Effect of Aluminum Content on Solidification Process and Microsegregation of δ-TRIP Steel
Abstract
1. Introduction
2. Thermodynamic Simulations
2.1. Thermodynamic Calculation
2.2. Microsegregation Model
2.3. Calculation Parameters of Microsegregation Model
2.3.1. Secondary Dendrite Spacing λSDAS and Local Solidification Time tf
2.3.2. Relationship Between fs and T
2.3.3. Equilibrium Partition Coefficient, ki
2.3.4. Diffusion Coefficients
3. Result and Discussion
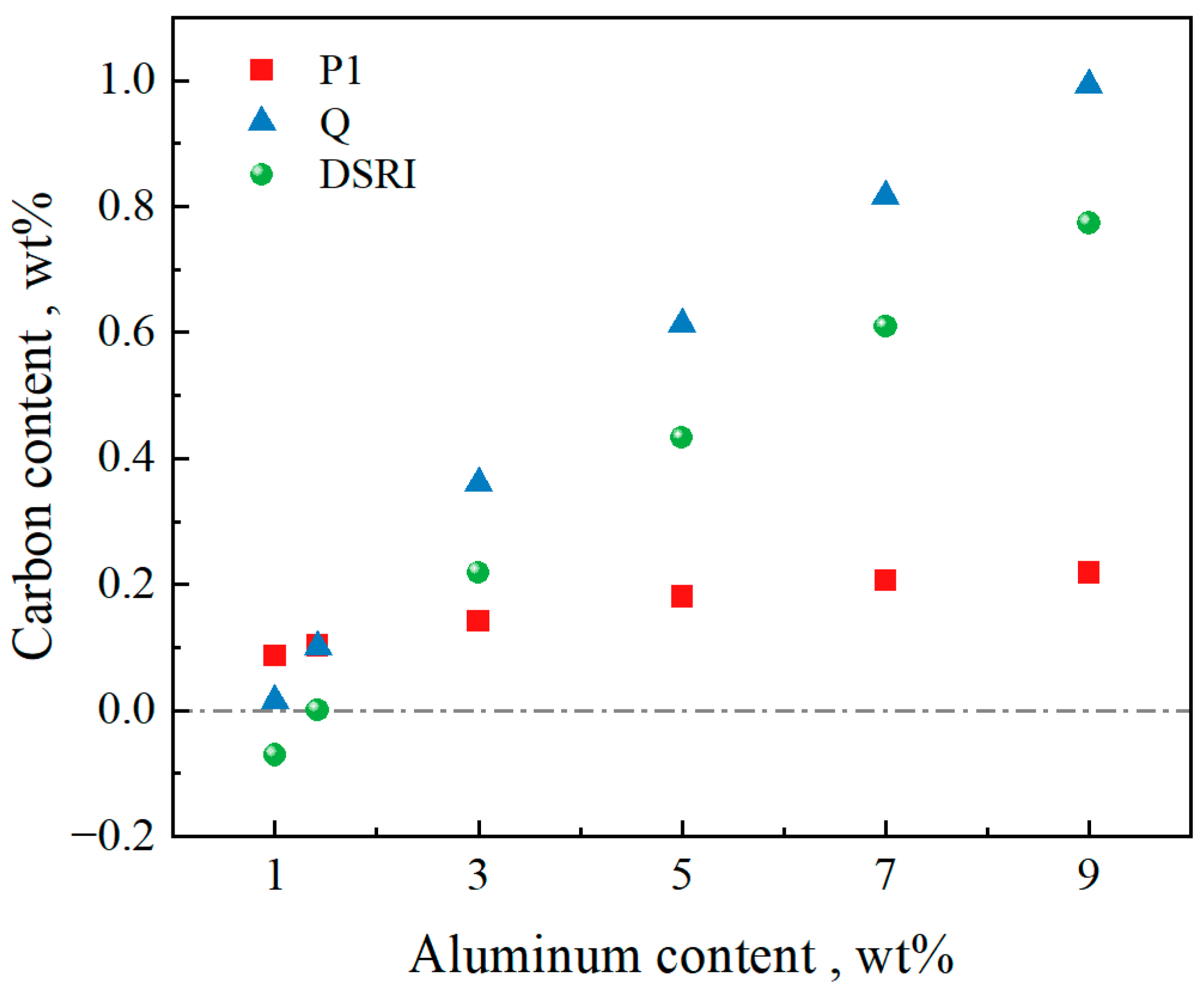
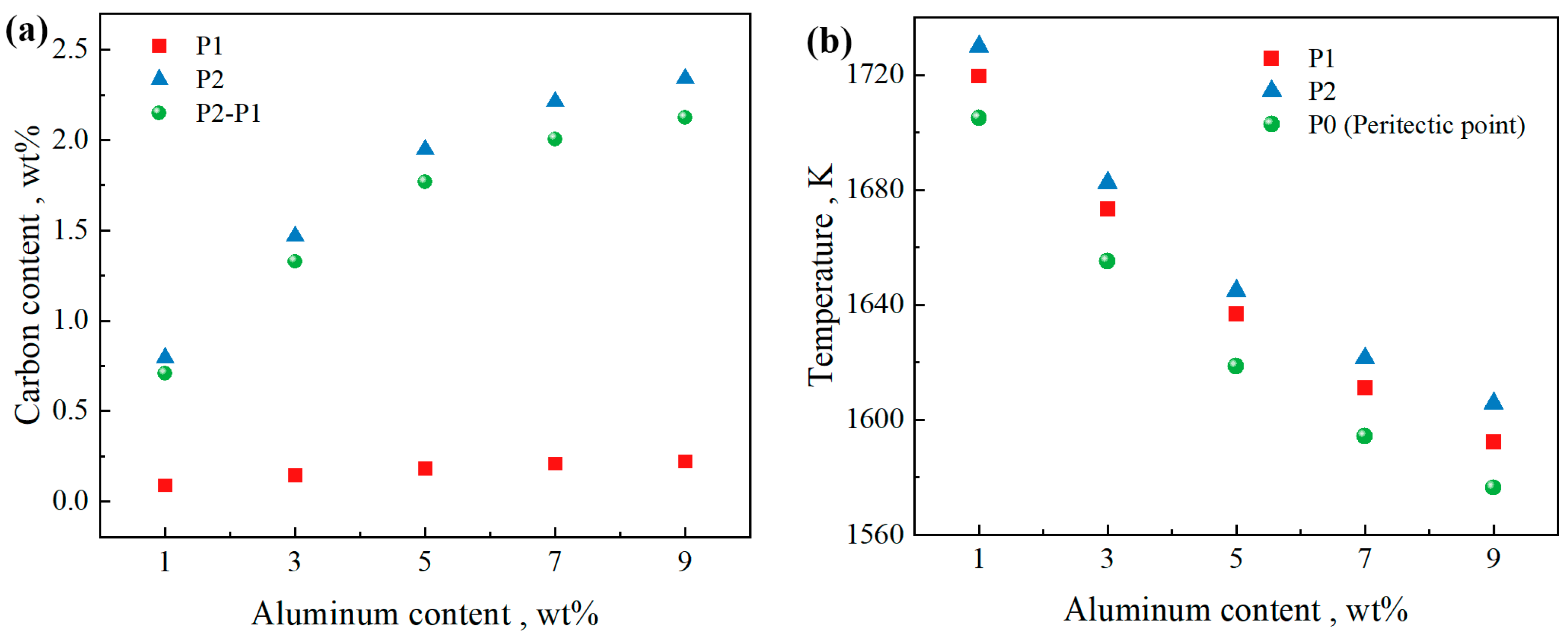
3.1. Effect of Al Content on Peritectic Range and δ Ferrite Solidification Retention Range
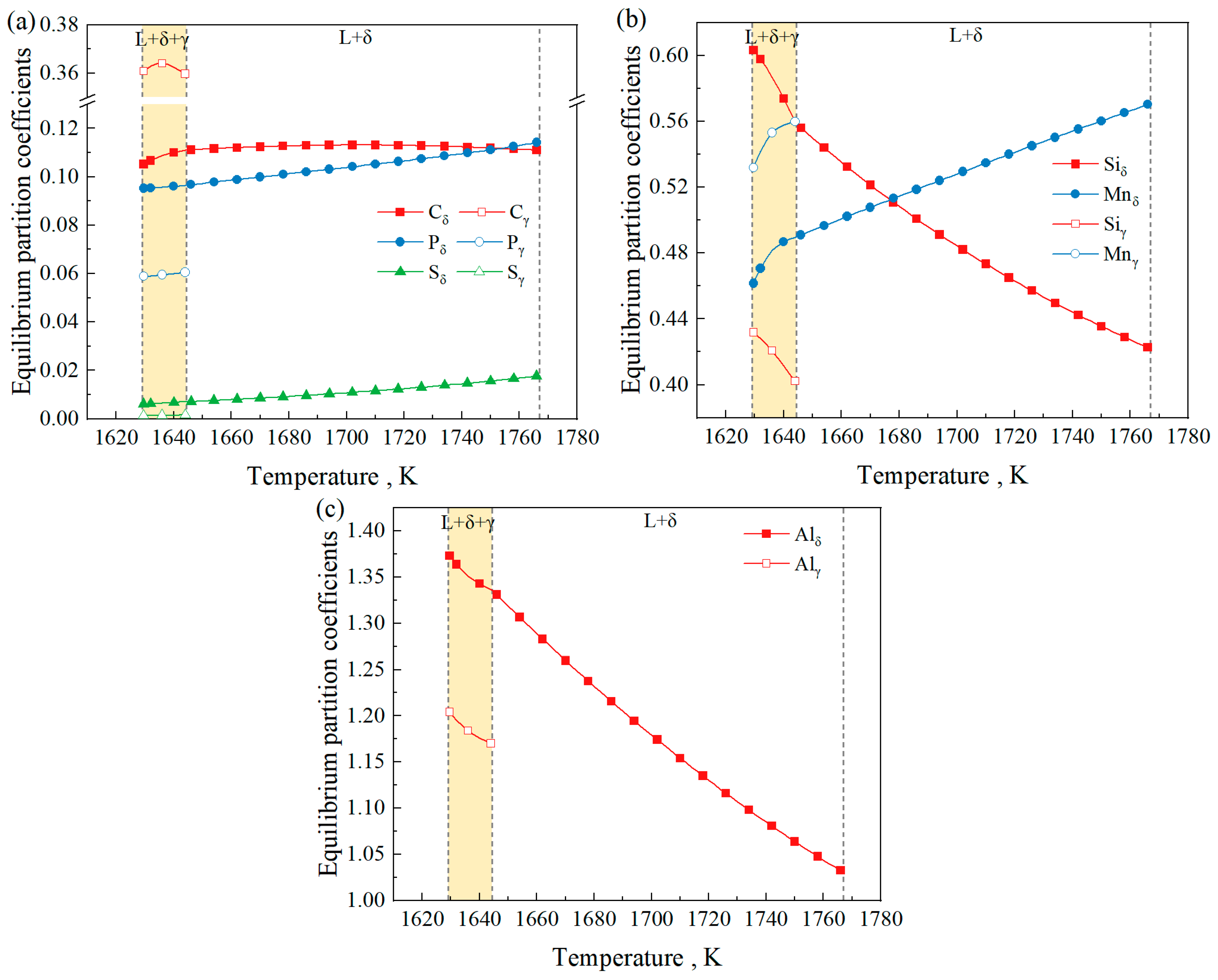
3.2. Effect of Temperature on the Phenomenon of Partitioning
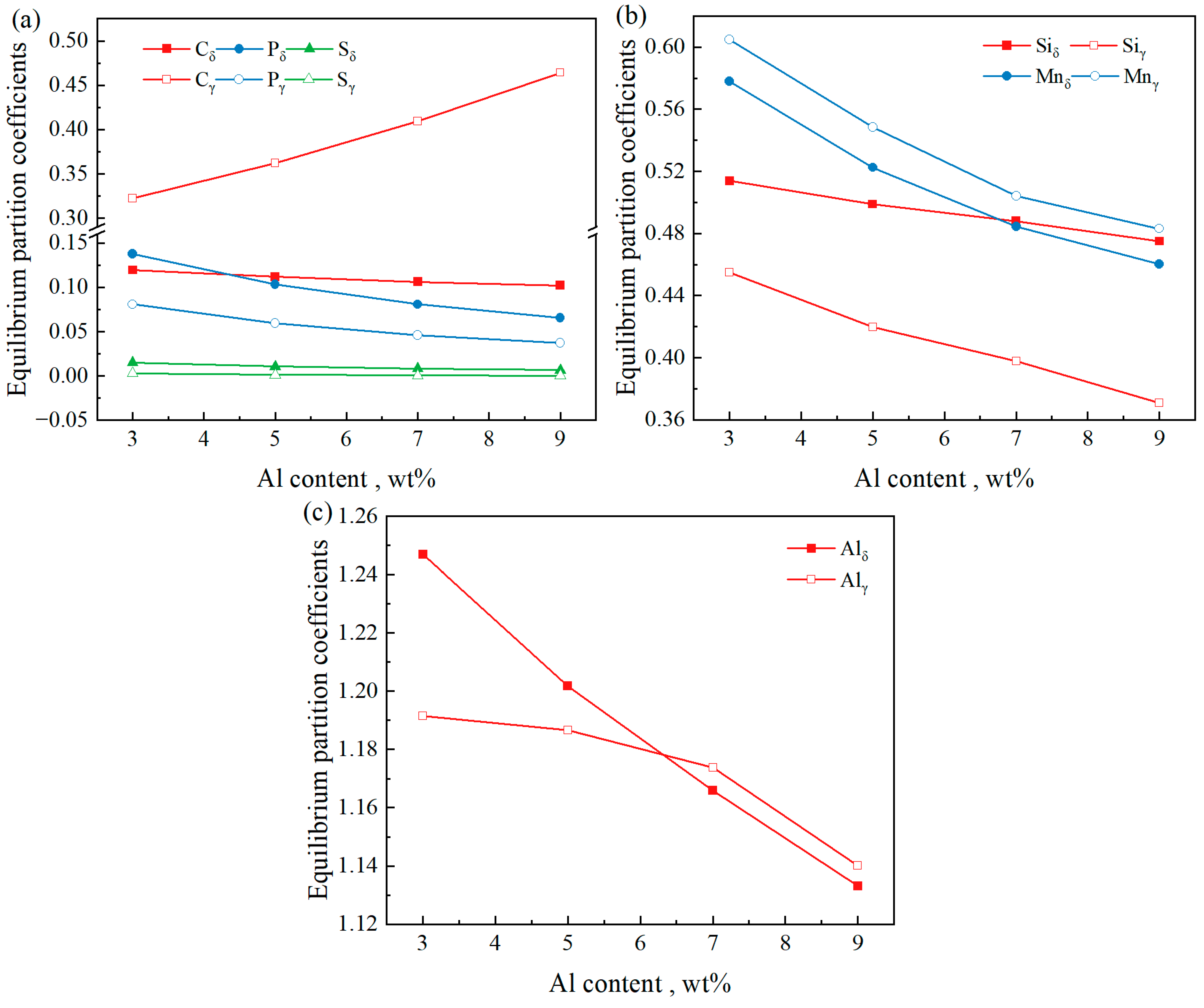
3.3. Effect of Al Content on Equilibrium Partition Coefficient
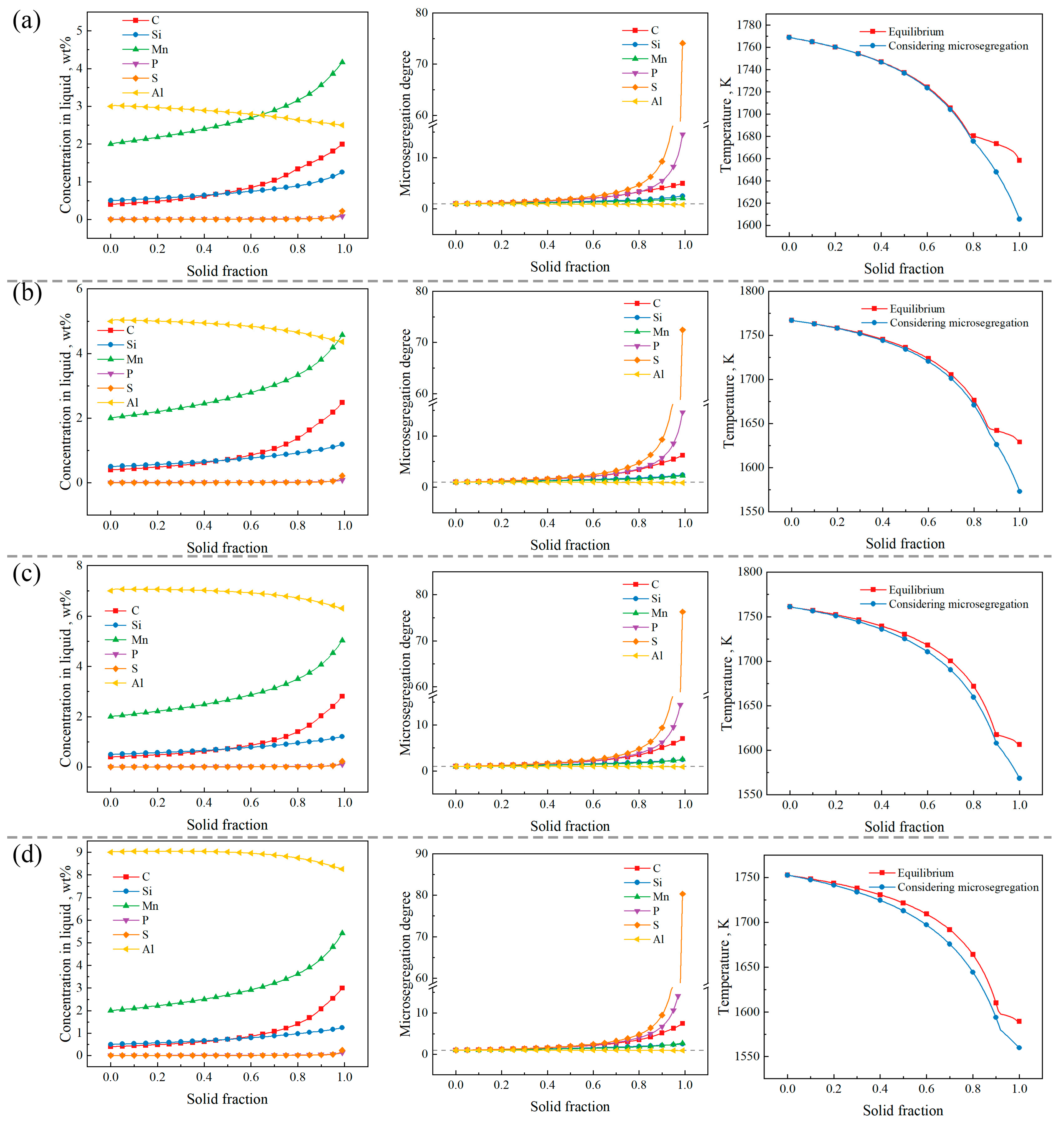
3.4. Effect of Al Content on Microsegregation and Solidification Path
4. Conclusions
- (1)
- For the steel composition Fe-0.4C-0.5Si-2Mn-0.006P-0.003S-(0–9)Al, when the Al content is 0 or 1 wt%, the solidification process passes through the γ single-phase region, and the δ ferrite cannot be retained. When the Al content is 3–9, the solidification path of L→L + BCC(δ)→L + BCC(δ) + FCC(γ)→BCC(δ or δ + α) + FCC(γ) appears in the phase diagram. Under this solidification process, there is ferrite that does not undergo δ→γ phase transformation. Additionally, when the Al content increases by 2 wt%, the range of carbon content where δ ferrite does not completely disappear increases by about 0.2 wt% during solid-state phase transition, and the carbon content range of the peritectic zone increases by about 0.35 wt%. Increasing the Al content shifts the peritectic point toward lower temperatures and a higher carbon content.
- (2)
- The influence of temperature on the equilibrium partition coefficients of Si, Mn, and Al is greater than that on C, P, and S. For different initial Al contents, the partition coefficients of C and Mn in the δ phase are smaller than those in the γ phase, while the partition coefficients of Si, P, and S in the δ phase are larger than those in the γ phase. Regarding the equilibrium partition coefficient of Al, kδ > kγ when the initial Al content is 3 wt% and 5 wt%, and kδ < kγ when the initial Al content is 7 wt% and 9 wt%.
- (3)
- In the steels with Al contents of 3 wt% and 5 wt%, the maximum microsegregation degree of each element follows the order: S > P > C > Si > Mn > Al. In the steels with Al contents of 7 wt% and 9 wt%, the maximum microsegregation degree of each element is ranked as S > P > C > Mn > Si > Al.
- (4)
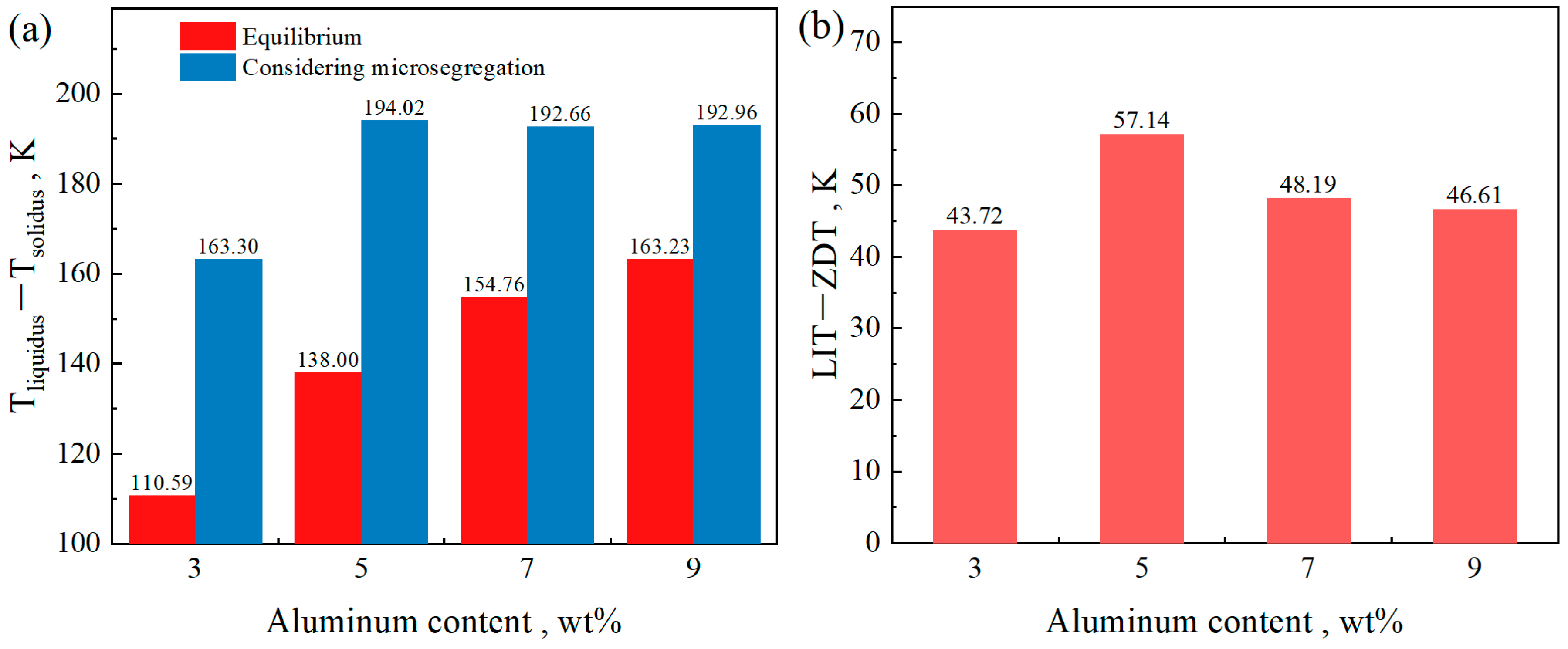
- Considering microsegregation, for the steel with the composition Fe-0.4C-0.5Si-2Mn-0.006P-0.003S-(3, 5, 7, 9)Al, as the Al content increases, the temperature range of the solid–liquid two-phase region and the brittle temperature range (ΔTb) initially increase, then decrease, and finally tend to stabilize. From the perspective of ΔTb, among the compositions studied, the steel with an Al content of 5 wt% exhibits the greatest cracking susceptibility.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, P.X.; Yan, Z.X.; Yang, Y.M. Progress in automobile body processing technology: Multi-material and lightweight strategies for saving energy and reducing emissions. J. Braz. Soc. Mech. 2024, 46, 324. [Google Scholar] [CrossRef]
- Chatterjee, S.; Murugananth, M.; Bhadeshia, H.K.D.H. δ TRIP steel. Mater. Sci. Technol. 2007, 23, 819–827. [Google Scholar] [CrossRef]
- Yi, H. δ-TRIP Steel; Pohang University of Science and Technology: Pohang, Republic of Korea, 2010. [Google Scholar]
- Yi, H.L.; Chen, P.; Hou, Z.Y.; Hong, N.; Cai, H.L.; Xu, Y.B.; Wu, D.; Wang, G.D. A novel design: Partitioning achieved by quenching and tempering (Q–T & P) in an aluminium-added low-density steel. Scr. Mater. 2013, 68, 370–374. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, C.; Mu, H.; Li, Y.; Jiang, M. Inclusion evolution after calcium addition in Al-killed steel with different sulphur content. Ironmak. Steelmak. 2018, 45, 447–456. [Google Scholar] [CrossRef]
- Fan, Y.W.; Hu, X.J.; Matsuura, H. Effect of Al and N Contents on the Characteristics of Non-metallic Inclusions and Precipitation Behaviors in Unidirectionally Solidified Fe-Mn-C-Al TWIP Alloys. Metall. Mater. Trans. B 2025, 56, 2070–2080. [Google Scholar] [CrossRef]
- Cao, L.; Han, L.L.; Wang, G.C.; Tao, K.; Xiao, Y.Y. Effects of Mn content on the formation of inclusions in high aluminum steel. Metall. Mater. Trans. B 2023, 54, 2680–2693. [Google Scholar] [CrossRef]
- Pinson, M.; Das, S.M.; Springer, H.; Depover, T.; Verbeken, K. The addition of aluminum to brittle martensitic steels in order to increase ductility by forming a grain boundary ferritic microfilm. Scr. Mater. 2022, 213, 114606. [Google Scholar] [CrossRef]
- Xu, B.; Chen, P.; Li, Z.; Wu, D.; Wang, G.; Guo, J.; Liu, R.; Misra, R.D.K.; Yi, H. The Significance of Optimizing Mn-Content in Tuning the Microstructure and Mechanical Properties of δ-TRIP Steels. Metals 2021, 11, 523. [Google Scholar] [CrossRef]
- Cui, H.; Chen, D.; Zhang, K. Effects of Al content on the high-temperature mechanical properties of Al-TRIP steel. Mater. Sci. Technol. 2020, 36, 484–491. [Google Scholar] [CrossRef]
- Yi, H.L.; Lee, K.Y.; Bhadeshia, H. Extraordinary ductility in Al-bearing δ-TRIP steel. Proc. R. Soc. A 2011, 467, 234–243. [Google Scholar] [CrossRef]
- Azizi, G.; Thomas, B.G.; Asle Zaeem, M. Review of Peritectic Solidification Mechanisms and Effects in Steel Casting. Metall. Mater. Trans. B 2020, 51, 1875–1903. [Google Scholar] [CrossRef]
- Li, Y.Q.; Liu, J.H.; Wang, Z.M.; Li, Y.Y.; He, Y.; Li, X.W. Solidification crack characteristics and sensitivity of peritectic steel. J. Iron. Steel Res. Int. 2024, 31, 1668–1679. [Google Scholar] [CrossRef]
- Song, R.; Huo, W.; Zhou, N.; Li, J.; Zhang, Z.; Wang, Y. Research progress and prospect of Fe-Mn-Al-C medium Mn steels. Chin. J. Eng. 2020, 42, 814–828. [Google Scholar] [CrossRef]
- Xu, J.; He, S.; Wu, T.; Long, X.; Wang, Q. Effect of elements on peritectic reaction in molten steel based on thermodynamic analysis. ISIJ Int. 2012, 52, 1856–1861. [Google Scholar] [CrossRef]
- Ren, Q.Q.; Liu, T.; Baik, S.I.; Mao, Z.G.; Krakauer, B.W.; Seidman, D.N. The effects of alloying elements on the peritectic range of Fe-C-Mn-Si steels. J. Mater. Sci 2021, 56, 6448–6464. [Google Scholar] [CrossRef]
- Luo, T.F.; Wang, W.L.; Shang, T.R.; Liu, H.L.; Luo, S.; Zhu, M.Y. Experimental and numerical investigations on solidification thermodynamics of H13 steel with multi components. Metall. Mater. Trans. B 2024, 55, 4001–4014. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.; Ren, R.J.; Xue, Z.X.; Wang, H.Z.; Zhang, Y.Z.; Wang, J.X.; Wang, J.; Chen, L.; Mu, W.Z. Ferrite formation and decomposition in 316H austenitic stainless steel electro slag remelting ingot for nuclear power applications. Mater. Charact. 2024, 218, 114581. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.; Yang, X.Y.; Zhang, Z.R.; Wang, J.; Li, Z.; Chen, L.; Mu, W.Z. Solidification modes and delta-ferrite of two types of 316L stainless steels: A combination of as-cast microstructure and HT-CLSM research. J. Iron. Steel Res. Int. 2025, 32, 426–436. [Google Scholar] [CrossRef]
- Gong, T.Z.; Chen, Y.; Li, S.S.; Cao, Y.F.; Li, D.Z.; Chen, X.Q.; Reinhart, G.; Nguyen-Thi, H. Revisiting dynamics and models of microsegregation during polycrystalline solidification of binary alloy. J. Mater. Sci. Technol. 2021, 74, 155–167. [Google Scholar] [CrossRef]
- Hou, Z.B.; Cheng, G.G.; Wu, C.C.; Chen, C. Time-Series Analysis Technologies Applied to the Study of Carbon Element Distribution along Casting Direction in Continuous-Casting Billet. Metall. Mater. Trans. B 2012, 43, 1517–1529. [Google Scholar] [CrossRef]
- Strzępek, P.; Mamala, A.; Boumerzoug, Z.; Baudin, T.; Brisset, F.; Zasadzińska, M.; Noga, P. Effect of horizontal continuous casting parameters on cyclic macrosegregation, microstructure, and properties of high-strength Cu–Mg alloy cast rod. Metall. Mater. Trans. A 2025, 56, 41–60. [Google Scholar] [CrossRef]
- Xu, H.; Wang, W.L.; Lyu, P.S.; Wang, L.K. New Method for the Production of Medium-Mn Steel with Micro-segregation Bands Induced by Sub-rapid Solidification. In Proceedings of the Characterization of Minerals, Metals, and Materials Symposium (MMMS), Orlando, FL, USA, 3 March 2024–7 March 2024; pp. 237–245. [Google Scholar]
- Dang, A.G.; Wang, M.Y.; Wang, H.D.; Feng, X.M.; Liu, W. Homogenization Path Based on 250 mm × 280 mm Bloom under Mixed Light and Heavy Presses: Simulation and Industrial Studies. Metals 2024, 14, 591. [Google Scholar] [CrossRef]
- Ennis, B.L.; Jimenez-Melero, E.; Mostert, R.; Santillana, B.; Lee, P.D. The role of aluminium in chemical and phase segregation in a TRIP-assisted dual phase steel. Acta Mater. 2016, 115, 132–142. [Google Scholar] [CrossRef]
- Scheil, E. Bemerkungen zur Schichtkristallbildung. Int. J. Mater. Res. 1942, 34, 70–72. [Google Scholar] [CrossRef]
- Brody, H.D.; Flemings, M.C. Solute redistribution in dendritic solidification. In Proceedings of the Merton C Flemings Symposium on Solidification and Materials Processing, Cambridge, MA, USA, 28–30 June 2000; pp. 13–21. [Google Scholar]
- Clyne, T.W.; Kurz, W. Solute redistribution during solidification with rapid solid state diffusion. Metall. Trans. A 1981, 12, 965–971. [Google Scholar] [CrossRef]
- Kobayashi, S. Solute redistribution during solidification with diffusion in solid phase: A theoretical analysis. J. Cryst. Growth 1988, 88, 87–96. [Google Scholar] [CrossRef]
- Ohnaka, I. Mathematical Analysis of Solute Redistribution during Solidification with Diffusion in Solid Phase. Trans. Iron Steel Inst. Jpn. 1986, 26, 1045–1051. [Google Scholar] [CrossRef]
- Voller, V.R.; Beckermann, C. A unified model of microsegregation and coarsening. Metall. Mater. Trans. A 1999, 30, 2183–2189. [Google Scholar] [CrossRef]
- You, D.; Bernhard, C.; Wieser, G.; Michelic, S. Microsegregation Model with Local Equilibrium Partition Coefficients During Solidification of Steels. Steel Res. Int. 2016, 87, 840–849. [Google Scholar] [CrossRef]
- Wolczynski, W. Pattern selection in the eutectic growth—Thermodynamic interpretation. Arch. Metall. Mater. 2020, 65, 653–666. [Google Scholar] [CrossRef]
- Han, L.; Li, H.; Santillana, B.; Zhuang, L. Microsegregation and solidification characteristics of an advanced high strength steel —Part I: Modelling and prediction on hot cracking susceptibility. Ironmak. Steelmak. 2022, 49, 101–108. [Google Scholar] [CrossRef]
- Won, Y.M.; Thomas, B.G. Simple model of microsegregation during solidification of steels. Metall. Mater. Trans. A 2001, 32, 1755–1767. [Google Scholar] [CrossRef]
- Huang, Y.; Long, M.; Liu, P.; Chen, D.; Chen, H.; Gui, L.; Liu, T.; Yu, S. Effects of partition coefficients, diffusion coefficients, and solidification paths on microsegregation in Fe-based multinary alloy. Metall. Mater. Trans. B 2017, 48, 2504–2515. [Google Scholar] [CrossRef]
- Gui, L.; Long, M.; Huang, Y.; Chen, D.; Chen, H.; Duan, H.; Yu, S. Effects of inclusion precipitation, partition coefficient, and phase transition on microsegregation for high-sulfur steel solidification. Metall. Mater. Trans. B 2018, 49, 3280–3292. [Google Scholar] [CrossRef]
- Gui, L.; Long, M.; Wu, S.; Dong, Z.; Chen, D.; Huang, Y.; Duan, H.; Vitos, L. Quantitative effects of phase transition on solute partition coefficient, inclusion precipitation, and microsegregation for high-sulfur steel solidification. J. Mater. Sci. Technol. 2019, 35, 2383–2395. [Google Scholar] [CrossRef]
- Jiang, L.; Liao, M.; Wang, Z.; Zhu, H.; Cui, H. Effect of cooling rate on the peritectic transformation of delta-TRIP steel. Chin. J. Eng. 2023, 45, 747–754. [Google Scholar] [CrossRef]
- Liu, K.; Cheng, S.; Li, Y. Study of Crack Sensitivity of Peritectic Steels. Coatings 2022, 12, 15. [Google Scholar] [CrossRef]
- Choudhary, S.K.; Ghosh, A. Mathematical Model for Prediction of Composition of Inclusions Formed during Solidification of Liquid Steel. ISIJ Int. 2009, 49, 1819–1827. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, J.; Xu, H.; Liu, H. Micro-segregation Study of Directional Solidified Fe–Mn–C–Al TWIP Steels. Metall. Mater. Trans. B 2020, 51, 2963–2975. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L. Thermodynamic calculation of solute equilibrium distribution coefficient in steel with full composition system. China Metall. 2023, 33, 44–52. [Google Scholar] [CrossRef]





| No. | C | Si | Mn | P | S | Al | Fe |
|---|---|---|---|---|---|---|---|
| c04a3 | 0.4 | 0.5 | 2 | 0.006 | 0.003 | 3 | 94.091 |
| c04a5 | 0.4 | 0.5 | 2 | 0.006 | 0.003 | 5 | 92.091 |
| c04a7 | 0.4 | 0.5 | 2 | 0.006 | 0.003 | 7 | 90.091 |
| c04a9 | 0.4 | 0.5 | 2 | 0.006 | 0.003 | 9 | 88.091 |
| Element | Dδ(×10−4 m2/s) | Dγ(×10−4 m2/s) |
|---|---|---|
| C | 0.0127 × exp(−19,450/(R*T)) | 0.0761 × exp(−32,160/(R*T)) |
| Si | 8 × exp(−59,500/(R*T)) | 0.3 × exp(−60,100/(R*T)) |
| Mn | 0.76 × exp(−53,640/(R*T)) | 0.055 × exp(−59,600/(R*T)) |
| P | 2.9 × exp(−55,000/(R*T)) | 0.01 × exp(−43,700/(R*T)) |
| S | 4.56 × exp(−51,300/(R*T)) | 2.4 × exp(−53,400/(R*T)) |
| Al | 5.9 × exp(−57,697/(R*T)) | 5.9 × exp(−57,697/(R*T)) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Sun, Y.; Cui, H. Effect of Aluminum Content on Solidification Process and Microsegregation of δ-TRIP Steel. Metals 2025, 15, 587. https://doi.org/10.3390/met15060587
Wang R, Sun Y, Cui H. Effect of Aluminum Content on Solidification Process and Microsegregation of δ-TRIP Steel. Metals. 2025; 15(6):587. https://doi.org/10.3390/met15060587
Chicago/Turabian StyleWang, Rudong, Yanhui Sun, and Heng Cui. 2025. "Effect of Aluminum Content on Solidification Process and Microsegregation of δ-TRIP Steel" Metals 15, no. 6: 587. https://doi.org/10.3390/met15060587
APA StyleWang, R., Sun, Y., & Cui, H. (2025). Effect of Aluminum Content on Solidification Process and Microsegregation of δ-TRIP Steel. Metals, 15(6), 587. https://doi.org/10.3390/met15060587






