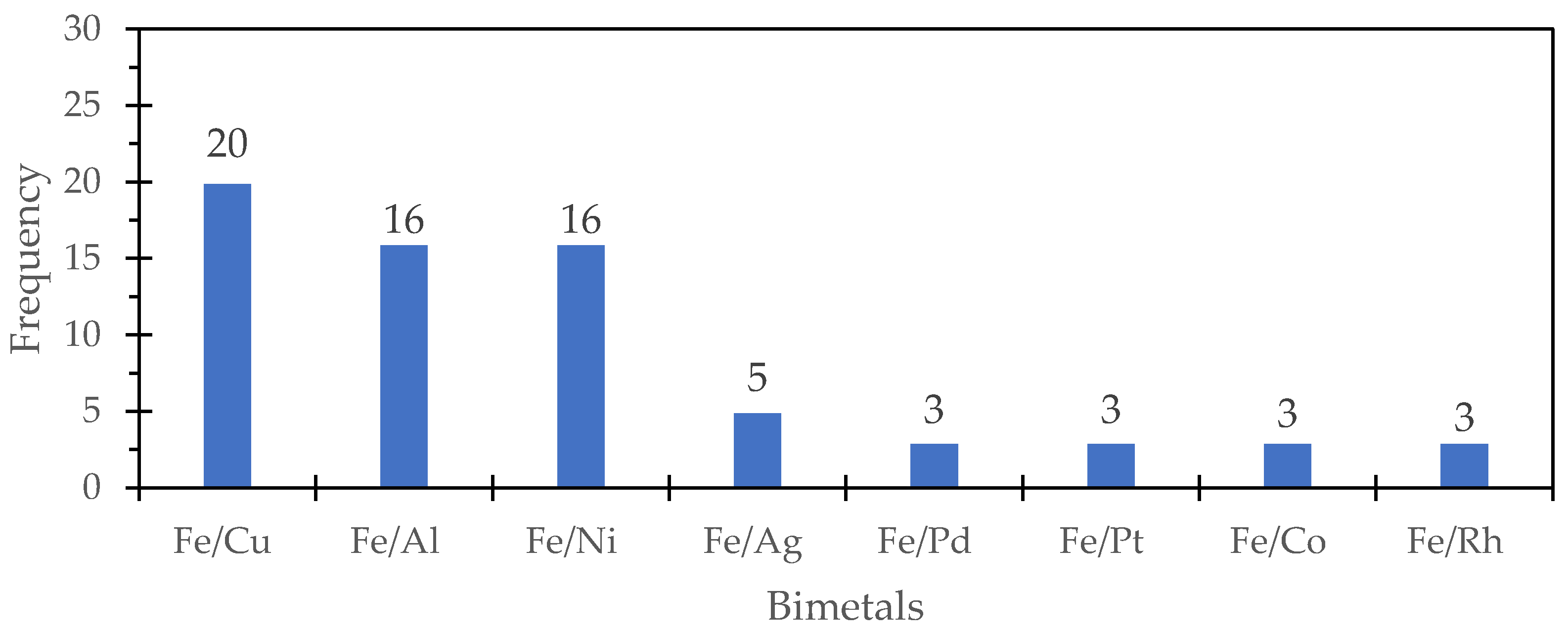
2.7. Most-Synthesized Iron-Based and Aluminum-Based Bimetals
3. Physical Methods for Synthesizing Iron-Based and Aluminum-Based Bimetals
3.1. Mechanical Alloying
| Bimetal System | Experimental Materials | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Fe-Cu/Al collar | Fe powder (99.9% pure @125 µm particle size), Cu powder (99.5% @100 µm particles size), aluminum collar substrate | Simple synthesis process Cost-effective Solid-state diffusion (non-melting process) Environmentally friendly process | Time-intensive in milling process Energy-intensive in milling process | [49] |
| Cu-Fe/CNT | Pure Fe powder, electrolytic Cu powder, and multi-walled carbon nanotubes (CNTs) | CNTs as reinforcement to Cu/Fe bimetals, application of controlled atmosphere (argon) | Time-intensive in milling process Energy-intensive in milling process Multi-step synthesis | [50] |
| Fe/Ag nanocomposite | Carbonyl Fe and Ag2O powders | Annealing process at 550 °C Vacuum-drying Controlled atmosphere (hexane + Ar) | Time intensive milling Energy-intensive milling Other energy requirements | [52] |
| Fe/Ag nanocomposite | Carbonyl Fe and Ag2O powders | Attrition milling in hexane under Ar Vacuum-drying Heat treatment at 550 °C in H2 | Time-intensive milling High-energy milling Other energy requirements | [53] |
| Fe/Ag, Fe/Cu nanocomposites | Carbonyl Fe, Ag nanoxide, Fe and cuprous oxide nanopowders | Attrition milling in hexane under Ar Consolidation process at 400 MPa Application of cold-sintering application of hydrogen treatment (450 °C) | Time-intensive milling High-energy milling Other energy requirements Material costs | [54] |
| Fe/Ag, Fe/Cu nanocomposites | Carbonyl Fe, silver oxide, Fe and cuprous oxide nanopowders | Attrition milling in hexane application of cold-sintering compression process at 3 GPa | Time-intensive milling High-energy milling Other energy requirements Material costs | [55] |
3.2. Electrical Explosion of Metal Wires
3.3. Radiolysis
3.4. Sonochemical Method
| Bimetal System | Experimental Materials | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Ni/Fe | FeSO4·7H2O, NiSO4·6H2O, NaBH4 (reducing agent) | Mild synthesis conditions Sequential reduction process Versatile synthesis process | Multiple washing steps using deionized water | [69] |
| s-Fe/Cu | Sponge iron (s-Fe) particles, CuSO4·5H2O | Simple and fast process Low energy requirement Magnetic recoverability | Ultrasound equipment dependency | [70] |
| Fe/Cu-GO | FeSO4·7H2O, CuSO4·5H2O, graphene oxide (GO), NaBH4 (reducing agent) | Magnetic recoverability Low-cost process Graphene oxide as a support pH-neutral synthesis | Multi-step process | [71] |
| Fe-Mn/KB | Mn(NO3)2, Fe(NO3)3, melamine, ketjenblack carbon | Mild calcination conditions Nitrogen atmosphere calcination Carbon support integration (ketjenblack) | Energy requirements in calcination, heating ang drying Long duration of heating and drying requirements Multiple processing steps | [72] |
| Fe-Mn@SCAs | MnSO4, C6H5Na3O7·2H2O, K3[Fe(CN)6], corn starch | Eco-friendly support material High porosity of the bimetallic material | Time-intensive synthesis process Energy requirements in the synthesis Multi-step process | [73] |
3.5. Magnetic Field-Assisted Laser Ablation in Liquid (MF-LAL)
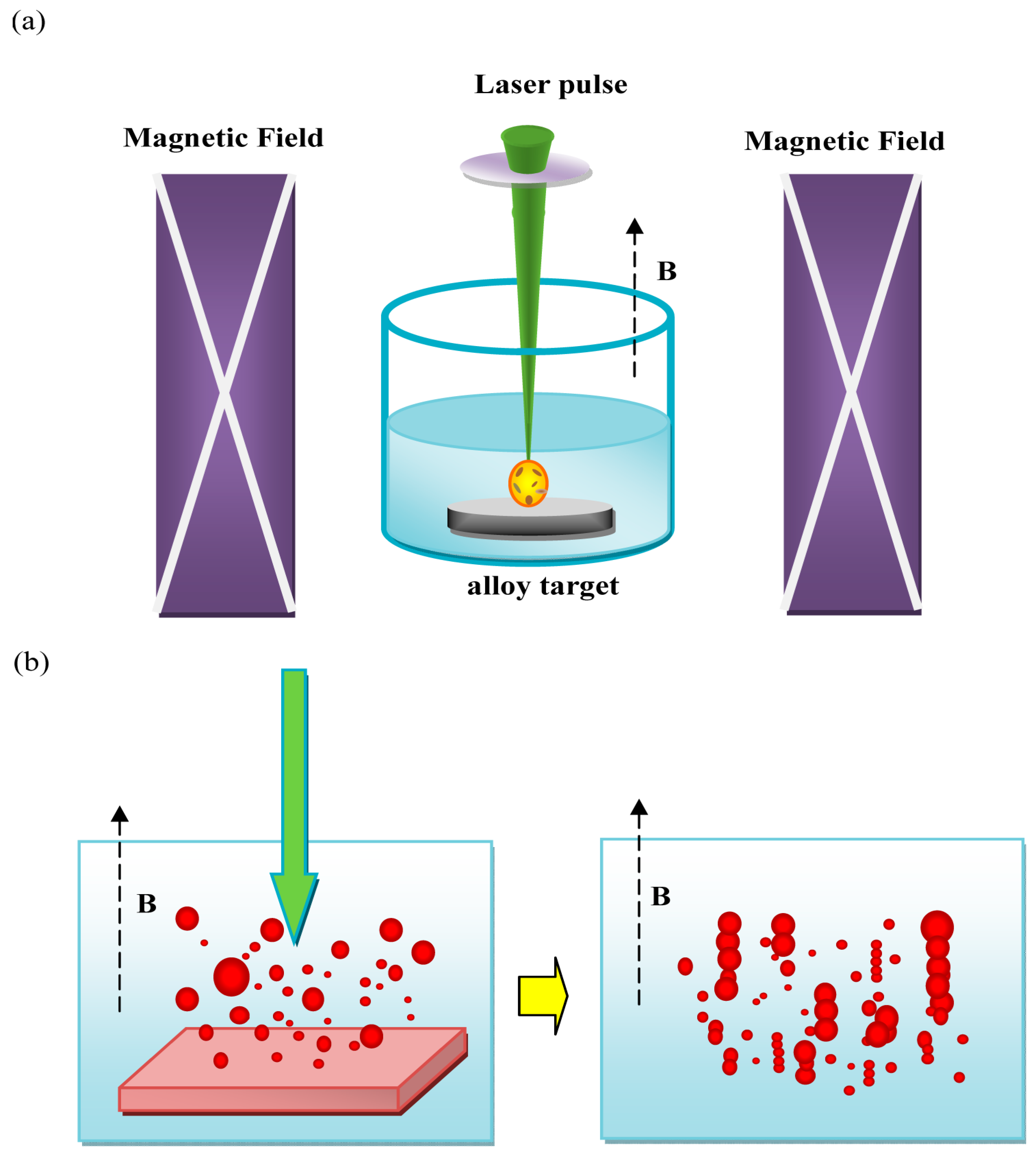
3.6. Influence of Characteristics of Synthesized Bimetals by Physical Methods to Their Properties
4. Chemical Methods for Synthesizing Iron-Based and Aluminum-Based Bimetals
4.1. Chemical Reduction

4.2. Chemical Dealloying
4.3. Seed-Mediated Growth
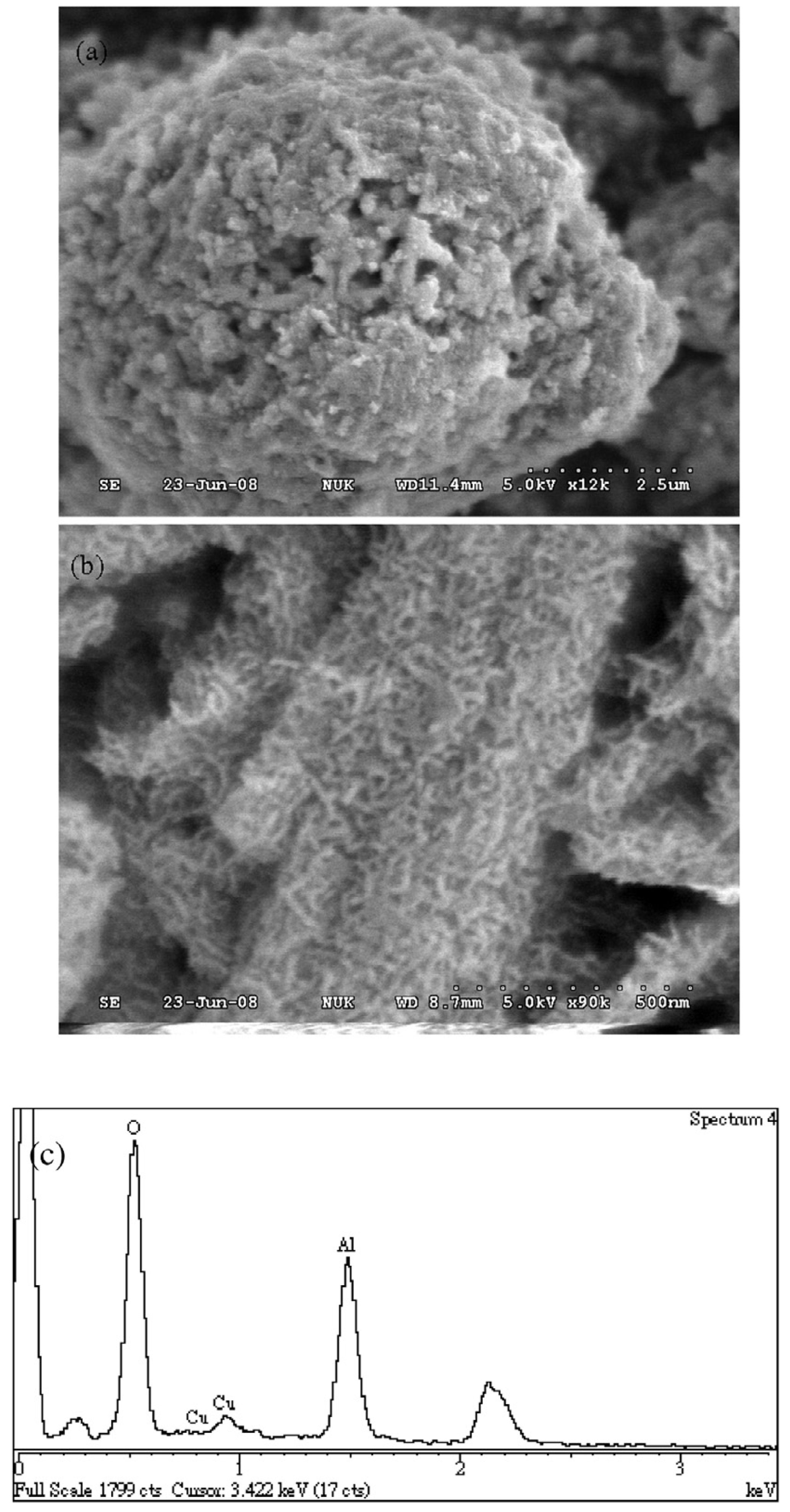
4.4. Electrochemical Synthesis
4.5. Galvanic Replacement
| Bimetal System | Experimental Materials | Advantages | Disadvantages | Reference |
|---|---|---|---|---|
| Fe/Cu | Metal precursors: CuSO4, ZVI | Simple synthesis process Controlled Cu mass loading | High Cu loading can be costly (e.g., 1.26 g Cu/g Fe) | [123] |
| Fe/Cu | Metal precursors: CuSO4·5H2O, nZVI | Simple and controllable synthesis Storage of particles under nitrogen atmosphere | Energy requirements in the overall process Long processing time | [124,125] |
| Fe/Cu | Metal precursors: CuSO4·5H2O, nZVI | Controlled Cu loading Efficient Cu deposition Use of nitrogen atmosphere in the process | Multi-step synthesis method Drying requirements at 80 °C | [126] |
| Fe/Cu | Metal precursors: CuSO4, CuCl2, ZVI | Wide temperature range studied Variable Cu2+ concentrations Controlled Cu loading pH influence studied Stirring speed was also varied | Longer coverage of study Time-intensive study Material and cost considerations | [127] |
| Fe/Cu | Metal precursors: CuSO4·5H2O, nZVI | Simplicity of the process Controlled Cu loading and mixing speed Mild operating temperature (40 °C) Fixed Cu2+ concentration | Energy requirements in drying (40 °C for 40 min) | [128] |
| Fe/Cu | Metal precursors: CuSO4, ZVI | Use of electroless plating in the synthesis Fixed copper concentration (11.25 g/L CuSO4·5H2O) Controlled temperature (70 ± 1 °C) in the process Controlled agitation | Complex chemical system involvement of many chemicals Processing temperature requirements (70 ± 1 °C) | [129] |
| Fe/Cu | Metal precursors: CuSO4·5H2O, ZVI | Versatile synthesis method Controlled Cu/Fe mass ratio Room-temperature drying Use of argon atmosphere | Long drying time Refrigeration storage adds complexity | [130] |
| Fe/Al | Metal precursors: FeSO4, ZVAl | Simple synthesis method Rapid synthesis (15 min reaction time) Controlled Fe mass loading | Use of concentrated HCl | [131,132] |
| Fe/Al | Metal precursors: FeSO4·7H2O, ZVAl | Simple synthesis method Controlled pH in the synthesis Controlled addition of NaBH4 solution Use of nitrogen atmosphere | Energy-intensive process Long processing time | [133] |
| Mg/Fe | Metal precursors: FeSO4·7H2O, ZVMg | Simple and versatile synthesis method Rapid synthesis process (2 min reaction time) Controlled Mg/Fe ratios | High energy requirement for freeze-drying Long drying time | [134] |
| Fe/Al | Metal precursors: FeCl3·6H2O, ZVAl | Simple synthesis method Involving acid-washing of ZVAl particles Good control over Fe/Al ratio Mild reaction conditions | Requiring acid (HCl) pretreatment | [135,136] |
| Fe/Al | Metal precursors: FeSO4, ZVAl | Controlled Fe loading Al powder is pretreated with HCl and deionized water Use of nitrogen atmosphere | Use of HCl in acid pretreatment Long drying time | [137] |
| Fe/Al | Metal precursors: FeCl3, ZVAl | Simple synthesis Al powder is pretreated with HCl Controlled Fe loading Processing under ambient conditions | Use of HCl in acid pretreatment | [138] |
| Fe/Cu | Metal precursors: CuSO4·5H2O, ZVI | Simple synthesis method Rapid synthesis (15 min reaction time) Pretreatment of ZVI with dilute HCl Room-temperature drying Controlled Cu/Fe ratio Use of argon atmosphere in the process | Use of HCl in acid pretreatment Long drying time | [139] |
| Fe/Al | Metal precursors: FeCl2, Al alloys (1050, 2024, 3003, 5083, 6061, and 7075) | Simplicity and versatility of the process Mild temperature conditions (25–50 °C) Shorter reaction times (15–60 min) Magnetic recoverability of the particles | Involving different types of Al alloys (adds material cost) Dependence on acid (HCl) concentration | [140] |
| Fe/Al | Metal precursors: Ferric chloride, Al scrap | Simple synthesis process Efficient Fe deposition Pretreatment of Al scrap with HCl Use of Al scrap as ZVAl source | Use of HCl in acid pretreatment | [141] |
| Fe/Al | Metal precursors: FeCl3, Al scraps | Versatile and innovative synthesis method Controlled Fe deposition Mild drying conditions (40 °C) Adjustable reaction durations (0.5 to 6 h) Magnetic recoverability of the particles Use of Al scraps as ZVAl source | Labor-intensive process Long processing time (up to 6 h) | [142] |
| Fe/Al | Metal precursors: ZVI (source of Fe2+), ZVAl | Simple process Room-temperature drying Use of argon atmosphere | Long drying time (24 h) Use of strong acid (HCl) in the process Refrigerator storage requirement for the particles | [144,145] |
| Fe/Al | Metal precursors: ZVI (source of Fe2+), ZVAl | Simple process Room-temperature drying No acid (HCl) treatment involved Use of argon atmosphere | Long drying time (24 h) Refrigerator storage requirement for the particles | [146] |
4.6. Thermogravimetric Method
4.7. Supported Particles
4.7.1. Carbon-Based Materials as Support
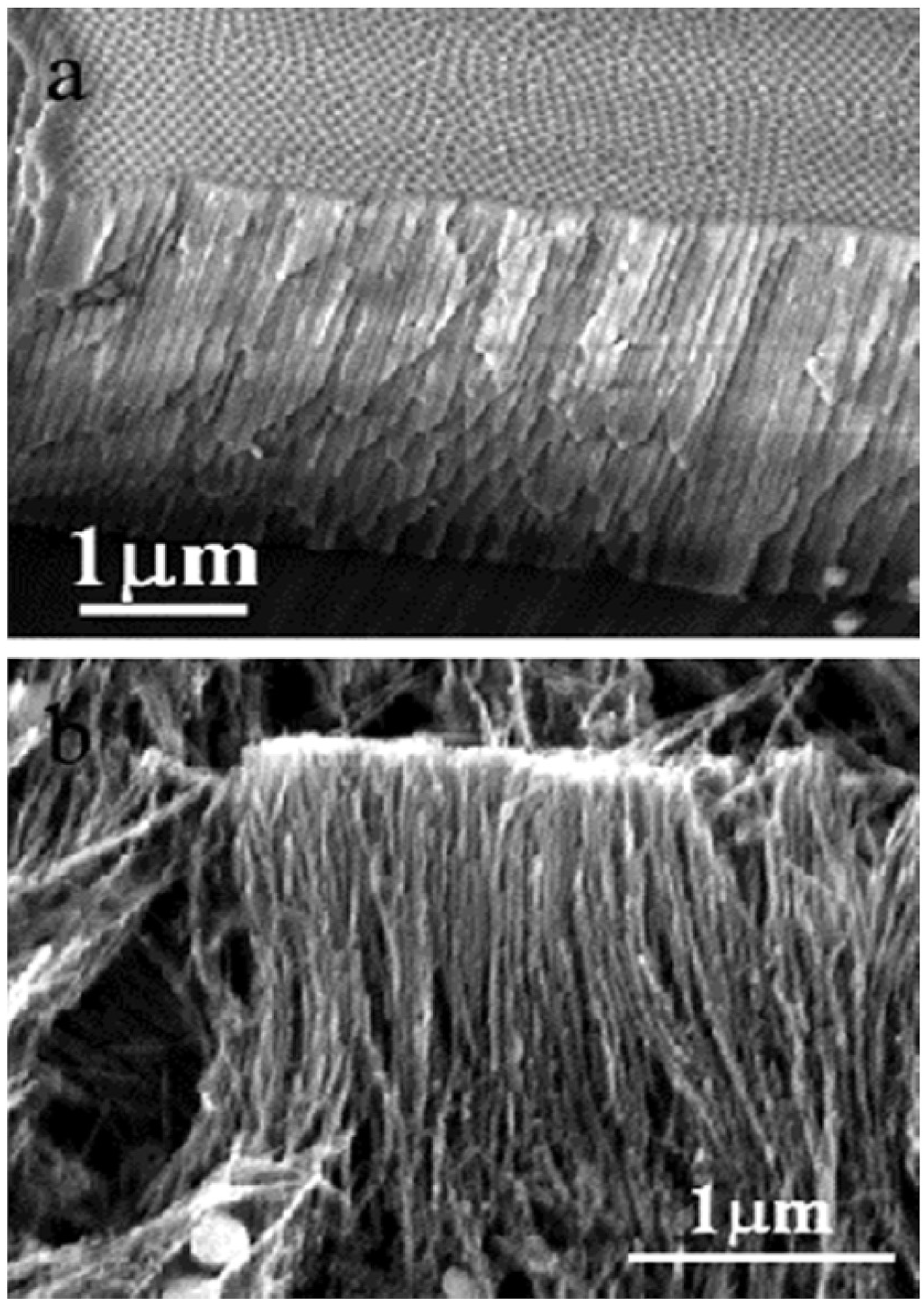
4.7.2. Alumina (Al2O3) as Support
4.7.3. Silica as Support
4.7.4. Minerals as Supports
| Bimetal System | Experimental Materials | Advantages | Disadvantages | Reference |
|---|---|---|---|---|
| Be@Fe-Cu | Metal precursors: FeCl3∙6H2O, CuSO4∙5H2O Others: Bentonite, NaBH4 (reducing agent) Support: Bentonite (Be) | Mild synthesis conditions Sequential metal impregnation for controlled deposition Use of an inert argon atmosphere Magnetic recoverability of the particles | Multi-step synthesis process Long processing time | [193] |
| B-Fe/Ni | Metal precursors: FeCl3∙6H2O, NiSO4∙6H2O Others: Bentonite, NaBH4 Support: Bentonite (B) | Scalable and simple synthesis process Controlled chemical reduction via NaBH4 Use of inert nitrogen atmosphere | Energy-intensive drying requirement Long processing time | [194] |
| K-Fe/Pd | Metal precursors: FeCl3∙6H2O, CuCl2∙2H2O Others: Natural kaolinite, NaBH4 Support: Kaolinite (K) | Versatile synthesis process Ultrasonic treatment improves Pd deposition Use of inert nitrogen atmosphere | Energy-intensive drying requirement Multiple ethanol washing steps Long processing time | [197] |
| Cu/Fe@zeolite | Metal precursors: FeSO4·7H2O, CuCl2·2H2O Other(s): Zeolite Support: Zeolite | Multiple synthesis routes provide flexibility Simplicity of the synthesis methods Controlled stirring and centrifugation in the syntheses | Time-Consuming Processes Energy-intensive drying requirement for Cu/Fe@zeolite-2 synthesis | [200] |
| Di-Fe/Ni | Metal precursors: FeCl3∙6H2O, NiSO4∙6H2O Others: Diatomite (Di), NaBH4 Support: Diatomite (Di) | Simplicity and versatility of the synthesis Controlled addition of NaBH4 solution Use of nitrogen atmosphere | Multi-step process Multiple ethanol washing steps Pre-processing of diatomite is required Moderate temperature (60 °C) drying is required Long processing time | [201] |
| Pal-Fe/Ni | Metal precursors: FeCl3∙6H2O, NiSO4∙6H2O Others: Palygorskite, NaBH4 Support: Palygorskite (Pal) | Versatility of the synthesis method Controlled addition of NaBH4 solution Controlled stirring and centrifugation in the process Use of nitrogen atmosphere | Multi-step procedure Pre-processing of palygorskite is required Drying requirements of the overall process Long processing time | [202] |
| Sep-Fe/Ni | Metal precursors: FeCl3, NiSO4∙6H2O Others: Sepiolite, NaBH4 Support: Sepiolite (Sep) | Versatility of the process Controlled Ni/Fe composition Controlled addition of NaBH4 solution Use of nitrogen atmosphere | Drying requirements of the overall process Pre-processing of sepiolite is required Long processing time | [203] |
4.7.5. Other Material Supports
4.8. Influence of Characteristics of Synthesized Bimetals by Chemical Methods to Their Properties
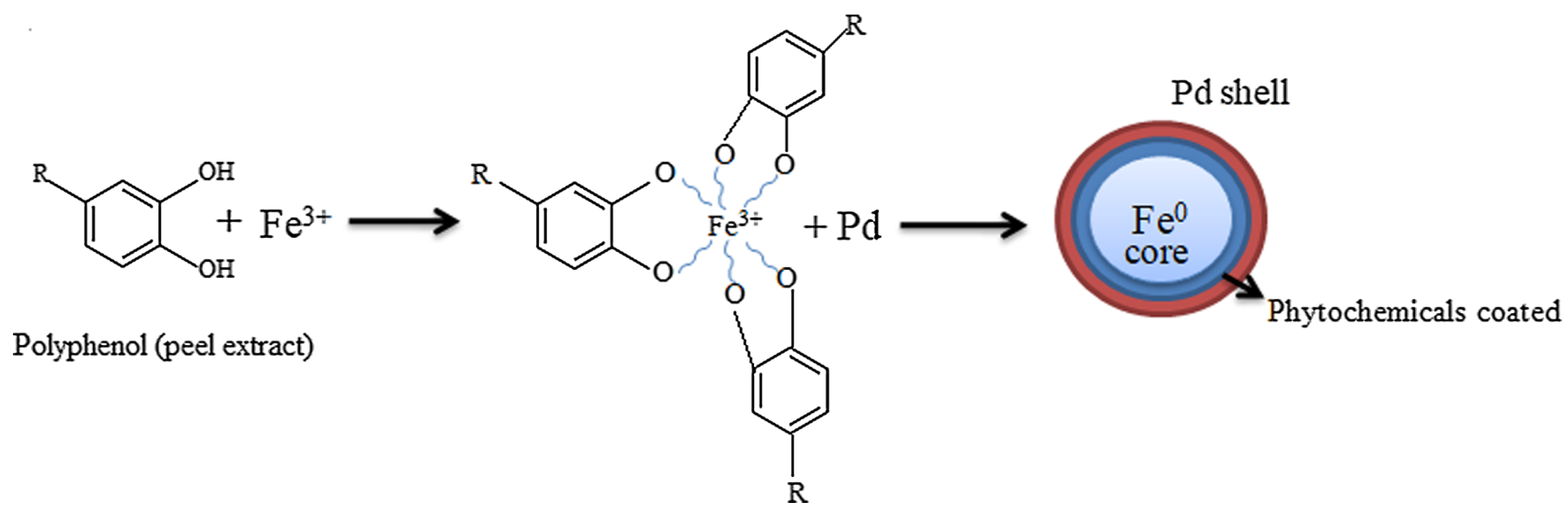
5. Biological Methods for Synthesizing Iron-Based and Aluminum-Based Bimetals
| Bimetal System | Experimental Materials | Advantages | Disadvantages | Reference |
|---|---|---|---|---|
| Fe-Ni | Metal precursors: Fe(NO3)3, Ni(NO3)2 Reducing agent: Pithecellobium dulce legume mesocarp extract | Eco-friendly synthesis Simple and cost-effective Efficient metal ion reduction Surfactant-assisted stability Controlled nanoparticle formation | Process variability Longer preparation time (multi-step process) | [215] |
| Fe-Pd | Metal precursors: FeCl3·6H2O, PdCl2 Reducing agent: Eucalyptus leaf extract | Green synthesis approach Simple process Enhanced stability Reduced contamination | Variability in leaf extract composition Time-intensive preparation Energy consumption Limited reduction efficiency | [216] |
| Fe-Cu | Metal precursors: FeSO4·7H2O, CuSO4 Reducing agent: Green tea extract | Eco-friendly synthesis Controlled copper loading Improved stability Simple and scalable process | Time-consuming process Batch-to-batch variability Energy consumption Limited control over particle size | [217] |
| Fe-Cu | Metal precursors: FeSO4·7H2O, CuSO4·5H2O Reducing agent: Green tea extract | Green synthesis approach Simple and efficient process | Potential batch variability Energy consumption Limited control over particle size | [218] |
| C-Fe-Ni | Metal precursors: FeCl3·6H2O, NiCl2·6H2O Reducing agent: Eucalyptus leaf extract | Eco-friendly synthesis Cost-effective materials Simple preparation method Improved stability via calcination | Time-intensive process Batch-to-batch variability Energy-intensive steps Potential agglomeration | [219] |
| Fe-Pd | Metal precursors: Fe (III) chloride, Potassium hexachloropalladate (IV) Reducing agent: Pomegranate peel extract | Eco-friendly synthesis Cost-effective and sustainable process Enhanced stability Controlled bimetallic composition | Time-intensive synthesis Batch-to-batch variability Energy consumption | [220] |
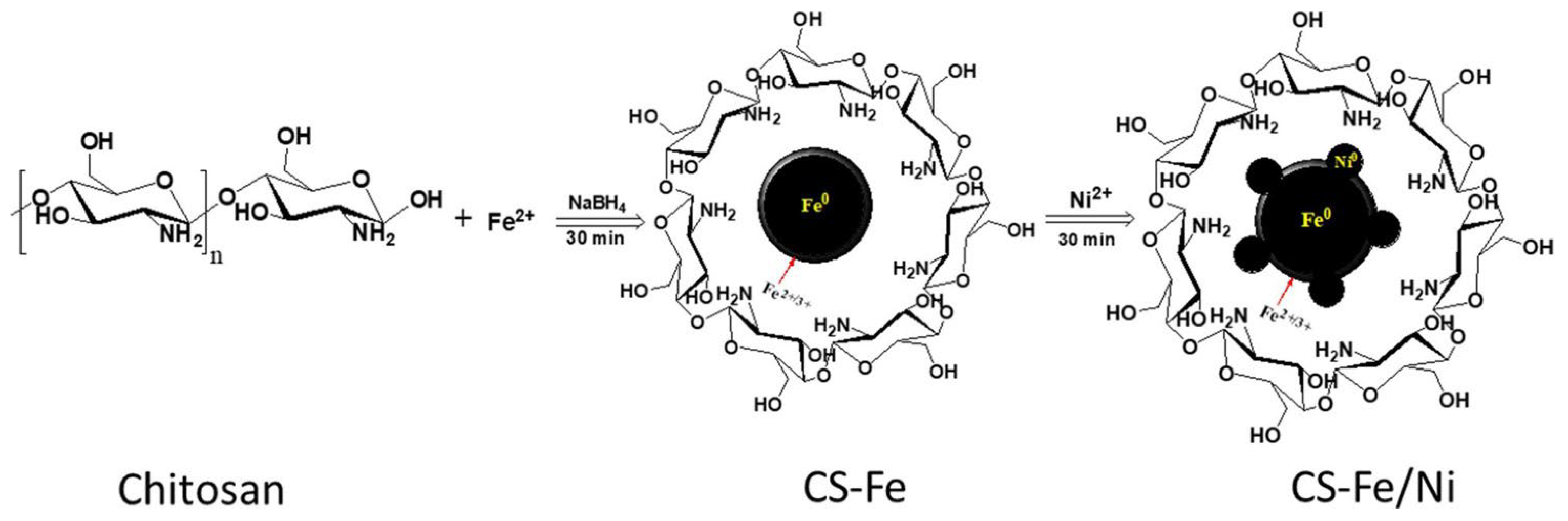
| Chitosan(CS)-stabilized Fe-Cu | Metal precursors: FeSO4·7H2O, CuSO4·5H2O Reducing agent: NaBH4 Stabilizing agent: Chitosan | Enhanced stability Controlled cu loading Efficient reduction process Oxygen-free synthesis | Energy-intensive process Complex synthesis procedure | [223] |
| Fe–Al bimetal chitosan bentonite (Fe–Al bimetal@bent) complex | Metal precursors: Fe2SO4, Al powder Stabilizing agents: Chitosan, Na-bentonite | Simple synthesis process Effective ph control Improved structural stability Vacuum-drying enhancing purity | Use of concentrated acid Energy and time-intensive drying process Centrifugation step complexity | [224] |
| Chitosan (CS)-Fe-Ni | Metal precursors: FeCl3·6H2O, NiSO4·6H2O Reducing agent: NaBH4 Stabilizing agent: Chitosan | Green synthesis approach Controlled reduction process Effective metal loading Lyophilization for long-term stability | Intricate synthesis method Labor-intensive process | [225] |
| Chitosan–Cu–Fe bimetal complex | Metal precursors: FeCl3·6H2O, NiSO4·6H2O Reducing agent: NaBH4 Stabilizing agent: Chitosan | Simple and efficient synthesis Good metal loading control Improved swelling properties | Chitosan involving acid dissolution Limited structural control | [226] |
| ZVFe–Cu/Alg–LS | Metal precursors: FeSO4·7H2O, CuSO4·5H2O Stabilizing agents: Sodium alginate, limestone | Environmentally friendly synthesis Improved stability Controlled release of zero-valent iron (ZVI) and copper | Multi-step process Requires multiple washing steps | [230] |
| Fe–Cu@MCC | Metal precursors: FeCl2·4H2O, CuCl2·2H2O Reducing agent: NaBH4 Stabilizing agent: Microcrystalline cellulose (MCC) | Magnetic recoverability of the catalysts Support material (MCC) improves stability MCC as biodegradable and nontoxic support | Complex multi-step process | [236] |
Influence of Characteristics of Synthesized Bimetals by Biological Methods to Their Properties
6. Summary and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ZVMs | Zero-valent metals |
| ZVI | Zero-valent iron |
| HOCs | Halogenated organic compounds |
| TCE | Trichloroethylene |
| PCP | Pentachlorophenol |
| ZVAl | Zero-valent aluminum |
| AMD | Acid mine drainage |
| MF-LAL | Magnetic field-assisted laser ablation in liquid |
| PVP | Polyvinylpyrrolidone |
| TCD | Trisodium citrate dehydrate |
| PSTT | Potassium sodium tartrate tetrahydrate |
| EDTA | Disodium ethylenediaminetetraacetate dehydrate |
| En | Ethylenediamine |
| TEA | Triethanolamine |
| AAS | Atomic absorption spectroscopy |
| ORR | Oxygen reduction reaction |
| LDH | Layered double hydroxide |
| CNTs | Carbon nanotubes |
| MWCNTs | Multi-walled carbon nanotubes |
| CNF | Carbon nanofibers |
| PAN | Polyacrylonitrile |
| DMF | Dimethylformamide |
| MC | Mesoporous carbon |
| MB | Modified biochar |
| BC | Biochar |
| FMBC | Fe–Co-modified biochar |
| nZVIC-SBC | Fe-Cu-municipal sludge-derived biochar nanoparticles |
| ZF@CBC | Zn/Fe nanoparticles on corncob biochar (CBC) |
| AC | Activated carbon |
| PAC | Powder-activated carbon |
| CAC | Commercial activated carbon |
| ALD | Atomic layer deposition |
| PVDF | Polyvinylidene difluoride |
| HMS | Hollow mesoporous silica sphere |
| TMOS | Tetramethyl orthosilicate |
| CEC | Cation exchange capacity |
| Be@Fe-Cu | Fe-Cu on bentonite |
| B-Fe/Ni | Fe/Ni on bentonite |
| K-Fe/Pd | K-Fe/Pd on kaolinite |
| Cu/Fe@zeolite | Cu/Fe on zeolite |
| Di-Fe/Ni | Fe/Ni on diatomite |
| Pal-Fe/Ni | Fe/Ni on palygorskite |
| Sep-Fe/Ni | Fe/Ni on sepiolite |
| ASF@NC | N-doped carbon layer functionalized on aluminum silicate fibers |
| PDA | Polydopamine |
| PAM | Polyacrylamide |
| PEI | Polyethylenimine |
| Cu/Fe-BM@FA | Cu/Fe bimetallic modified fly ash |
| GT-nZVI/Cu | Green tea extract-based nZVI/Cu particles |
| C–Fe/Ni NPs | Calcined Fe/Ni nanoparticles |
| CMC | Carboxymethyl cellulose |
| CS | Chitosan |
| CS-Fe-Cu | Chitosan (CS)-stabilized Fe/Cu |
| Fe–Al bimetal @ bent | Fe–Al bimetal chitosan bentonite complex |
| Alg–LS | Alginate–limestone |
| Fe–Cu@MCC | Fe-Cu immobilized on microcrystalline cellulose |
References
- Yan, Z.; Ouyang, J.; Wu, B.; Liu, C.; Wang, H.; Wang, A.; Li, Z.A.B. Nonmetallic modified zero-valent iron for remediating halogenated organic compounds and heavy metals: A comprehensive review. Environ. Sci. Ecotechnol. 2024, 21, 100417. [Google Scholar] [CrossRef] [PubMed]
- Silwamba, M.; Ito, M.; Tabelin, C.B.; Park, I.; Jeon, S.; Takada, M.; Kubo, Y.; Hokari, N.; Tsunekawa, M.; Hiroyoshi, N. Simultaneous extraction and recovery of lead using citrate and micro-scale zero-valent iron for decontamination of polluted shooting range soils. Environ. Adv. 2021, 5, 100115. [Google Scholar] [CrossRef]
- Silwamba, M.; Ito, M.; Hiroyoshi, N.; Tabelin, C.B.; Fukushima, T.; Park, I.; Jeon, S.; Igarashi, T.; Sato, T.; Nyambe, I.; et al. Detoxification of lead-bearing zinc plant leach residues from Kabwe, Zambia by coupled extraction-cementation method. J. Environ. Chem. Eng. 2020, 8, 104197. [Google Scholar] [CrossRef]
- Silwamba, M.; Ito, M.; Hiroyoshi, N.; Tabelin, C.B.; Hashizume, R.; Fukushima, T.; Park, I.; Jeon, S.; Igarashi, T.; Sato, T.; et al. Alkaline leaching and concurrent cementation of dissolved Pb and Zn from zinc plant leach residues. Minerals 2022, 12, 393. [Google Scholar] [CrossRef]
- Seng, S.; Tabelin, C.B.; Kojima, M.; Hiroyoshi, N.; Ito, M. Galvanic microencapsulation (GME) using zero-valent aluminum and zero-valent iron to suppress pyrite oxidation. Mater. Trans. 2019, 60, 277–286. [Google Scholar] [CrossRef]
- Seng, S.; Tabelin, C.B.; Makino, Y.; Chea, M.; Phengsaart, T.; Park, I.; Hiroyoshi, N.; Ito, M. Improvement of flotation and suppression of pyrite oxidation using phosphate-enhanced galvanic microencapsulation (GME) in a ball mill with steel ball media. Miner. Eng. 2019, 143, 105931. [Google Scholar] [CrossRef]
- Choi, S.; Jeon, S.; Park, I.; Tabelin, C.B.; Ito, M.; Hiroyoshi, N. Enhanced cementation of Cd2+, Co2+, Ni2+, and Zn2+ on Al from sulfate solutions by activated carbon addition. Hydrometallurgy 2021, 201, 105580. [Google Scholar] [CrossRef]
- Jeon, S.; Bright, S.; Park, I.; Tabelin, C.B.; Ito, M.; Hiroyoshi, N. A simple and efficient recovery technique for gold ions from ammonium thiosulfate medium by galvanic interactions of zero-valent aluminum and activated carbon: A parametric and mechanistic study of cementation. Hydrometallurgy 2022, 208, 105815. [Google Scholar] [CrossRef]
- Chiu, P.C. Applications of zero-valent iron (ZVI) and nanoscale ZVI to municipal and decentralized drinking water systems—A review. Nov. Solut. Water Pollut. 2013, 237–249. [Google Scholar] [CrossRef]
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef]
- Mueller, N.C.; Nowack, B. Nanoparticles for remediation: Solving big problems with little particles. Elements 2010, 6, 395–400. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, W.; Cai, Z.; Han, B.; Qian, T.; Zhao, D. An overview of preparation and applications of stabilized zero-valent iron nanoparticles for soil and groundwater remediation. Water Res. 2016, 100, 245–266. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Ito, M.; Tabelin, C.B.; Pongsumrankul, R.; Tanaka, S.; Kitajima, N.; Saito, A.; Park, I.; Hiroyoshi, N. A physical separation scheme to improve ammonium thiosulfate leaching of gold by separation of base metals in crushed mobile phones. Miner. Eng. 2019, 138, 168–177. [Google Scholar] [CrossRef]
- Jeon, S.; Tabelin, C.B.; Park, I.; Nagata, Y.; Ito, M.; Hiroyoshi, N. Ammonium thiosulfate extraction of gold from printed circuit boards (PCBs) of end-of-life mobile phones and its recovery from pregnant leach solution by cementation. Hydrometallurgy 2020, 191, 105214. [Google Scholar] [CrossRef]
- Chen, S.Y.; Chen, W.H.; Shih, C.J. Heavy metal removal from wastewater using zero-valent iron nanoparticles. Water Sci. Technol. 2008, 58, 1947–1954. [Google Scholar] [CrossRef]
- Wang, C.B.; Zhang, W.X. Synthesizing nanoscale iron particles for rapid and complete dechlorination of TCE and PCBs. Environ. Sci. Technol. 1997, 31, 2154–2156. [Google Scholar] [CrossRef]
- Cheng, S.F.; Wu, S.C. The enhancement methods for the degradation of TCE by zero-valent metals. Chemosphere 2000, 41, 1263–1270. [Google Scholar] [CrossRef]
- Sun, Y.; Li, J.; Huang, T.; Guan, X. The influences of iron characteristics, operating conditions and solution chemistry on contaminants removal by zero-valent iron: A review. Water Res. 2016, 100, 277–295. [Google Scholar] [CrossRef]
- Zaleska-Medynska, A.; Marchelek, M.; Diak, M.; Grabowska, E. Noble metal-based bimetallic nanoparticles: The effect of the structure on the optical, catalytic and photocatalytic properties. Adv. Colloid Interface Sci. 2016, 229, 80–107. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Goodman, D.W. The nature of the metal-metal bond in bimetallic surfaces. Science 1992, 257, 897–903. [Google Scholar] [CrossRef]
- Kitchin, J.R.; Nørskov, J.K.; Barteau, M.A.; Chen, J.G. Modification of the surface electronic and chemical properties of Pt(111) by subsurface 3d transition metals. J. Chem. Phys. 2004, 120, 10240–10246. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Sun, Y.; Qin, H.; Li, J.; Lo, I.M.; He, D.; Dong, H. The limitations of applying zero-valent iron technology in contaminants sequestration and the corresponding countermeasures: The development in zero-valent iron technology in the last two decades (1994–2014). Water Res. 2015, 75, 224–248. [Google Scholar] [CrossRef]
- Huo, X.; Zhou, P.; Liu, Y.; Cheng, F.; Liu, Y.; Cheng, X.; Zhang, Y.; Wang, Q. Removal of contaminants by activating peroxymonosulfate (PMS) using zero valent iron (ZVI)-based bimetallic particles (ZVI/Cu, ZVI/Co, ZVI/Ni, and ZVI/Ag). RSC Adv. 2020, 10, 28232–28242. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Khatri, J.; Singh, T.A.; Gandhimathi, R.; Ramesh, S.T. Review of zero-valent aluminium based water and wastewater treatment methods. Chemosphere 2018, 200, 621–631. [Google Scholar] [CrossRef]
- Fu, F.; Cheng, Z.; Lu, J. Synthesis and use of bimetals and bimetal oxides in contaminants removal from water: A review. RSC Adv. 2015, 5, 85395–85409. [Google Scholar] [CrossRef]
- Sankar, M.; Dimitratos, N.; Miedziak, P.J.; Wells, P.P.; Kiely, C.J.; Hutchings, G.J. Designing bimetallic catalysts for a green and sustainable future. Chem. Soc. Rev. 2012, 41, 8099–8139. [Google Scholar] [CrossRef]
- Yu, W.; Porosoff, M.D.; Chen, J.G. Review of Pt-based bimetallic catalysis: From model surfaces to supported catalysts. Chem. Rev. 2012, 112, 5780–5817. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Zero-valent aluminum for oxidative degradation of aqueous organic pollutants. Environ. Sci. Technol. 2009, 43, 7130–7135. [Google Scholar] [CrossRef]
- Kim, J.; Choi, H.; Kim, D.; Park, J.Y. Operando surface studies on metal-oxide interfaces of bimetal and mixed catalysts. ACS Catal. 2021, 11, 8645–8677. [Google Scholar] [CrossRef]
- Scaria, J.; Nidheesh, P.V.; Kumar, M.S. Synthesis and applications of various bimetallic nanomaterials in water and wastewater treatment. J. Environ. Manag. 2020, 259, 110011. [Google Scholar] [CrossRef]
- Quiton, K.G.N.; Lu, M.C.; Huang, Y.H. Synthesis and catalytic utilization of bimetallic systems for wastewater remediation: A review. Chemosphere 2021, 262, 128371. [Google Scholar] [CrossRef]
- Liu, W.J.; Qian, T.T.; Jiang, H. Bimetallic Fe nanoparticles: Recent advances in synthesis and application in catalytic elimination of environmental pollutants. Chem. Eng. J. 2014, 236, 448–463. [Google Scholar] [CrossRef]
- Aryee, A.A.; Liu, Y.; Han, R.; Qu, L. Bimetallic adsorbents for wastewater treatment: A review. Environ. Chem. Lett. 2023, 21, 1811–1835. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Andrews, R. The place of systematic reviews in education research. Br. J. Educ. Stud. 2005, 53, 399–416. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. bmj 2021, 372, n71. [Google Scholar] [CrossRef]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef]
- Imawaka, K.; Sugita, M.; Takewaki, T.; Tanaka, S. Mechanochemical synthesis of bimetallic CoZn-ZIFs with sodalite structure. Polyhedron 2019, 158, 290–295. [Google Scholar] [CrossRef]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef]
- Chen, J.C.; Lin, Z.H.; Ma, X.X. Evidence of the production of silver nanoparticles via pretreatment of Phoma sp. 3.2883 with silver nitrate. Lett. Appl. Microbiol. 2003, 37, 105–108. [Google Scholar] [CrossRef]
- Li, Y.; Duan, X.; Qian, Y.; Yang, L.; Liao, H. Nanocrystalline silver particles: Synthesis, agglomeration, and sputtering induced by electron beam. J. Colloid Interface Sci. 1999, 209, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, J.S. Dispersion strengthened superalloys by mechanical alloying. Metall. Trans. 1970, 1, 2943–2951. [Google Scholar] [CrossRef]
- Lyu, H.; Gao, B.; He, F.; Ding, C.; Tang, J.; Crittenden, J.C. Ball-milled carbon nanomaterials for energy and environmental applications. ACS Sustain. Chem. Eng. 2017, 5, 9568–9585. [Google Scholar] [CrossRef]
- Wang, P.; Hu, J.; Liu, T.; Han, G.; Ma, W.M.; Li, J. New insights into ball-milled zero-valent iron composites for pollution remediation: An overview. J. Clean. Prod. 2023, 385, 135513. [Google Scholar] [CrossRef]
- Ai, D.; Wei, T.; Meng, Y.; Chen, X.; Wang, B. Ball milling sulfur-doped nano zero-valent iron@ biochar composite for the efficient removal of phosphorus from water: Performance and mechanisms. Bioresour. Technol. 2022, 357, 127316. [Google Scholar] [CrossRef]
- Gao, J.; Wang, W.; Rondinone, A.J.; He, F.; Liang, L. Degradation of trichloroethene with a novel ball milled Fe–C nanocomposite. J. Hazard. Mater. 2015, 300, 443–450. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, B.; He, F.; Bradley, M.J.; Tratnyek, P.G. Mechanochemically sulfidated microscale zero valent iron: Pathways, kinetics, mechanism, and efficiency of trichloroethylene dechlorination. Environ. Sci. Technol. 2017, 51, 12653–12662. [Google Scholar] [CrossRef]
- Friščić, T.; Mottillo, C.; Titi, H.M. Mechanochemistry for synthesis. Angew. Chem. 2020, 132, 1030–1041. [Google Scholar] [CrossRef]
- Hawili, A.A.; Ghommem, M.; Alami, A.H.; Alasad, S.; Egilmez, M.; Zaid, W.A. Utilizing aluminum sheets with FeCu deposits as cheap water cleaning electrodes. Appl. Surf. Sci. Adv. 2022, 7, 100193. [Google Scholar] [CrossRef]
- Vishlaghi, M.B.; Ataie, A. Investigation on solid solubility and physical properties of Cu–Fe/CNT nano-composite prepared via mechanical alloying route. Powder Technol. 2014, 268, 102–109. [Google Scholar] [CrossRef]
- Schoenitz, M.; Dreizin, E.L. Structure and properties of Al–Mg mechanical alloys. J. Mater. Res. 2003, 18, 1827–1836. [Google Scholar] [CrossRef]
- Sharipova, A.; Swain, S.K.; Gotman, I.; Starosvetsky, D.; Psakhie, S.G.; Unger, R.; Gutmanas, E.Y. Mechanical, degradation and drug-release behavior of nano-grained Fe-Ag composites for biomedical applications. J. Mech. Behav. Biomed. Mater. 2018, 86, 240–249. [Google Scholar] [CrossRef]
- Sharipova, A.; Gotman, I.; Psakhie, S.G.; Gutmanas, E.Y. Biodegradable nanocomposite Fe–Ag load-bearing scaffolds for bone healing. J. Mech. Behav. Biomed. Mater. 2019, 98, 246–254. [Google Scholar] [CrossRef]
- Sharipova, A.F.; Psakhie, S.G.; Gotman, I.; Gutmanas, E.Y. Smart nanocomposites based on Fe–Ag and Fe–Cu nanopowders for biodegradable high-strength implants with slow drug release. Phys. Mesomech. 2020, 23, 128–134. [Google Scholar] [CrossRef]
- Sharipova, A.F.; Psakhye, S.G.; Gotman, I.; Lerner, M.I.; Lozhkomoev, A.S.; Gutmanas, E.Y. Cold Sintering of Fe–Ag and Fe–Cu Nanocomposites by Consolidation in the High-Pressure Gradient. Russ. J. Non-Ferr. Met. 2019, 60, 162–168. [Google Scholar] [CrossRef]
- Romanova, V.M.; Ivanenkov, G.V.; Mingaleev, A.R.; Ter-Oganesyan, A.E.; Shelkovenko, T.A.; Pikuz, S.A. Electric explosion of fine wires: Three groups of materials. Plasma Phys. Rep. 2015, 41, 617–636. [Google Scholar] [CrossRef]
- Sahai, A.; Goswami, N.; Kaushik, S.D.; Tripathi, S. Cu/Cu2O/CuO nanoparticles: Novel synthesis by exploding wire technique and extensive characterization. Appl. Surf. Sci. 2016, 390, 974–983. [Google Scholar] [CrossRef]
- Park, G.H.; Lee, G.Y.; Kim, H.A.; Lee, A.Y.; Oh, H.R.; Kim, S.Y.; Kim, D.H.; Lee, M.H. Evaluation of alloying effect on the formation of Ni-Fe nanosized powders by pulsed wire discharge. Mater. Sci. Eng. B 2016, 212, 24–29. [Google Scholar] [CrossRef]
- Bland, S.N.; Krasik, Y.E.; Yanuka, D.; Gardner, R.; MacDonald, J.; Virozub, A.; Efimov, S.; Gleizer, S.; Chaturvedi, N. Generation of highly symmetric, cylindrically convergent shockwaves in water. Phys. Plasmas 2017, 24, 082702. [Google Scholar] [CrossRef]
- Shelkovenko, T.A.; Pikuz, S.A.; Hammer, D.A. A review of projection radiography of plasma and biological objects in X-pinch radiation. Plasma Phys. Rep. 2016, 42, 226–268. [Google Scholar] [CrossRef]
- Tokoi, Y.; Orikawa, T.; Suzuki, T.; Nakayama, T.; Suematsu, H.; Niihara, K. Phase Control of Ti–Fe Nanoparticles Prepared by Pulsed Wire Discharge. Jpn. J. Appl. Phys. 2011, 50, 01BJ06. [Google Scholar] [CrossRef]
- Ishihara, S.; Koishi, T.; Orikawa, T.; Suematsu, H.; Nakayama, T.; Suzuki, T.; Niihara, K. Synthesis of intermetallic NiAl compound nanoparticles by pulsed wire discharge of twisted Ni and Al wires. Intermetallics 2012, 23, 134–142. [Google Scholar] [CrossRef]
- Pervikov, A.; Glazkova, E.; Lerner, M. Energy characteristics of the electrical explosion of two intertwined wires made of dissimilar metals. Phys. Plasmas 2018, 25, 070701. [Google Scholar] [CrossRef]
- Lerner, M.I.; Psakhie, S.G.; Lozhkomoev, A.S.; Sharipova, A.F.; Pervikov, A.V.; Gotman, I.; Gutmanas, E.Y. Fe–Cu nanocomposites by high pressure consolidation of powders prepared by electric explosion of wires. Adv. Eng. Mater. 2018, 20, 1701024. [Google Scholar] [CrossRef]
- Chau, J.L.H.; Chen, C.Y.; Yang, C.C. Facile synthesis of bimetallic nanoparticles by femtosecond laser irradiation method. Arab. J. Chem. 2017, 10, S1395–S1401. [Google Scholar] [CrossRef]
- Suslick, K.S.; Fang, M.; Hyeon, T. Sonochemical synthesis of iron colloids. J. Am. Chem. Soc. 1996, 118, 11960–11961. [Google Scholar] [CrossRef]
- Gogate, P.R.; Sutkar, V.S.; Pandit, A.B. Sonochemical reactors: Important design and scale up considerations with a special emphasis on heterogeneous systems. Chem. Eng. J. 2011, 166, 1066–1082. [Google Scholar] [CrossRef]
- Shirsath, S.R.; Pinjari, D.V.; Gogate, P.R.; Sonawane, S.H.; Pandit, D.A. Ultrasound assisted synthesis of doped TiO2 nano-particles: Characterization and comparison of effectiveness for photocatalytic oxidation of dyestuff effluent. Ultrason. Sonochem. 2013, 20, 277–286. [Google Scholar] [CrossRef]
- Zhao, D.; Zheng, Y.; Li, M.; Baig, S.A.; Wu, D.; Xu, X. Catalytic dechlorination of 2, 4-dichlorophenol by Ni/Fe nanoparticles prepared in the presence of ultrasonic irradiation. Ultrason. Sonochem. 2014, 21, 1714–1721. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, Z.; Deng, D.; Ju, Y.; Ren, L.; Xiang, M.; Li, L.; Li, H. Synthesis of millimeter-scale sponge Fe/Cu bimetallic particles removing TBBPA and insights of degradation mechanism. Chem. Eng. J. 2017, 325, 279–288. [Google Scholar] [CrossRef]
- Tabrizian, P.; Ma, W.; Bakr, A.; Rahaman, M.S. pH-sensitive and magnetically separable Fe/Cu bimetallic nanoparticles supported by graphene oxide (GO) for high-efficiency removal of tetracyclines. J. Colloid Interface Sci. 2019, 534, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Shi, P.; Wu, J.; Qi, X.; Liu, Y.; Li, G. Trace Bimetallic Iron/Manganese Co-Doped N-Ketjenblack Carbon Electrocatalyst for Robust Oxygen Reduction Reaction. J. Electrochem. Soc. 2021, 168, 060502. [Google Scholar] [CrossRef]
- Geng, C.; Lin, R.; Yang, P.; Liu, P.; Guo, L.; Fang, Y.; Cui, B. Mild routine to prepare Fe-Mn bimetallic nano-cluster (Fe-Mn NCs) and its magnetic starch-based composite adsorbent (Fe-Mn@ SCAs) for wide pH range adsorption for Hg (II) sewage. J. Taiwan Inst. Chem. Eng. 2023, 144, 104768. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, P.; Xiao, J.; Li, H.B.; Wang, C.X.; Yang, G.W. A general strategy for one-step fabrication of one-dimensional magnetic nanoparticle chains based on laser ablation in liquid. Laser Phys. Lett. 2014, 11, 056001. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, P.; Xiao, J.; Li, H.; Wang, C.; Yang, G. A microfibre assembly of an iron-carbon composite with giant magnetisation. Sci. Rep. 2013, 3, 3051. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, P.; Yang, G. Fabrication of one-dimensional chain of iron-based bimetallic alloying nanoparticles with unique magnetizations. Cryst. Growth Des. 2014, 14, 5847–5855. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y. Bimetallic nanocrystals: Liquid-phase synthesis and catalytic applications. Adv. Mater. 2011, 23, 1044–1060. [Google Scholar] [CrossRef]
- Gilroy, K.D.; Ruditskiy, A.; Peng, H.C.; Qin, D.; Xia, Y. Bimetallic nanocrystals: Syntheses, properties, and applications. Chem. Rev. 2016, 116, 10414–10472. [Google Scholar] [CrossRef]
- Raut, S.S.; Shetty, R.; Raju, N.M.; Kamble, S.P.; Kulkarni, P.S. Screening of zero valent mono/bimetallic catalysts and recommendation of Raney Ni (without reducing agent) for dechlorination of 4-chlorophenol. Chemosphere 2020, 250, 126298. [Google Scholar] [CrossRef]
- Ou, J.H.; Sheu, Y.T.; Tsang, D.C.; Sun, Y.J.; Kao, C.M. Application of iron/aluminum bimetallic nanoparticle system for chromium-contaminated groundwater remediation. Chemosphere 2020, 256, 127158. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, C.; Shi, B.; Wang, D.; Feng, C. Efficiency and mechanism of micro-and nano-plastic removal with polymeric Al-Fe bimetallic coagulants: Role of Fe addition. J. Hazard. Mater. 2023, 448, 130978. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, P.; Rubio, M.A.; Baltazar, S.E.; Rojas-Nunez, J.; Llamazares, J.S.; Garcia, A.G.; Arancibia-Miranda, N. As (V) removal capacity of FeCu bimetallic nanoparticles in aqueous solutions: The influence of Cu content and morphologic changes in bimetallic nanoparticles. J. Colloid Interface Sci. 2018, 524, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Ulucan-Altuntas, K.; Kuzu, S.L. Modelling and optimization of dye removal by Fe/Cu bimetallic nanoparticles coated with different Cu ratios. Mater. Res. Express 2019, 6, 1150a4. [Google Scholar] [CrossRef]
- Abdel-Aziz, A.B.; Mohamed, N.; El-taweel, R.M.; Husien, S.; Fahim, I.S.; Said, L.A.; Radwan, A.G. Crystal violet removal using bimetallic Fe0–Cu and its composites with fava bean activated carbon. Results Eng. 2023, 20, 101420. [Google Scholar] [CrossRef]
- Muradova, G.G.; Gadjieva, S.R.; Di Palma, L.; Vilardi, G. Nitrates removal by bimetallic nanoparticles in water. Chem. Eng. Trans. 2016, 47, 205–210. [Google Scholar]
- Torres-Blancas, T.; Roa-Morales, G.; Ureña-Núñez, F.; Barrera-Díaz, C.; Dorazco-González, A.; Natividad, R. Ozonation enhancement by Fe–Cu biometallic particles. J. Taiwan Inst. Chem. Eng. 2017, 74, 225–232. [Google Scholar] [CrossRef]
- Naser, R.; Shahwan, T. Comparative assessment of the decolorization of aqueous bromophenol blue using Fe nanoparticles and Fe-Ni bimetallic nanoparticles. Desalination Water Treat. 2019, 159, 64416. [Google Scholar] [CrossRef]
- Weng, X.; Sun, Q.; Lin, S.; Chen, Z.; Megharaj, M.; Naidu, R. Enhancement of catalytic degradation of amoxicillin in aqueous solution using clay supported bimetallic Fe/Ni nanoparticles. Chemosphere 2014, 103, 80–85. [Google Scholar] [CrossRef]
- Zhou, X.; Jing, G.; Lv, B.; Zhou, Z.; Zhu, R. Highly efficient removal of chromium (VI) by Fe/Ni bimetallic nanoparticles in an ultrasound-assisted system. Chemosphere 2016, 160, 332–341. [Google Scholar] [CrossRef]
- Zhou, S.; Li, Y.; Chen, J.; Liu, Z.; Wang, Z.; Na, P. Enhanced Cr (VI) removal from aqueous solutions using Ni/Fe bimetallic nanoparticles: Characterization, kinetics and mechanism. RSC Adv. 2014, 4, 50699–50707. [Google Scholar] [CrossRef]
- Mansouriieh, N.; Sohrabi, M.R.; Khosravi, M. Adsorption kinetics and thermodynamics of organophosphorus profenofos pesticide onto Fe/Ni bimetallic nanoparticles. Int. J. Environ. Sci. Technol. 2016, 13, 1393–1404. [Google Scholar] [CrossRef]
- Liao, H.; Zhu, W.; Duan, T.; Zhang, Y.; He, G.; Wei, Y.; Zhou, J. Beaded segments like bi-metallic nano-zero-valent iron-titanium for the fast and efficient adsorption and reduction of U (VI) in aqueous solutions. Colloids Surf. A Physicochem. Eng. Asp. 2021, 613, 126080. [Google Scholar] [CrossRef]
- Koryam, A.A.; El-Wakeel, S.T.; Radwan, E.K.; Darwish, E.S.; Abdel Fattah, A.M. One-step room-temperature synthesis of bimetallic nanoscale zero-valent FeCo by hydrazine reduction: Effect of metal salts and application in contaminated water treatment. ACS Omega 2022, 7, 34810–34823. [Google Scholar] [CrossRef]
- Qureshi, S.S.; Memon, S.A.; Ram, N.; Saeed, S.; Mubarak, N.M.; Karri, R.R. Rapid adsorption of selenium removal using iron manganese-based micro adsorbent. Sci. Rep. 2022, 12, 17207. [Google Scholar] [CrossRef]
- Guo, D.J.; Ding, Y. Porous nanostructured metals for electrocatalysis. Electroanalysis 2012, 24, 2035–2043. [Google Scholar] [CrossRef]
- Shen, W.N.; Dunn, B.; Moore, C.D.; Goorsky, M.S.; Radetic, T.; Gronsky, R. Synthesis of nanoporous bismuth films by liquid-phase deposition. J. Mater. Chem. 2000, 10, 657–662. [Google Scholar] [CrossRef]
- Luo, H.; Sun, L.; Lu, Y.; Yan. Electrodeposition of mesoporous semimetal and magnetic metal films from lyotropic liquid crystalline phases. Langmuir 2004, 20, 10218–10222. [Google Scholar] [CrossRef]
- Wang, X.; Qi, Z.; Zhao, C.; Wang, W.; Zhang, Z. Influence of alloy composition and dealloying solution on the formation and microstructure of monolithic nanoporous silver through chemical dealloying of Al−Ag alloys. J. Phys. Chem. C 2009, 113, 13139–13150. [Google Scholar] [CrossRef]
- Chen, L.; Guo, H.; Fujita, T.; Hirata, A.; Zhang, W.; Inoue, A.; Chen, M. Nanoporous PdNi bimetallic catalyst with enhanced electrocatalytic performances for electro-oxidation and oxygen reduction reactions. Adv. Funct. Mater. 2011, 21, 4364–4370. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, M. Nanoporous metals for catalytic and optical applications. MRS Bull. 2009, 34, 569–576. [Google Scholar] [CrossRef]
- Han, B.; Xu, C. Nanoporous PdFe alloy as highly active and durable electrocatalyst for oxygen reduction reaction. Int. J. Hydrogen Energy 2014, 39, 18247–18255. [Google Scholar] [CrossRef]
- Tian, C.; Chen, D.; Lu, N.; Li, Y.; Cui, R.; Han, Z.; Zhang, G. Electrochemical bisphenol A sensor based on nanoporous PtFe alloy and graphene modified glassy carbon electrode. J. Electroanal. Chem. 2018, 830, 27–33. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, Y.W.; Tao, F.F. Shape control of bimetallic nanocatalysts through well-designed colloidal chemistry approaches. Chem. Soc. Rev. 2012, 41, 8050–8065. [Google Scholar] [CrossRef]
- Chen, S.; Jenkins, S.V.; Tao, J.; Zhu, Y.; Chen, J. Anisotropic seeded growth of Cu–M (M= Au, Pt, or Pd) bimetallic nanorods with tunable optical and catalytic properties. J. Phys. Chem. C 2013, 117, 8924–8932. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, W.; Fennell, D.E. Rapid dechlorination of 1, 2, 3, 4-TCDD by Ag/Fe bimetallic particles. Chem. Eng. J. 2015, 273, 465–471. [Google Scholar] [CrossRef]
- Huang, C.C.; Lo, S.L.; Lien, H.L. Vitamin B12-mediated hydrodechlorination of dichloromethane by bimetallic Cu/Al particles. Chem. Eng. J. 2015, 273, 413–420. [Google Scholar] [CrossRef]
- Zhou, X.J.; Harmer, A.J.; Heinig, N.F.; Leung, K.T. Parametric study on electrochemical deposition of copper nanoparticles on an ultrathin polypyrrole film deposited on a gold film electrode. Langmuir 2004, 20, 5109–5113. [Google Scholar] [CrossRef]
- Yancey, D.F.; Carino, E.V.; Crooks, R.M. Electrochemical synthesis and electrocatalytic properties of Au@ Pt dendrimer-encapsulated nanoparticles. J. Am. Chem. Soc. 2010, 132, 10988–10989. [Google Scholar] [CrossRef]
- Tremont, R.J.; Cruz, G.; Cabrera, C.R. Pt electrodeposition on a copper surface modified with 3-mercaptopropyltrimethoxysilane and 1-propanethiol. J. Electroanal. Chem. 2003, 558, 65–74. [Google Scholar] [CrossRef]
- Stiger, R.M.; Gorer, S.; Craft, B.; Penner, R.M. Investigations of electrochemical silver nanocrystal growth on hydrogen-terminated silicon (100). Langmuir 1999, 15, 790–798. [Google Scholar] [CrossRef]
- Ortega, J.M. Electrodeposition of copper on poly (o-aminophenol) modified platinum electrode. Thin Solid Film. 2000, 360, 159–165. [Google Scholar] [CrossRef]
- Claussen, J.C.; Franklin, A.D.; ul Haque, A.; Porterfield, D.M.; Fisher, T.S. Electrochemical biosensor of nanocube-augmented carbon nanotube networks. ACS Nano 2009, 3, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, W.D.; Gunasekaran, S. An amperometric non-enzymatic glucose sensor by electrodepositing copper nanocubes onto vertically well-aligned multi-walled carbon nanotube arrays. Biosens. Bioelectron. 2010, 26, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, L.; Wang, X.; Yao, W.; Zhang, Q. Recent advances in noble metal based composite nanocatalysts: Colloidal synthesis, properties, and catalytic applications. Nanoscale 2015, 7, 10559–10583. [Google Scholar] [CrossRef]
- Hyeon, T. Chemical synthesis of magnetic nanoparticles. Chem. Commun. 2003, 9, 927–934. [Google Scholar] [CrossRef]
- Kang, K.M.; Kim, H.W.; Shim, I.W.; Kwak, H.Y. Catalytic test of supported Ni catalysts with core/shell structure for dry reforming of methane. Fuel Process. Technol. 2011, 92, 1236–1243. [Google Scholar] [CrossRef]
- Duan, S.; Wang, R. Bimetallic nanostructures with magnetic and noble metals and their physicochemical applications. Prog. Nat. Sci. Mater. Int. 2013, 23, 113–126. [Google Scholar] [CrossRef]
- Riva, J.S.; Pozo-López, G.; Condo, A.M.; Fabietti, L.M.; Urreta, S.E. Low temperature ferromagnetism in Rh-rich Fe-Rh granular nanowires. J. Alloys Compd. 2018, 747, 1008–1017. [Google Scholar] [CrossRef]
- Riva, J.S.; Pozo-López, G.; Condo, A.M.; Viqueira, M.S.; Urreta, S.E.; Cornejo, D.R.; Fabietti, L.M. Biphasic FeRh nanowires synthesized by AC electrodeposition. J. Alloys Compd. 2016, 688, 804–813. [Google Scholar] [CrossRef]
- Riva, J.S.; Pozo-López, G.; Condó, A.M.; Levingston, J.M.; Fabietti, L.M.; Urreta, S.E. Magnetic viscosity in iron-rhodium nanowires. J. Alloys Compd. 2017, 709, 531–534. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, J.; Xia, Q.; Zhang, X.; Song, Y.; Yao, Z. Accelerating Fe (iii)/Fe (ii) redox cycling by Zn 0 in micro-nano dendritic Fe–Zn alloy for enhanced Fenton-like degradation of phenol. New J. Chem. 2023, 47, 17508–17516. [Google Scholar] [CrossRef]
- Zhang, H.; Jin, M.; Wang, J.; Li, W.; Camargo, P.H.; Kim, M.J.; Yang, D.; Xie, Z.; Xia, Y. Synthesis of Pd−Pt bimetallic nanocrystals with a concave structure through a bromide-induced galvanic replacement reaction. J. Am. Chem. Soc. 2011, 33, 6078–6089. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Li, H.; Lai, B.; Yang, P.; Gou, M.; Zhou, Y.; Sun, G. Removal of high-concentration CI acid orange 7 from aqueous solution by zerovalent iron/copper (Fe/Cu) bimetallic particles. Ind. Eng. Chem. Res. 2014, 53, 2605–2613. [Google Scholar] [CrossRef]
- Mahmoud, M.S.; Mahmoud, A.S. Wastewater treatment using nano bimetallic iron/copper, adsorption isotherm, kinetic studies, and artificial intelligence neural networks. Emergent Mater. 2021, 4, 1455–1463. [Google Scholar] [CrossRef]
- Mahmoud, A.S.; Mohamed, N.Y.; Mostafa, M.K.; Mahmoud, M.S. Effective chromium adsorption from aqueous solutions and tannery wastewater using bimetallic Fe/Cu nanoparticles: Response surface methodology and artificial neural network. Air Soil Water Res. 2021, 14, 11786221211028162. [Google Scholar] [CrossRef]
- Lai, B.; Zhang, Y.; Chen, Z.; Yang, P.; Zhou, Y.; Wang, J. Removal of p-nitrophenol (PNP) in aqueous solution by the micron-scale iron–copper (Fe/Cu) bimetallic particles. Appl. Catal. B Environ. 2014, 144, 816–830. [Google Scholar] [CrossRef]
- Lai, B.; Zhang, Y.H.; Yuan, Y.; Chen, Z.Y.; Yang, P. Influence of preparation conditions on characteristics, reactivity, and operational life of microsized Fe/Cu bimetallic particles. Ind. Eng. Chem. Res. 2014, 53, 12295–12304. [Google Scholar] [CrossRef]
- Xiong, Z.; Lai, B.; Yang, P.; Zhou, Y.; Wang, J.; Fang, S. Comparative study on the reactivity of Fe/Cu bimetallic particles and zero valent iron (ZVI) under different conditions of N2, air or without aeration. J. Hazard. Mater. 2015, 297, 261–268. [Google Scholar] [CrossRef]
- Ren, Y.; Lai, B. Comparative study on the characteristics, operational life and reactivity of Fe/Cu bimetallic particles prepared by electroless and displacement plating process. RSC Adv. 2016, 6, 58302–58314. [Google Scholar] [CrossRef]
- Liu, X.; Fan, J.H.; Ma, L.M. Simultaneously degradation of 2, 4-Dichlorophenol and EDTA in aqueous solution by the bimetallic Cu–Fe/O2 system. Environ. Sci. Pollut. Res. 2015, 22, 1186–1198. [Google Scholar] [CrossRef]
- Fu, F.; Cheng, Z.; Dionysiou, D.D.; Tang, B. Fe/Al bimetallic particles for the fast and highly efficient removal of Cr (VI) over a wide pH range: Performance and mechanism. J. Hazard. Mater. 2015, 298, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Fu, F.; Dionysiou, D.D.; Tang, B. Adsorption, oxidation, and reduction behavior of arsenic in the removal of aqueous As (III) by mesoporous Fe/Al bimetallic particles. Water Res. 2016, 96, 22–31. [Google Scholar] [CrossRef]
- Xiang, S.; Cheng, W.; Nie, X.; Ding, C.; Yi, F.; Asiri, A.M.; Marwani, H.M. Zero-valent iron-aluminum for the fast and effective U (VI) removal. J. Taiwan Inst. Chem. Eng. 2018, 85, 186–192. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, X.; Pu, S.; Ni, R.; Lin, Y.; Liu, Y. Novel Fenton-like system (Mg/Fe-O2) for degradation of 4-chlorophenol. Environ. Pollut. 2019, 250, 906–913. [Google Scholar] [CrossRef]
- Aghaei, E.; Wang, Z.; Tadesse, B.; Tabelin, C.B.; Quadir, Z.; Alorro, R.D. Performance evaluation of Fe-Al bimetallic particles for the removal of potentially toxic elements from combined acid mine drainage-effluents from refractory gold ore processing. Minerals 2021, 11, 590. [Google Scholar] [CrossRef]
- Aghaei, E.; Tadesse, B.; Tabelin, C.B.; Alorro, R.D. Mercury sequestration from synthetic and real gold processing wastewaters using Fe–Al bimetallic particles. J. Clean. Prod. 2022, 372, 133482. [Google Scholar] [CrossRef]
- He, Y.; Sun, H.; Liu, W.; Yang, W.; Lin, A. Study on removal effect of Cr (VI) and surface reaction mechanisms by bimetallic system in aqueous solution. Environ. Technol. 2020, 41, 1867–1876. [Google Scholar] [CrossRef]
- Yeh, L.; Yen, C.H.; Kao, Y.L.; Lien, H.L.; Chang, S.M. Inactivation of Escherichia coli by dual-functional zerovalent Fe/Al composites in water. Chemosphere 2022, 299, 134371. [Google Scholar] [CrossRef]
- Liu, X.; Fan, J.H.; Hao, Y.; Ma, L.M. The degradation of EDTA by the bimetallic Fe–Cu/O2 system. Chem. Eng. J. 2014, 250, 354–365. [Google Scholar] [CrossRef]
- Park, I.; Ito, M.; Jeon, S.; Tabelin, C.B.; Phengsaart, T.; Silwamba, M.; Hiroyoshi, N. A novel recycling route for aluminum alloys: Synthesis of Fe/Al bimetallic materials and magnetic separation. Miner. Eng. 2023, 201, 108202. [Google Scholar] [CrossRef]
- Lien, H.L.; Yu, C.H.; Kamali, S.; Sahu, R.S. Bimetallic Fe/Al system: An all-in-one solid-phase Fenton reagent for generation of hydroxyl radicals under oxic conditions. Sci. Total Environ. 2019, 673, 480–488. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Resabal, V.J.T.; Park, I.; Villanueva, M.G.B.; Choi, S.; Ebio, R.; Cabural, P.J.; Villacorte-Tabelin, M.; Orbecido, A.; Alorro, R.D.; et al. Repurposing of aluminum scrap into magnetic Al0/ZVI bimetallic materials: Two-stage mechanical-chemical synthesis and characterization of products. J. Clean. Prod. 2021, 317, 128285. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, Y. Mechanistic study on the replacement reaction between silver nanostructures and chloroauric acid in aqueous medium. J. Am. Chem. Soc. 2004, 126, 3892–3901. [Google Scholar] [CrossRef]
- Liu, X.; Fan, J.H.; Ma, L.M. Elimination of 4-chlorophenol in aqueous solution by the bimetallic Al–Fe/O2 at normal temperature and pressure. Chem. Eng. J. 2014, 236, 274–284. [Google Scholar] [CrossRef]
- Fan, J.H.; Liu, X.; Ma, L.M. EDTA enhanced degradation of 4-bromophenol by Al0–Fe0–O2 system. Chem. Eng. J. 2015, 263, 71–82. [Google Scholar] [CrossRef]
- Fan, J.; Wang, H.; Ma, L. Oxalate-assisted oxidative degradation of 4-chlorophenol in a bimetallic, zero-valent iron–aluminum/air/water system. Environ. Sci. Pollut. Res. 2016, 23, 16686–16698. [Google Scholar] [CrossRef]
- Khoshandam, B.; Kumar, R.V.; Jamshidi, E. Simulation of non-catalytic gas–solid reactions: Application of grain model for the reduction of cobalt oxide with methane. Miner. Process. Extr. Metall. 2005, 114, 10–22. [Google Scholar] [CrossRef]
- Khoshandam, B.; Kumar, R.V.; Jamshidi, E. Kinetics of reduction of manganese oxide by methane. Can. Metall. Q. 2007, 46, 365–371. [Google Scholar] [CrossRef]
- Yape, E.O.; Anacleto, N.M. Direct smelting of chromite and laterite ores with carbon under argon atmosphere. Adv. Mater. Res. 2014, 849, 170–176. [Google Scholar] [CrossRef]
- Balangao, J.K.B.; Podiotan, F.J.C.; Ambolode, A.E.C.; Anacleto, N.M.; Namoco, C.S., Jr. Production of Iron-Chromium-Nickel Metal Alloys via Reduction of Mixed Chromite Ore from Zambales and Laterite Ore from Taganito, Surigao del Norte under Argon Atmosphere. Indian J. Sci. Technol. 2018, 11, 1–11. [Google Scholar]
- Balangao, J.K.B.; Podiotan, F.J.C.; Ambolode, A.E.C.; Anacleto, N.M. Isothermal Reduction Smelting of Mixed Chromite-Laterite Samples with Coconut Charcoal as Reductant under Argon Atmosphere in a Vertical Electric Arc Furnace. Int. J. Mech. Eng. Technol. 2022, 13, 46–53. [Google Scholar]
- Meshkini Far, R.; Ischenko, O.V.; Dyachenko, A.G.; Bieda, O.; Gaidai, S.V.; Lisnyak, V.V. CO2 hydrogenation into CH4 over Ni–Fe catalysts. Funct. Mater. Lett. 2018, 11, 1850057. [Google Scholar] [CrossRef]
- Tang, H.; Li, S.; Gong, D.; Guan, Y.; Liu, Y. Bimetallic Ni-Fe catalysts derived from layered double hydroxides for CO methanation from syngas. Front. Chem. Sci. Eng. 2017, 11, 613–623. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, J.; Sun, K.; Gao, G.; Li, C.; Zhang, L.; Zhang, S.; Xu, L.; Hu, G.; Hu, X. Selective hydrogenation of furfural and its derivative over bimetallic NiFe-based catalysts: Understanding the synergy between Ni sites and Ni–Fe alloy. Renew. Energy 2021, 170, 1114–1128. [Google Scholar] [CrossRef]
- Li, D.; Koike, M.; Wang, L.; Nakagawa, Y.; Xu, Y.; Tomishige, K. Regenerability of hydrotalcite-derived nickel–iron alloy nanoparticles for syngas production from biomass tar. ChemSusChem 2014, 7, 510–522. [Google Scholar] [CrossRef]
- Zhao, J.; Shi, R.; Waterhouse, G.I.; Zhang, T. Selective photothermal CO2 reduction to CO, CH4, alkanes, alkenes over bimetallic alloy catalysts derived from layered double hydroxide nanosheets. Nano Energy 2022, 102, 107650. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, J.; An, K.; Zhang, S.; Liu, G.; Liu, Y. Highly dispersed Ni–Fe alloy catalysts on MgAl2O4 derived from hydrotalcite for direct ethanol synthesis from syngas. Energy Technol. 2020, 8, 2000205. [Google Scholar] [CrossRef]
- De Masi, D.; Asensio, J.M.; Fazzini, P.F.; Lacroix, L.M.; Chaudret, B. Engineering iron–nickel nanoparticles for magnetically induced CO2 methanation in continuous flow. Angew. Chem. Int. Ed. 2020, 59, 6187–6191. [Google Scholar] [CrossRef]
- Ferrando, R.; Jellinek, J.; Johnston, R.L. Nanoalloys: From theory to applications of alloy clusters and nanoparticles. Chem. Rev. 2008, 108, 845–910. [Google Scholar] [CrossRef]
- Zhang, L.; Lepper, M.; Stark, M.; Menzel, T.; Lungerich, D.; Jux, N.; Hieringer, W.; Steinrück, H.P.; Marbach, H. On the critical role of the substrate: The adsorption behaviour of tetrabenzoporphyrins on different metal surfaces. Phys. Chem. Chem. Phys. 2017, 19, 20281–20289. [Google Scholar] [CrossRef]
- Wang, J.; Wu, A.; Qiu, Z.; Li, A.; Qin, W.; Huang, H. N-CNT supported Fe/Ce bimetallic catalyst for Al-air aqueous batteries. Appl. Surf. Sci. 2023, 608, 155185. [Google Scholar] [CrossRef]
- Tian, F.; Zhong, S.; Nie, W.; Zeng, M.; Chen, B.; Liu, X. Multi-walled carbon nanotubes prepared with low-cost Fe-Al bimetallic catalysts for high-rate rechargeable Li-ion batteries. J. Solid State Electrochem. 2020, 24, 667–674. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Li, J.; Luo, R.; Hu, X.; Sun, X.; Shen, J.; Han, W.; Wang, L. In-situ incorporation of iron-copper bimetallic particles in electrospun carbon nanofibers as an efficient Fenton catalyst. Appl. Catal. B Environ. 2017, 207, 316–325. [Google Scholar] [CrossRef]
- Nam, G.; Park, J.; Choi, M.; Oh, P.; Park, S.; Kim, M.G.; Park, N.; Cho, J.; Lee, J.S. Carbon-coated core–shell Fe–Cu nanoparticles as highly active and durable electrocatalysts for a Zn–air battery. ACS Nano 2015, 9, 6493–6501. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, H.; Zhao, G. Iron-copper bimetallic nanoparticles embedded within ordered mesoporous carbon as effective and stable heterogeneous Fenton catalyst for the degradation of organic contaminants. Appl. Catal. B Environ. 2015, 164, 396–406. [Google Scholar] [CrossRef]
- Wu, H.; Feng, Q.; Lu, P.; Chen, M.; Yang, H. Degradation mechanisms of cefotaxime using biochar supported Co/Fe bimetallic nanoparticles. Environ. Sci. Water Res. Technol. 2018, 4, 964–975. [Google Scholar] [CrossRef]
- Wu, H.; Feng, Q. Fabrication of bimetallic Ag/Fe immobilized on modified biochar for removal of carbon tetrachloride. J. Environ. Sci. 2017, 54, 346–357. [Google Scholar] [CrossRef]
- Xing, X.; Ren, X.; Alharbi, N.S.; Chen, C. Biochar-supported Fe/Ni bimetallic nanoparticles for the efficient removal of Cr (VI) from aqueous solution. J. Mol. Liq. 2022, 359, 119257. [Google Scholar] [CrossRef]
- Hao, J.; Wu, L.; Lu, X.; Zeng, Y.; Jia, B.; Luo, T.; He, S.; Liang, L. A stable Fe/Co bimetallic modified biochar for ofloxacin removal from water: Adsorption behavior and mechanisms. RSC Adv. 2022, 12, 31650–31662. [Google Scholar] [CrossRef]
- Ji, J.; Xu, S.; Ma, Z.; Mou, Y. Trivalent antimony removal using carbonaceous nanomaterial loaded with zero-valent bimetal (iron/copper) and their effect on seed growth. Chemosphere 2022, 296, 134047. [Google Scholar] [CrossRef]
- Xia, H.; Zhang, Y.; Chen, Q.; Liu, R.; Wang, H. Unraveling adsorption characteristics and removal mechanism of novel Zn/Fe-bimetal-loaded and starch-coated corn cobs biochar for Pb (II) and Cd (II) in wastewater. J. Mol. Liq. 2023, 391, 123375. [Google Scholar] [CrossRef]
- Bose, S.; Mukherjee, T.; Rahaman, M. Simultaneous adsorption of manganese and fluoride from aqueous solution via bimetal impregnated activated carbon derived from waste tire: Response surface method modeling approach. Environ. Prog. Sustain. Energy 2021, 40, e13600. [Google Scholar] [CrossRef]
- Danmaliki, G.I.; Saleh, T.A. Effects of bimetallic Ce/Fe nanoparticles on the desulfurization of thiophenes using activated carbon. Chem. Eng. J. 2017, 307, 914–927. [Google Scholar] [CrossRef]
- Tian, H.; Chen, C.; Zhu, T.; Zhu, B.; Sun, Y. Characterization and degradation mechanism of bimetallic iron-based/AC activated persulfate for PAHs-contaminated soil remediation. Chemosphere 2021, 267, 128875. [Google Scholar] [CrossRef]
- Kakavandi, B.; Kalantary, R.R.; Farzadkia, M.; Mahvi, A.H.; Esrafili, A.; Azari, A.; Yari, A.R.; Javid, A.B. Enhanced chromium (VI) removal using activated carbon modified by zero valent iron and silver bimetallic nanoparticles. J. Environ. Health Sci. Eng. 2014, 12, 115. [Google Scholar] [CrossRef]
- Fong, W.M.; Affam, A.C.; Chung, W.C. Synthesis of Ag/Fe/CAC for colour and COD removal from methylene blue dye wastewater. Int. J. Environ. Sci. Technol. 2020, 17, 3485–3494. [Google Scholar] [CrossRef]
- Rahaman, M.; Das, A.; Bose, S. Development of copper—Iron bimetallic nanoparticle impregnated activated carbon derived from coconut husk and its efficacy as a novel adsorbent toward the removal of chromium (VI) from aqueous solution. Water Environ. Res. 2021, 93, 1417–1427. [Google Scholar] [CrossRef]
- Zhao, R.; Du, X.; Cao, K.; Gong, M.; Li, Y.; Ai, J.; Ye, R.; Chen, R.; Shan, B. Highly dispersed Fe-decorated Ni nanoparticles prepared by atomic layer deposition for dry reforming of methane. Int. J. Hydrogen Energy 2023, 48, 28780–28791. [Google Scholar] [CrossRef]
- Mutz, B.; Belimov, M.; Wang, W.; Sprenger, P.; Serrer, M.A.; Wang, D.; Pfeifer, P.; Kleist, W.; Grunwaldt, J.D. Potential of an alumina-supported Ni3Fe catalyst in the methanation of CO2: Impact of alloy formation on activity and stability. ACS Catal. 2017, 7, 6802–6814. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, Z.; Tian, P.; Sheng, Y.; Xu, J.; Han, Y.F. Oxidative degradation of nitrobenzene by a Fenton-like reaction with Fe-Cu bimetallic catalysts. Appl. Catal. B Environ. 2019, 244, 1–10. [Google Scholar] [CrossRef]
- Liuzzi, D.; Pérez-Alonso, F.J.; Rojas, S. Ru-M (M= Fe or Co) Catalysts with high Ru surface concentration for Fischer-Tropsch synthesis. Fuel 2021, 293, 120435. [Google Scholar] [CrossRef]
- Zhang, L.; Meng, Z.; Zang, S. Preparation and characterization of Pd/Fe bimetallic nanoparticles immobilized on Al2O3/PVDF membrane: Parameter optimization and dechlorination of dichloroacetic acid. J. Environ. Sci. 2015, 31, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.C.; Sahoo, M.K.; Bellamkonda, S.; Parida, K.M.; Rao, G.R. Enhanced photodegradation of dyes and mixed dyes by heterogeneous mesoporous Co–Fe/Al2O3–MCM-41 nanocomposites: Nanoparticles formation, semiconductor behavior and mesoporosity. RSC Adv. 2016, 6, 94263–94277. [Google Scholar] [CrossRef]
- Munoz, M.; de Pedro, Z.M.; Casas, J.A.; Rodriguez, J.J. Improved γ-alumina-supported Pd and Rh catalysts for hydrodechlorination of chlorophenols. Appl. Catal. A Gen. 2014, 488, 78–85. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Tong, L.; Li, J.; Luo, R.; Qi, J.; Li, Y.; Wang, L. Iron–copper bimetallic nanoparticles supported on hollow mesoporous silica spheres: An effective heterogeneous Fenton catalyst for orange II degradation. RSC Adv. 2015, 5, 69593–69605. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Hussain, I.; Li, C.; Li, J.; Sun, X.; Shen, J.; Han, W.; Wang, L. Iron–copper bimetallic nanoparticles supported on hollow mesoporous silica spheres: The effect of Fe/Cu ratio on heterogeneous Fenton degradation of a dye. RSC Adv. 2016, 6, 54623–54635. [Google Scholar] [CrossRef]
- Lin, H.; Zhong, X.; Ciotonea, C.; Fan, X.; Mao, X.; Li, Y.; Deng, B.; Zhang, H.; Royer, S. Efficient degradation of clofibric acid by electro-enhanced peroxydisulfate activation with Fe-Cu/SBA-15 catalyst. Appl. Catal. B Environ. 2018, 230, 1–10. [Google Scholar] [CrossRef]
- Yan, H.; Qu, H.; Bai, H.; Zhong, Q. Property, active species and reaction mechanism of NO and NH3 over mesoporous Fe-Al-SBA-15 via microwave assisted synthesis for NH3-SCR. J. Mol. Catal. A Chem. 2015, 403, 1–9. [Google Scholar] [CrossRef]
- Kurniawan, A.; Sutiono, H.; Ju, Y.H.; Soetaredjo, F.E.; Ayucitra, A.; Yudha, A.; Ismadji, S. Utilization of rarasaponin natural surfactant for organo-bentonite preparation: Application for methylene blue removal from aqueous effluent. Microporous Mesoporous Mater. 2011, 142, 184–193. [Google Scholar] [CrossRef]
- Campos, B.; Aguilar-Carrillo, J.; Algarra, M.; Gonçalves, M.A.; Rodríguez-Castellón, E.; da Silva, J.C.E.; Bobos, I. Adsorption of uranyl ions on kaolinite, montmorillonite, humic acid and composite clay material. Appl. Clay Sci. 2013, 85, 53–63. [Google Scholar] [CrossRef]
- Zhou, C.H.; Zhang, D.; Tong, D.S.; Wu, L.M.; Yu, W.H.; Ismadji, S. Paper-like composites of cellulose acetate–organo-montmorillonite for removal of hazardous anionic dye in water. Chem. Eng. J. 2012, 209, 223–234. [Google Scholar] [CrossRef]
- Murray, H.H. Applied Clay Mineralogy: Occurrences, Processing and Applications of Kaolins, Bentonites, Palygorskite–Sepiolite, and Common Clays; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Sabouri, M.R.; Sohrabi, M.R.; Zeraatkar Moghaddam, A. Response surface method Optimization of the Dyes Degradation using Zero-Valent Iron based Bimetallic Nanoparticle on the Bentonite Clay Surface. Pollution 2020, 6, 581–595. [Google Scholar]
- Weng, X.; Cai, W.; Lan, R.; Sun, Q.; Chen, Z. Simultaneous removal of amoxicillin, ampicillin and penicillin by clay supported Fe/Ni bimetallic nanoparticles. Environ. Pollut. 2018, 236, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.Q.; Jin, X.Y.; Lu, X.Q.; Chen, Z.L. Adsorption of Pb (II), Cd (II), Ni (II) and Cu (II) onto natural kaolinite clay. Desalination 2010, 252, 33–39. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, S.; Chen, Z.; Megharaj, M.; Naidu, R. Kaolinite-supported nanoscale zero-valent iron for removal of Pb2+ from aqueous solution: Reactivity, characterization and mechanism. Water Res. 2011, 45, 3481–3488. [Google Scholar] [CrossRef]
- Jin, X.; Chen, Z.; Wang, T.; Chen, Z.; Megharaj, M.; Naidu, R. Simultaneous removal of co-contaminants: Acid brilliant violet and Cu 2+ by functional bimetallic Fe/Pd nanoparticles. J. Nanopart. Res. 2014, 16, 2657. [Google Scholar] [CrossRef]
- Masters, A.F.; Maschmeyer, T. Zeolites—From curiosity to cornerstone. Microporous Mesoporous Mater. 2011, 142, 423–438. [Google Scholar] [CrossRef]
- Rahman, R.O.A.; El-Kamash, A.M.; Hung, Y.T. Applications of nano-zeolite in wastewater treatment: An overview. Water 2022, 14, 137. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, L.; Mao, Y.; Zhou, Z.; Wu, D. Enhancing the degradation of bisphenol A by dioxygen activation using bimetallic Cu/Fe@ zeolite: Critical role of Cu (I) and superoxide radical. Sep. Purif. Technol. 2020, 253, 117550. [Google Scholar] [CrossRef]
- Ezzatahmadi, N.; Bao, T.; Liu, H.; Millar, G.J.; Ayoko, G.A.; Zhu, J.; Zhu, R.; Liang, X.; He, H.; Xi, Y. Catalytic degradation of Orange II in aqueous solution using diatomite-supported bimetallic Fe/Ni nanoparticles. RSC Adv. 2018, 8, 7687–7696. [Google Scholar] [CrossRef]
- Ezzatahmadi, N.; Millar, G.J.; Ayoko, G.A.; Zhu, J.; Zhu, R.; Liang, X.; He, H.; Xi, Y. Degradation of 2,4-dichlorophenol using palygorskite-supported bimetallic Fe/Ni nanocomposite as a heterogeneous catalyst. Appl. Clay Sci. 2019, 168, 276–286. [Google Scholar] [CrossRef]
- Ezzatahmadi, N.; Marshall, D.L.; Hou, K.; Ayoko, G.A.; Millar, G.J.; Xi, Y. Simultaneous adsorption and degradation of 2, 4-dichlorophenol on sepiolite-supported bimetallic Fe/Ni nanoparticles. J. Environ. Chem. Eng. 2019, 7, 102955. [Google Scholar] [CrossRef]
- Svarovskaya, N.; Bakina, O.; Glazkova, E.; Rodkevich, N.; Lerner, M.; Vornakova, E.; Chzhou, V.; Naumova, L. Synthesis of novel hierarchical micro/nanostructures AlOOH/AlFe and their application for As (V) removal. Environ. Sci. Pollut. Res. 2022, 29, 1246–1258. [Google Scholar] [CrossRef]
- Hina, R.; Arafa, I.; Al-Khateeb, F. Gas phase hydrodechlorination of CCl4 over Pd–Cu and Pd–Fe bimetallic catalysts supported on an AlF3 matrix. Prog. React. Kinet. Mech. 2016, 41, 29–38. [Google Scholar] [CrossRef]
- Xi, J.; Wang, Q.; Duan, X.; Zhang, N.; Yu, J.; Sun, H.; Wang, S. Continuous flow reduction of organic dyes over Pd-Fe alloy based fibrous catalyst in a fixed-bed system. Chem. Eng. Sci. 2021, 231, 116303. [Google Scholar] [CrossRef]
- Aftab, T.B.; Hussain, A.; Li, D. Application of a novel bimetallic hydrogel based on iron and cobalt for the synergistic catalytic degradation of Congo Red dye. J. Chin. Chem. Soc. 2019, 66, 919–927. [Google Scholar] [CrossRef]
- Shi, D.; Ouyang, Z.; Zhao, Y.; Xiong, J.; Shi, X. Catalytic reduction of hexavalent chromium using iron/palladium bimetallic nanoparticle-assembled filter paper. Nanomaterials 2019, 9, 1183. [Google Scholar] [CrossRef]
- Ge, W.; Li, S.; Jiang, M.; He, G.; Zhang, W. Cu/Fe bimetallic modified fly ash: An economical adsorbent for enhanced phosphorus removal from aqueous solutions. Water Air Soil Pollut. 2022, 233, 182. [Google Scholar] [CrossRef]
- Balangao, J.K.B.; Ramos, M.S.K. Production of Autoclaved Aerated Concretes with Fly Ash from a Coal Power Plant in Misamis Oriental, Philippines. Indian J. Sci. Technol. 2019, 12, 1–16. [Google Scholar] [CrossRef]
- Balangao, J.K.B.; Caingles, V.K.S.; Baguhin, I.A. Morphological and environmental characterization of lime sludge/fly ash stabilized sub-base materials. Sci. Int. 2023, 35, 283–290. [Google Scholar]
- Deplanche, K.; Caldelari, I.; Mikheenko, I.P.; Sargent, F.; Macaskie, L.E. Involvement of hydrogenases in the formation of highly catalytic Pd (0) nanoparticles by bioreduction of Pd (II) using Escherichia coli mutant strains. Microbiology 2010, 156, 2630–2640. [Google Scholar] [CrossRef] [PubMed]
- Pantidos, N.; Horsfall, L.E. Biological synthesis of metallic nanoparticles by bacteria, fungi and plants. J. Nanomed. Nanotechnol. 2014, 5, 1000233. [Google Scholar] [CrossRef]
- Yong, P.; Rowson, N.A.; Farr, J.P.G.; Harris, I.R.; Macaskie, L.E. Bioaccumulation of palladium by Desulfovibrio desulfuricans. J. Chem. Technol. Biotechnol. 2002, 77, 593–601. [Google Scholar] [CrossRef]
- Alruqi, S.S.; Al-Thabaiti, S.A.; Khan, Z. Iron-nickel bimetallic nanoparticles: Surfactant assisted synthesis and their catalytic activities. J. Mol. Liq. 2019, 282, 448–455. [Google Scholar] [CrossRef]
- Lin, Y.; Jin, X.; Khan, N.I.; Owens, G.; Chen, Z. Efficient removal of As (III) by calcined green synthesized bimetallic Fe/Pd nanoparticles based on adsorption and oxidation. J. Clean. Prod. 2021, 286, 124987. [Google Scholar] [CrossRef]
- Zhang, J.; Niu, Y.; Zhou, Y.; Ju, S.; Gu, Y. Green preparation of nano-zero-valent iron-copper bimetals for nitrate removal: Characterization, reduction reaction pathway, and mechanisms. Adv. Powder Technol. 2022, 33, 103807. [Google Scholar] [CrossRef]
- Zhu, F.; Ma, S.; Liu, T.; Deng, X. Green synthesis of nano zero-valent iron/Cu by green tea to remove hexavalent chromium from groundwater. J. Clean. Prod. 2018, 174, 184–190. [Google Scholar] [CrossRef]
- Lin, Y.; Jin, X.; Khan, N.I.; Owens, G.; Chen, Z. Bimetallic Fe/Ni nanoparticles derived from green synthesis for the removal of arsenic (V) in mine wastewater. J. Environ. Manag. 2022, 301, 113838. [Google Scholar] [CrossRef]
- Gopal, G.; Ravikumar, K.V.G.; Salma, M.; Chandrasekaran, N.; Mukherjee, A. Green synthesized Fe/Pd and in-situ Bentonite-Fe/Pd composite for efficient tetracycline removal. J. Environ. Chem. Eng. 2020, 8, 104126. [Google Scholar] [CrossRef]
- Kazemi, M.; Jahanshahi, M.; Peyravi, M. Hexavalent chromium removal by multilayer membrane assisted by photocatalytic couple nanoparticle from both permeate and retentate. J. Hazard. Mater. 2018, 344, 12–22. [Google Scholar] [CrossRef]
- Liu, T.; Yang, X.; Wang, Z.L.; Yan, X. Enhanced chitosan beads-supported Fe0-nanoparticles for removal of heavy metals from electroplating wastewater in permeable reactive barriers. Water Res. 2013, 47, 6691–6700. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Huang, D.; Lai, C.; Xu, P.; Zeng, G.; Wan, J.; Tang, L.; Dong, H.; Huang, B.; Hu, T. Difunctional chitosan-stabilized Fe/Cu bimetallic nanoparticles for removal of hexavalent chromium wastewater. Sci. Total Environ. 2018, 644, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Zhu, Q.; Wang, A.; Weng, M.; Xing, Z. Composite of chitosan and bentonite cladding Fe–Al bimetal: Effective removal of nitrate and by-products from wastewater. Environ. Res. 2020, 184, 109336. [Google Scholar] [CrossRef]
- Anju, R.P.P.; Sunil, J.T.; Dinoop, L. Chitosan stabilized Fe/Ni bimetallic nanoparticles for the removal of cationic and anionic triphenylmethane dyes from water. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100295. [Google Scholar]
- Rashid, S.; Shen, C.; Chen, X.; Li, S.; Chen, Y.; Wen, Y.; Liu, J. Enhanced catalytic ability of chitosan–Cu–Fe bimetal complex for the removal of dyes in aqueous solution. RSC Adv. 2015, 5, 90731–90741. [Google Scholar] [CrossRef]
- Silva, E.C.; Soares, V.R.; Fajardo, A.R. Removal of pharmaceuticals from aqueous medium by alginate/polypyrrole/ZnFe2O4 beads via magnetic field enhanced adsorption. Chemosphere 2023, 316, 137734. [Google Scholar] [CrossRef]
- Wasilewska, M.; Deryło-Marczewska, A. Adsorption of non-steroidal anti-inflammatory drugs on alginate-carbon composites—Equilibrium and kinetics. Materials 2022, 15, 6049. [Google Scholar] [CrossRef]
- Ragab, A.H.; Hussein, H.S.; Ahmed, I.A.; Abualnaja, K.M.; AlMasoud, N. An efficient strategy for enhancing the adsorption of antibiotics and drugs from aqueous solutions using an effective limestone-activated carbon–alginate nanocomposite. Molecules 2021, 26, 5180. [Google Scholar] [CrossRef]
- Ahmed, I.A.; Hussein, H.S.; ALOthman, Z.A.; ALanazi, A.G.; Alsaiari, N.S.; Khalid, A. Green synthesis of Fe–Cu bimetallic supported on alginate-limestone nanocomposite for the removal of drugs from contaminated water. Polymers 2023, 15, 1221. [Google Scholar] [CrossRef]
- Wu, X.; Lu, C.; Zhang, W.; Yuan, G.; Xiong, R.; Zhang, X. A novel reagentless approach for synthesizing cellulose nanocrystal-supported palladium nanoparticles with enhanced catalytic performance. J. Mater. Chem. A 2013, 1, 8645–8652. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Baran, N.Y.; Baran, T.; Menteş, A. Fabrication and application of cellulose Schiff base supported Pd (II) catalyst for fast and simple synthesis of biaryls via Suzuki coupling reaction. Appl. Catal. A Gen. 2017, 531, 36–44. [Google Scholar] [CrossRef]
- Mandal, B.H.; Rahman, M.L.; Yusoff, M.M.; Chong, K.F.; Sarkar, S.M. Bio-waste corn-cob cellulose supported poly (hydroxamic acid) copper complex for Huisgen reaction: Waste to wealth approach. Carbohydr. Polym. 2017, 156, 175–181. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Sahi, S.; Mahajan, H.; Paul, S.; Clark, J.H. Novel heterogeneous catalyst systems based on Pd (0) nanoparticles onto amine functionalized silica-cellulose substrates [Pd (0)-EDA/SCs]: Synthesis, characterization and catalytic activity toward C–C and C–S coupling reactions in water under limiting basic conditions. J. Mol. Catal. A Chem. 2015, 408, 48–59. [Google Scholar]
- Karami, S.; Zeynizadeh, B.; Shokri, Z. Cellulose supported bimetallic Fe–Cu nanoparticles: A magnetically recoverable nanocatalyst for quick reduction of nitroarenes to amines in water. Cellulose 2018, 25, 3295–3305. [Google Scholar] [CrossRef]


| Country | Frequency | % (n = 122) | C/D |
|---|---|---|---|
| China | 76 | 62.3 | 45.2 |
| Russia | 7 | 5.7 | 16.0 |
| Australia | 6 | 4.9 | 23.8 |
| India | 6 | 4.9 | 16.7 |
| Taiwan | 5 | 4.1 | 27.2 |
| Iran | 5 | 4.1 | 64.0 |
| Egypt | 4 | 3.3 | 14.0 |
| Israel | 4 | 3.3 | 12.8 |
| Argentina | 3 | 2.4 | 4.3 |
| Saudi Arabia | 3 | 2.4 | 95.0 |
| Journal | Frequency | % (n = 122) | C/D | Country | Journal Rank |
|---|---|---|---|---|---|
| RSC Advances | 8 | 6.6 | 48.0 | UK | Q1 |
| Chemical Engineering Journal | 7 | 5.7 | 70.4 | The Netherlands | Q1 |
| Chemosphere | 7 | 5.7 | 41.6 | UK | Q1 |
| Applied Catalysis B: Environmental | 5 | 4.1 | 203.0 | The Netherlands | Q1 |
| Journal of Cleaner Production | 4 | 3.3 | 59.0 | UK | Q1 |
| Environmental Science and Pollution Research | 3 | 2.4 | 10.3 | Germany | Q1 |
| Journal of the Taiwan Institute of Chemical Engineers | 3 | 2.4 | 17.3 | Taiwan | Q1 |
| Journal of Hazardous Materials | 3 | 2.4 | 80.7 | The Netherlands | Q1 |
| Journal of Alloys and Compounds | 3 | 2.4 | 14.3 | The Netherlands | Q1 |
| Journal of Molecular Liquids | 3 | 2.4 | 8.3 | The Netherlands | Q1 |
| Institution | Frequency | % (n = 122) | Country |
|---|---|---|---|
| Tongji University | 7 | 5.7 | China |
| Institute of Strength Physics and Materials Science | 6 | 4.9 | Russia |
| Fujian Normal University | 5 | 4.1 | China |
| Sichuan University | 5 | 4.1 | China |
| Nanjing University of Science and Technology | 4 | 3.3 | China |
| Technion–Israel Institute of Technology | 4 | 3.3 | Israel |
| Tianjin University | 3 | 2.4 | China |
| China University of Mining and Technology | 3 | 2.4 | China |
| Chinese Academy of Sciences | 3 | 2.4 | China |
| Queensland University of Technology | 3 | 2.4 | Australia |
| Consejo Nacional de Investigaciones Científicas y Tecnicas (CONICET) | 3 | 2.4 | Argentina |
| Author | Frequency | % (n = 122) | Country |
|---|---|---|---|
| A. Sharipova | 4 | 3.3 | Israel/Russia |
| Xin Liu | 3 | 2.4 | China |
| Jing Wang | 3 | 2.4 | China |
| J.S. Riva | 3 | 2.4 | Argentina |
| Naeim Ezzatahmadi | 3 | 2.4 | Australia |
| Xiulan Weng | 2 | 1.6 | China |
| Hongwei Wu | 2 | 1.6 | China |
| Bo Lai | 2 | 1.6 | China |
| Yuanqiong Lin | 2 | 1.6 | China |
| Jin-Hong Fan | 2 | 1.6 | China |
| Elham Aghaei | 2 | 1.6 | Australia |
| Bimetal System | Method | Characteristics of Synthesized Bimetals | Properties | Reference |
|---|---|---|---|---|
| Fe-Cu/aluminum collar | Mechanical Alloying | Presence of Fe and Cu seen deposited on aluminum substrate; coating of up to 500 nm layer thickness | Magnetic | [49] |
| Cu-Fe/CNT | Mechanical Alloying | CNT additions to Cu-Fe at 2, 5, and 10 (wt.%); Cu-Fe shown as agglomerate microparticles, larger at 2 and 5 wt.% CNT, decreased in size at 10 wt.% CNT | Magnetic | [50] |
| Fe/Ag nanocomposite | Mechanical Alloying | Nanocomposite structure, Fe-5Ag, Fe-10Ag (vol.%) | Optimal combined strength and ductility after annealing at 550 °C, biomedical suitability | [52] |
| Fe/Ag nanocomposite | Mechanical Alloying | 70% and 75% Macroporous, Fe-5Ag and Fe-10Ag (vol%) | High compressive strength, high bending strength, high ductility, biodegradability | [53] |
| Fe/Ag, Fe/Cu nanocomposites | Mechanical Alloying | Nanocomposite structure, Fe–10% Ag, Fe–20% Ag, and Fe–25% Cu (vol%) | High strength and ductility, biodegradability | [54] |
| Fe/Ag, Fe/Cu nanocomposites | Mechanical Alloying | Nanocomposite structure, Fe–10% Ag, Fe–20% Ag, and Fe–25% Cu (vol%) | Densities close to theoretical values, High plasticity, bending strength | [55] |
| Fe/Cu | Electrical Explosion of Metal Wires | Nanostructured, near-fully dense, 72 Fe–28 Cu, 47 Fe–53 Cu, and 28 Fe–72 Cu (wt%) | High yield strength (Fe-rich, 72 Fe–28 Cu): 700 MPa; high bending strength (Fe-rich, 72 Fe–28 Cu): 920 MPa; greater ductility (Cu-rich, 28 Fe–72 Cu); lower electrical resistivity (Cu-rich, 28 Fe–72 Cu) | [64] |
| Fe/Pt | Radiolysis | Nanometer-sized fine particles | Eco-friendly, improved dispersibility with PVP addition, controllability of mean particle size with PVP addition | [65] |
| Ni/Fe | Sonochemical | Spherically shaped nanoparticles | Adsorptive and catalytic capability, enhanced dispersity, reduced agglomeration | [69] |
| s-Fe/Cu | Sonochemical | Irregularly-shaped microparticles | Magnetic | [70] |
| Fe/Cu-GO | Sonochemical | Fe/Cu nanoparticles integrated into a GO matrix forming Fe/Cu-GO nanocomposites | Magnetically recoverable, good dispersion | [71] |
| Fe-Mn/KB | Sonochemical | Uniform nanoparticles with the mean diameters of ∼41 nm, with distinct mesopores | Superior oxygen reduction reaction (ORR) activity, performance comparable to commercial Pt/C electrocatalyst, stable 1.50 V voltage platform, durability over 20 h in Al–air battery tests | [72] |
| Fe-Mn@SCAs | Sonochemical | Fe-Mn nanoclusters with porous carbon structure | Adsorptive capability, high surface area and porosity | [73] |
| Fe/Pt, Fe/Co, and Fe/Ni | MF-LAL | Nano-sized, one-dimensional (1D) chains | Ferromagnetism, high saturation magnetization, low coercivity, low remanent magnetization | [76] |
| Bimetal System | Experimental Materials | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Fe/Al | Metal precursors: FeSO4·7H2O, aluminum chloride Reducing agent: concentrated HCl | Simple and cost-effective synthesis Efficient bimetallic composition Controlled synthesis conditions | Use of concentrated hydrochloric acid (HCl) Energy-intensive drying process | [79] |
| Fe/Al | Metal precursors: ferric chloride, aluminum chloride Reducing agent: NaBH4 | Controlled sequential reduction Versatile metal coating configurations Improved stability of bimetallic nanoparticles Washing and lyophilization improve purity | Complex and lengthy synthesis process High consumption of reducing agent | [80] |
| Fe/Al | Metal precursors: FeCl3·6H2O, AlCl3·6H2O Reducing agent: NaOH solution | Controlled coagulation process Adjustable Al/Fe ratio Stable storage conditions Effective coagulant formation | Slow preparation process Storage requirements | [81] |
| Fe/Cu | Metal precursors: FeCl3·6H2O and CuCl2·2H2O Reducing agent: NaBH4 | Tunable Fe/Cu ratios Efficient reduction method Ultracentrifugation for separation Lyophilization for stability | Complex processing steps High energy consumption | [82] |
| Fe/Cu | Metal precursors: FeSO4·7H2O, CuSO4 Reducing agent: NaBH4 | Controlled Fe/Cu ratios Modified borohydride method Dropwise addition of NaBH4 Addition of ethanol in the modified borohydride method | Complex multi-step process Additional cost for ethanol usage | [83] |
| Fe/Cu | Metal precursors: FeCl3·6H2O, CuCl2·2H2O Reducing agent: NaBH4 | Simple and cost-effective Homogeneity of the mixture Efficient reduction process Mild reaction conditions | Energy-intensive drying process | [84] |
| Fe/Cu | Metal precursors: CuCl2, colloidal solution of nZVI particles Reducing agent: NaBH4 | Simple synthesis at room temperature Efficient copper deposition Customizable Cu content Rapid reaction time | Discontinuous Cu shell | [85] |
| Fe/Cu | Metal precursors: iron (II) sulfate, copper sulfate Reducing agent: NaBH4 | Simple process Controlled pH adjustment Rapid reduction process Effective removal of impurities Reaction under nitrogen atmosphere | Excess reducing agent usage | [86] |
| Fe/Ni | Metal precursors: FeSO4·7H2O, NiCl2·6H2O Reducing agent: NaBH4 | Simple and efficient process Rapid reaction time Controlled addition of NaBH4 | Energy-intensive drying process Multiple ethanol washing of nanoparticles | [87] |
| Fe/Ni | Metal precursors: FeCl3·6H2O, NiSO4·6H2O Reducing agent: NaBH4 | Efficient reduction process Prevention of oxidation by use of N2 atmosphere improved purity of nanoparticles | Energy-intensive drying process Multiple ethanol washing steps of nanoparticles | [88] |
| Fe/Ni | Metal precursors: FeCl3·6H2O, NiSO4·6H2O Reducing agent: KBH4 | Controlled reduction process Prevention of oxidation by use of N2 atmosphere Efficient mixing and homogeneity Controlled particle recovery Improved purity of nanoparticles | Energy-intensive centrifugation step | [89] |
| Fe/Ni | Metal precursors: FeSO4·7H2O, NiSO4·7H2O Reducing agent: KBH4 | Controlled reduction process Efficient washing and purification of nanoparticles Stable storage conditions of nanoparticles Magnetic recoverability of nanoparticles | Energy-intensive drying process Multiple washing steps for nanoparticles with ethanol and distilled water Longer synthesis time | [90] |
| Fe/Ni | Metal precursors: FeCl3·6H2O, Ni(NO3)2·6H2O Reducing agent: NaBH4 | Simple and scalable process Room-temperature processing Mechanical stirring for homogeneity Controlled NaBH4 addition Efficient washing and purification Use of N2 atmosphere | Multi-step process | [91] |
| Fe/Ti | Metal precursors: FeSO4·7H2O, Ti(SO4)2 Reducing agent: NaBH4 | Efficient reduction process Short reaction time Centrifugal selection of Fe-Ti nanoparticles Use of vacuum-drying to preserve nanoparticles Use of N2 atmosphere in the process | Long vacuum-drying time | [92] |
| Fe/Co | Metal precursors: Fe(NO3)3·9H2O, FeCl3·6H2O, Co(NO3)2·6H2O, CoCl2·6H2O, CoSO4·7H2O Reducing agents: NaOH and hydrazine hydrate (N2H4·7H2O) | Simple and efficient synthesis Controlled Fe/Co ratio Magnetic recoverability of nanoparticles Low-temperature drying | Multiple ethanol washing steps | [93] |
| Fe/Mn | Metal precursors: iron (II) chloride hexahydrate, manganese chloride Reducing agent: sodium tetraborate | Simple and cost-effective method Controlled bimetallic composition Controlled addition of sodium tetraborate solution Moderate drying temperature | Long drying time | [94] |
| Bimetal System | Experimental Materials | Advantages | Disadvantages | Reference |
|---|---|---|---|---|
| Fe-Ce/NCNT | Metal precursors: FeCl3·6H2O, Ce(NO3)3·9H2O Other(s): melamine Support: nitrogen-doped carbon nanotubes (NCNT) | Controlled bimetallic composition High-temperature pyrolysis in the process Ultrasonication with HCl Protective argon atmosphere | Time-consuming process High energy consumption Use of strong acid (HCl) for purification Equipment-dependent process | [161] |
| Fe-Al/MWCNTs | Metal precursors: Fe(NO3)3·9H2O, Al(NO3)3·9H2O Other(s): citric acid Support: multi-walled carbon nanotubes (MWCNTs) | Simple catalyst preparation Controlled bimetallic composition Involvement of calcination Use of N2 atmosphere | High energy consumption | [162] |
| Fe-Cu/CNF | Metal precursors: iron (III) acetylacetonate (Fe(acac)3), copper (II) acetate monohydrate (Cu(ac)2·H2O) Others: polyacrylonitrile (PAN), dimethylformamide (DMF) Support: carbon nanofibers (CNFs) | Controlled morphology and composition Strong metal-support interaction Good thermal stability Enhanced mechanical properties | Complex synthesis process High energy consumption | [163] |
| Fe-Cu/Graphitic carbon | Metal precursors: Fe(II) acetylacetonate, chlorophyllin (Cu precursor) Support: graphitic carbon | Simple and scalable synthesis Use of natural precursor (chlorophyllin) Controlled heating profile Use of high-temperature treatment Use of argon (Ar) atmosphere | High energy consumption | [164] |
| Cu-Fe/MC | Metal precursors: Fe(NO3)3·9H2O, Cu(NO3)2·3H2O Others: Pluronic F127, phenol and formalin solution (carbon precursors) Support: mesoporous carbon (MC) | Controlled metal loading Improved thermal stability Porous carbon structure | High energy consumption Long processing time | [165] |
| Co-Fe/MB | Metal precursors: FeSO4·7H2O, CoSO4·7H2O Others: pristine sawdust biochar, PEG-4000 (dispersant), NaBH4 (reducing agent) Support: modified biochar (MB) | Enhanced stability of Co/Fe nanoparticles Controlled reduction process Use of biochar as support Enhanced metal–support interaction | Multiple ethanol washing steps Long processing time Energy-intensive drying | [166] |
| Ag-Fe/MB | Metal precursors: FeSO4·7H2O, AgNO3 Others: original biochar (OB), NaBH4 (reducing agent), PEG-4000 Support: modified biochar (MB) | Versatile synthesis approach Controlled stirring of the mixture Controlled addition of NaBH4 solution Use of biochar as support | Complex multi-step process High energy consumption Long processing time | [167] |
| BC@Fe/Ni | Metal precursors: FeCl2, NiCl2 Others: ground straw (biochar source), NaBH4 (reducing agent), polyethylene glycol (PEG) (dispersant) Support: biochar (BC) | Biochar as a support material Controlled metal deposition Controlled biochar loading Magnetic recoverability of the particles | Multi-step synthesis procedure High energy consumption | [168] |
| Fe–Co-modified biochar (FMBC) | Metal precursors: Fe(NO3)3 9H2O, Co(NO3)2·6H2O Other(s): cedar bark (biochar source) Support: modified biochar (MBC) | Forestry waste (cedar bark) as a precursor for biochar Biochar as a support material High temperature pyrolysis Magnetic recoverability of the adsorbents | High energy consumption | [169] |
| nZVIC (Fe-Cu)-municipal sludge-derived biochar (SBC) | Metal precursors: FeSO4·7H2O, CuSO4·5H2O Others: modified sewage sludge (biochar source), NaBH4 Support: municipal sludge-derived biochar (SBC) | Low-temperature synthesis Biochar as a support material Controlled reduction conditions Municipal sludge utilization | Use of excess NaBH4 solution | [170] |
| Zn-Fe/CBC | Metal precursors: FeCl3·6H2O, ZnSO4·7H2O Other(s): corncob (biochar source) Support: corncob biochar (CBC) | Use of corncob as biochar source Biochar as a support material Moderate-temperature pyrolysis (450 °C) Centrifugation and washing ensuring selection of Zn-Fe/CBC | Long processing time Energy-intensive drying for biochar preparation Multiple washing steps Energy-intensive drying synthesis requirement Pyrolysis energy demand | [171] |
| Fe-Co/AC | Metal precursors: FeSO4·7H2O, CoCl2·6H2O Other(s): scrap tires (activated carbon source), NaBH4 Support: activated carbon (AC) | Use of scrap tires as activated carbon source Versatile synthesis method Controlled reduction with NaBH4 Uses water as the primary solvent in the process | High temperature requirements during synthesis Long processing time | [172] |
| Fe-Ce/AC | Metal precursors: Fe (NO3)3·9H2O, Ce (NO3)3·6H2O Other(s): waste rubber tires (WRT) (activated carbon source) Support: activated carbon (AC) | Use of waste rubber tires (wrt) as activated carbon source ensured metal–carbon interaction Controlled precipitation conditions Moderate calcination temperature (350 °C) | Energy-intensive process Long processing time | [173] |
| nZVI-Ni/AC | Metal precursors: FeSO4·7H2O, NiCl2·6H2O Others: KBH4 (reducing agent), PEG-4000 Support: activated carbon (AC) | Controlled reduction process Improved dispersion with peg-4000 Use of inert gas environment in the synthesis | Multi-step process High energy requirements Long processing time | [174] |
| PAC-Fe/Ag | Metal precursors: FeSO4·7H2O, AgNO3 Others: powder activated carbon, NaBH4 Support: powder activated carbon (PAC) | Versatile synthesis method Controlled reduction with NaBH4 Improved Ag adhesion onto ZVI/PAC Magnetic recoverability of the bimetallic nanoparticles | Muti-step process High energy requirements Use of excess NaBH4 solution | [175] |
| Ag-Fe/CAC | Metal precursors: FeSO4·7H2O, AgNO3 Others: commercial activated carbon (CAC), NaBH4 (reducing agent), polyethylene glycol 600 (PEG-600) Support: commercial activated carbon (CAC) | Simple versatile synthesis procedure Mild synthesis conditions | Involves uncontrolled stirring | [176] |
| Fe-Cu/CAC | Metal precursors: FeSO4, FeCl3, CuSO4 Others: coconut husk (activated carbon source), NaBH4 (reducing agent) Support: coconut husk-derived activated carbon (CAC) | Use of coconut husk as activated carbon source Versatile synthesis procedure (Sequential metal impregnation) | Muti-step synthesis procedure Process complexity High energy requirements Multiple chemical consumption | [177] |
| Bimetal System | Method | Characteristics of Synthesized Bimetals | Properties | Reference |
|---|---|---|---|---|
| Fe-Al | Chemical reduction | Spherically shaped nanoparticles | Adsorptive and catalytic capability | [80] |
| Fe-Cu | Chemical reduction | Nanoclusters, Fe/Cu mass ratios (0.9:0.1, 0.75:0.25, and 0.5:0.5) | Magnetic recoverability, adsorptive removal capacity | [82] |
| Fe-Cu | Chemical reduction | Core–shell structure, discontinuous Cu shell on an nZVI core | Catalytic capability | [85] |
| Fe-Cu | Chemical reduction | Spherically shaped nanoparticles | Degradation capability | [86] |
| Fe-Ni | Chemical reduction | Nanoparticles with chain-like structure | Magnetic recoverability, adsorption capability | [87] |
| Fe-Ni | Chemical reduction | Ni particles dispersed on Fe nanoparticle surface | Magnetic recoverability, adsorptive and reductive capability | [90] |
| Fe-Co | Chemical reduction | Spherically shaped nanoparticles | Magnetic recoverability, adsorptive capability | [93] |
| NP-Pd/Fe | Chemical dealloying | Open nanosponge structure | Electrocatalytic capability | [101] |
| NP-Pt/Fe alloy | Chemical dealloying | Interconnected strips with nanoporous morphology with pore size of about several nanometer | Electrocatalytic capability | [102] |
| Ag/Fe | Seed-mediated growth | Microparticles with diameters of 2–10 μm | Catalytic capability | [105] |
| Cu/Al | Seed-mediated growth | Core–shell structure, Cu particles deposited as rod-like aggregations on the aluminum surfaces | Degradation capability | [106] |
| Fe/Rh | Electrochemical synthesis | Dispersed nanowires about 18 nm in diameter and 1 mm long | Low temperature magnetic capability | [118] |
| Fe/Rh | Electrochemical synthesis | polycrystalline nanowire arrays, 20 nm in diameter, and about 1–3 mm in length | Magnetic properties | [119] |
| Fe-Cu | Galvanic replacement | Dispersed Cu particles on Fe surface | Catalytic capability | [123] |
| Fe-Cu | Galvanic replacement | Nanoparticles had an irregular surface structure with particle sizes ranging from 20 to 30 nm | Adsorptive capability | [124] |
| Fe-Al | Galvanic replacement | Fine Fe particles (about 200–400 nm) on the surface of Al were necklace-like or ball-like, the size of the bimetal was about 20–30 μm | Adsorptive and catalytic capability | [131] |
| Fe-Al | Galvanic replacement | Core–shell structure, Fe particles deposited on Al surface | Adsorptive capability | [133] |
| Mg-Fe | Galvanic replacement | Mg/Fe particles were indicated by many sheet crystal particles | Degradation capability, electrochemical property | [134] |
| Fe-Al | Galvanic replacement | Core–shell structure, Fe particles deposited on Al surface | Adsorptive capability | [137] |
| Fe-Cu | Galvanic replacement | Micro-scale particles, Cu particles deposited on Fe surface | Degradation capability | [139] |
| Ni/Fe | Thermogravimetric method | Nanoporous structure | Catalytic capability | [152] |
| Ni/Fe | Thermogravimetric method | Spherically shaped nanoparticles | Catalytic capability | [157] |
| Fe/Ni NPs | Thermogravimetric method | Nanoparticles have an average size of 18.6 ± 2.4 nm, with Ni observed on their surface | Catalytic capability, magnetic property | [158] |
| Fe-Ce/NCNT | Supported particles | Hollow CNTs encapsulated nanocrystals structure was evident, along with the ordered carbon layer distribution; and CNTs diameter distribution was between 100 and 200 nm | Electrocatalytic capability | [161] |
| Fe-Al/MWCNTs | Supported particles | Straight and long carbon nanotubes (MWNTs) appear on the Fe-Al catalyst, MWNTs have a graphite interlayer spacing of 0.34 nm | Catalytic capability, electronic conductivity | [162] |
| Ag-Fe/MB | Supported particles | Dispersion of Ag/Fe NPs (estimated diameter equal to 51 nm) on biochar surface, as well as the formation of small globular structures | Adsorptive and reductive capability | [167] |
| BC@Fe/Ni | Supported particles | Fe/Ni NPs existing in chain forms and distributed in some pores or other places of BC | Magnetic recoverability, adsorptive capability | [168] |
| Ni3Fe/Al2O3 | Supported particles | Ni-Fe nanoparticles are well dispersed on the Al2O3 support | Catalytic capability | [179] |
| Pd-Fe/Al2O3/PVDF | Supported particles | Pd/Fe NPs seen on the surface of the Al2O3/PVDF membrane, exhibiting a smooth, spherical morphology with particle sizes ranging from approximately 50 to 100 nm | Degradation capability | [182] |
| FeCu/HMS | Supported particles | Fe and Cu evenly dispersed throughout the silica matrix, metal nanoparticles in the Fe-Cu/HMS having an average size of approximately 18 nm | Catalytic and degradation capability | [185] |
| Fe-Al-SBA-15 | Supported particles | Well-ordered hexagonal arrays of mesopores with one-dimensional channels, also agglomeration of Fe seen clearly, indicating considerable FexOy clusters inside the channel | Catalytic capability | [188] |
| B-Fe/Ni | Supported particles | Fe/Ni spherical particles ranging in size from 30 to 60 nm well dispersed on bentonite | Adsorptive and catalytic capability | [194] |
| K-Fe/Pd | Supported particles | Fe/Pd particles with a diameter of about 20–70 nm, and consisting of short chain-like spherical particles seen on kaolinite | Catalytic capability | [197] |
| Di-Fe/Ni | Supported particles | porous structure of Di-Fe/Ni, dispersion of Fe/Ni spherical nanoparticles (in the range of 50–80 nm) into the pores and on the surface of diatomite | Catalytic and degradation capability | [201] |
| Pal-Fe/Ni | Supported particles | Fe/Ni spherical nanoparticles, with a diameter range of 20–60 nm, well dispersed and stabilized onto the palygorskite surface | Catalytic and degradation capability | [202] |
| Fe/Pd-assembled filter paper | Supported particles | Fe/Pd white, quasi-spherical NPs (mean diameter of 10.1 ± 1.7 nm) distributed homogeneously onto the filter paper surface | Catalytic and reduction capability | [208] |
| Cu/Fe-BM@FA | Supported particles | Fe and Cu detected on FA spherical microparticles | Adsorptive and catalytic capability | [209] |
| Bimetal System | Method | Characteristics of Synthesized Bimetals | Properties | Reference |
|---|---|---|---|---|
| Fe-Ni | Biological | Core–shell structure, Nanosphere with some irregularly-shaped nanoparticles | Adsorptive capacity | [215] |
| Fe-Pd | Biological | Spherically shaped nanoparticles | Catalytic capability, adsorptive capacity | [216] |
| Fe-Cu | Biological | Spherically shaped nanoparticles | Adsorptive capacity, reduction capability | [217] |
| Fe-Cu | Biological | Spherically shaped nanoparticles | Adsorptive capacity | [218] |
| C-Fe-Ni | Biological | Polydisperse regular spherical nanoparticles | Catalytic capability | [219] |
| Fe-Pd | Biological | core–shell structure Spherically shaped nanoparticles | Catalytic capability | [220] |
| Chitosan (CS)-stabilized Fe-Cu | Biological | Spherically shaped nanoparticles | Catalytic capability | [223] |
| Fe–Al bimetal chitosan bentonite (Fe–Al bimetal@bent) complex | Biological | Plenty of bimetal surrounded by chitosan (Cs)–bentonite | Adsorptive capability | [224] |
| Chitosan (CS)-Fe-Ni | Biological | Nanoparticle, Fe as core, chitosan as shell | Catalytic capability | [225] |
| Chitosan–Cu–Fe bimetal complex | Biological | Irregular and relatively nonporous complex with presence of Fu and Cu | Catalytic capability | [231] |
| ZVFe–Cu/Alg–LS | Biological | Nanocomposite exhibiting multilayer structure and rough surface | Adsorptive and catalytic capability | [230] |
| Fe–Cu@MCC | Biological | Nanocomposite particles within range of 27–35 nm | Catalytic capability, magnetic recoverability | [236] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balangao, J.K.B.; Tabelin, C.B.; Phengsaart, T.; Zoleta, J.B.; Arima, T.; Park, I.; Mufalo, W.; Ito, M.; Alorro, R.D.; Orbecido, A.H.; et al. Synthesis of Iron-Based and Aluminum-Based Bimetals: A Systematic Review. Metals 2025, 15, 603. https://doi.org/10.3390/met15060603
Balangao JKB, Tabelin CB, Phengsaart T, Zoleta JB, Arima T, Park I, Mufalo W, Ito M, Alorro RD, Orbecido AH, et al. Synthesis of Iron-Based and Aluminum-Based Bimetals: A Systematic Review. Metals. 2025; 15(6):603. https://doi.org/10.3390/met15060603
Chicago/Turabian StyleBalangao, Jeffrey Ken B., Carlito Baltazar Tabelin, Theerayut Phengsaart, Joshua B. Zoleta, Takahiko Arima, Ilhwan Park, Walubita Mufalo, Mayumi Ito, Richard D. Alorro, Aileen H. Orbecido, and et al. 2025. "Synthesis of Iron-Based and Aluminum-Based Bimetals: A Systematic Review" Metals 15, no. 6: 603. https://doi.org/10.3390/met15060603
APA StyleBalangao, J. K. B., Tabelin, C. B., Phengsaart, T., Zoleta, J. B., Arima, T., Park, I., Mufalo, W., Ito, M., Alorro, R. D., Orbecido, A. H., Beltran, A. B., Promentilla, M. A. B., Jeon, S., Haga, K., & Resabal, V. J. T. (2025). Synthesis of Iron-Based and Aluminum-Based Bimetals: A Systematic Review. Metals, 15(6), 603. https://doi.org/10.3390/met15060603















