Abstract
To explore the impact of niobium (Nb) addition on the austenitization behavior of Fe-Mn-Al-C lightweight steels, the effects were examined through Thermo-Calc thermodynamic simulations, optical microscopy, transmission electron microscopy (TEM), and the development of austenite grain growth models. Three distinct Fe-Mn-Al-C steel compositions, each containing different Nb contents (0.38%, and 0.56%), were subjected to various austenitization temperatures and aging conditions, and a kinetic model for austenite grain growth was established. The results demonstrate that for heating temperatures below 950 °C, the austenite grain growth rate of the steels was similar. However, at temperatures above 950 °C, the grain growth rate of the steel without Nb (Steel No. 1) increased significantly compared to the niobium-containing alloys. Austenite grain size increased with higher heating temperatures. At constant heating temperatures, longer holding times resulted in larger grain sizes, though the rate of grain size growth diminished over time. Based on the experimental data and the kinetic theory of austenite grain growth, a grain growth model of No. 2 Steel (which contained 0.38% Nb) was established. The predicted grain size values derived from this model closely matched the experimental measurements, indicating a strong correlation and providing valuable insights for future studies.
1. Introduction
In recent years, the automotive industry has experienced rapid growth, leading to increasing concerns regarding the environmental impact of energy consumption and tailpipe emissions. Research has shown that optimizing vehicle structure design and developing advanced lightweight, high-strength steels are effective strategies for enhancing fuel efficiency and reducing harmful emissions. Among various steel grades, Fe-Mn-Al-C steels, which were first developed in the 1950s, have gained significant attention due to their combination of low density and excellent mechanical properties, making them promising candidates for automotive structural applications [1,2,3]. Despite their potential, Fe-Mn-Al-C steels face several challenges in practical applications, including limited control over strengthening mechanisms, complex processing techniques, and suboptimal corrosion resistance during production. As a result, significant research efforts are focused on overcoming these limitations and developing new Fe-Mn-Al-C steel grades that exhibit higher strength, improved toughness, and enhanced durability, which are essential for meeting the increasingly stringent requirements of the automotive industry [4,5,6,7,8,9].
Fine grain strengthening is a crucial approach for enhancing both the strength and toughness of steel materials. For Fe-Mn-Al-C steel subjected to hot working, parameters such as the austenitization temperature and the holding time significantly influence the austenite grain size and the dissolution of precipitated phases. Grain size, in turn, plays a critical role in determining the subsequent phase transformations and the overall microstructure [10,11,12,13,14]. Consequently, it is essential to identify an effective heat treatment process and establish a reliable mathematical model to predict austenite grain growth, which will help in controlling the material’s properties and microstructure. In addition to thermal processing, introducing small quantities of alloying elements like Nb, V, and Ti can promote the formation of stable carbides, nitrides, and other second-phase particles [15]. These particles contribute to grain refinement by exerting a pinning effect on the grain boundaries. Among these alloying elements, Nb stands out due to its high stability and is commonly used in steel alloying. A significant body of research has been devoted to studying the kinetics of niobium’s role in grain refinement. Many studies have highlighted how the presence of Nb influences the overall grain structure and mechanical properties of the steel. Park C et al. [16] investigated the effect of Nb on the grain growth behavior of 10Cr-3Co-2W martensitic heat-resistant steel, and the results showed that Nb could refine the grains, and a MatCalc microstructure model was developed, and the predicted results were consistent with the experimental results. V.S.A. Challa et al. [17] explored the impact of temperature on the microstructure and mechanical properties of niobium-titanium microalloyed steels. They concluded that at lower temperatures, the microstructure was predominantly bainitic, while at higher temperatures, ferrite became the dominant phase. The optimum combination of strength and toughness was observed at lower temperatures, primarily due to the uniform distribution of NbC, TiC, and (Nb, Ti) C particles, which, combined with the higher dislocation density in bainitic structures, contributed to superior toughness. Guang et al. [18] examined the austenite grain growth in high-carbon steels containing niobium under varying austenitization temperatures. They found that the austenite grain growth rate in steels without niobium was significantly higher compared to those containing Nb. This difference was attributed to the formation of Nb (C,N) second-phase particles in niobium-containing steels, which effectively pinned the grain boundaries, thereby hindering grain growth and promoting grain refinement. Tian et al. [19] investigated the influence of niobium on austenite grain growth and the mechanical properties of high-strength stainless steel. Their findings indicated that as the temperature increases, the drag effect of NbC phases and dissolved atoms slows down the migration rate of austenite grain boundaries, effectively hindering grain growth. Furthermore, the introduction of Nb enhances the refinement of the martensitic microstructure by increasing the nucleation sites for residual austenite, dispersing its distribution, and preventing crack propagation, all of which contribute to improved toughness. Huo et al. [20] demonstrated that incorporating 0.5% Nb into Fe-28Mn-10Al-1C austenitic mild steel significantly reduces the austenite grain size from 39.49 µm to 13.67 µm. This grain refinement is closely associated with marked improvements in mechanical properties, such as a yield strength of 171 MPa, ultimate tensile strength of 64 MPa, and elongation of 11.5%. However, despite these improvements, the effects of niobium on the morphology and growth mechanisms of austenite grains, particularly during the austenitization process in low-density Fe-Mn-Al-C steels, remain insufficiently studied.
The present investigation focuses on the austenite grain growth characteristics in three Fe-Mn-Al-C steels, two of which have distinct niobium contents. These steels were subjected to various austenitizing temperatures and holding durations. A comprehensive kinetic model was formulated to accurately forecast grain growth, providing essential insights that can guide the optimization of heat treatment parameters and enhance the resulting material properties.
2. Materials and Methods
2.1. Experimental Materials
In this study, the Fe-Mn-Al-C-Nb low-density steels were processed using a 50 kg vacuum induction furnace (KJ-M1200-12LZ, Zhengzhou Kejia Electric Furnace Co., Zhengzhou, China) for melting. Subsequently (vacuum level of 10 Pa), the heating procedure was performed at a steady temperature of 1150 °C and maintained for a duration of 2 h. After forging, the material was shaped into billets with a cross-sectional area of 80 mm × 40 mm. The specific composition of these steels is provided in Table 1, with the steels being designated as No. 1, No. 2, and No. 3. The billets were then heated at a rate of 5 °C/min to 1200 °C and held at this temperature for 2 h to obtain 5 mm thick hot-rolled slabs. The opening rolling temperature was set at 1150 °C, while the final rolling temperature was 880 °C. For the experimental specimens, 10 mm × 10 mm samples were cut from the test steel billets. The forging and hot rolling process used for these steels is illustrated in Figure 1.

Table 1.
Chemical composition of Fe-Mn-Al-C-Nb steels (mass percent, %).
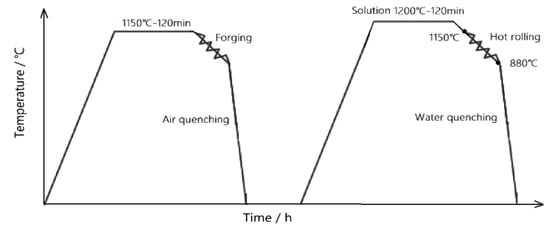
Figure 1.
Forging and hot rolling process flow of test steel.
2.2. Experimental Methods
In this experimental investigation, three distinct types of specimens were subjected to a controlled heat treatment process using a muffle furnace (SGM·M10/13, Sigma (Shanghai) High Temperature Electric Furnace Co., Shanghai, China). The specimens were heated at a constant rate of 10 °C/min, gradually reaching temperatures of 800 °C, 850 °C, 900 °C, 950 °C, 1000 °C, 1050 °C, 1100 °C, and 1150 °C. At each target temperature, the specimens were held for a duration of 1 h to ensure uniform heating. After the designated heat treatment, the specimens were rapidly quenched in water to room temperature to capture the immediate effects of the different heating temperatures on the austenite grain size. To further investigate the influence of holding time, specimen No. 2 was subjected to a series of temperature treatments at 950 °C, 1000 °C, 1050 °C, and 1100 °C, with each treatment followed by a quenching process after varying holding times of 5 min, 30 min, 60 min, and 120 min. This experimental setup was designed to explore how both temperature and time impact the austenite grain structure. After heat treatment, the specimens were meticulously ground and polished using a series of sandpapers with progressively finer grits, starting from 320# and progressing through 500#, 800#, 1200#, and finishing with 2000# grit paper. Once the specimens were mechanically polished to a mirror-like finish, they were etched with a 4% nitric acid-alcohol solution to reveal the microstructure. The etched specimens were then rinsed with water and dried with alcohol to remove any residual etchant. The austenite grain morphology was examined under a Zeiss metallurgical microscope (Axiovert 200MAT, Carl Zeiss AG, Oberkochen, Germany), which allowed for high-precision imaging of the grain structure. Image-Pro Plus software (Version 6.0) was used to calculate the average grain size, with a minimum of 400 grains being measured per specimen to ensure statistical reliability. In addition to optical microscopy, the microstructure of the experimental steels was further characterized at the nanoscale using a Tecnai G2 F20 S-TWIN TEM (FEI Company, Hillsboro, OR, USA), providing detailed insights into the finer structural features of the materials. For TEM (FEI Talos F200S G2, Brno, Czech Republic) sample preparation, 1 mm-thick slices were initially cut from the flat area of the experimental steel. These slices were then sanded using abrasive papers of various grits (120#, 320#, 500#, 800#, and 1200#) to a final thickness of 0.06 mm, washed with alcohol, and punched into 3 mm-diameter discs. The samples were then subjected to electrolytic double-jet thinning in a 10% perchloric acid ethanol electrolyte. The thinning process was performed at an applied voltage of 20 V AC, with the entire procedure being cooled by liquid nitrogen to ensure the electrolyte temperature remained below −35 °C.
3. Results
3.1. Thermodynamic Calculations of Equilibrium Phases in Steel
The incorporation of Nb into steel plays a crucial role in modifying the material’s microstructure and properties. Nb interacts with C to form NbC, which is not only highly stable but also uniformly distributed in the steel matrix. This second-phase precipitation mechanism enhances the strength of the matrix and contributes to grain refinement, thus improving the mechanical properties of the material [21,22]. To explore the effect of Nb on the precipitation behavior of phases in Fe-Mn-Al-C low-density steel, equilibrium calculations were conducted using the Thermo-Calc software (Version 2019a, TCFE8). These calculations were applied to three different steel compositions with varying Nb contents, namely No. 1 Steel, No. 2 Steel, and No. 3 Steel. The equilibrium phase diagrams for these steels, as shown in Figure 2a–c, were analyzed to understand the precipitation patterns of the primary and secondary phases under varying conditions. The phase diagrams indicate that the steel compositions with different Nb contents exhibit a complex array of phases including the liquid phase (L), austenite (γ), low-temperature ferrite (α), κ-carbide (κ), and M23C6 carbide. The liquid phase begins to solidify into austenite at around 1250 °C, and a stable two-phase region (α + γ) forms between 800 °C and 1200 °C. Ferrite precipitation typically takes place at lower temperatures, with the temperature range for maximum ferrite (α) precipitation occurring between 400 °C and 800 °C. This temperature interval represents the optimal conditions for ferrite formation, where the transformation is most pronounced. As the temperature increases, the steel begins to form more austenite. Interestingly, the introduction of Nb has a distinct effect on the precipitation of these phases. While the precipitation temperatures of α-ferrite and κ-carbide remain relatively unchanged with increasing Nb content, the amount of these phases decreases with a higher Nb concentration. For example, as the Nb content increases from 0 to 0.56%, the maximum content of κ-carbide in the low-density steel decreases from 13.87% to 13.71%, and the maximum content of α-ferrite decreases from 86.28% to 85.77%, as detailed in Table 2. This suggests that the presence of Nb in the equilibrium state plays a role in stabilizing the austenite phase, which inhibits the decomposition of austenite and the occurrence of eutectic reactions. Consequently, the presence of Nb reduces the overall volume fractions of low-temperature α-ferrite and κ-carbide in the steel, further indicating its role in controlling phase transitions and enhancing the steel’s properties. In addition, the formation of NbC particles in the steel matrix contributes to grain refinement. This precipitation behavior, which is directly influenced by the Nb content, limits the grain growth during heat treatment, improving the overall toughness and strength of the material. The findings highlight the significant role that Nb plays not only in modifying the precipitation behavior but also in enhancing the material’s mechanical performance by stabilizing the austenitic structure and refining the microstructure.
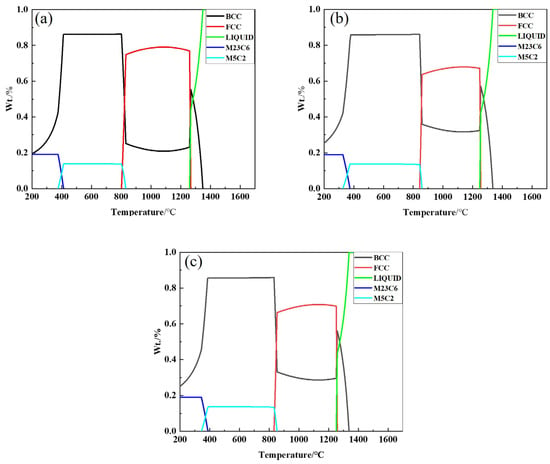
Figure 2.
Phase diagram of Fe-28Mn-10Al-C-xNb low-density steel calculated by Thermo-Calc. (a) Fe-28Mn-10Al-C; (b) Fe-28Mn-10Al-C-0.38Nb; (c) Fe-28Mn-10Al-C-0.56Nb.

Table 2.
Ultimate content of κ-carbide and low temperature α-ferrite in Fe-28Mn-10Al-C low density steel at different Nb contents.
Under equilibrium conditions, the variation in the mass fraction of NbC in steels with different Nb contents (Steel No. 2 and Steel No. 3) as a function of temperature is presented in Figure 3. From the phase diagram, it is evident that the complete dissolution temperatures of NbC in these steels occur at 1340 °C and 1400 °C, respectively. This implies that as the temperature increases, the NbC phase gradually dissolves, leading to a significant reduction in its mass fraction. Notably, at 850 °C, the limiting mass fractions of NbC in Steel No. 2 and Steel No. 3 are 0.00403 and 0.00595, respectively. These values indicate that, at this temperature, almost all the Nb added to the steels will remain in the form of precipitated NbC rather than dissolving into the matrix. Thermodynamic calculations confirm that the presence of Nb in the steel promotes the formation of a stable and uniformly distributed NbC phase, formed by the interaction of Nb with carbon and nitrogen. Furthermore, this process inhibits the decomposition of austenite and suppresses the precipitation of κ-carbide. This suggests that the NbC phase plays a key role in enhancing the material’s microstructure by controlling the phase transitions and improving phase stability. The uniform distribution and stability of the NbC phase ensure that the added Nb does not just dissolve into the matrix but remains in the form of finely dispersed precipitates, which significantly contributes to the refinement of the steel’s grain structure and overall mechanical properties. Thus, the thermodynamic calculations indicate that Nb significantly influences the phase behavior of the steel. The presence of NbC not only affects the precipitation dynamics but also acts to stabilize the austenitic structure, suppressing unwanted phase transformations and leading to improved material performance.
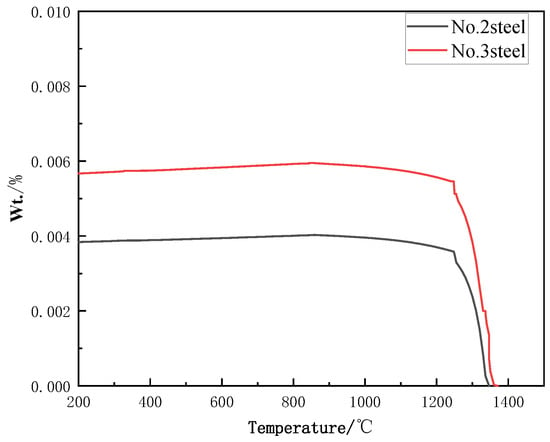
Figure 3.
Localized magnification of NbC precipitation phases of Fe-28Mn-10Al-C-xNb low-density steel with different Nb contents.
3.2. Influence of Nb Precipitation Phase Properties in Fe-Mn-Al-C-Nb Low-Density Steels
Thermodynamic calculations suggest that in Fe-Mn-Al-C steels, the Nb element predominantly exists in the form of NbC. To further examine the characteristics of NbC, including its composition, size, and distribution in low-density steels, the precipitates formed in the three test steels after austenitizing at 950 °C for 60 min were analyzed using TEM. The results are shown in Figure 4. In Figure 4a, the TEM image of the steel without niobium is presented, where no precipitate phases are visible within the crystal structure or at the grain boundaries, which is consistent with the absence of niobium in the alloy. In contrast, Figure 4b,c show the TEM images and energy spectrum analysis of precipitates from the Nb-containing steels. These images reveal that the NbC precipitates are finely distributed at the grain boundaries of austenite, and the main constituents of the precipitates are Nb and C. The precipitates in all the low-density steels predominantly consist of Nb and C. The selected area electron diffraction (SAED) pattern of a submicron-sized particle shown in Figure 4d, Interestingly, when the Nb content was increased from 0.38% to 0.56%, the size of the precipitated NbC phase remained largely unchanged. Statistical analysis of the precipitate size in both steels indicated an average size of approximately 34 nm, suggesting that the size of the NbC precipitates is unaffected by the amount of Nb at the same austenitization temperature. However, there was a noticeable increase in the number of NbC precipitates in the No. 3 experimental steel compared to the No. 2 Steel, despite similar precipitate sizes. This observation aligns with the thermodynamic predictions made using Thermo-Calc, where an increase in Nb content resulted in a higher number of NbC precipitates, but not a change in their size.
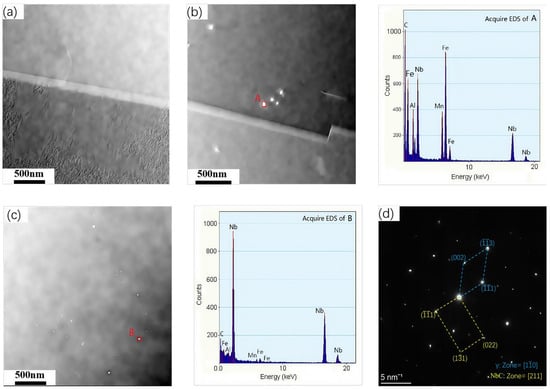
Figure 4.
TEM bright field and precipitated phase energy spectrum analysis of Fe-Mn-Al-C-xNb low density steel after solid solution treatment at 950 °C for 60 min. (a) 0 Nb; (b) 0.38 Nb; (c) 0.56 Nb; (d) selected area electron diffraction (SAED) pattern of the submicron-sized intra-granular particle.
To better understand the composition, morphology, size, and distribution of precipitates in Nb-containing low-density steel, after 60 min of solid solution treatment at 950 °C, test Steel No. 3 was observed using a higher magnification transmission electron microscope. The results, as illustrated in Figure 5, show that ellipsoidal precipitates were formed within the Fe-Mn-Al-C-Nb low-density steel. These precipitates are evenly dispersed within the austenite grains and along the grain boundaries, exhibiting a fine and uniform distribution. Notably, the precipitate density was found to be higher near the grain boundaries, where the number of precipitates per unit area was also significantly increased. This distribution pattern suggests that the grain boundaries play a crucial role in the nucleation and growth of precipitates in this particular steel composition.
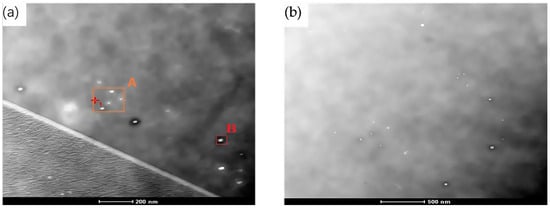
Figure 5.
TEM bright field image of precipitated phase of Fe-28Mn-10Al-C-0.56Nb low density steel after solid solution treatment at 950 °C for 60 min. (a) Higher magnification TEM brightfield image; (b) TEM bright field image.
To gain a deeper understanding of the phase formation, elemental distribution, and morphology of the precipitated phases in Nb-containing low-density steel, advanced TEM techniques were employed. The focus of the analysis was on the No. 3 experimental steel after austenitizing at 950 °C for 60 min. A localized region (Region A) was selected for higher magnification imaging, and both TEM bright-field images and corresponding elemental EDS scans were performed. The results are presented in Figure 6, where the TEM image in Figure 6a illustrates the overall structure of the matrix and the precipitates. The elemental distribution maps for various elements (shown in Figure 6b–g) reveal that the elements are finely distributed within the matrix. However, a significant concentration of the Nb element is observed in the areas corresponding to the precipitates, highlighting that Nb is preferentially concentrated in these regions. The analysis was further extended to another region, designated as Region B, which was observed at an even higher magnification to gain a more detailed insight into the precipitate structure and elemental distribution. The results of these higher-magnification observations are displayed in Figure 7. In this figure, Figure 7a shows the TEM image of the precipitates at the grain boundaries, where elliptical precipitates are evident. These precipitates are smaller in size compared to those observed in other regions, likely due to the higher magnification and smaller selected area for analysis. The elemental energy dispersive spectra confirm the composition of these precipitates, with a clear increase in the C content relative to the surrounding matrix. The distribution of elements in the vicinity of the precipitates also shows important trends. Elements such as Fe, Mn, and Al are evenly spread throughout the matrix, while the Nb element is notably concentrated in the precipitate phases. The increased concentration of C in the precipitate regions is consistent with the high affinity between Nb and C, which results in the preferential accumulation of C at the Nb-rich areas. This behavior inhibits the diffusion of C and directs its localization at sites with higher Nb content. Additionally, the distribution of other elements like Fe, Mn, and Al remains uniform in the matrix, indicating their relatively stable positions outside the precipitate regions. An interesting observation is the low content of N in the steel, which is shown in Figure 7g. This low N content has a minimal impact on the precipitation process, suggesting that Nb is not contributing to the formation of NbN precipitates in the steel. Instead, the primary precipitate observed is NbC, which is consistent with the thermodynamic predictions. The results further confirm that the precipitation of NbC occurs predominantly at the austenite grain boundaries. This localized precipitation at the grain boundaries is a significant finding, as it aligns with the thermodynamic calculations and provides important insights into the role of Nb in the phase evolution of low-density steels. The formation of NbC precipitates at these boundaries is expected to have a considerable effect on the material’s mechanical properties, such as strength and hardness, as it inhibits grain growth and enhances the overall stability of the steel.
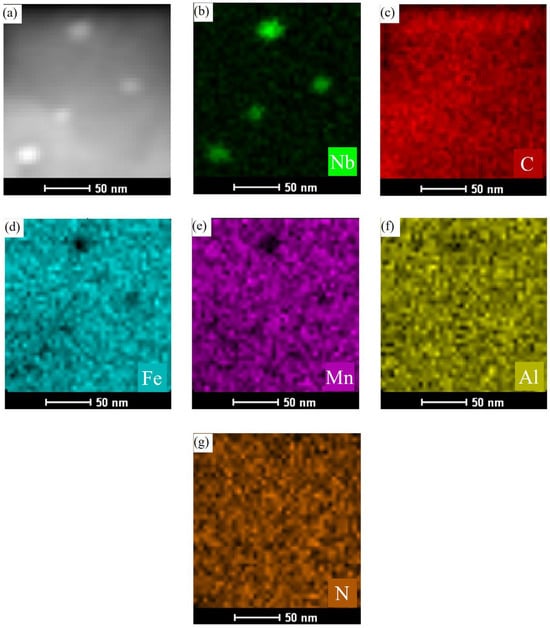
Figure 6.
High magnification STEM image and energy spectrum surface scanning analysis of precipitated phases of Fe-28Mn-10Al-C-0.56Nb low-density steel after austenitizing treatment at 950 °C for 60 min. (a) STEM; (b) Nb-element distribution; (c) C-element distribution; (d) Fe-element distribution; (e) Mn-element distribution; (f) Al-element distribution; (g) N-element distribution.
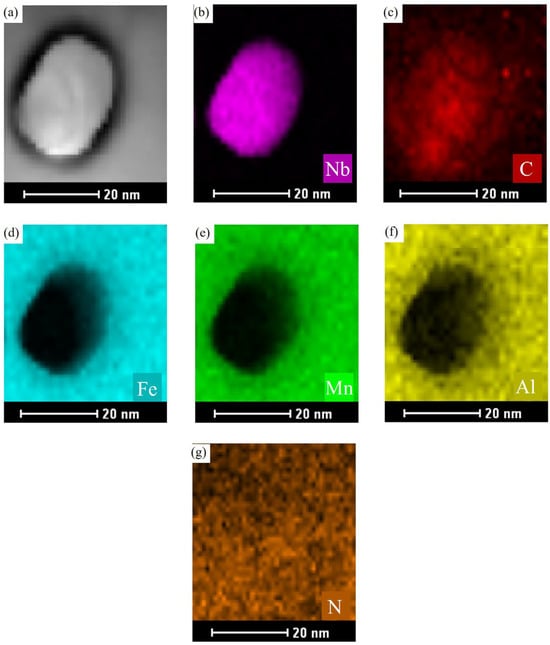
Figure 7.
Highly magnified STEM image and energy spectrum surface scanning analysis of the precipitated phase of Fe-28Mn-10Al-C-0.56Nb low-density steel after austenitizing treatment at 950 °C for 60 min. (a) STEM; (b) Nb-element distribution; (c) C-element distribution; (d) Fe-element distribution; (e) Mn-element distribution; (f) Al-element distribution; (g) N-element distribution.
3.3. Effect of Heating Temperature on Austenite Grain Size
To explore the impact of heating temperature on the austenite grain size in steels numbered 1, 2, and 3, specimens were held at various temperatures between 800 °C and 1150 °C in 50 °C increments for 1 h before undergoing rapid water quenching. Taking Steel No. 3 as a representative case, the changes in austenite grain morphology at different holding temperatures are presented in Figure 8. The results demonstrate a clear correlation between increasing holding temperature and grain size enlargement. Within the temperature range of 800° C to 950 °C, grain growth occurs gradually, but the increase is relatively small. However, when the temperature reaches 1000 °C, a more significant grain growth is observed, with the average grain size increasing to 15.96 μm. Upon further heating from 1000 °C to 1100 °C, the austenite grains undergo a transformation from an irregular shape to a more well-defined polygonal structure, and the average grain size increases further to 17.15 μm. Above 1100 °C, the grain size grows rapidly, reaching an average size of 24.29 μm.
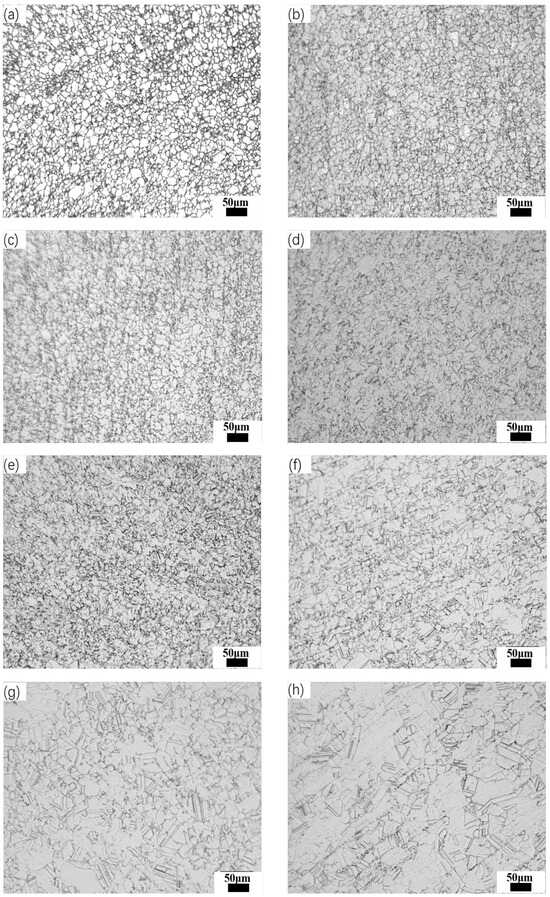
Figure 8.
Austenite grain morphology of Steel No. 3 at different homogenization temperatures under optical microscope (OM) (holding time 1 h). (a) 800 °C; (b) 850 °C; (c) 900 °C; (d) 950 °C; (e) 1000; (f) 1050 °C; (g) 1100 °C; (h) 1150 °C.
Figure 9 illustrates how the austenite grain size changes as a function of heating temperature for the three steels used in this study. The data clearly show that as the temperature increases, the size of the austenite grains also increases in all three steels, with a similar trend observed across the samples. Specifically, within the temperature range of 800 °C to 950 °C, the grain size increases, but the rate of growth is relatively slow during this period. When the heating temperature exceeds 950 °C, Steel No. 1, which contains no niobium, exhibits a more pronounced increase in grain size compared to the steels containing niobium. At temperatures higher than 1100 °C, the grain size of both Steel No. 2 and No. 3 shows a significant increase. This indicates that the NbC phase in these steels has fully dissolved, leading to a weakening of its pinning effect on the grain boundaries. The thermodynamic software (Version 2019a, TCFE8) used for equilibrium calculations suggests that the NbC phase should fully solidify at a temperature of 1300 °C. However, the actual solidification temperature observed during the experiment is lower than this predicted value. This discrepancy is due to the fact that the thermodynamic calculations are based on equilibrium conditions, while the actual experimental process was conducted for only 1 h, meaning that equilibrium was not reached during this time. Additionally, external factors during the experiment may have contributed to this deviation, leading to the observed differences between the theoretical and practical solidification temperatures.
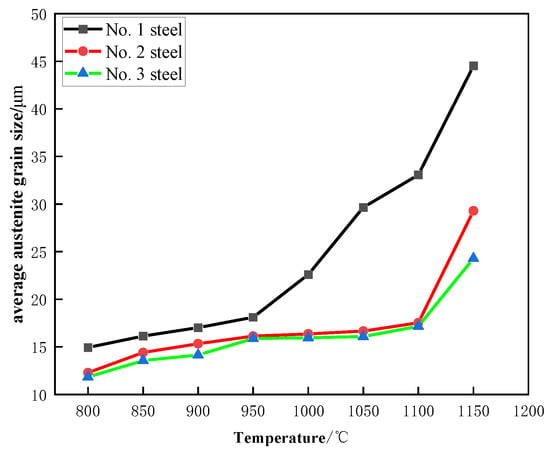
Figure 9.
Variation of austenite average grain size with averaging temperature in test steel (holding time 1 h).
3.4. Effect of Holding Time on Austenite Grain Size
Steel No. 2 was heated at different temperatures (950 °C, 1000 °C, 1050 °C, and 1100 °C) for various durations, including 5, 30, 60, and 120 min, and subsequently quenched rapidly to room temperature. Figure 10 depicts how the austenite grain size is influenced by holding time at each of these temperatures. Additionally, Figure 11 shows the microstructure of Steel No. 2 after being held at 1100 °C for different time intervals. From the data presented in these figures, it is clear that when the holding time remains constant, an increase in heating temperature leads to larger austenite grains. Similarly, at a fixed heating temperature, prolonging the holding time results in a gradual enlargement of the grains. Notably, the rate of grain growth slows over time, suggesting that the effect of extended holding periods diminishes as the process continues.
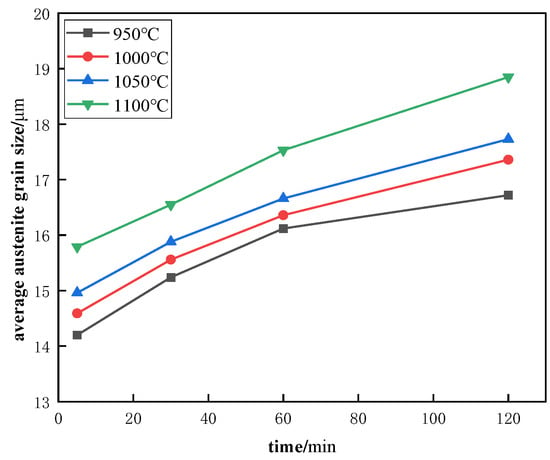
Figure 10.
Variation curves of austenite grain size with holding time for No. 2 Steel at different heating temperatures.
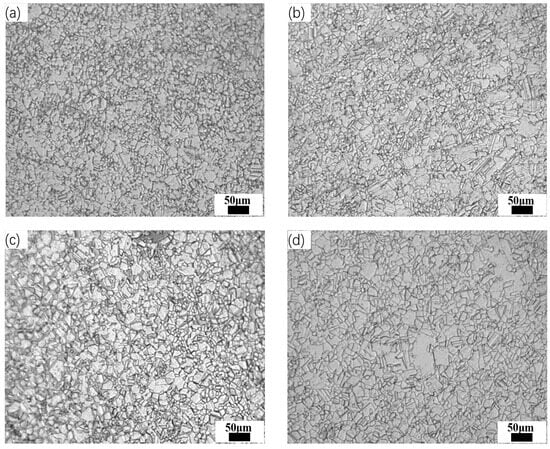
Figure 11.
Optical microscope (OM) morphology of No. 2 Steel at austenitizing temperature 1100 °C for different time periods. (a) 5 min; (b) 30 min; (c) 60 min; (d) 120 min.
3.5. Modeling of Austenite Grain Growth
The model for austenite grain growth serves as a valuable method for forecasting the grain size of austenite under different combinations of temperature and holding time. Both the temperature at which the steel is heated and the duration of the holding period play critical roles in determining the final austenite grain size. In the case of isothermal processing, the grain growth behavior is commonly described by a kinetic model, which is typically expressed through an empirical Equation (1) [23].
In this model, Dt denotes the average austenite grain size (in micrometers) at a given insulation temperature and holding time, t, while D0 represents the initial austenite grain size when the holding time, t, equals zero. The parameter t indicates the duration of the holding period (measured in minutes), and K stands for the rate constant. The time exponent, n, is related to the rate of grain growth. Furthermore, the rate constant, K, described in Equation (1), is governed by the Arrhenius equation:
A is a constant related only to the material; Q is the austenite grain growth activation energy, J/mol; R is the gas constant, taken as 8.314 J/(mol·K); When the test steel is solid solution treated, D0 is much smaller than Dt, and the time parameter n of niobium-containing steels is generally larger than 4, so D0 is negligible, and Equation (1) can be converted to:
Taking the logarithm of both sides of Equation (3) simultaneously yields:
From Equation (4), it can be seen that the relationship between ln(Dt) and ln(t) demonstrates a linear pattern, with the slope of the line being represented by the exponent n. Figure 12 displays the curve that fits the average austenite grain size as a function of time in a logarithmic coordinate system for Steel No. 2, which was subjected to various heating temperatures. The plotted data highlights how the grain size changes with time under different thermal conditions, providing valuable insights into the kinetics of grain growth. The corresponding values of n are summarized in the Table 3, with the average n across the four heating temperatures being 0.0527. Additionally, from Equation (3), the fitting curve of versus time t is shown in Figure 13. The slope of this curve, obtained from the experimental data, corresponds to the rate constant K at different heating temperatures. The resulting values of K are listed in Table 3. Taking logarithms on both sides of Equation (2), we can get , is linearly related to , as shown in Figure 14, and substituting the data in the table, we can calculate the slope of the straight line Q to be 171,104.8876, and based on the calculated values of Q, K, and n, as well as Equation (2), we can calculate to find out that A = .
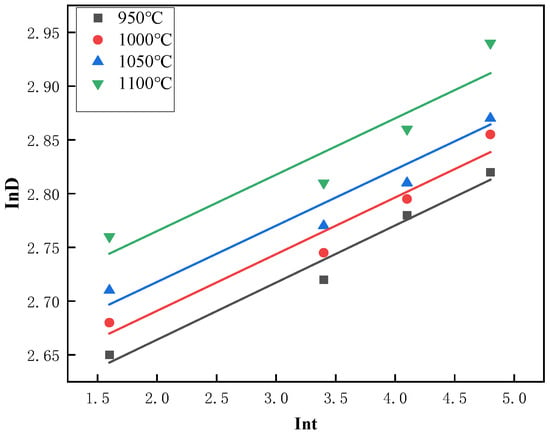
Figure 12.
Logarithmic coordinate fit of InD vs. Int for No. 2 Steel at different austenitic temperatures.

Table 3.
n and k values of No. 2 Steel at different heating temperatures.
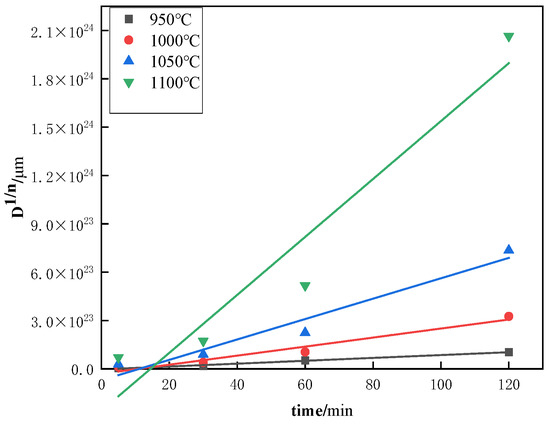
Figure 13.
Fitted curves of versus holding time t for No. 2 Steel at different austenite temperatures.
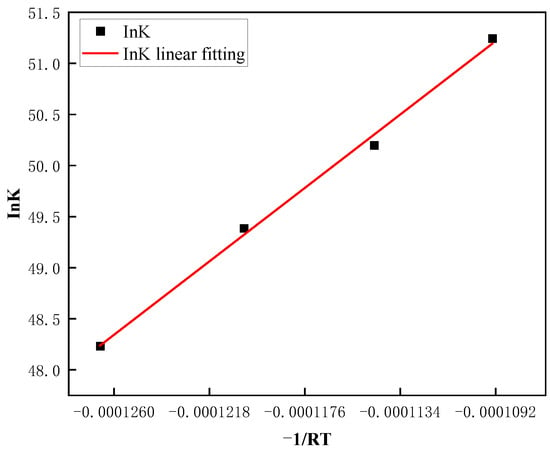
Figure 14.
Fitted curve of lnK versus −1/RT relationship.
At this point, the austenite grain growth model for test Steel No. 2 can be obtained as follows:
4. Discussion
The thermodynamic calculations and TEM analysis results show that in Fe-Mn-Al-C steel, Nb element mainly exists in the form of NbC. Based on the theory of solid solubility of the second phase of iron and steel materials, it can be known that the solubility of Nb in steel is extremely low, and the solid solubility of the Nb element will be affected by the C content in the steel and the heat treatment temperature, usually, in the heat treatment temperature of 800~1200 °C, the solid solubility of the Nb element in the austenite of the steel can be expressed as follows [24,25]:
In the equation:
—carbon content in steel, %;
—niobium (Nb) solubility in steel, %;
—heat treatment temperature, K.
Under the designed experimental conditions, the C content in the low-density steel is 1.15%, and the solid solubility of Nb can be calculated from Equation (6) to be 0.000022–0.002% at a solid solution temperature of 800 °C–1150 °C, as shown in Figure 15. It can be seen that, in the fully austenitic Fe-Mn-Al-C-Nb low-density steel designed in this experiment, the solid solubility of the added Nb element in the austenite is extremely low, even in the austenite single-phase region after high-temperature homogenization treatment and then cooled sharply to room temperature, the Nb element added to the steel is difficult to dissolve in the austenite matrix to form a supersaturated solid solution, and most of the Nb will precipitate out of the steel in the second-phase form. That is, the Nb element in Fe-Mn-Al-C-Nb low-density steel is almost entirely in the form of precipitated phase.
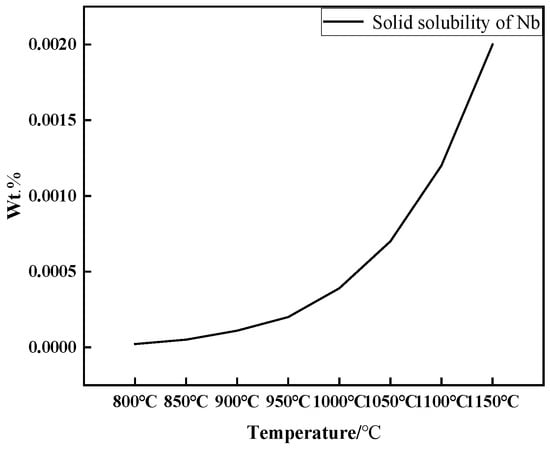
Figure 15.
Solid solubility product of niobium in steel at 800 °C–1150 °C.
Moreover, due to the extremely high thermodynamic stability of the NbC phase, the coarsening of the NbC particle size during deformation and heat treatment is extremely slow. Earlier studies [26] have shown that the NbC phase can still maintain nanoscale particle size during thousands of hours of aging treatment. Therefore, in this study, it can be seen that as the heat treatment temperature increases, the grain size in the test steel increases, but the NbC particle size remains basically unchanged, which indicates that the NbC generated by Fe-Mn-Al-C steel alloyed with Nb is thermodynamically more stable, which is consistent with the conclusion of the study by Wang et al. [27].
As can be seen from Figure 9, the austenite grain size gradually increases with the increase of holding temperature; however, the growth rate decreases in the later stages. In Section 3.4, we investigated the effect of holding time on the austenite grain size, showing that for the same holding time the austenite grain size becomes larger with increasing heating temperature, a behavior that can be attributed to the enhanced mobility of the grain boundaries. As the grain size increases, the boundaries between the grains become more flattened, reducing the energy associated with these boundaries. This reduction in boundary energy limits the driving force for further boundary migration, thus slowing down the growth of the grains. Essentially, while longer holding times provide more opportunity for grain boundary movement, the process eventually slows due to the decreased energy gradient that is required for further migration [22,28]. At the same time, the aforementioned results demonstrate that Nb added to steel can strengthen the steel matrix by solid solution or by combining with the C element in the steel to generate a highly stable form of carbide (NbC). in the process of deformation and heat treatment, the thermodynamic stability of the NbC phase is extremely high, and the coarsening of the particle size is extremely slow, which can effectively play a role in nailing the rolling of grain boundaries and stabilizing the organization [29,30,31]. When the NbC particles, which were initially fine, dissolve into the matrix at higher temperatures, they lose their pinning effect on the grain boundaries, allowing the grains to grow more quickly. However, if the NbC phases remain undissolved, they continue to restrict grain boundary movement, effectively limiting grain growth. This finding is consistent with the study by An et al. [32].
The prediction of austenite grain size for No. 2 Steel was performed using Equation (5), and the predicted grain sizes were compared with the actual measurements. The results, shown in Figure 16, indicate that the predicted values closely match the experimentally obtained values. Additionally, the deviation of the predicted grain sizes was quantified using Equation (7), which resulted in an E-value of 0.56. This small deviation suggests that the model provides accurate predictions for No. 2 Steel within the temperature range of 950 °C to 1100 °C. Therefore, the prediction model is considered reliable and offers valuable insights for further applications.
where: N is the number of austenite grains counted, Dm is the actual measured value of austenite grains in Steel No. 2, and Dp is the predicted value of austenite grains
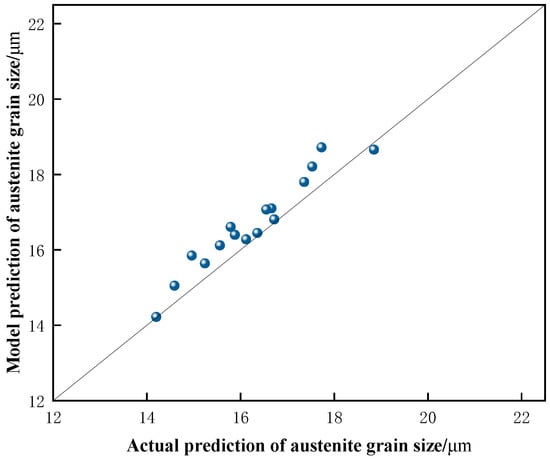
Figure 16.
Comparison of measured and predicted austenite grains of No. 2 Steel.
5. Conclusions
- (1)
- The incorporation of Nb with C in Fe-Mn-Al-C low-density steel leads to the formation of a highly stable and uniformly distributed NbC phase. This phase, particularly at the grain boundaries, plays a significant role in restricting the movement of austenite grain boundaries, effectively preventing the grains from growing excessively. The NbC phase acts as a barrier, hindering grain boundary migration and, as a result, retarding the overall growth of austenite grains.
- (2)
- At temperatures below 950 °C, the austenite grain growth rates for all test steels are similar. However, when the temperature exceeds 950 °C, No. 1 Steel, which does not contain niobium, exhibits a significantly higher rate of grain size increase compared to the niobium-containing steels.
- (3)
- The austenite grain growth behavior in the specimens demonstrates notable differences based on the applied heating temperature. Within the temperature range of 800 °C to 950 °C, the grains exhibit slow expansion. This period is characterized by a gradual increase in grain size, with minimal changes occurring during this stage. However, once the temperature exceeds 1100 °C, a dramatic acceleration in grain growth is observed.
- (4)
- At a constant heating temperature, the austenite grain size increases with prolonged holding time, while the rate of grain growth progressively decreases. Based on the experimental data and the kinetic theory of austenite grain growth, the corresponding kinetic model for austenite grain growth was derived for No. 2 Steel, which contains 0.38% Nb. .
Author Contributions
Conceptualization, L.H. and T.M.; methodology, T.M.; software, L.H.; validation, J.G., Y.L. and H.Z.; formal analysis, T.M.; investigation, Y.L.; resources, Y.L.; data curation, L.H.; writing—original draft preparation, L.H.; writing—review and editing, T.M.; visualization, H.Z.; supervision, H.Z.; project administration, W.G. and J.G.; funding acquisition, Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the High-level Talent Project of Hebei (Grant No. E2019100007), the National Natural Science Foundation of China (No. 51974129), and the Tangshan Science and Technology Program (No. 22130223H).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chen, S.; Rana, R.; Haldar, A.; Ray, R.K. Current state of Fe-Mn-Al-C low density steels. Prog. Mater. Sci. 2017, 89, 345–391. [Google Scholar] [CrossRef]
- Shivkumar, K.; Raju, B.G.; Prasad, V.S. A review on the current status of Fe–Al based ferritic lightweight steel. Def. Technol. 2023, 26, 1–22. [Google Scholar]
- Bai, S.; Chen, Y.; Liu, X.; Lu, H.; Bai, P.; Li, D.; Huang, Z.; Li, J. Research status and development prospect of Fe–Mn–C–Al system low-density steels. J. Mater. Res. Technol. 2023, 25, 1537–1559. [Google Scholar] [CrossRef]
- Gomez, A.; Banis, A.; Avella, M.; Molina, M.J.; Petrov, R.; Dutta, A.; Sabirov, I. The effect of κ-carbides on high cycle fatigue behavior of a Fe-Mn-Al-C lightweight steel. Int. J. Fatigue 2024, 184, 108306. [Google Scholar] [CrossRef]
- Sozańska-Jędrasik, L.; Mazurkiewicz, J.; Matus, K.; Borek, W. Structure of Fe-Mn-Al-C Steels after Gleeble Simulations and Hot-Rolling. Materials 2020, 13, 739. [Google Scholar] [CrossRef]
- Alexandros, B.; Andrea, G.; Aniruddha, D.; Ilchat, S.; Roumen, H.P. The effect of nano-sized κ-carbides on the mechanical properties of an Fe-Mn-Al-C alloy. Mater. Charact. 2023, 205, 113364. [Google Scholar]
- Barona-Osorio, G.M.; Teran, L.A.; Rodríguez, S.A.; Coronado, J.J. On the Tribocorrosion Behavior of Fe-Mn-Al-C Alloys in Ringer’s Solution. Metals 2022, 12, 1339. [Google Scholar] [CrossRef]
- Li, G.; Kong, L.; Liu, E.; Zhang, X.; Cao, W.; Wang, Y. Effect of Aging Effect of Aging Treatment on the Microstructure and Mechanical Properties of Fe-Mn-Al-C Low Density Steel. IOP Conf. Ser. Mater. Sci. Eng. 2022, 1249, 12053. [Google Scholar] [CrossRef]
- Riaz, T.; Das, S.; Sahu, T.; Chakraborti, P.; Sahu, P. Dislocation substructures in tensile deformed Fe–Mn–Al–C steel. Mater. Lett. 2021, 282, 128691. [Google Scholar] [CrossRef]
- An, J.; Cai, Z.; Cheng, B.; Zhu, M. Nb–Ti composite precipitation behaviour and its effect on the growth of austenite grains in peritectic steel. Ironmak. Steelmak. 2023, 50, 410–417. [Google Scholar] [CrossRef]
- Chamanfar, A.; Chentouf, S.; Jahazi, M.; Lapierre-Boire, L. Austenite grain growth and hot deformation behavior in a medium carbon low alloy steel. J. Mater. Res. Technol. 2020, 9, 12102–12114. [Google Scholar] [CrossRef]
- Meng, F.; Wang, J.; Guo, J.; Fu, K.; Zhao, M.; Wang, Z.; Xu, Z. Growth behavior and kinetics of austenite grain in low-carbon high-strength steel with copper. Mater. Res. Express 2021, 8, 096504. [Google Scholar] [CrossRef]
- Kotan, H.; Darling, A. Phase transformation and grain growth behavior of a nanocrystalline 18/8 stainless steel. Mater. Sci. Eng. A 2017, 686, 168–175. [Google Scholar] [CrossRef]
- Gorbachev, I.I.; Korzunova, E.I.; Popov, V.V.; Khabibulin, D.M.; Urtsev, N.V. Simulation of Austenite Grain Growth in Low-Alloyed Steels upon Austenitization. Phys. Met. Metallogr. 2023, 124, 290–295. [Google Scholar] [CrossRef]
- Raj, R.; Wiskel, B.; Gaudet, J.; Ivey, D.; Henein, H. Model and validation of NbC nanoprecipitation during TMCP of X70 microalloyed steels. J. Mater. Res. Technol. 2025, 36, 4750–4760. [Google Scholar] [CrossRef]
- Park, C.B.; Kim, D.S.; Park, I.; Shin, J.; Jang, J.; Kang, N. Effect of Nb on Austenite Grain Growth in 10Cr-3Co-2W Martensitic Heat-Resistant Steel. Met. Mater. Int. 2024, 30, 3311–3319. [Google Scholar] [CrossRef]
- Challa, V.S.A.; Zhou, W.; Misra, R.D.K.; O’Malley, R.; Jansto, S.G. The effect of coiling temperature on the microstructure and mechanical properties of a niobium–titanium microalloyed steel processed via thin slab casting. Mater. Sci. Eng. A 2014, 595, 143–153. [Google Scholar] [CrossRef]
- Ji, G.; Gao, X.; Liu, Z.; Zhang, K. In situ observation and modeling of austenite grain growth in a Nb–Ti-bearing high carbon steel. J. Iron Steel Res. Int. 2019, 26, 292–300. [Google Scholar] [CrossRef]
- Tian, S.; Liu, Z.; Fu, R.; Wang, X. Effect of niobium alloying on the austenite grain growth and mechanical properties of ultrahigh-strength stainless steel. Mater. Res. Express 2022, 9, 026511. [Google Scholar] [CrossRef]
- Huo, L.; Gao, J.; Li, Y.; Xu, P.; Wei, X.; Ma, T. Effect of Nb Alloying and Solution Treatment on the Mechanical Properties of Cold-Rolled Fe-Mn-Al-C Low-Density Steel. Metals 2025, 15, 102. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Wan, X.; Zhou, S.; Wei, R.; Zhu, C.; Li, G. Impacts of Nb on grain refinement in a simulated coarse-grained-heat-affected-zone of ultra-high-strength steels. Sci. Technol. Weld. Join. 2023, 28, 608–618. [Google Scholar] [CrossRef]
- Genki, S.; Norihito, S.; Kiyotaka, M.; Taichi, S.; Takuya, Y. Effects of Normalizing Temperature on the Precipitation of Fine Particles and Austenite Grain Growth during Carburization of Al- and Nb-Microalloyed Case-Hardening Steel. ISIJ Int. 2022, 63, 727–736. [Google Scholar]
- Dong, Y.; Xu, R.; Xia, D.; Yang, X.; Wu, S.; Li, S.; Sun, X.; Hu, J. Study on Austenite Grain Growth Behavior of GCr15 Bearing Steel. J. Phys. Conf. Ser. 2024, 2694, 12027. [Google Scholar] [CrossRef]
- Liu, P.; Xu, X.; Liu, Q.; Li, J.; Liu, D.; Yan, Z.; Sun, M.; Wang, X. Solid solution precipitation behavior of Nb in high alumina ferritic steel. J. Eng. Sci. 2019, 41, 882–888. [Google Scholar]
- Yong, Q.; Zheng, L.; Sun, Z. Preliminary study on ordered NbC in Nb-containing microalloyed steels. J. Met. 1986, 6, 81–83. [Google Scholar]
- Powwll, D.; Pilkington, R.; Miller, D. The precipitation characteristics of 20% Cr/25% Ni-Nb stabilised stainless steel. Acta Metall. 1988, 36, 713–724. [Google Scholar] [CrossRef]
- Wang, M.; Sun, H.; Phaniraj, M.; NamHan, H.; Jiang, J.; Zhou, J. Evolution of microstructure and tensile properties of Fe–18Ni–12Cr based AFA steel during aging at 700 C. Mater. Sci. Eng. A 2016, 672, 23–31. [Google Scholar] [CrossRef]
- Wu, D.; Wang, F.; Cheng, J.; Li, C. Effect of Nb and V on Austenite Grain Growth Behavior of the Cr-Mo-V Steel for Brake Discs. High Temp. Mater. Process. 2018, 37, 899–907. [Google Scholar] [CrossRef]
- Zhang, C.; Wen, L.; Liu, Z. Effect of Precipitates on Austenite Grain Growth Behavior in a Low-Carbon Nb-V Microalloyed Steel. Mater. Sci. Forum 2017, 4502, 783–790. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, C.; Ma, Q.; Zheng, Y.; Li, H.; Gao, Q. Precipitation behavior and strengthening mechanism of NbC phase in alumina-forming austenitic steel. Mater. Sci. Eng. A 2025, 936, 148421. [Google Scholar] [CrossRef]
- Alogab, A.; Matlock, K.; Speer, G. The influence of niobium microalloying on austenite grain coarsening behavior of Ti-modified SAE 8620 steel. ISIJ Int. 2007, 47, 307–316. [Google Scholar] [CrossRef]
- An, X.; Cao, W.; Zhang, X.; Yu, J. Suppress Austenite Grain Coarsening by Nb Alloying in High–Temperature–Pseudo–Carburized Bearing Steel. Materials 2024, 17, 2962. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).