Prediction of Reduction Recovery of Iron and Vanadium Oxides in Low-Grade Vanadium–Titanium Magnetite Based on Response Surface Model
Abstract
1. Introduction
2. Experimental Design
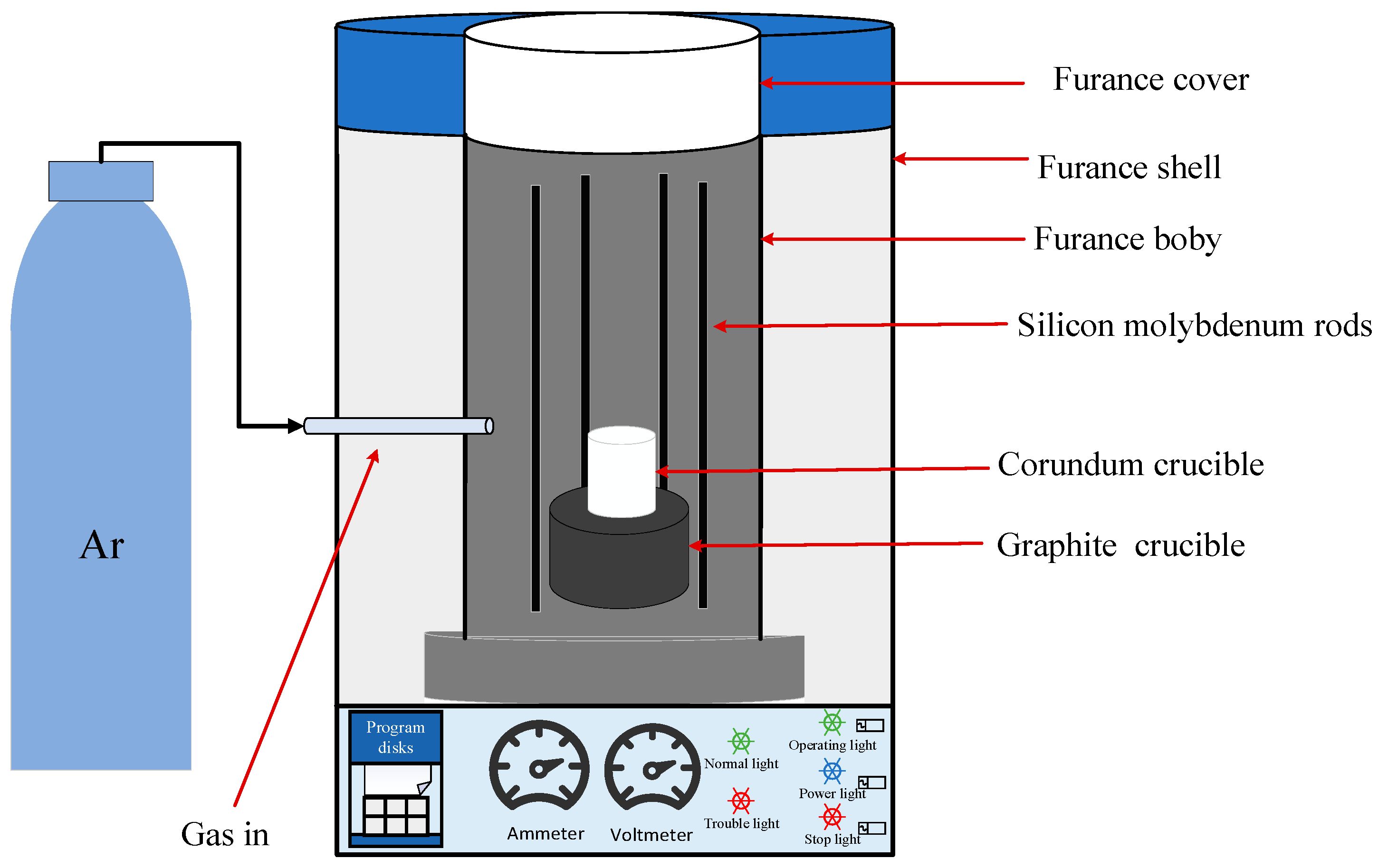
2.1. Experimental Materials and Equipment
2.2. Experimental Method
- (1)
- Raw material pretreatment: Weigh 100 g of VTM fine powder and place it in a crusher for crushing treatment. Use a standard inspection sieve for screening to ensure that the final particle sizes are all less than 200 mesh (about 74 μm) so as to increase the contact area and reaction efficiency of the subsequent reaction.
- (2)
- Raw material ratio and mixing: Accurately calculate the amount of reducing medium used based on the stoichiometric relationship in the material reaction equation. Meanwhile, weigh 2% of the iron concentrate powder, 1% of the sodium carboxymethyl cellulose binder, and 5% of pure water. Place all the above raw materials in a stirring container and stir thoroughly to ensure uniform mixing of each component, thereby obtaining a VTM mixture. The specific specifications, manufacturers, and other details of the chemical reagents used in the experiment are detailed in Table 2.
- (3)
- Briquetting and drying: Transfer the mixed materials to the briquetting mold and maintain pressure at 20 MPa for 3 to 5 min to complete the briquetting process. Subsequently, place the pressing blocks in a constant temperature drying oven, with the temperature set at 105 °C, and dry them for 6 h to fully cure the pressing blocks and enhance their physical strength and stability.
- (4)
- Reduction reaction: This experiment is conducted under normal pressure conditions, and the reduction process is controlled by adjusting the reduction temperature and reduction time. Select a corundum crucible as the reaction vessel and carefully place the dried and solidified briquettes into the crucible. Turn on the muffle furnace and slowly heat it up to the specified temperature according to the preset program (with the heating rate controlled at 7 °C/min). After the furnace temperature stabilizes, quickly place the crucible containing the sample into the center of the muffle furnace chamber that has been filled with Ar protective gas. The Ar gas flow rate should be controlled at 100–200 mL/min to ensure that the reaction proceeds fully in an inert atmosphere. The reaction duration should be set according to the experimental requirements.
- (5)
- Cooling and post-treatment: After the reaction is completed, quickly remove the crucible and place it in a cooling device filled with Ar gas. Let it cool naturally to room temperature under a protective atmosphere. After the cooling is completed, completely take the sample out of the crucible. Mechanically crush achieve effective separation of the slag phase and the iron phase. Then, crush and grind the slag phase, and detect its composition. Calculate the reduction rates of iron and vanadium metal oxides in the raw ore, and analyze the characteristics of the reactants under microscopic conditions to enhance the understanding of the reduction reaction process for subsequent component analysis and performance testing.
2.3. Experimental Design Based on Response Surface Method
3. Result and Discussion
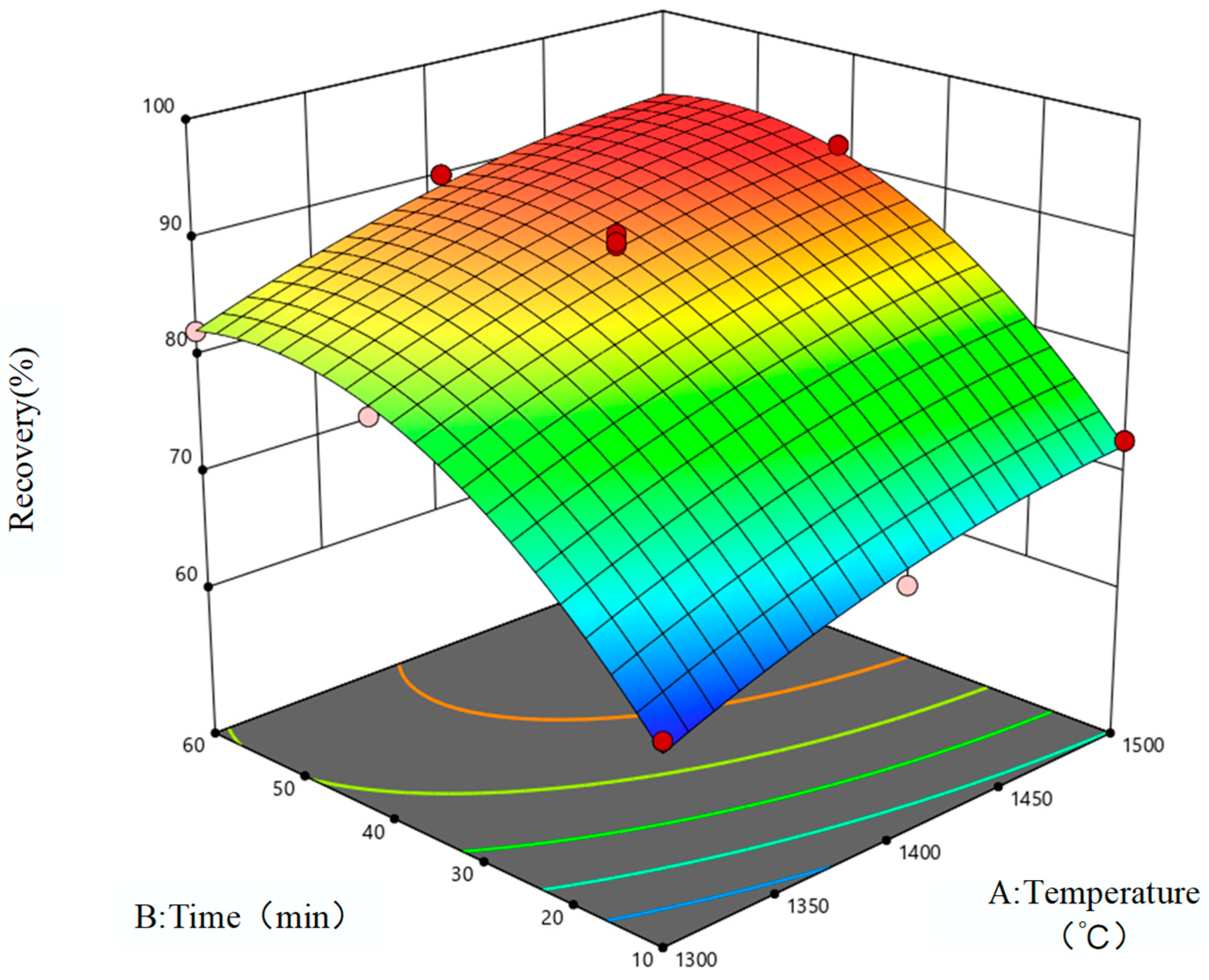
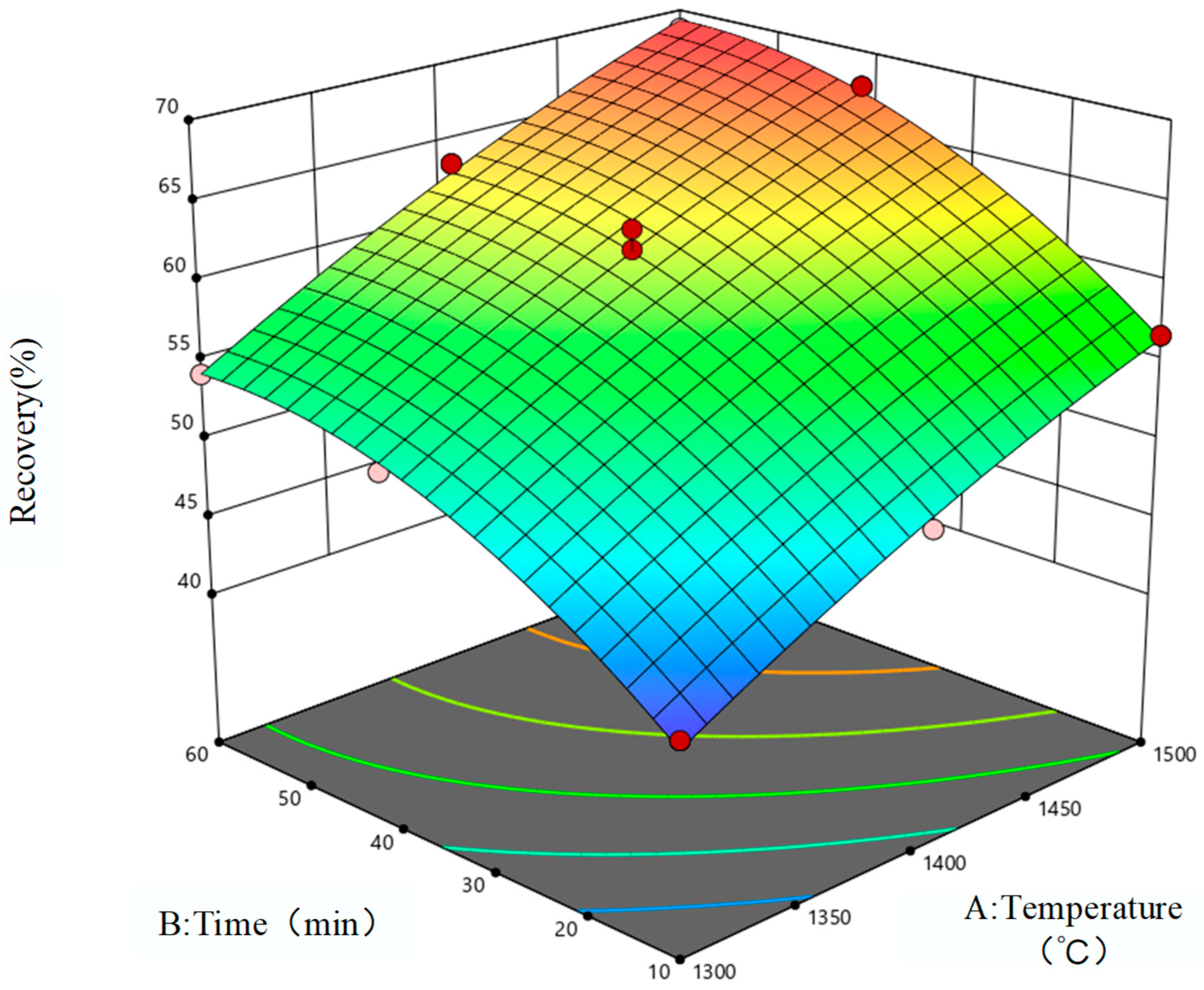
3.1. Response Surface Model
- Y: calculate model response parameters, %;
- A: reduction temperature, °C;
- B: reduction time, min.
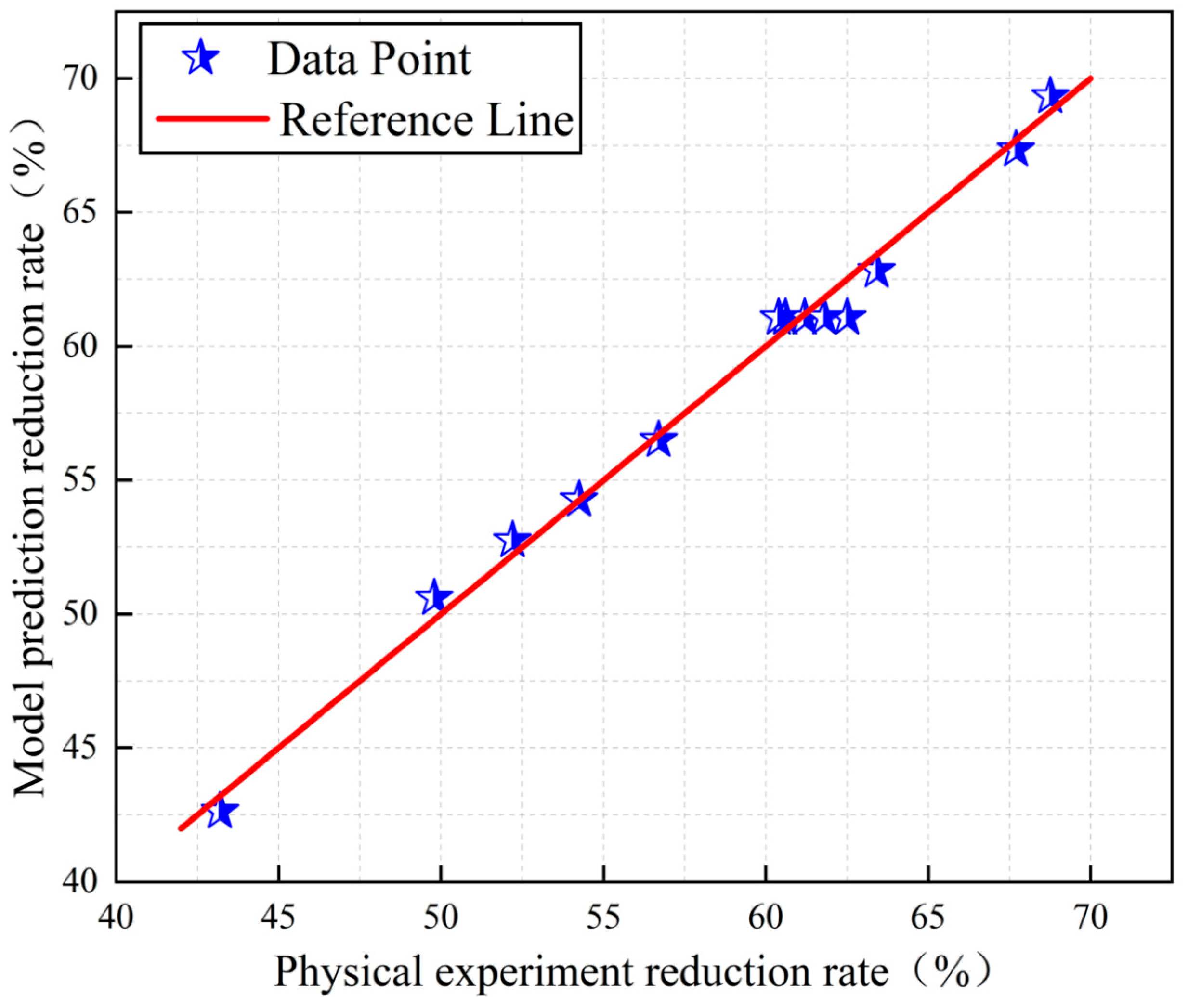
3.2. Response Surface Model Evaluation and Analysis
3.3. Model Optimization
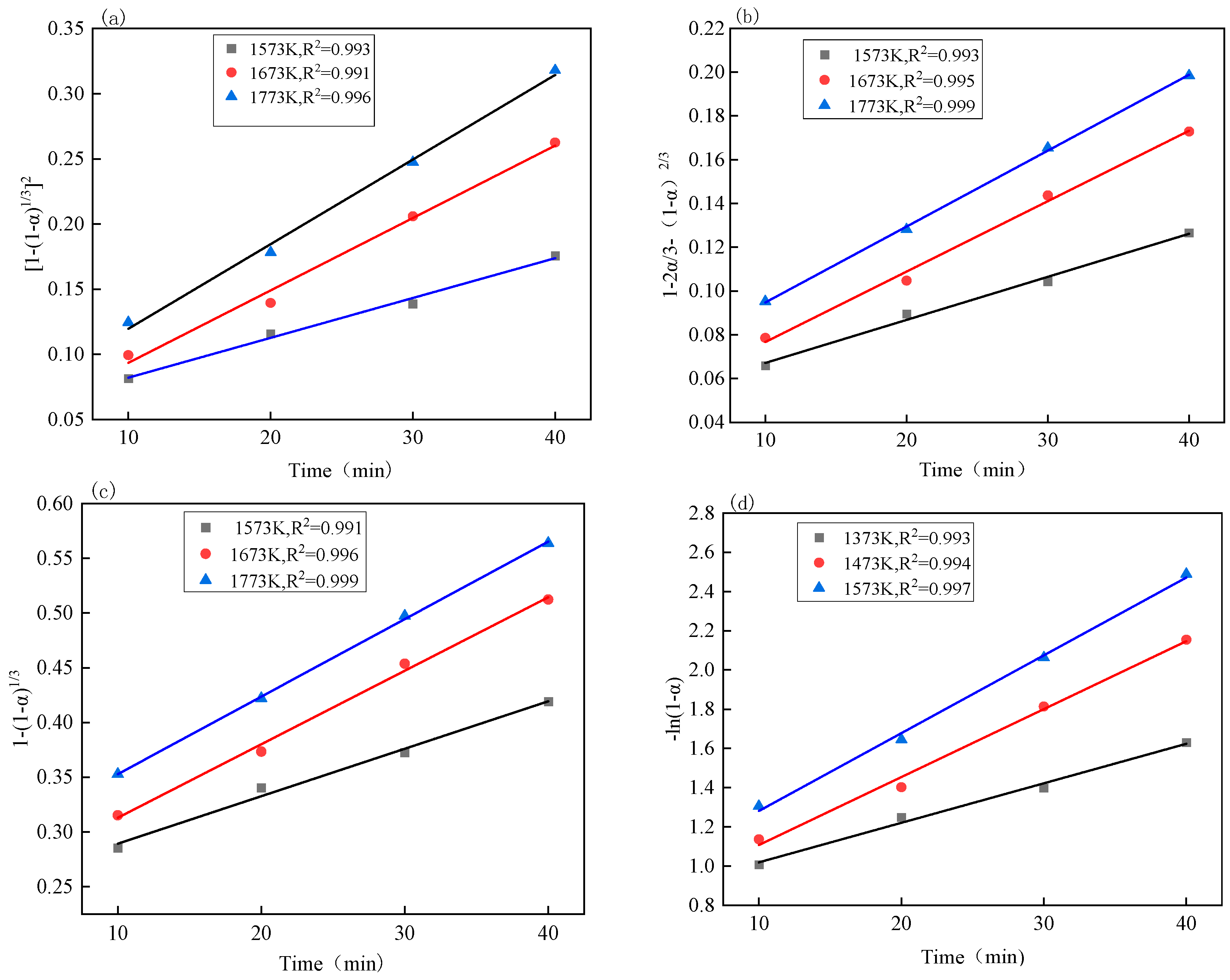
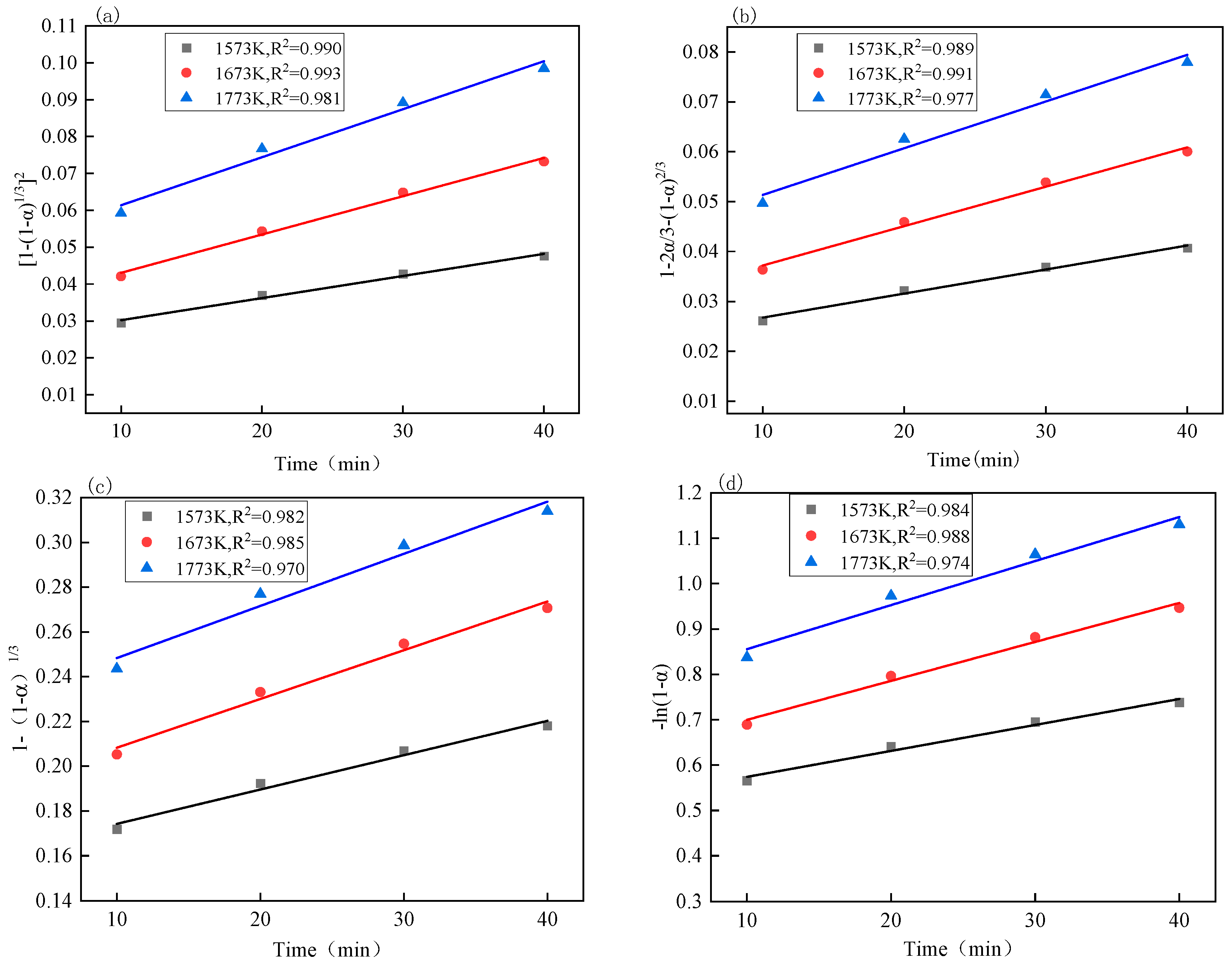
3.4. Reaction Kinetics Analysis
- : reaction mechanism function;
- : the integral form of the reaction mechanism function;
- : reaction time, ;
- : reaction rate constant, .
- A: pre-exponential factor, s−1;
- E: apparent activation energy, kJ/mol;
- R: standard molar gas constant, 8.314 J/(mol·K);
- T: temperature, K.
3.5. Analysis of Microscopic Characteristics of Reactive Slag
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zagainov, S.A.; Smirnov, L.A.; Zazhigaev, P.A.; Mironov, K.V.; Forshev, A.A. Improvement of Technology for Processing Vanadium-Containing Titanomagnetites. Steel Transl. 2021, 50, 882–887. [Google Scholar] [CrossRef]
- Zhang, L.H.; Yang, S.T.; Tang, W.D.; Yang, H.; Xue, X.X. Effect of coke breeze content on sintering mechanism and metallurgical properties of high-chromium vanadium-titanium magnetite. Ironmak. Steelmak. 2019, 47, 1–7. [Google Scholar] [CrossRef]
- Kurniawan, K.; Lee, H.; Bae, M.; Jong, W.C.; Sookyung, K. Intensified extraction of vanadium from vanadium-bearing titanomagnetite (VTM) concentrate via one-stage leaching and solvent extraction using acidic organophosphorus extractant. Miner. Eng. 2024, 217, 108961. [Google Scholar] [CrossRef]
- Ou, Y.; Sun, Y.S.; Yu, J.W.; Li, Y.J. Research status and development prospect of utilization of vanadium-titanium magnetite. J. Iron. Steel Res. 2021, 33, 267–278. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhao, H.Q.; Qi, Y.H.; Wang, F. Research and development for direct reduction of vanadium-bearing titanomagnetite. China. Metall. 2024, 34, 1–8+51. [Google Scholar] [CrossRef]
- Zhang, S.; Zong, Y.B.; Huang, Y.; Lei, D.; Zhang, J.L.; Gan, X.; Wang, Z.Y.; Zhang, S.S.; Lu, P.Y. Investigation of Basicity on Compressive Strength and Oxidation Induration Mechanism of Vanadium–Titanium Magnetite Pellets. Steel Res. Int. 2024, 95, 2400461. [Google Scholar] [CrossRef]
- Zhao, W.; Chu, M.S.; Liu, Z.G.; Wang, H.T.; Tang, J.; Ying, Z.W. High-Temperature Interactions Between Vanadium-Titanium Magnetite Carbon Composite Hot Briquettes and Pellets Under Simulated Blast Furnace Conditions. Metall. Mater. Trans. B 2019, 50, 1878–1895. [Google Scholar] [CrossRef]
- Gao, Y.J.; Liu, R.; Yan, G.S.; Tian, P.; Liu, Y.T.; Lan, C.C.; Lv, C. Analysing the HIsmelt process for smelting vanadium–titanium magnetite. Ironmak. Steelmak. 2023, 50, 1645–1651. [Google Scholar] [CrossRef]
- Liu, S.S.; Guo, Y.F.; Qiu, G.Z.; Jiang, T.; Cheng, F. Solid-state reduction kinetics and mechanism of pre-oxidized vanadium-titanium magnetite concentrate. Trans. Nonferrous Met. Soc. China 2014, 24, 3372–3377. [Google Scholar] [CrossRef]
- Chen, F.; Li, H.; Guo, Y.F.; Liu, K.; Wang, S.; Yang, L.Z.; Wen, Y.K.; Zheng, M. Reduction disintegration behavior and crystallographic transformation of vanadium–titanium magnetite pellets during hydrogen-based reduction. J. Mater. Res. Technol. 2025, 34, 152–163. [Google Scholar] [CrossRef]
- Shi, Q.; Tang, J.; Chu, M.S. Optimal Process Parameters for Direct Carbothermal Reduction of Vanadium–Titanium Magnetite in a Rotary Kiln. Steel Res. Int. 2023, 94, 1–12. [Google Scholar] [CrossRef]
- Zhan, R.N.; Guo, Z.Q.; Zhu, D.Q.; Pan, J.; Yang, C.C.; Huang, X.Z. Co-disposal of zinc-containing slag and dust based on coal-based rotary kiln direct reduction method. J. Iron. Steel Res. 2023, 35, 219–231. [Google Scholar] [CrossRef]
- Shi, Y.; Zhu, D.Q.; Pan, J.; Guo, Z.Q.; Lu, S.H.; Xue, Y.X. Investigation into the coal-based direct reduction behaviors of various vanadium titanomagnetite pellets. J. Mater. Res. Technol. 2022, 19, 243–262. [Google Scholar] [CrossRef]
- Cheng, G.J.; Liu, J.X.; Liu, Z.G.; Chu, M.S.; Xue, X.X. Non-isothermal reduction mechanism and kinetics of high chromium vanadium-titanium magnetite pellets. Ironmak. Steelmak. 2015, 42, 17–26. [Google Scholar] [CrossRef]
- Qin, J.; Wang, Y.; You, Z.X.; Wen, L.Y.; Lv, X.Y. Carbonization and nitridation of vanadium-bearing titanomagnetite during carbothermal reduction with coal. J. Mater. Res. Technol. 2020, 9, 4272–4282. [Google Scholar] [CrossRef]
- Dishwar, R.K.; Mandal, A.K.; Sinha, O.P. Studies on reduction behaviour of highly fluxed iron ore pellets for application in steelmaking. Mater. Today Proc. 2021, 46, 1471–1475. [Google Scholar] [CrossRef]
- Sarkar, B.K.; Dastidar, M.G.; Dey, R.; Das, G.C. A study on isothermal reduction kinetics of titaniferous magnetite ore using coke dust, an industrial waste. Can. Metall. Q. 2019, 58, 299–307. [Google Scholar] [CrossRef]
- Kumar, A.; Ramadas, H.; Kumar, S.C.; Nath, A.K. Laser polishing of additive manufactured stainless-steel parts by line focused beam: A response surface method for improving surface finish. J. Manuf. Process. 2025, 133, 1310–1328. [Google Scholar] [CrossRef]
- Belouettar, K.; Louar, A.M. Experimental investigation of the influence of electrical discharge machining process parameters using response surface methodology. Int. J. Adv. Manuf. Technol. 2024, 135, 5287–5299. [Google Scholar] [CrossRef]
- Wang, Z.M.; Lu, W.B. Machining accuracy reliability optimization of three-axis CNC machine tools using doubly-weighted vector projection response surface method. Int. J. Adv. Manuf. Technol. 2024, 132, 1019–1030. [Google Scholar] [CrossRef]
- Box, G.E.P.; Wilson, K.B. On the experimental attainment of optimum conditions. J. R. Stat. Soc. 1951, 13, 270–310. [Google Scholar] [CrossRef]
- Gullón, B.; Gullón, P.; Lú-Chau, T.A.; Moreiraet, M.T.; Lema, J.M.; Eibes, C. Optimization of sol-vent extraction of antioxidants from eucalyptus globulus leavesby response surface methodology: Characterization and assess-ment of their bioactive properties. Ind. Crop. Prod. 2017, 108, 649–659. [Google Scholar] [CrossRef]
- Rajendran, C.; Sonar, T.; Ivanov, M.; Kumar, P.S.; Amarnath, V.; Loknandham, R. Optimization of friction stir spot welding parameters for joining dissimilar AZ31B magnesium alloy and AA6061 aluminium alloy using response surface methodology. Int. J. Interact. Des. Manuf. (IJIDeM) 2023, 19, 115–126. [Google Scholar] [CrossRef]
- Alexandre, E.M.C.; Araújo, P.; Duarte, M.F.; Freitas, V.D.; Pintado, M.; Saraiva, J.A. Experimental design, modeling, and optimization of high-pressure-assisted extraction of bioactive compounds from pomegranate peel. Food Bioprocess Technol. 2017, 10, 886–900. [Google Scholar] [CrossRef]
- Lu, X.H.; Wang, F.R.; Wang, X.X.; Lu, Y.J.; Si, L.K. A surface roughness prediction model using response surface methodology in micro-milling Inconel 718. Int. J. Mach. Mach. Mater. 2017, 19, 230–245. [Google Scholar] [CrossRef]
- Teferi, T.F.; Kolhe, P.K.; Tsegaw, A.A.; Fetoba, O.S. Optimization of SiC reinforced Al7039/Cu composites for enhanced thermal conductivity and hardness via response surface methodology. Mater. Res. Express 2025, 12, 015501. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Q.; He, Q.; Hu, X.B. Application of Response Surface Methodology in Experiment Design and Optimization. Res. Explor. Lab. 2015, 34, 41–45. [Google Scholar] [CrossRef]
- Han, W.J.; Zhu, Y.M.; Li, W.B.; Liu, J.; Han, Y.X. Hydrogen reduction kinetics and microstructure evolution of Hainan Shilu iron ore during hydrogen-based ore phase transformation. J. Iron. Steel Res. Int. 2022, 34, 1352–1360. [Google Scholar] [CrossRef]
- Cheng, G.J.; Han, T.; Song, H.L.; Gao, M.L.; Yang, H.; Xue, X.X. Enhancing Reduction Separation and Efficient Recovery of Iron, Vanadium, and Titanium for Ultra-High-Titanium Magnetite. Metall. Mater. Trans. B 2024, 55, 287–300. [Google Scholar] [CrossRef]
- Yu, S.; Mao, R.; Wang, F.; Yao, H.W. Kinetics and reduction mechanism of high-basicity electric arc furnace dust by carbothermal reduction. J. Iron. Steel Res. Int. 2024, 36, 256–264. [Google Scholar] [CrossRef]
- Li, W.; Fu, G.Q.; Chu, M.S.; Zhu, M.Y. Oxidation induration process and kinetics of Hongge vanadium titanium-bearing magnetite pellets. Ironmak. Steelmak. 2017, 44, 294–303. [Google Scholar] [CrossRef]
- Pan, J.; Duan, Z.; Zhu, D.Q.; Guo, Z.Q.; Yang, C.C.; Cou, J.L. Kinetics of coal-based direct reduction of stainless steel dust pellets. J. Iron. Steel Res. Int. 2022, 34, 1067–1077. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, H.T.; Liu, Z.G.; Chu, M.S.; Ying, M.W.; Tang, J. Investigation of Reduction Mechanism and Kinetics of Vanadium Titanomagnetite Carbon Composite Hot Briquette at 1173–1373K. Steel Res. Int. 2017, 88, 1600306. [Google Scholar] [CrossRef]
- Zhao, W.; Chu, M.S.; Wang, H.T.; Liu, Z.G.; Tang, J.; Ying, Z.W. Volumetric Shrinkage Characteristics and Kinetics Analysis of Vanadium Titanomagnetite Carbon Composite Hot Briquette during Isothermal Reduction. ISIJ Int. 2018, 58, 823–832. [Google Scholar] [CrossRef]
- Bao, J.W.; Chu, M.S.; Tang, J.; Zhang, L.F. Isothermal Reduction Kinetics of the Mixture of Iron Carbon Agglomerates and Sinter: Regular Article. ISIJ Int. 2024, 64, 988–999. [Google Scholar] [CrossRef]
- Elzohiery, M.; Sohn, Y.H.; Mohassab, Y. Kinetics of Hydrogen Reduction of Magnetite Concentrate Particles in Solid State Relevant to Flash Ironmaking. Steel Res. Int. 2017, 88, 290–303. [Google Scholar] [CrossRef]
- Zhang, A.; Monaghan, J.B.; Longbottom, J.R.; Nusheh, M.; Bumby, C.W. Reduction Kinetics of Oxidized New Zealand Ironsand Pellets in H2 at Temperatures up to 1443K. Metall. Mater. Trans. B 2020, 51, 492–504. [Google Scholar] [CrossRef]
- Mohamed, E.; Deqiu, F.; Yousef, M.; Sohn, H.Y. Kinetics of hydrogen reduction of magnetite concentrate particles at 1623–1873 K relevant to flash ironmaking. Ironmak. Steelmak. 2021, 48, 485–492. [Google Scholar] [CrossRef]
- Yang, S.T.; Zhou, M.; Xue, X.X.; Jiang, T.; Sun, C.Y. Isothermal Reduction Kinetics of Chromium-Bearing Vanadium–Titanium Sinter Reduced with CO Gas at 1173K. JOM 2019, 71, 2812–2820. [Google Scholar] [CrossRef]
- Bai, Y.Y.; Ding, X.Y.; Qi, Y.H.; Ju, T.H.; Liu, J.S. Non-isothermal kinetics of mullite with Fe prepared by Sol-Gel method. J. Iron. Steel Res. Int. 2022, 34, 317–325. [Google Scholar] [CrossRef]
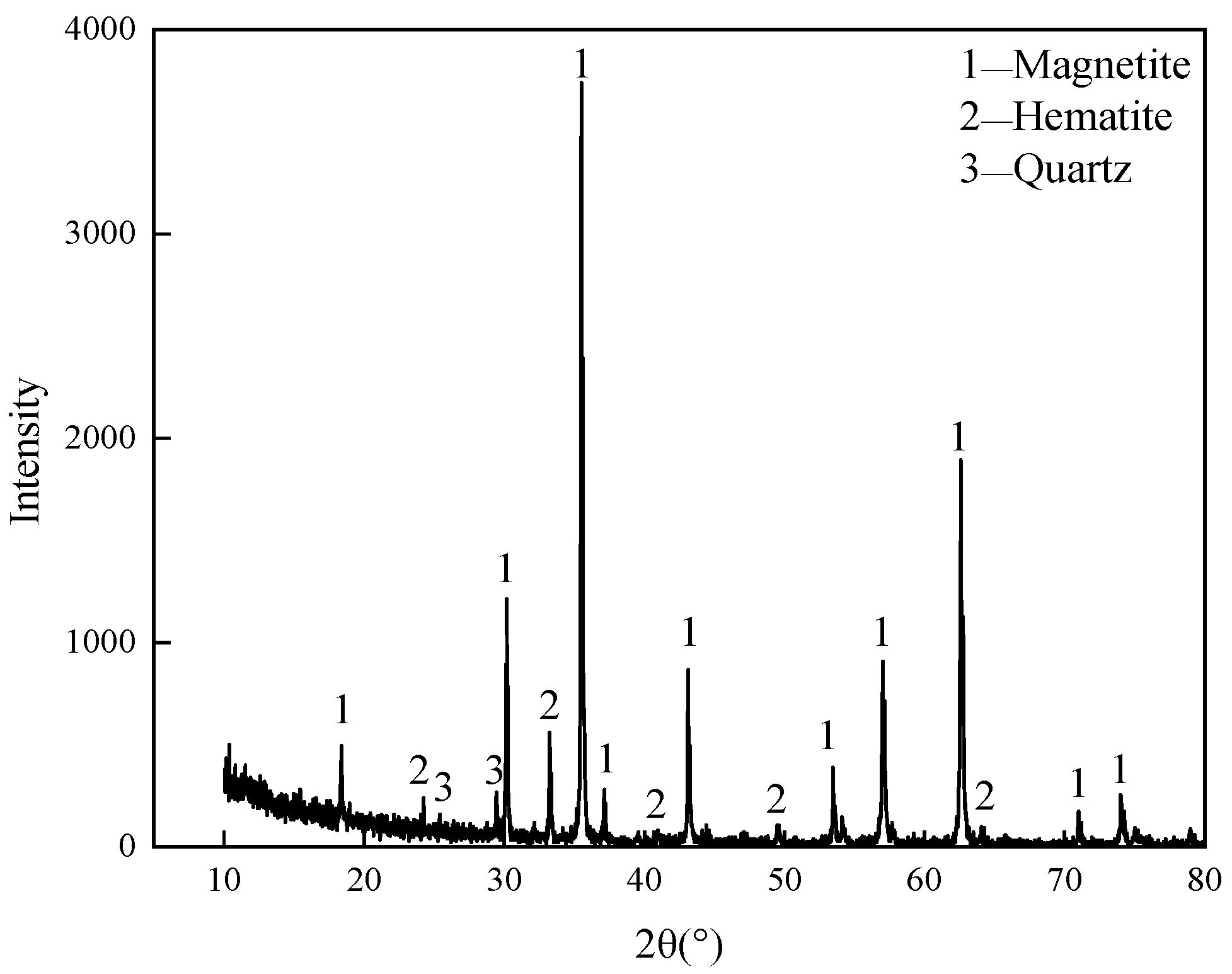





| Chemical Component | TFe | FeO | V2O5 | TiO2 | CaO | MgO | SiO2 | Al2O3 | MnO | Magnetite | Hematite | Quartz |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content/% | 67.07 | 26.18 | 0.362 | 0.557 | 0.715 | 0.828 | 2.06 | 1.24 | 0.306 | 90.53 | 2.81 | 0.46 |
| Name of Experimental Reagent | Producer | Reagent Status | Reagent Code |
|---|---|---|---|
| CaO | Sinopharm Group Chemical Reagent Co., Ltd., Shanghai, China | Powder form | 10005928 |
| Sodium carboxymethyl cellulose | 30036328 | ||
| C | C134372500 |
| Factors | Unit | Lower Limit | Upper Limit |
|---|---|---|---|
| Reduction temperature | °C | 1300 | 1500 |
| Reduction time | min | 10 | 60 |
| Number | Experimental Variable | Reduction Rate of Fe (%) | Reduction Rate of V (%) | |
|---|---|---|---|---|
| Reduction Temperature (°C) | Reduction Time (min) | |||
| 1 | 1300 | 10 | 63.5 | 43.2 |
| 2 | 1500 | 10 | 72.9 | 56.7 |
| 3 | 1300 | 60 | 82.3 | 54.2 |
| 4 | 1500 | 60 | 91.4 | 68.7 |
| 5 | 1300 | 40 | 80.4 | 52.2 |
| 6 | 1500 | 40 | 91.7 | 67.7 |
| 7 | 1400 | 10 | 67.9 | 49.8 |
| 8 | 1400 | 60 | 90.1 | 63.4 |
| 9 | 1400 | 40 | 88.4 | 61.2 |
| 10 | 1400 | 40 | 87.6 | 60.8 |
| 11 | 1400 | 40 | 89.4 | 62.5 |
| 12 | 1400 | 40 | 88.7 | 60.4 |
| 13 | 1400 | 40 | 86.9 | 60.6 |
| Reducing Medium | Response Parameter | Prediction Model Expression |
|---|---|---|
| C | Iron oxide reduction rate | 86.41 + 4.97A + 9.93B − 0.0043AB − 1.83A2 − 7.16B2 |
| Vanadium oxide reduction rate | 59.97 + 7.23A + 6.12B + 0.2984AB − 1.02A2 − 3.27B2 |
| Reducing Medium | Response Parameter | Mean | Standard Deviation | CV % | R2 | Adjusted R2 | Predicted R2 |
|---|---|---|---|---|---|---|---|
| C | Iron oxide reduction rate | 83.17 | 1.18 | 1.42 | 0.9908 | 0.9842 | 0.9331 |
| Vanadium oxide reduction rate | 58.58 | 0.8356 | 1.43 | 0.9921 | 0.9865 | 0.9572 |
| Response | Source | Sum of Square | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|---|
| C-Fe | Model | 1043.78 | 5 | 208.76 | 150.05 | <0.0001 |
| A-Temperature | 147.15 | 1 | 147.15 | 150.77 | <0.0001 | |
| B-Time | 591.23 | 1 | 591.23 | 424.97 | <0.0001 | |
| AB | 0.0001 | 1 | 0.0001 | 0.0001 | 0.9944 | |
| A2 | 9.23 | 1 | 9.23 | 6.63 | 0.0367 | |
| B2 | 128.27 | 1 | 128.27 | 92.20 | <0.0001 | |
| Residual | 9.74 | 7 | 1.39 | |||
| Lack of fit | 5.96 | 3 | 1.99 | 2.10 | 0.2427 | |
| Pure error | 3.78 | 4 | 0.9450 | |||
| C-V | Model | 616.41 | 5 | 123.28 | 176.19 | <0.0001 |
| A-Temperature | 311.74 | 1 | 311.74 | 445.52 | <0.0001 | |
| B-Time | 224.73 | 1 | 224.73 | 321.16 | <0.0001 | |
| AB | 0.3608 | 1 | 0.3608 | 0.5156 | 0.4960 | |
| A2 | 2.86 | 1 | 2.86 | 4.09 | 0.0827 | |
| B2 | 26.73 | 1 | 26.73 | 38.20 | 0.0005 | |
| Residual | 4.90 | 7 | 0.6997 | |||
| Lack of fit | 2.10 | 3 | 0.6994 | 0.9991 | 0.4793 | |
| Pure error | 2.80 | 4 | 0.7000 |
| Number | Function Name | Reaction Mechanism | Integral Form | Differential Form |
|---|---|---|---|---|
| 1 | Jander equation | Three-dimensional diffusion | ||
| 2 | G-B equation | Three-dimensional diffusion | ||
| 3 | Shrink ball (volume) | Interfacial reaction | ||
| 4 | First-order reaction | Gasification reaction |
| Kinetic Equation | Iron Oxide: Apparent Activation Energy (kJ/mol) | Vanadium Oxide: Apparent Activation Energy (kJ/mol) |
|---|---|---|
| 86.76 | 90.30 | |
| 65.38 | 78.08 | |
| 66.34 | 50.28 | |
| 80.54 | 62.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Wu, H.; Deng, A. Prediction of Reduction Recovery of Iron and Vanadium Oxides in Low-Grade Vanadium–Titanium Magnetite Based on Response Surface Model. Metals 2025, 15, 595. https://doi.org/10.3390/met15060595
Jiang X, Wu H, Deng A. Prediction of Reduction Recovery of Iron and Vanadium Oxides in Low-Grade Vanadium–Titanium Magnetite Based on Response Surface Model. Metals. 2025; 15(6):595. https://doi.org/10.3390/met15060595
Chicago/Turabian StyleJiang, Xueting, Hao Wu, and Aijun Deng. 2025. "Prediction of Reduction Recovery of Iron and Vanadium Oxides in Low-Grade Vanadium–Titanium Magnetite Based on Response Surface Model" Metals 15, no. 6: 595. https://doi.org/10.3390/met15060595
APA StyleJiang, X., Wu, H., & Deng, A. (2025). Prediction of Reduction Recovery of Iron and Vanadium Oxides in Low-Grade Vanadium–Titanium Magnetite Based on Response Surface Model. Metals, 15(6), 595. https://doi.org/10.3390/met15060595






