Copper–Ammonia–Thiosulfate Leaching of High-Sulfide Concentrates: Process Optimization and Additive Effects on Gold Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Leaching Experiments
3. Results and Discussion
3.1. Leaching of Gold from Flotation Concentrate
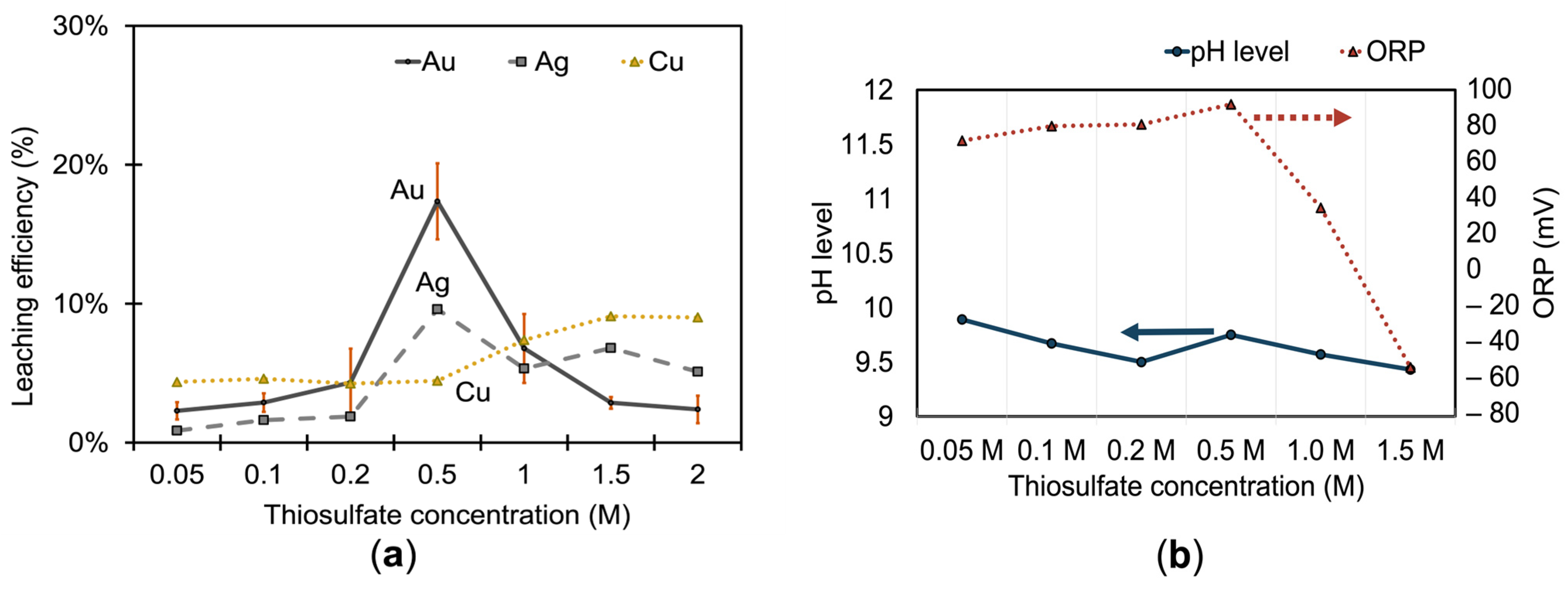
3.1.1. Effect of Thiosulfate on Metal Extraction
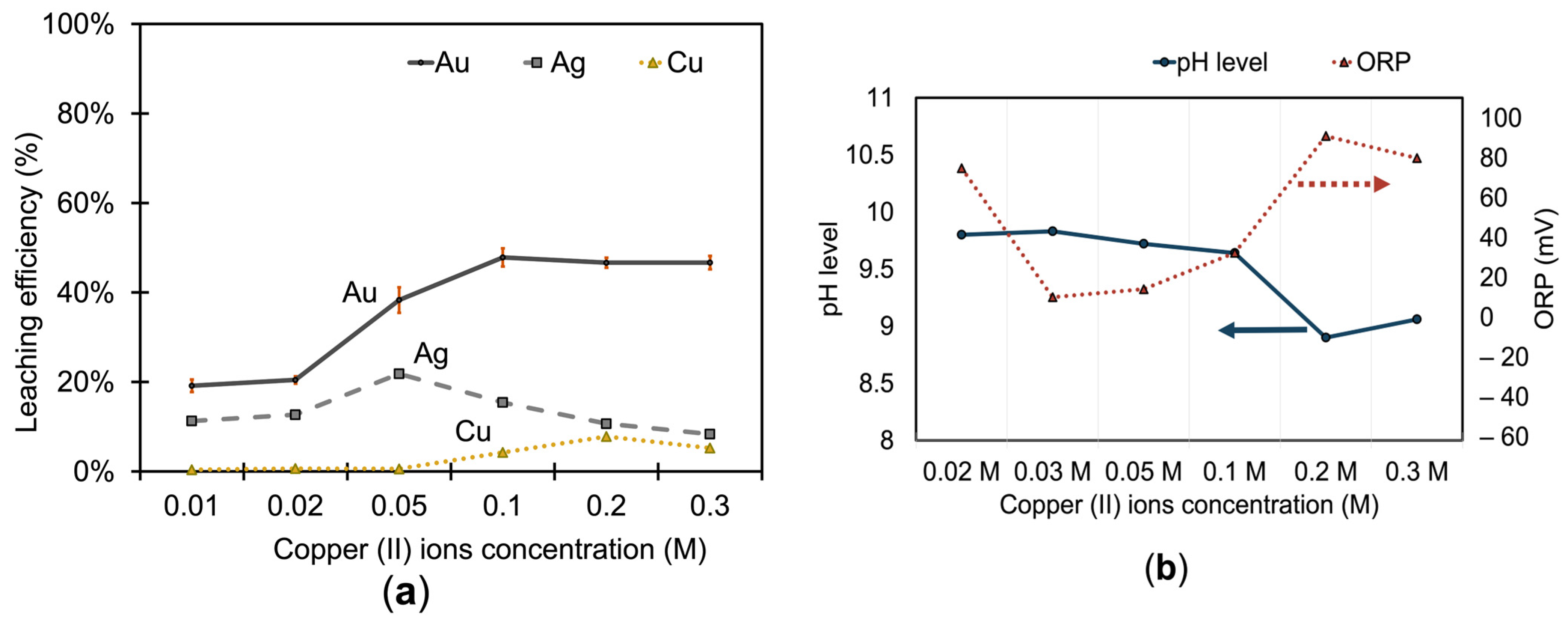
3.1.2. Effect of Copper (II) Ions on Metal Extraction
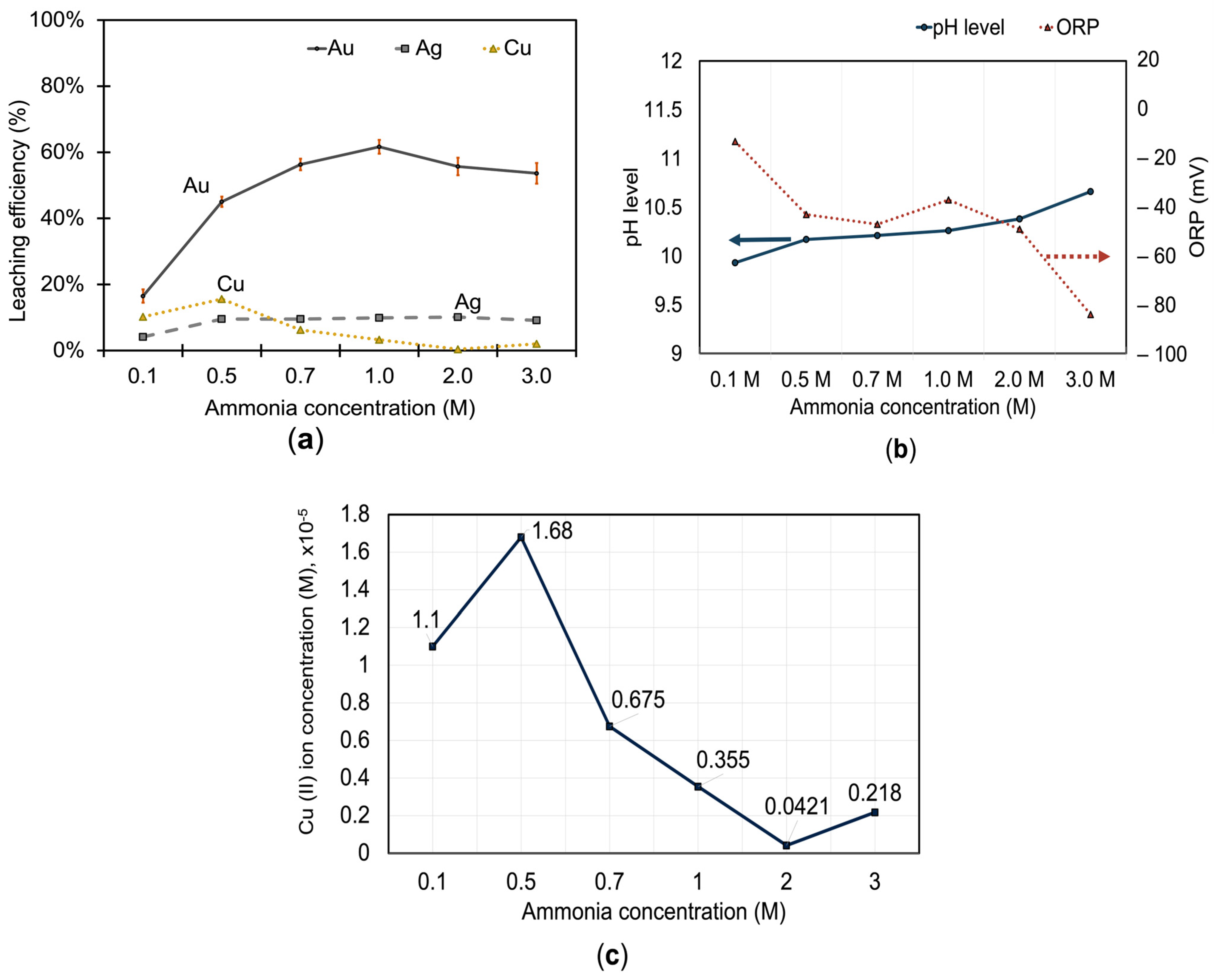
3.1.3. Effect of Ammonia on Metal Extraction
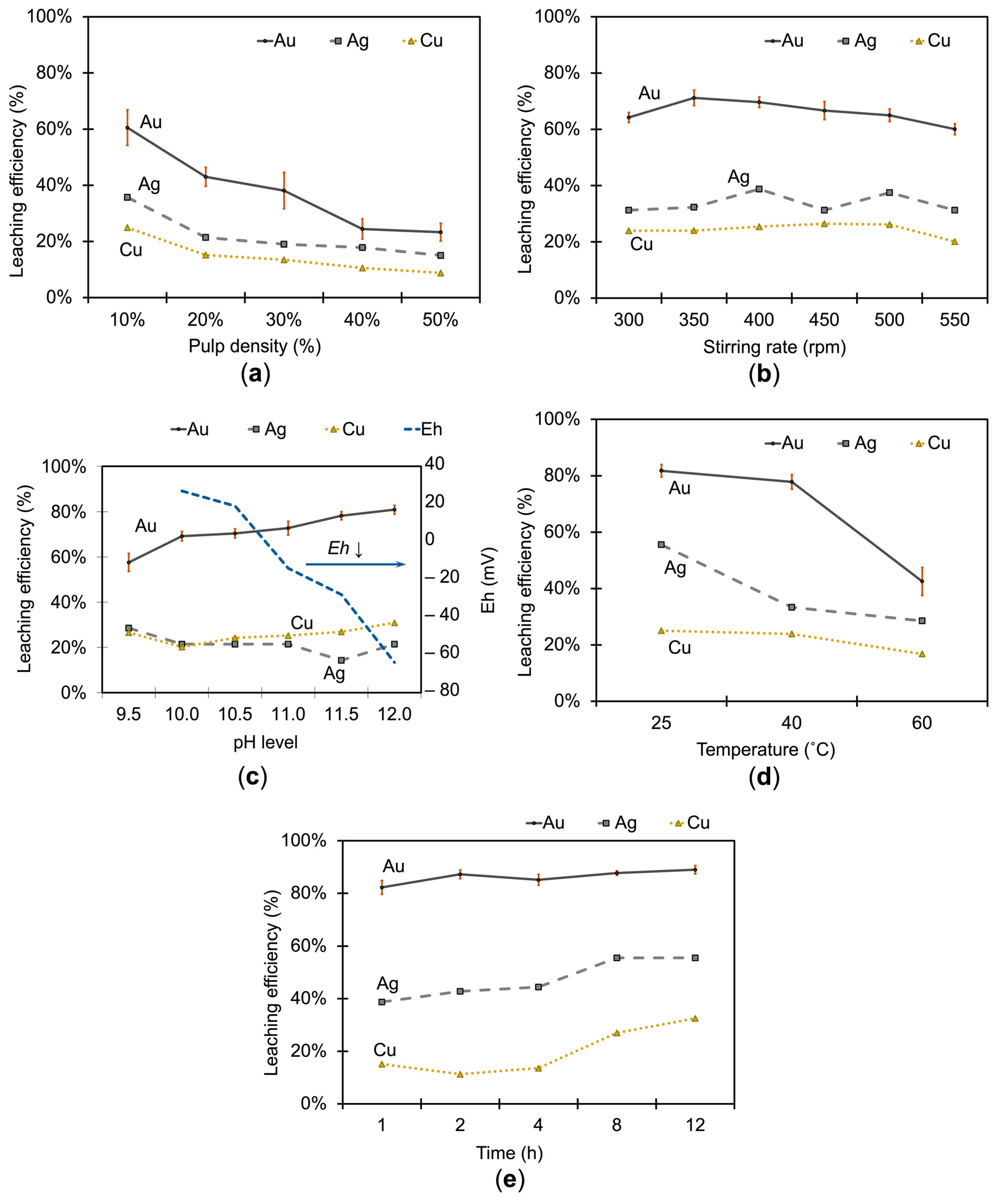
3.1.4. Effect of the Solid-to-Liquid Ratio on Metal Extraction
3.1.5. Effect of the Stirring Rate on Metal Extraction
3.1.6. Effect of the pH Level on Metal Extraction
3.1.7. Effect of Temperature on Metal Extraction
3.1.8. Effect of Time on Metal Extraction
| Subfigure | Variable Studied | Values Tested | Constant Conditions |
|---|---|---|---|
| Figure 7a | Pulp density | 10–50% | 0.5 M S2O32−, 0.1 M Cu2+, 1.0 M NH3, a stirring rate of 400 rpm, a leaching time of 1 h, a temperature of 25 °C, and a pH of 10 |
| Figure 7b | Stirring rate | 300–550 rpm | 0.5 M S2O32−, 0.1 M Cu2+, 1.0 M NH3, a pulp density of 10%, a leaching time of 1 h, a temperature of 25 °C, and a pH of 10 |
| Figure 7c | pH level | 9.5–12.0 | 0.5 M S2O32−, 0.1 M Cu2+, 1.0 M NH3, a pulp density of 10%, a stirring rate of 350 rpm, a leaching time of 1 h, and a temperature of 25 °C |
| Figure 7d | Temperature | 25–60 °C | 0.5 M S2O32−, 0.1 M Cu2+, 1.0 M NH3, a pulp density of 10%, a stirring rate of 350 rpm, a leaching time of 1 h, and a pH of 12 |
| Figure 7e | Time | 1–12 h | 0.5 M S2O32−, 0.1 M Cu2+, 1.0 M NH3, a pulp density of 10%, a stirring rate of 350 rpm, a temperature of 25 °C, and a pH of 12 |
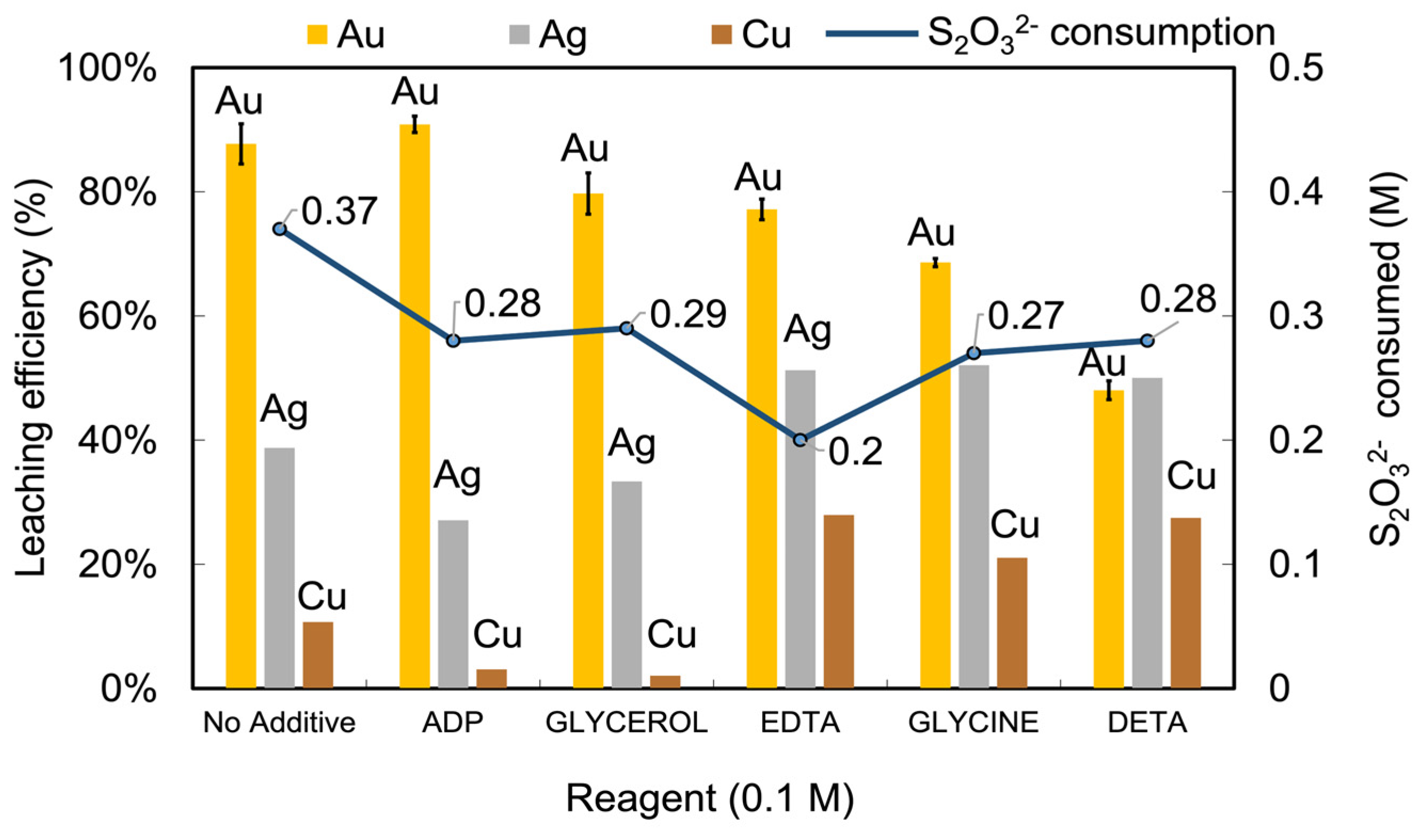
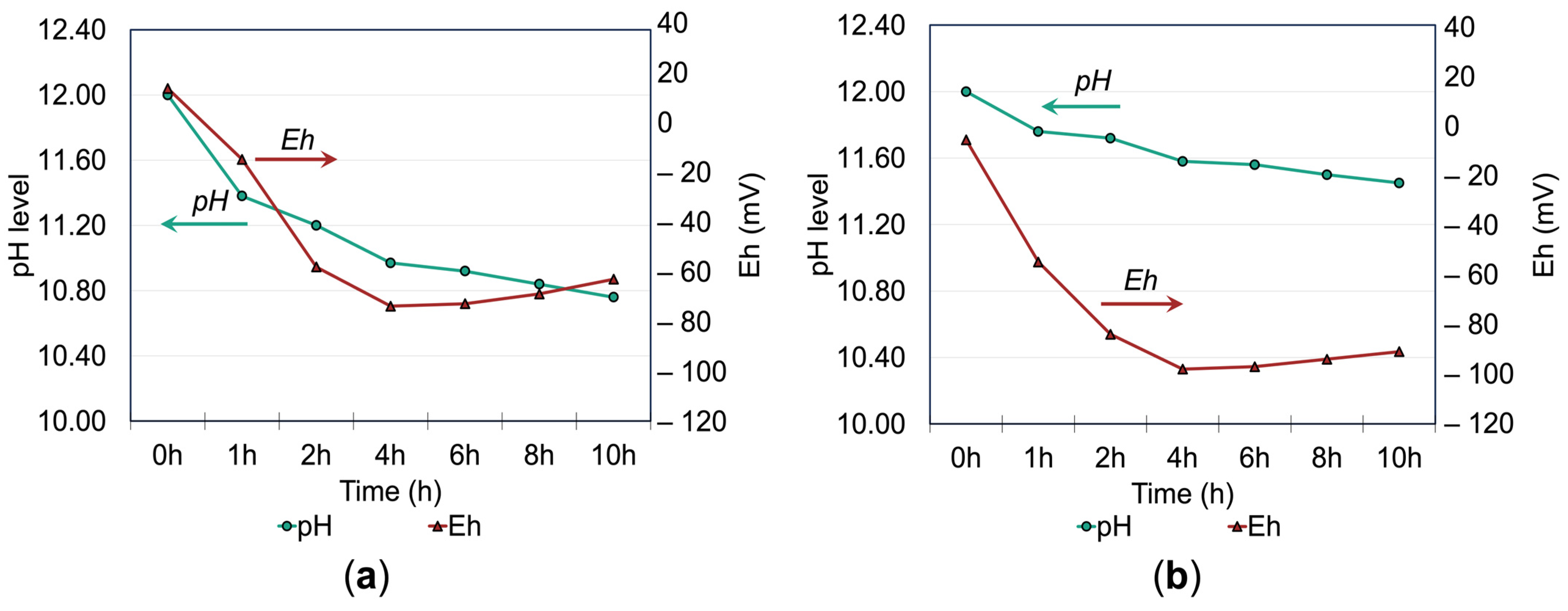
3.2. Effects of Additives on Thiosulfate Decomposition
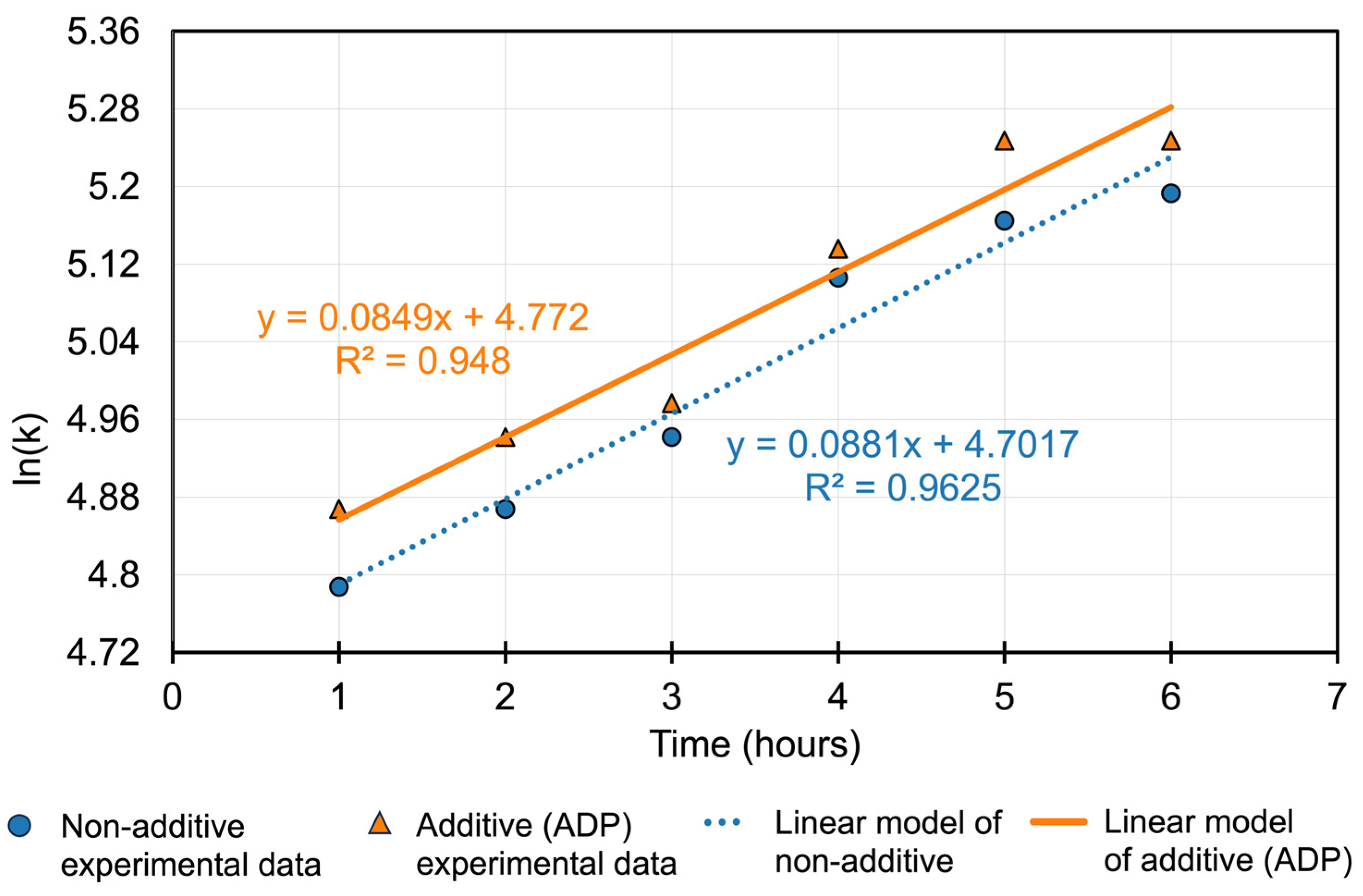
3.3. Kinetic Analysis of Gold Leaching
- Non-additive system: y = 0.0881x + 4.7017 (R2 = 0.9625);
- Additive (ADP) system: y = 0.0849x + 4.772 (R2 = 0.948).
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hegerl, G.C.; Brönnimann, S.; Cowan, T.; Friedman, A.R.; Hawkins, E.; Iles, C.; Müller, W.; Schurer, A.; Undorf, S. Causes of Climate Change over the Historical Record. Environ. Res. Lett. 2019, 14, 123006. [Google Scholar] [CrossRef]
- Chen, L.; Msigwa, G.; Yang, M.; Osman, A.I.; Fawzy, S.; Rooney, D.W.; Yap, P.S. Strategies to Achieve a Carbon Neutral Society: A Review. Environ. Chem. Lett. 2022, 20, 2277–2310. [Google Scholar] [CrossRef] [PubMed]
- Karakurt, O.; Alemdar, E.; Erer, M.C.; Cevher, D.; Gulmez, S.; Taylan, U.; Cevher, S.C.; Hizalan Ozsoy, G.; Ortac, B.; Cirpan, A. Boosting the Efficiency of Organic Solar Cells via Plasmonic Gold Nanoparticles and Thiol Functionalized Conjugated Polymer. Dye. Pigment. 2022, 208, 110818. [Google Scholar] [CrossRef]
- Savory, C.N.; Ganose, A.M.; Travis, W.; Atri, R.S.; Palgrave, R.G.; Scanlon, D.O. An Assessment of Silver Copper Sulfides for Photovoltaic Applications: Theoretical and Experimental Insights. J. Mater. Chem. A 2016, 4, 12648–12657. [Google Scholar] [CrossRef]
- Dissanayake, M.A.K.L.; Umair, K.; Senadeera, G.K.R.; Jaseetharan, T.; Weerasinghe, A.M.J.S.; Wijayasinghe, H.W.M.A.C. Plasmonic Gold Nanoparticle Incorporated MgO-Coated SnO2 Photoanode for Efficiency Enhancement in Dye-Sensitized Solar Cells. Sol. Energy 2022, 233, 363–377. [Google Scholar] [CrossRef]
- Jha, M.C. Refractoriness of Certain Gold Ores to Cyanidation: Probable Causes and Possible Solutions. Miner. Process. Extr. Metall. Rev. 1987, 2, 331–352. [Google Scholar] [CrossRef]
- Akcil, A. A New Global Approach of Cyanide Management: International Cyanide Management Code for the Manufacture, Transport, and Use of Cyanide in the Production of Gold. Miner. Process. Extr. Metall. Rev. 2010, 31, 135–149. [Google Scholar] [CrossRef]
- Udupa, A.R.; Kawatra, S.K.; Prasad, M.S. Developments in Gold Leaching: A Literature Survey. Miner. Process. Extr. Metall. Rev. 1990, 7, 115–135. [Google Scholar] [CrossRef]
- Arslan, F.; Sayiner, B. Extraction of Gold and Silver from Turkish Gold Ore by Ammoniacal Thiosulphate Leaching. Miner. Process. Extr. Metall. Rev. 2008, 29, 68–82. [Google Scholar] [CrossRef]
- Konyratbekova, S.S.; Baikonurova, A.; Akcil, A. Non-Cyanide Leaching Processes in Gold Hydrometallurgy and Iodine-Iodide Applications: A Review. Miner. Process. Extr. Metall. Rev. 2015, 36, 198–212. [Google Scholar] [CrossRef]
- Li, J.; Miller, J.D. A Review of Gold Leaching in Acid Thiourea Solutions. Miner. Process. Extr. Metall. Rev. 2006, 27, 177–214. [Google Scholar] [CrossRef]
- Mohammadi, E.; Pourabdoli, M.; Ghobeiti-Hasab, M.; Heidarpour, A. Ammoniacal Thiosulfate Leaching of Refractory Oxide Gold Ore. Int. J. Miner. Process. 2017, 164, 6–10. [Google Scholar] [CrossRef]
- Jeon, S.; Tabelin, C.B.; Park, I.; Nagata, Y.; Ito, M.; Hiroyoshi, N. Ammonium Thiosulfate Extraction of Gold from Printed Circuit Boards (PCBs) of End-of-Life Mobile Phones and Its Recovery from Pregnant Leach Solution by Cementation. Hydrometallurgy 2020, 191, 105214. [Google Scholar] [CrossRef]
- Aylmore, M.G. Thiosulfate as an Alternative Lixiviant to Cyanide for Gold Ores. In Gold Ore Processing: Project Development and Operations; Elsevier: Amsterdam, The Netherlands, 2016; pp. 485–523. ISBN 9780444636584. [Google Scholar]
- Aylmore, M.G.; Muir, D.M. Thiosulfate leaching of gold—A review. Miner. Eng. 2001, 14, 135–174. [Google Scholar] [CrossRef]
- Xia, C.; Yen, W.T.; Deschenes, G. Improvement of Thiosulfate Stability in Gold Leaching. Miner. Metall. Process. 2003, 20, 68–72. [Google Scholar] [CrossRef]
- Xu, B.; Kong, W.; Li, Q.; Yang, Y.; Jiang, T.; Liu, X. A Review of Thiosulfate Leaching of Gold: Focus on Thiosulfate Consumption and Gold Recovery from Pregnant Solution. Metals 2017, 7, 222. [Google Scholar] [CrossRef]
- Feng, D.; van Deventer, J.S.J. Thiosulphate Leaching of Gold in the Presence of Orthophosphate and Polyphosphate. Hydrometallurgy 2011, 106, 38–45. [Google Scholar] [CrossRef]
- Jian, W.; Feng, X.; Wei, W.; Yunlong, B.; Yan, F.; David, D. Eco-friendly leaching of gold from a carbonaceous gold concentrate in copper-citrate-thiosulfate solutions. Hydrometallurgy 2020, 191, 105204. [Google Scholar] [CrossRef]
- Senanayake, G. Gold Leaching by Copper(II) in Ammoniacal Thiosulphate Solutions in the Presence of Additives. Part I: A Review of the Effect of Hard-Soft and Lewis Acid-Base Properties and Interactions of Ions. Hydrometallurgy 2012, 115–116, 1–20. [Google Scholar] [CrossRef]
- Income, K.; Boonpo, S.; Kruatian, T.; Sooksamiti, P.; Kungwankunakorn, S. Gold Recovery from Copper-Gold Tailings by Ammoniacal Thiosulphate Leaching; ThaiJO: Bangkok, Thailand, 2021; Volume 21. [Google Scholar]
- Feng, D.; van Deventer, J.S.J. Ammoniacal Thiosulphate Leaching of Gold in the Presence of Pyrite. Hydrometallurgy 2006, 82, 126–132. [Google Scholar] [CrossRef]
- Xie, F.; Chen, J.-N.; Wang, J.; Wang, W. Review of Gold Leaching in Thiosulfate-Based Solutions. Trans. Nonferrous Met. Soc. China Engl. Ed. 2021, 31, 3506–3529. [Google Scholar] [CrossRef]
- Navarro, P.; Vargas, C.; Villarroel, A.; Alguacil, F.J. On the Use of Ammoniacal/Ammonium Thiosulphate for Gold Extraction from a Concentrate. Hydrometallurgy 2002, 65, 37–42. [Google Scholar] [CrossRef]
- Sitando, O.; Dai, X.; Senanayake, G.; Nikoloski, A.N.; Breuer, P. A Fundamental Study of Gold Leaching in a Thiosulfate-oxygen-copper System in the Presence of Activated Carbon. Hydrometallurgy 2020, 192, 105232. [Google Scholar] [CrossRef]
- Yener Yazıcı, E.; Celep, O.; Deveci, H.; Deniz BAS, A.; Ozdemir, E.; Yazici, E.Y. Ammoniacal thiosulphate leaching of a copper-rich gold ore. In Proceedings of the 15th Conference on Environment and Mineral Processing, Ostrava, Czech Republic, 8–10 June 2011. [Google Scholar]
- Oraby, E.A.; Browner, R.E.; Nikraz, H.R. Effect of Silver in Thiosulfate Leaching of Gold-Silver Alloys in the Presence of Copper and Ammonia Relative to Pure Gold and Silver. Miner. Process. Extr. Metall. Rev. 2014, 35, 136–147. [Google Scholar] [CrossRef]
- Lampinen, M.; Laari, A.; Turunen, I. Ammoniacal Thiosulfate Leaching of Pressure Oxidized Sulfide Gold Concentrate with Low Reagent Consumption. Hydrometallurgy 2015, 151, 1–9. [Google Scholar] [CrossRef]
- Abbruzzese, C.; Massidda, R.; Vegli, F.; Ubaldini, S. Thiosulphate Leaching for Gold Hydrometallurgy. Hydrometallurgy 1995, 39, 265–276. [Google Scholar] [CrossRef]
- Bae, M.; Kim, S.; Sohn, J.; Yang, D.; Lee, H. Leaching Behavior of Gold and Silver from Concentrated Sulfide Ore Using Ammonium Thiosulfate. Metals 2020, 10, 1029. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Dong, K.; Fu, Y.; Xie, F. Research on Leaching of Carbonaceous Gold Ore with Copper-Ammonia-Thiosulfate Solutions. Miner. Eng. 2019, 137, 232–240. [Google Scholar] [CrossRef]
- Zhang, X.M.; Senanayake, G. A Review of Ammoniacal Thiosulfate Leaching of Gold: An Update Useful for Further Research in Non-Cyanide Gold Lixiviants. Miner. Process. Extr. Metall. Rev. 2016, 37, 385–411. [Google Scholar] [CrossRef]
- Yazıcı, E.Y.; Celep, O.; Deveci, H.; Ahlatcı, F.; Yazici, E.Y.; Celep, O.; Deveci, H. Thiosulphate Leaching of Gold/Silver from a Copper-Bearing Pyritic Gold Concentrate. In Proceedings of the International Mineral Processing Congress (IMPC), Moscow, Russia, 17–21 September 2018. [Google Scholar]
- Yu, H.; Zi, F.; Hu, X.; Zhong, J.; Nie, Y.; Xiang, P. The Copper-Ethanediamine-Thiosulphate Leaching of Gold Ore Containing Limonite with Cetyltrimethyl Ammonium Bromide as the Synergist. Hydrometallurgy 2014, 150, 178–183. [Google Scholar] [CrossRef]
- Wang, J.; Xie, F.; Wang, W.; Bai, Y.; Fu, Y.; Chang, Y. Leaching of Gold from a Free Milling Gold Ore in Copper-Citrate-Thiosulfate Solutions at Elevated Temperatures. Miner. Eng. 2020, 155, 106476. [Google Scholar] [CrossRef]
- Xu, B.; Li, K.; Li, Q.; Yang, Y.; Liu, X.; Jiang, T. Kinetic Studies of Gold Leaching from a Gold Concentrate Calcine by Thiosulfate with Cobalt-Ammonia Catalysis and Gold Recovery by Resin Adsorption from Its Pregnant Solution. Sep. Purif. Technol. 2019, 213, 368–377. [Google Scholar] [CrossRef]
- Oraby, E.A.; Eksteen, J.J. The Leaching of Gold, Silver and Their Alloys in Alkaline Glycine-Peroxide Solutions and Their Adsorption on Carbon. Hydrometallurgy 2015, 152, 199–203. [Google Scholar] [CrossRef]
- Agorhom, E.A.; Skinner, W.; Zanin, M. Post-Regrind Selective Depression of Pyrite in Pyritic Copper-Gold Flotation Using Aeration and Diethylenetriamine. Miner. Eng. 2015, 72, 36–46. [Google Scholar] [CrossRef]
- Xiang, P.Z.; Ye, G.H. Electrochemical Analysis of Copper-EDTA-Ammonia-Gold Thiosulfate Dissolution System. Green Process. Synth. 2023, 12, 20230110. [Google Scholar] [CrossRef]
- Aazami, M.; Lapidus, G.T.; Azadeh, A. The Effect of Solution Parameters on the Thiosulfate Leaching of Zarshouran Refractory Gold Ore. Int. J. Miner. Process. 2014, 131, 43–50. [Google Scholar] [CrossRef]
- Godigamuwa, K.; Okibe, N. Gold Leaching from Printed Circuit Boards Using a Novel Synergistic Effect of Glycine and Thiosulfate. Minerals 2023, 13, 1270. [Google Scholar] [CrossRef]
- O’Connor, G.M.; Lepkova, K.; Eksteen, J.J.; Oraby, E.A. Electrochemical Behaviour of Copper in Alkaline Glycine Solutions. Hydrometallurgy 2018, 181, 221–229. [Google Scholar] [CrossRef]
- Soap & Detergent Association. Glycerine: An Overview Terms Technical Data Properties Performance; Soap & Detergent Association (SDA): Washington, DC, USA, 1990.
- Feng, D.; van Deventer, J.S.J. Thiosulphate Leaching of Gold in the Presence of Ethylenediaminetetraacetic Acid (EDTA). Miner. Eng. 2010, 23, 143–150. [Google Scholar] [CrossRef]
- Munive, G.T.; Encinas, M.A.; Salazar Campoy, M.M.; Álvarez, V.E.; Vazquez, V.M.; Choque, D.C. Leaching Gold and Silver with an Alternative System: Glycine and Thiosulfate from Mineral Tailings. JOM 2020, 72, 918–924. [Google Scholar] [CrossRef]
- Oraby, E.A.; Eksteen, J.J. The Selective Leaching of Copper from a Gold-Copper Concentrate in Glycine Solutions. Hydrometallurgy 2014, 150, 14–19. [Google Scholar] [CrossRef]
- Tanda, B.C.; Eksteen, J.J.; Oraby, E.A. An Investigation into the Leaching Behaviour of Copper Oxide Minerals in Aqueous Alkaline Glycine Solutions. Hydrometallurgy 2017, 167, 153–162. [Google Scholar] [CrossRef]
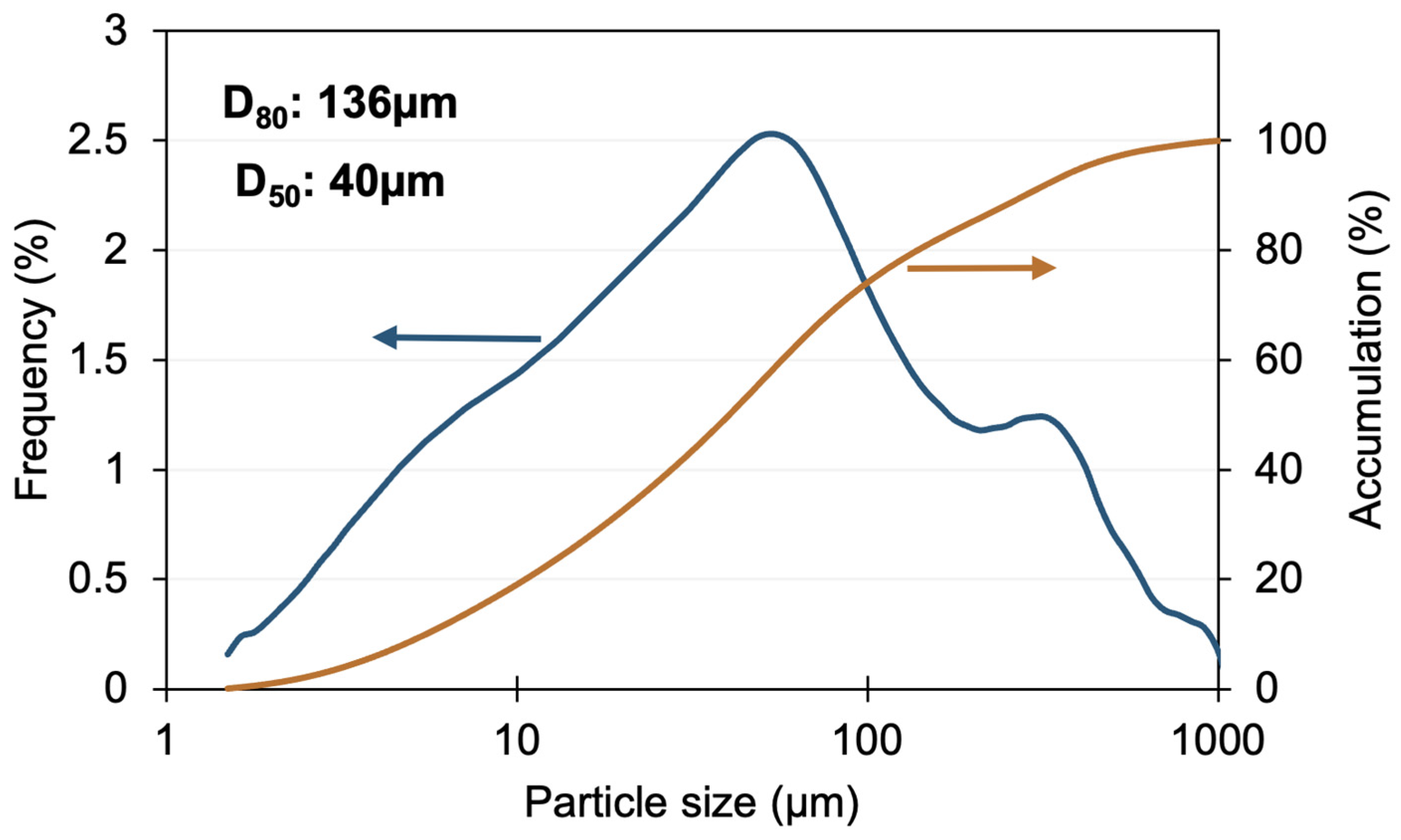
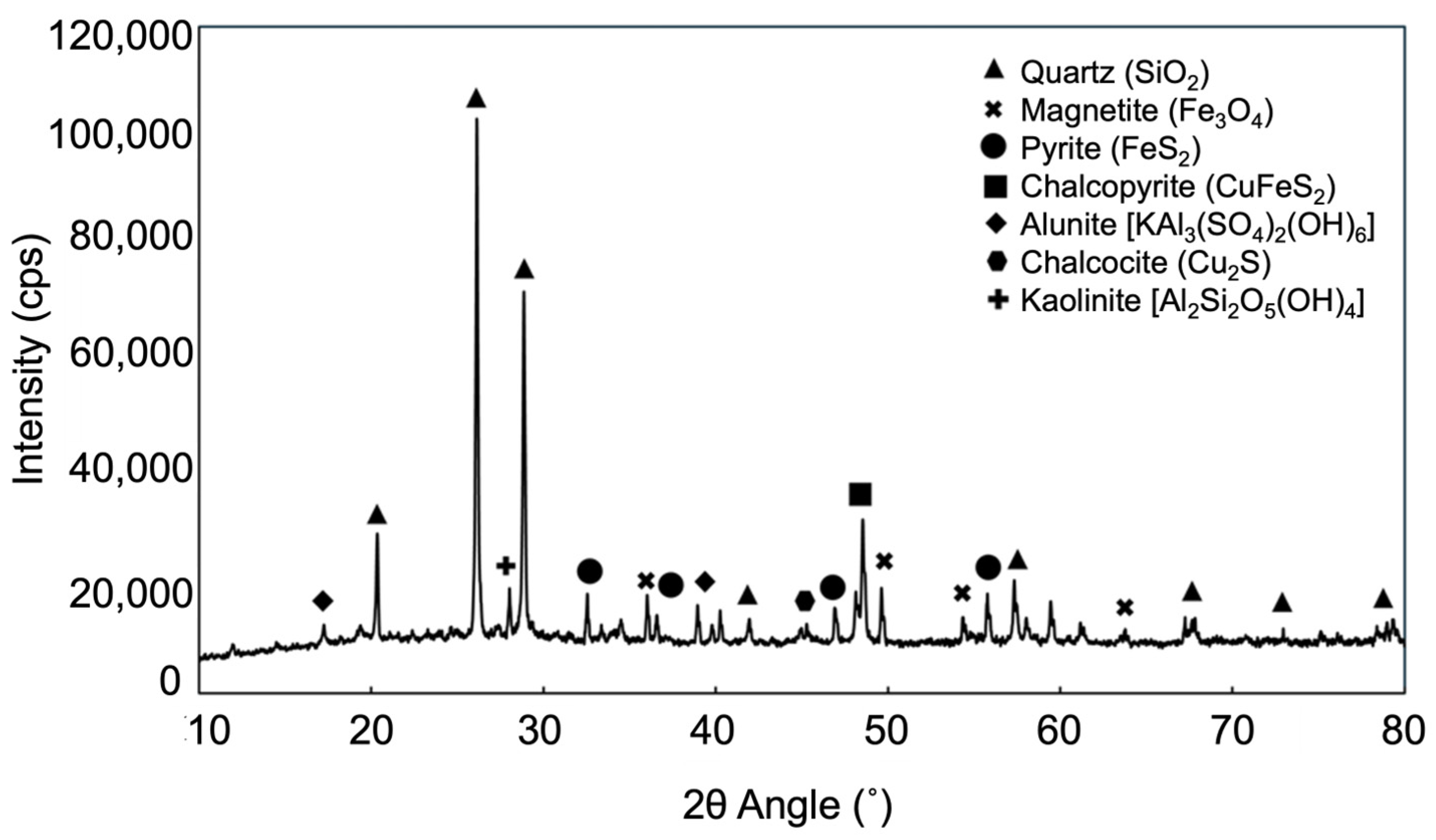



| Element | O | Si | Fe | S | Cu | Al | C | Zn | Pb | Ag | Au |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Contents (wt.%) | 36.17 | 18.73 | 11.30 | 11.06 | 6.89 | 6.55 | 4.08 | 0.98 | 0.29 | 160 1 | 190 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buronov, A.B.; Yayabu, B.K.; Godirilwe, L.L.; Altansukh, B.; Jeon, S.; Haga, K.; Shibayama, A. Copper–Ammonia–Thiosulfate Leaching of High-Sulfide Concentrates: Process Optimization and Additive Effects on Gold Extraction. Metals 2025, 15, 572. https://doi.org/10.3390/met15060572
Buronov AB, Yayabu BK, Godirilwe LL, Altansukh B, Jeon S, Haga K, Shibayama A. Copper–Ammonia–Thiosulfate Leaching of High-Sulfide Concentrates: Process Optimization and Additive Effects on Gold Extraction. Metals. 2025; 15(6):572. https://doi.org/10.3390/met15060572
Chicago/Turabian StyleBuronov, Azizbek Bolikulovich, Blackie Korul Yayabu, Labone Lorraine Godirilwe, Batnasan Altansukh, Sanghee Jeon, Kazutoshi Haga, and Atsushi Shibayama. 2025. "Copper–Ammonia–Thiosulfate Leaching of High-Sulfide Concentrates: Process Optimization and Additive Effects on Gold Extraction" Metals 15, no. 6: 572. https://doi.org/10.3390/met15060572
APA StyleBuronov, A. B., Yayabu, B. K., Godirilwe, L. L., Altansukh, B., Jeon, S., Haga, K., & Shibayama, A. (2025). Copper–Ammonia–Thiosulfate Leaching of High-Sulfide Concentrates: Process Optimization and Additive Effects on Gold Extraction. Metals, 15(6), 572. https://doi.org/10.3390/met15060572









