Experimental Investigation and Molecular Dynamics Modeling of the Effects of K2O on the Structure and Viscosity of SiO2-CaO-Al2O3-MgO-K2O Slags at High Temperatures
Abstract
1. Introduction
2. Experimental Part
2.1. Slag Sample Preparation
2.2. Molecular Dynamics Simulation
2.3. Viscosity Test
2.4. Slag Structural Analysis
3. Results and Discussion
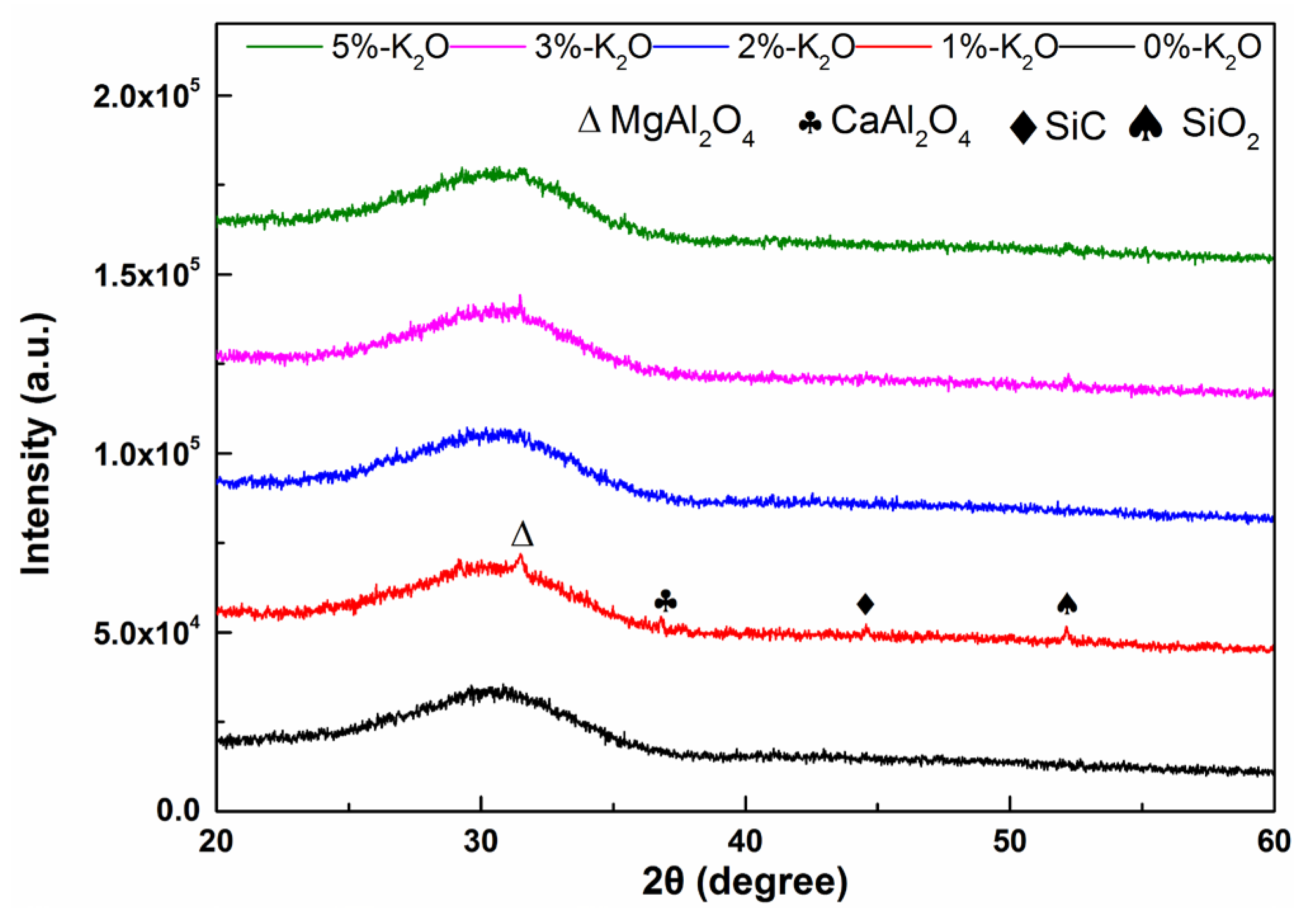
3.1. XRD Analysis
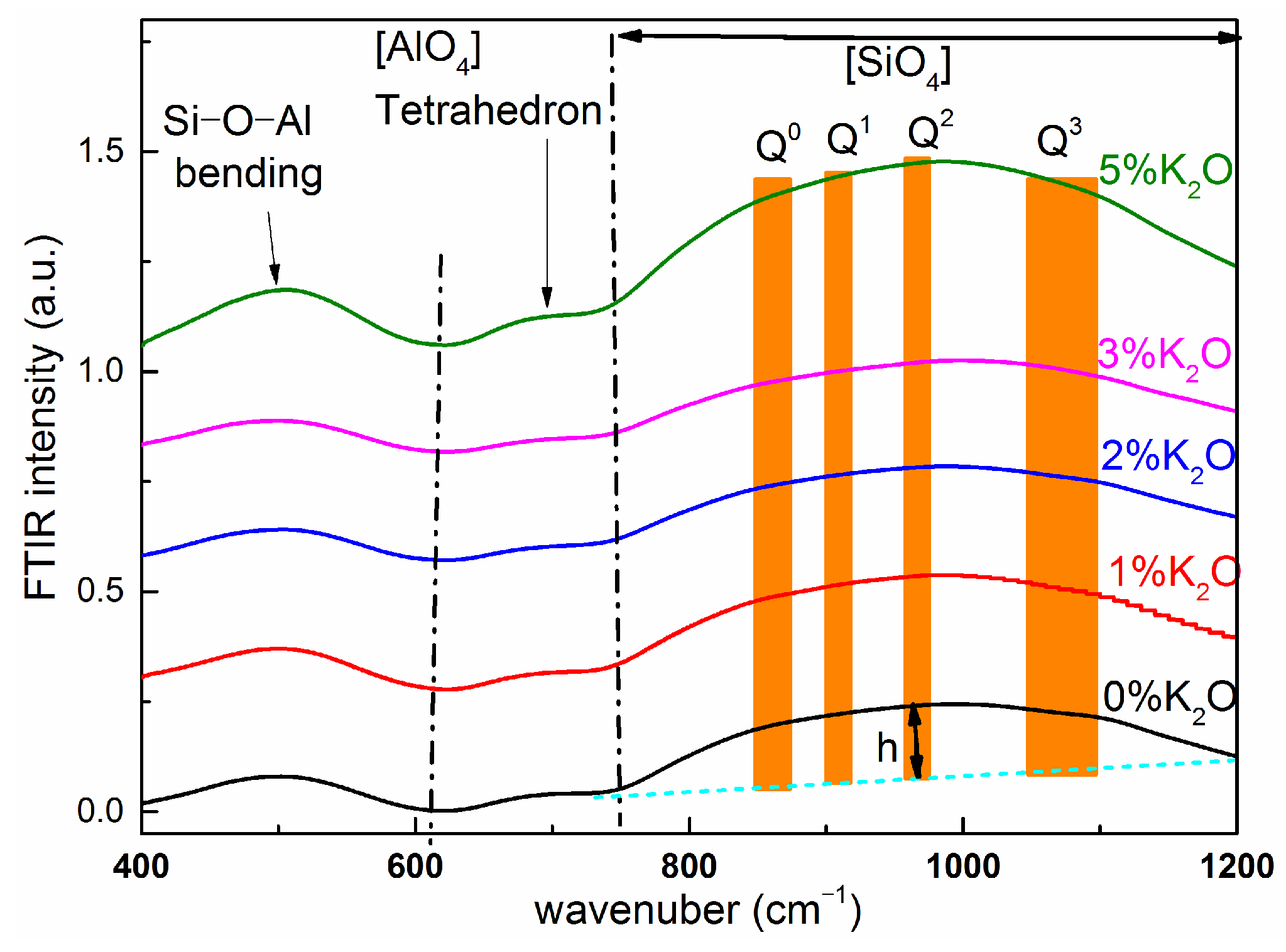
3.2. FTIR Spectroscopic Analysis
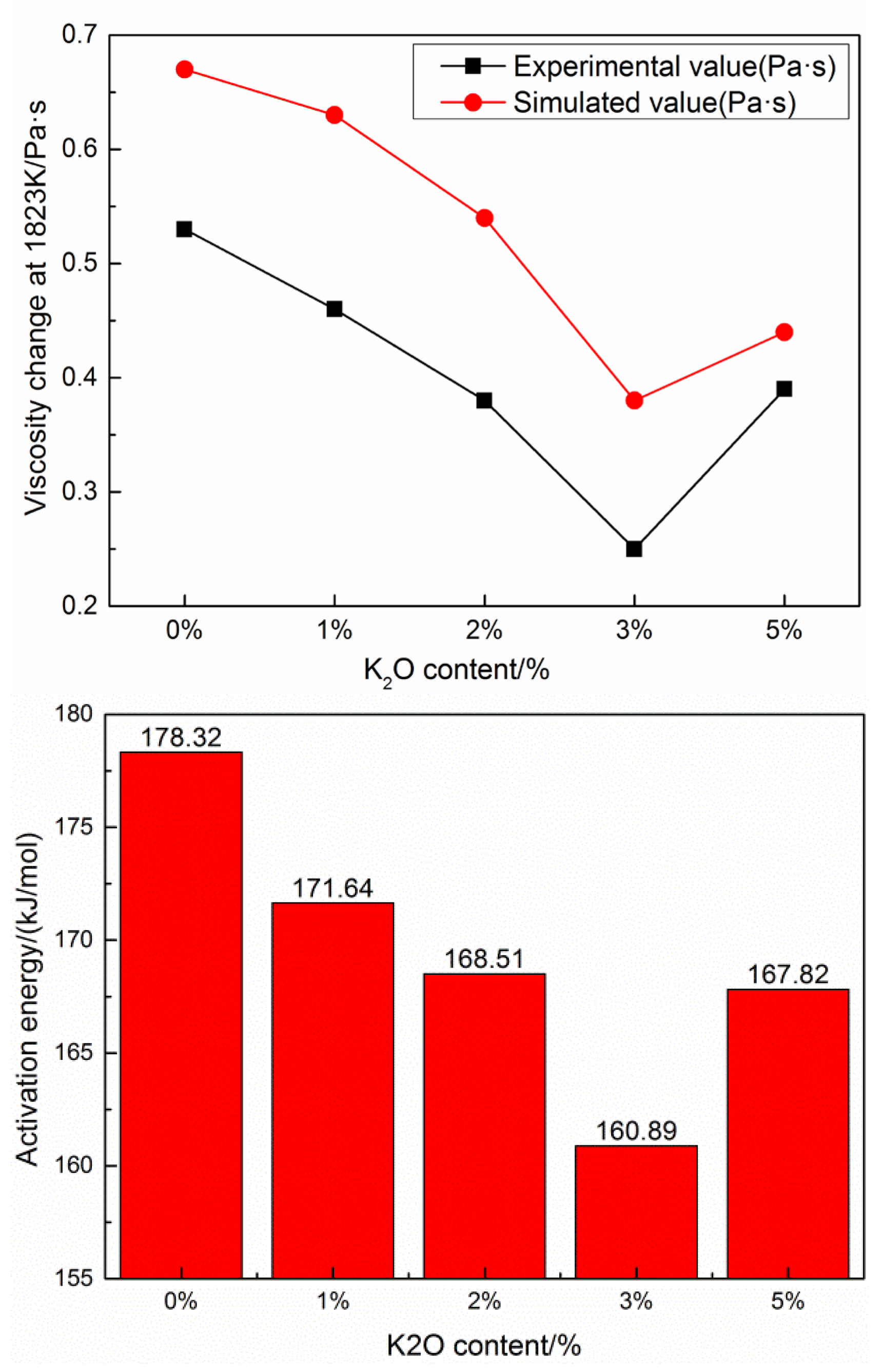
3.3. Viscosity Change
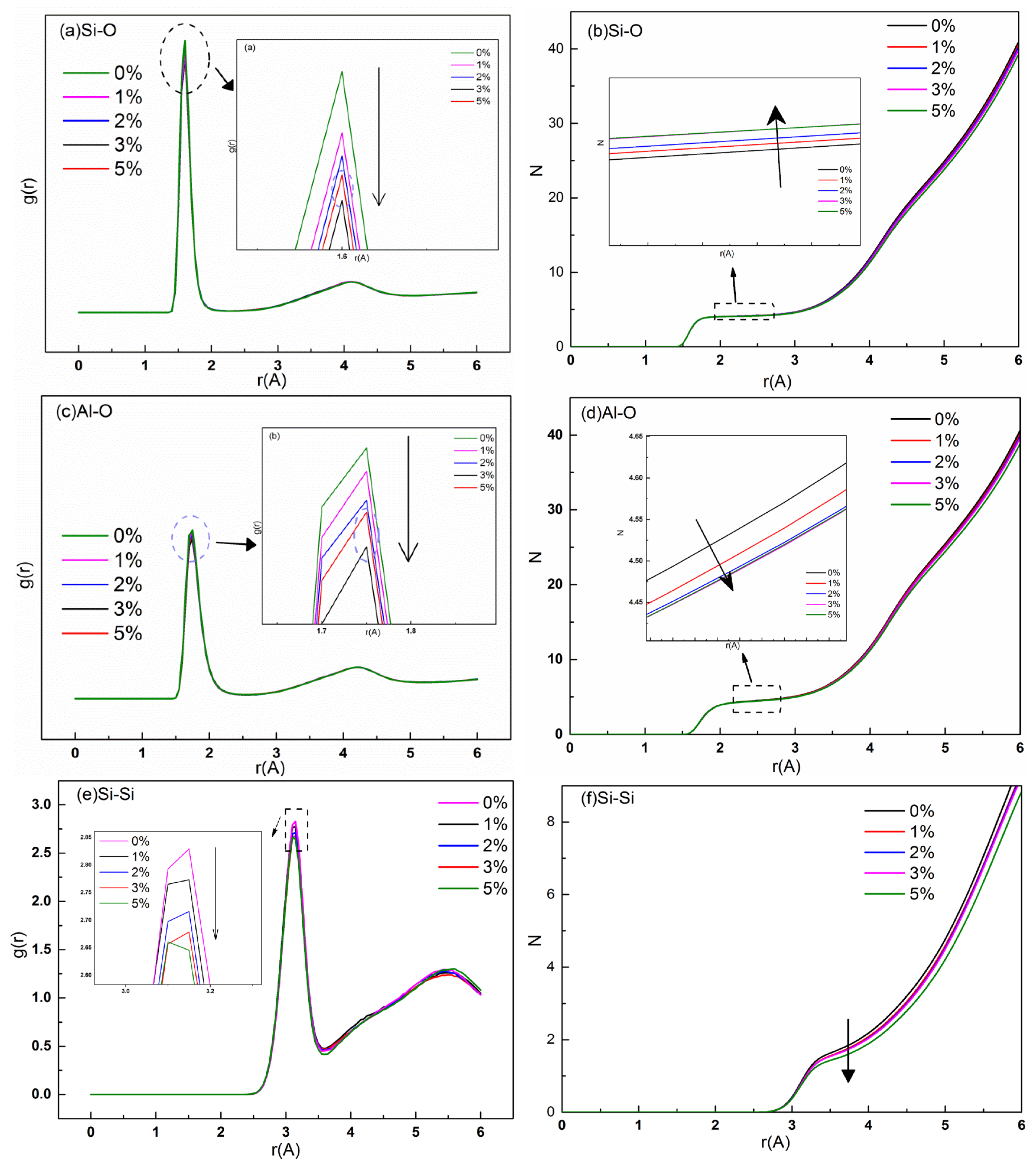
3.4. Radial Distribution Functions (RDFs) and Coordination Numbers (CNs)
3.5. Mean Square Displacements (MSDs) and [SiO4] Tetrahedral Population Variations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Partyka, J.; Sitarz, M.; Leśniak, M.; Gasek, K.; Jeleń, P. The effect of SiO2/Al2O3 ratio on the structure and microstructure of the glazes from SiO2-Al2O3-CaO-MgO-Na2O-K2O system. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 134, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Meng, Z.; Liu, L.; Wang, H.; Sun, Y.; Wang, X. Structural and Viscous Insight into Impact of MoO3 on Molten Slags. Metall. Mater. Trans. B 2021, 52, 3730–3743. [Google Scholar] [CrossRef]
- Kim, H.; Kim, W.H.; Park, J.H.; Min, D.J. A Study on the Effect of Na₂O on the Viscosity for Ironmaking Slags. Steel Res. Int. 2010, 81, 17–24. [Google Scholar] [CrossRef]
- Blenau, L.W.; Sander, S.A.; Fuhrmann, S.; Charitos, A. Holistic valorization of fayalitic slag to pig iron and glass fibers. J. Clean. Prod. 2023, 396, 136575. [Google Scholar] [CrossRef]
- Li, Y.; Dai, W.-B. Modifying hot slag and converting it into value-added materials: A review. J. Clean. Prod. 2018, 175, 176–189. [Google Scholar] [CrossRef]
- Tesovnik, A.; Horvat, B. Rapid Immobilisation of Chemical Reactions in Alkali-Activated Materials Using Solely Microwave Irradiation. Minerals 2024, 14, 1219. [Google Scholar] [CrossRef]
- Elzeadani, M.; Bompa, D.V.; Elghazouli, A.Y. One part alkali activated materials: A state-of-the-art review. J. Build. Eng. 2022, 57, 104871. [Google Scholar] [CrossRef]
- Kupczewska-Dobecka, M.; Konieczko, K.; Czerczak, S. Occupational risk resulting from exposure to mineral wool when installing insulation in buildings. Int. J. Occup. Med. Environ. Health 2020, 33, 757–769. [Google Scholar] [CrossRef]
- Rawlings, R.D.; Wu, J.P.; Boccaccini, A.R. Glass-ceramics: Their production from wastes—A Review. J. Mater. Sci. 2006, 41, 729–744. [Google Scholar] [CrossRef]
- Jin, Z.N.; Lv, J.F.; Yang, H.Y. Effect of K₂O on Phase Relation and Viscosity of the CaO–SiO2—ZnO—FeO–Al2O3 System Slags. Russ. J. Non-Ferr. Met. 2020, 61, 153–161. [Google Scholar]
- Chang, Z.Y.; Jiao, K.X.; Ning, X.J.; Zhang, J.L. Novel Approach to Studying Influences of Na2O and K2O Additions on Viscosity and Thermodynamic Properties of BF Slags. Metall. Mater. Trans. B 2019, 50, 1399–1406. [Google Scholar] [CrossRef]
- Perez-Pariente, J.; Martens, J.A.; Jacobs, P.A. Crystallization mechanism of zeolite beta from (TEA)2O, Na2O and K2O containing aluminosilicate gels. Appl. Catal. 1987, 31, 35–64. [Google Scholar] [CrossRef]
- Michalski, J.R.; Kraft, M.D.; Sharp, T.G.; Williams, L.B. Mineralogical constraints on the high-silica martian surface component observed by TES. Icarus 2005, 174, 161–177. [Google Scholar] [CrossRef]
- Klima, K.M.; Luo, Y.; Brouwers, H.J.H.; Yu, Q. Effects of mineral wool waste in alkali activated-artificial aggregates for high-temperature applications. Constr. Build. Mater. 2023, 401, 132937. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Chen, Y.; Çopuroğlu, O. The effect of slag chemistry on the reactivity of synthetic and commercial slags. Constr. Build. Mater. 2022, 335, 127493. [Google Scholar] [CrossRef]
- Koroleva, O.N.; Shtenberg, M.V.; Osipov, A.A. Structural Features of K2O-SiO2 Melts: Modeling and High-Temperature Experiments. Minerals 2023, 13, 94. [Google Scholar] [CrossRef]
- Xuan, W.; Guhl, S.; Zhang, Y.; Zhang, J.; Meyer, B. A Structural Viscosity Model for Silicate Slag Melts Based on MD Simulation. Metals 2022, 12, 1123. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Wang, G. Influence of MgO on Viscosity and Structure of CaO-SiO2-MgO-Al2O3 Slags. Metals 2021, 11, 789–796. [Google Scholar]
- Higo, T.; Sukenaga, S.; Kanehashi, K.; Shibata, H.; Osugi, T.; Saito, N.; Nakashima, K. Effect of Potassium Oxide Addition on Viscosity of Calcium Aluminosilicate Melts at 1673–1873 K. ISIJ Int. 2014, 54, 2039–2044. [Google Scholar] [CrossRef]
- Kim, W.H.; Sohn, I.; Min, D.J. A Study on the Viscous Behaviour with K2O Additions in the CaO-SiO2-Al2O3-MgO-K2O Quinary Slag System. Steel Res. Int. 2010, 81, 735–741. [Google Scholar] [CrossRef]
- Liu, W.; Zuo, H. Mixed influence of Na2O and K2O on the viscous properties of aluminosilicate melt. Ceram. Int. 2025, in press. [CrossRef]
- He, C.; Fan, F.F.; Guo, J.; Yuan, M.; Qin, Y.; Wei, Y.; Yan, J. Viscosity determination of the biomass slag in the SiO2-CaO-K2O system based on the bond distribution. Fuel 2024, 356, 129642. [Google Scholar] [CrossRef]
- Xing, X.; Pang, Z.; Zheng, J.; Du, Y.; Ren, S.; Ju, J. Effect of MgO and K₂O on High-Al Silicon–Manganese Alloy Slag Viscosity and Structure. Minerals 2020, 10, 810. [Google Scholar] [CrossRef]
- Wu, G.; Yazhenskikh, E.; Hack, K.; Wosch, E.; Müller, M. Viscosity model for oxide melts relevant to fuel slags. Part 1: Pure oxides and binary systems in the system SiO2–Al2O3–CaO–MgO–Na2O–K2O. Fuel Process. Technol. 2015, 137, 93–103. [Google Scholar] [CrossRef]
- Fang, J.; Pang, Z.; Xing, X.; Xu, R. Thermodynamic Properties, Viscosity, and Structure of CaO-SiO2-MgO-Al2O3-TiO2-Based Slag. Materials 2020, 14, 124. [Google Scholar] [CrossRef]
- Jak, E. Viscosity Model for Slags in the Al2O3-CaO-FeO-K2O-Na2O-MgO-SiO2 System. In Proceedings of the VIII International Conference on Molten Slags, Fluxes and Salts, Santiago, Chile, 18–21 January 2009; pp. 1–10. [Google Scholar]
- Wu, G.; Yazhenskikh, E.; Hack, K.; Müller, M. Viscosity model for oxide melts relevant to fuel slags. Part 2: The system SiO2–Al2O3–CaO–MgO–Na2O–K2O. Fuel Process. Technol. 2015, 138, 520–533. [Google Scholar] [CrossRef]
- Heikkilä, A.; Iljana, M.; Heikkinen, E.; Koskela, A.; Fabritius, T. Effect of Coal and Coke Ash on Blast Furnace Slag Properties: A Comparison Between Pulverized Coal, Charcoal, Fossil-Based Coke, and Biocoke. Steel Res. Int. 2022, 93, 2100188. [Google Scholar] [CrossRef]
- Mysen, B.O.; Virgo, D.; Harrison, W.J.; Scarfe, C.M. Solubility mechanisms of H₂O in silicate melts at high pressures and temperatures; a Raman spectroscopic study. Am. Mineral. 1980, 65, 900–914. [Google Scholar]
- Mysen, B.O.; Virgo, D.; Seifert, F.A. The structure of silicate melts: Implications for chemical and physical properties of natural magma. Rev. Geophys. 1982, 20, 353–383. [Google Scholar] [CrossRef]
- Belashchenko, D.; Ostrovski, O. Computer Simulation of Noncrystalline Ionic-Covalent Oxides in the SiO2-CaO-FeO System. Inorg. Mater. 2002, 38, 799–804. [Google Scholar] [CrossRef]
- Belashchenko, D.; Skvortsov, L. Molecular Dynamics Study of CaO-Al2O3 Melts. Inorg. Mater. 2001, 37, 476–481. [Google Scholar] [CrossRef]
- Bauchy, M. Structural, vibrational, and elastic properties of a calcium aluminosilicate glass from molecular dynamics simulations: The role of the potential. J. Chem. Phys. 2014, 141, 024507. [Google Scholar] [CrossRef] [PubMed]
- Bouhadja, M.; Jakse, N.; Pasturel, A. Stokes-Einstein violation and fragility in calcium aluminosilicate glass formers: A molecular dynamics study. Mol. Simul. 2014, 40, 251–259. [Google Scholar] [CrossRef]
- Sukenaga, S.; Saito, N.; Kawakami, K.; Nakashima, K. Viscosities of CaO-SiO2-Al2O3-(R2O or RO) Melts. ISIJ Int. 2006, 46, 352–358. [Google Scholar] [CrossRef]
- Liu, W.; Qin, J.; Xing, X.; Wang, J.; Zuo, H. Viscosity and Structure Evolution of CaO-SiO2-MgO-Al2O3-BaO Slag with the CaO/SiO2 Mass Ratio of 0.9. Ceram. Int. 2021, 47, 33337–33343. [Google Scholar] [CrossRef]
- Zhou, X.P.; Cheng, H.F.; Wang, Y. FTIR spectroscopic study of montmorillonite with different exchanged cations. J. Non-Cryst. Solids 2023, 598, 121543. [Google Scholar]
- Lodesani, F.; Menziani, M.C.; Hijiya, H.; Takato, Y.; Urata, S.; Pedone, A. Structural origins of the Mixed Alkali Effect in Alkali Aluminosilicate Glasses: Molecular Dynamics Study and its Assessment. Sci. Rep. 2020, 10, 2906. [Google Scholar] [CrossRef]
- Halász, I.; Agarwal, M.; Li, R.; Miller, N. What can vibrational spectroscopy tell about the structure of dissolved sodium silicates? Microporous Mesoporous Mater. 2010, 135, 74–81. [Google Scholar] [CrossRef]
- Dalby, K.; Topsøe, H.; King, P.L. A new approach to determine and quantify structural units in silicate glasses using micro Fourier-Transform Infrared spectroscopy. Am. Mineral. 2006, 91, 1783–1793. [Google Scholar] [CrossRef]
- Yang, F.; Han, Y.; Zhu, L. Analysis of basic network structure of continuous casting mould powder in electromagnetic field. Ironmak. Steelmak. 2019, 46, 827–834. [Google Scholar] [CrossRef]







| No. | CaO | SiO2 | MgO | Al2O3 | K2O | R |
|---|---|---|---|---|---|---|
| 1 | 42.63 | 32.29 | 10 | 15 | 0 | 1.32 |
| 2 | 41.79 | 31.66 | 10 | 15 | 1 | 1.32 |
| 3 | 41.53 | 31.46 | 10 | 15 | 2 | 1.32 |
| 4 | 40.68 | 30.99 | 10 | 15 | 3 | 1.32 |
| 5 | 39.79 | 30.14 | 10 | 15 | 5 | 1.32 |
| Ionic | Ionic | Aij | Bij | Cij | Dij |
|---|---|---|---|---|---|
| K | K | 5.0665 × 10−20 | 6.25 | 1.5632 × 10−25 | 0 |
| K | Ca | 1.6346 × 10−20 | 6.25 | 1.0421 × 10−25 | 0 |
| Ca | Ca | 5.2737 × 10−22 | 6.25 | 6.9467 × 10−26 | 0 |
| Mg | K | 3.3626 × 10−21 | 6.25 | 2.0843 × 10−26 | 0 |
| Mg | Ca | 1.0849 × 10−21 | 6.25 | 1.3895 × 10−26 | 0 |
| Si | K | 1.3251 × 10−21 | 6.25 | 0 | 0 |
| Mg | Mg | 2.2318 × 10−22 | 6.25 | 2.7791 × 10−27 | 0 |
| Si | Ca | 4.2751 × 10−22 | 6.25 | 0 | 0 |
| Al | K | 1.8339 × 10−21 | 6.25 | 0 | 0 |
| Mg | Si | 8.7947 × 10−23 | 6.25 | 0 | 0 |
| Al | Ca | 5.9169 × 10−20 | 6.25 | 0 | 0 |
| Si | Si | 3.4656 × 10−23 | 6.25 | 0 | 0 |
| O | K | 3.4457 × 10−20 | 6.06 | 2.0843 × 10−25 | 0 |
| Mg | Al | 1.2172 × 10−22 | 6.25 | 0 | 0 |
| O | Ca | 1.1505 × 10−20 | 6.06 | 1.38950 × 10−25 | 0 |
| Al | Si | 4.7966 × 10−23 | 6.25 | 0 | 0 |
| O | Mg | 2.4829 × 10−21 | 6.06 | 2.7791 × 10−26 | 0 |
| Al | Al | 6.3869 × 10−23 | 6.25 | 0 | 0 |
| O | Si | 1.0064 × 10−21 | 6.06 | 0 | 0 |
| O | Al | 1.3792 × 10−21 | 6.06 | 0 | 0 |
| O | O | 2.3993 × 10−20 | 5.88 | 2.7791 × 10−21 | 0 |
| No. | Particle Count | Box Side Length/A | Density | |||||
|---|---|---|---|---|---|---|---|---|
| Si | Al | Ca | K | Mg | O | |||
| 1 | 802 | 464 | 1074 | 0 | 287 | 3374 | 42.291 | 3.052 |
| 2 | 793 | 465 | 1061 | 34 | 288 | 3360 | 42.353 | 3.047 |
| 3 | 783 | 465 | 1049 | 67 | 288 | 3347 | 42.416 | 3.041 |
| 4 | 774 | 466 | 1036 | 101 | 289 | 3334 | 42.484 | 3.035 |
| 5 | 754 | 467 | 1010 | 172 | 291 | 3306 | 42.614 | 3.024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Xue, Q.; Zuo, H.; Liu, Y.; Wang, J. Experimental Investigation and Molecular Dynamics Modeling of the Effects of K2O on the Structure and Viscosity of SiO2-CaO-Al2O3-MgO-K2O Slags at High Temperatures. Metals 2025, 15, 590. https://doi.org/10.3390/met15060590
Yang F, Xue Q, Zuo H, Liu Y, Wang J. Experimental Investigation and Molecular Dynamics Modeling of the Effects of K2O on the Structure and Viscosity of SiO2-CaO-Al2O3-MgO-K2O Slags at High Temperatures. Metals. 2025; 15(6):590. https://doi.org/10.3390/met15060590
Chicago/Turabian StyleYang, Fan, Qingguo Xue, Haibin Zuo, Yu Liu, and Jingsong Wang. 2025. "Experimental Investigation and Molecular Dynamics Modeling of the Effects of K2O on the Structure and Viscosity of SiO2-CaO-Al2O3-MgO-K2O Slags at High Temperatures" Metals 15, no. 6: 590. https://doi.org/10.3390/met15060590
APA StyleYang, F., Xue, Q., Zuo, H., Liu, Y., & Wang, J. (2025). Experimental Investigation and Molecular Dynamics Modeling of the Effects of K2O on the Structure and Viscosity of SiO2-CaO-Al2O3-MgO-K2O Slags at High Temperatures. Metals, 15(6), 590. https://doi.org/10.3390/met15060590






