Nanoporous CuAuPtPd Quasi-High-Entropy Alloy Prism Arrays for Sustainable Electrochemical Nitrogen Reduction
Abstract
1. Introduction
2. Materials and Methods
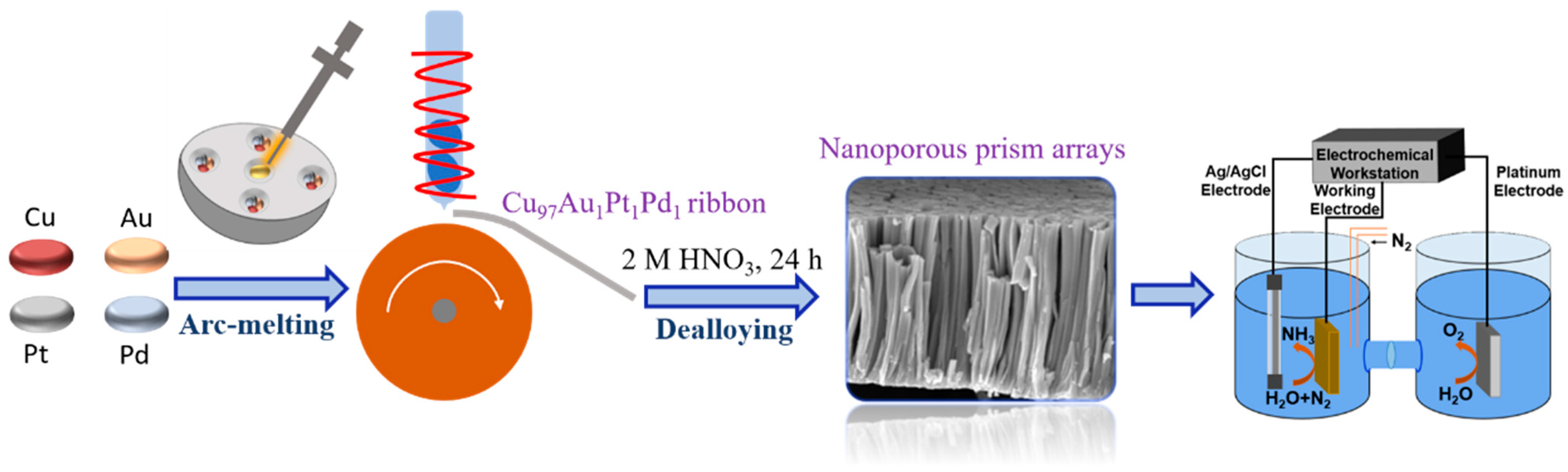
2.1. Preparation of Materials
2.2. Preparation of Electrodes
2.3. Electrochemical Tests
3. Results and Discussion
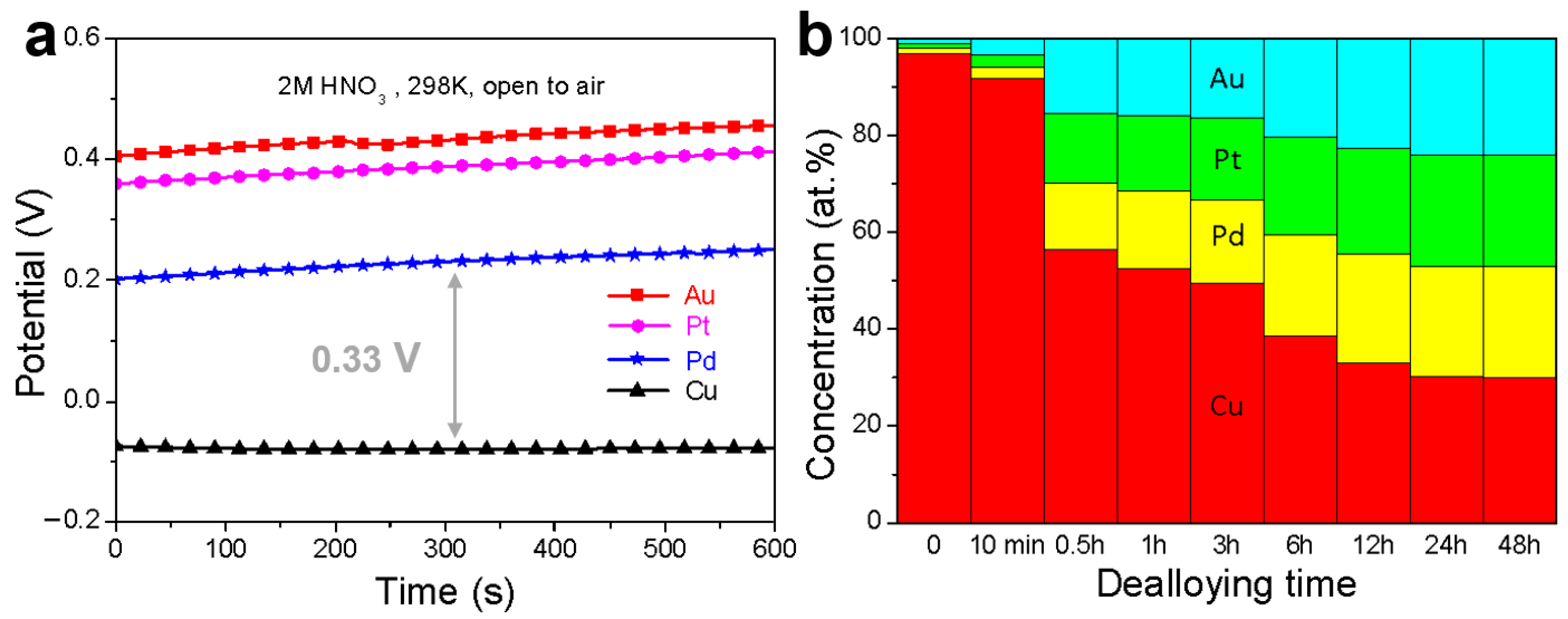
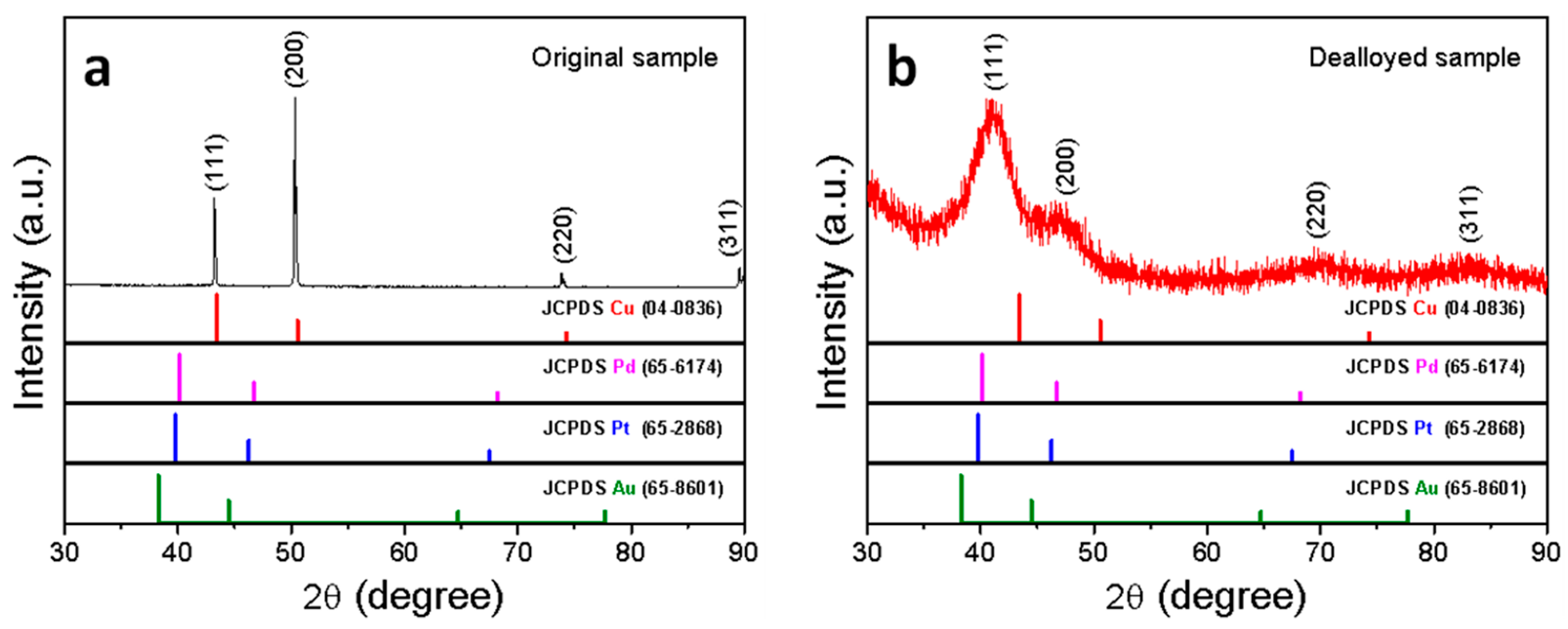
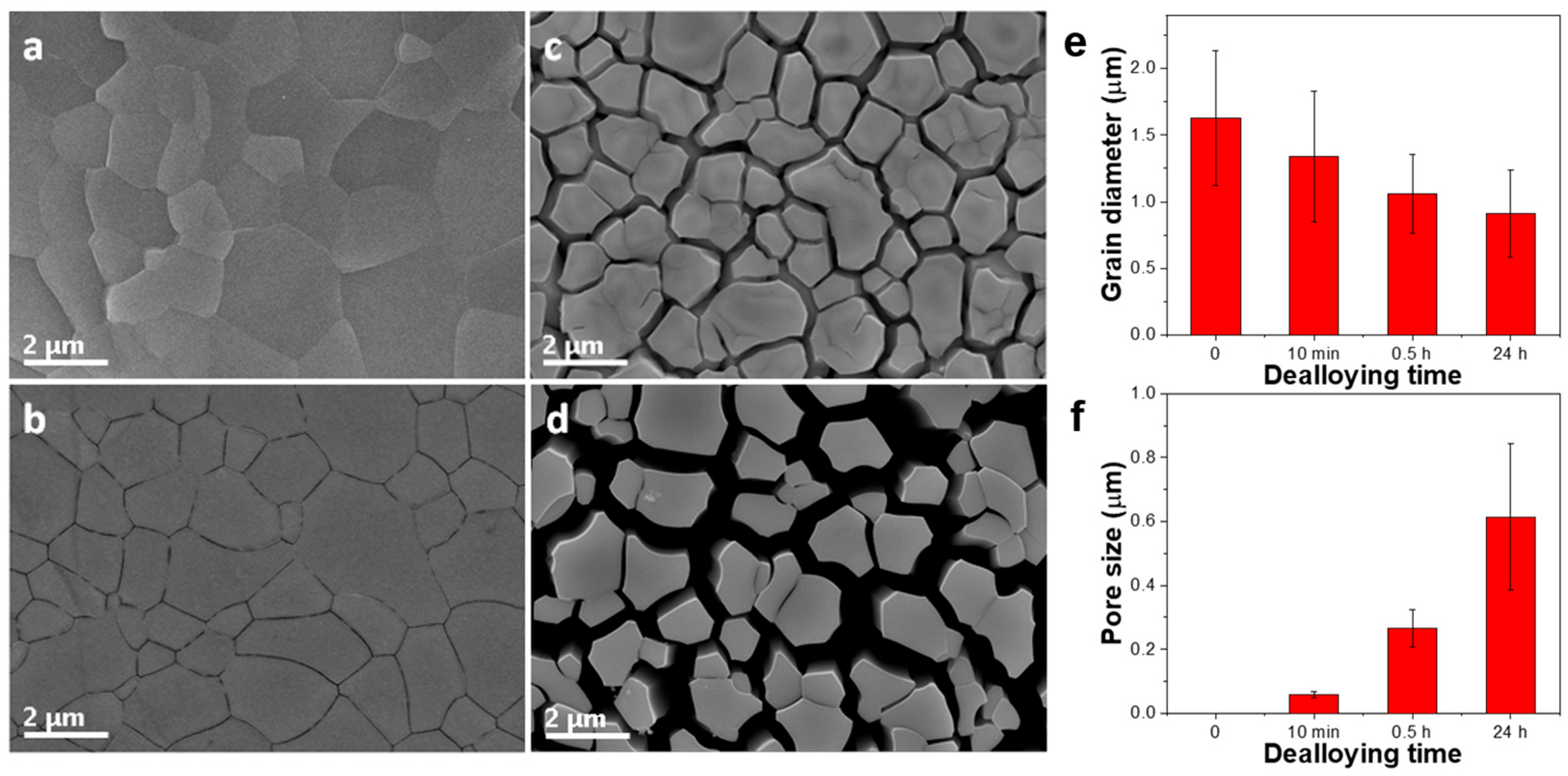
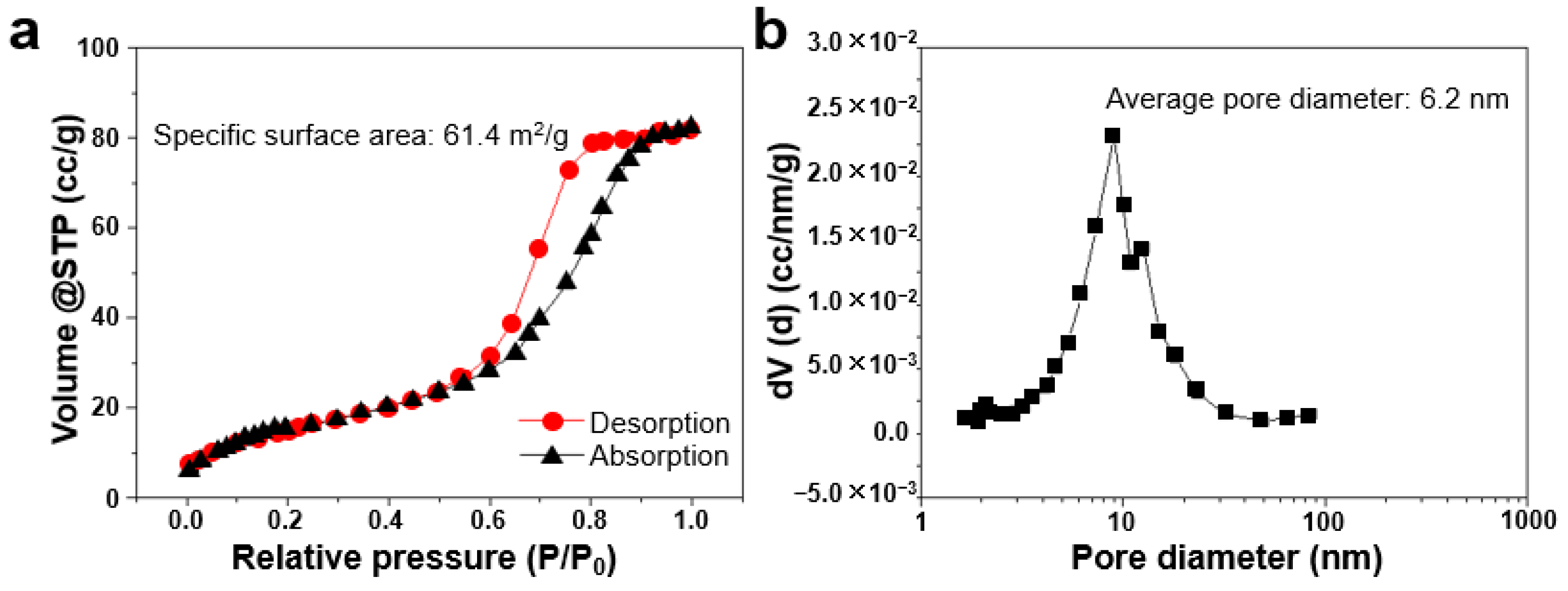
3.1. Fabrication and Characterization of np-CuAuPtPd-1
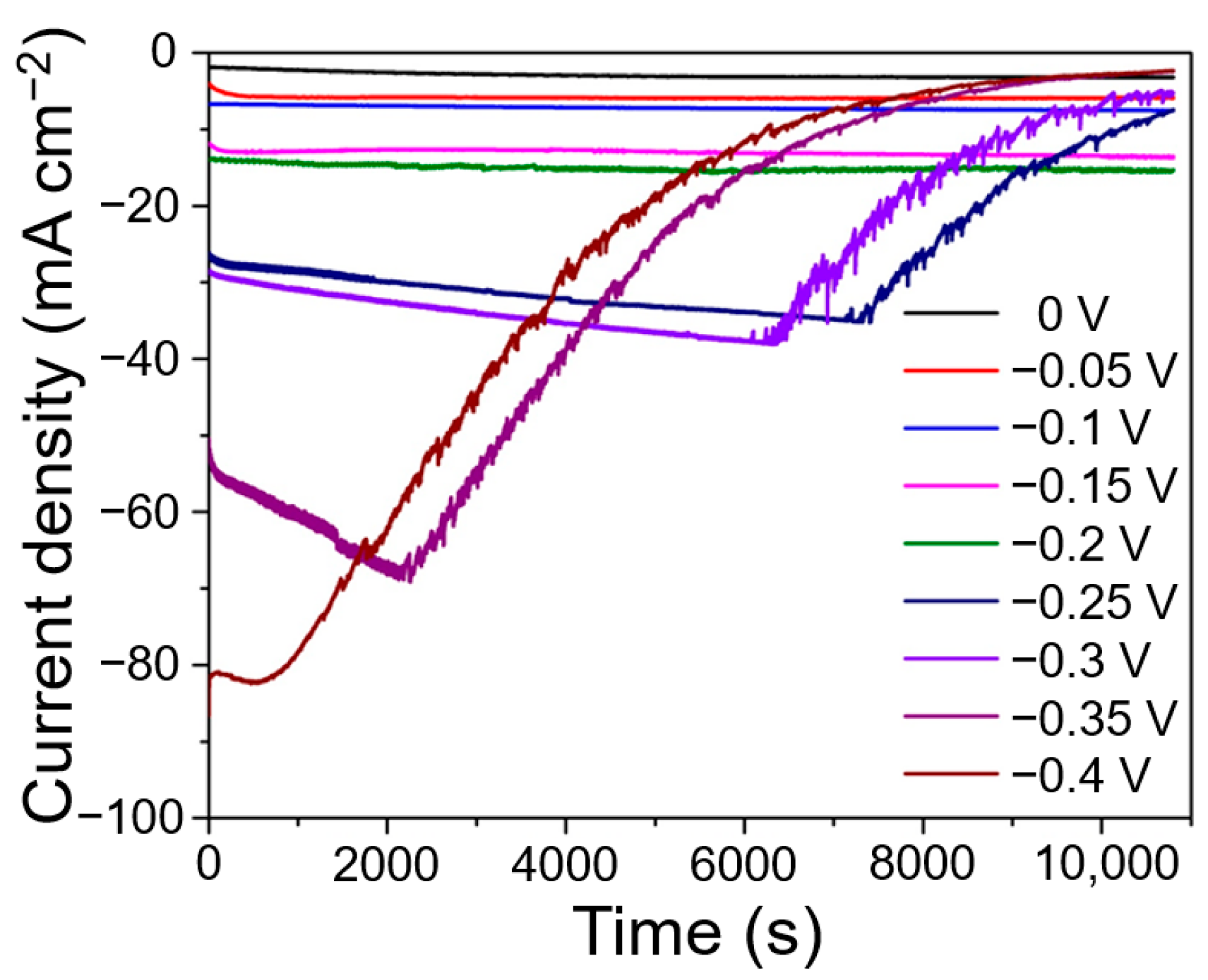
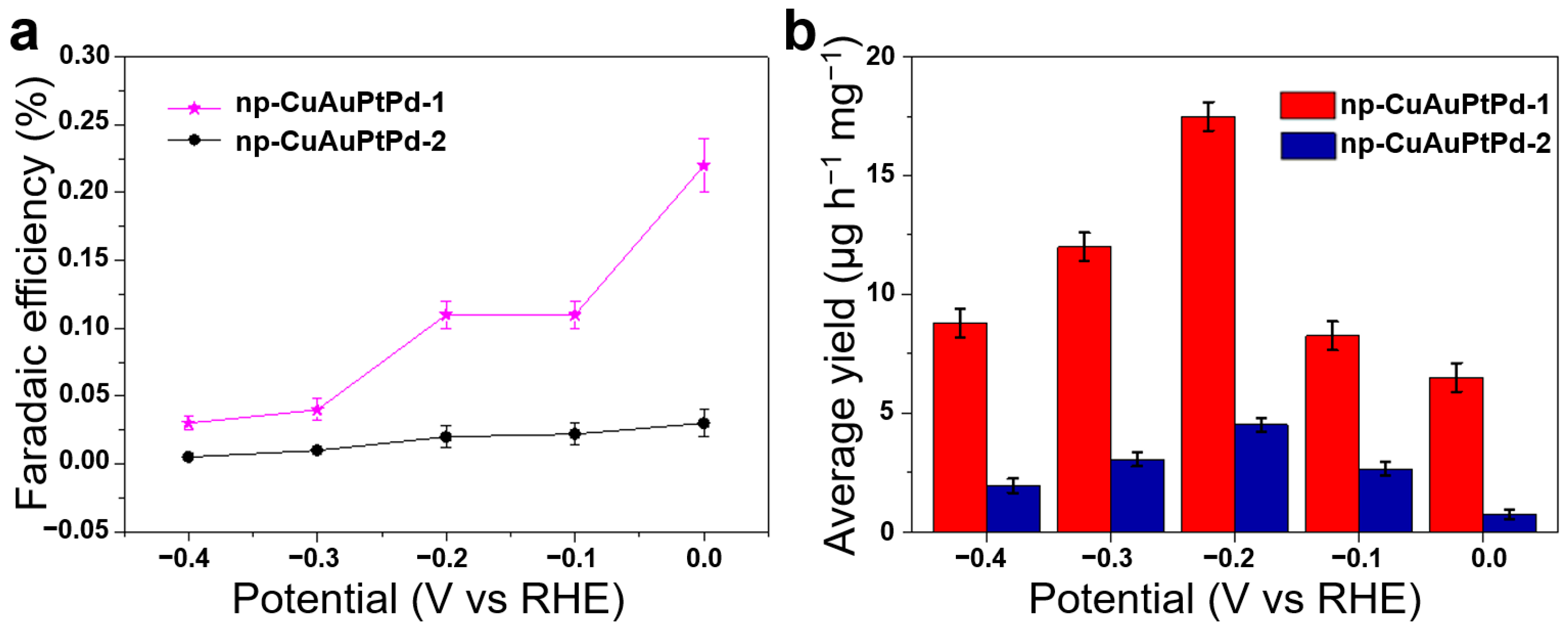
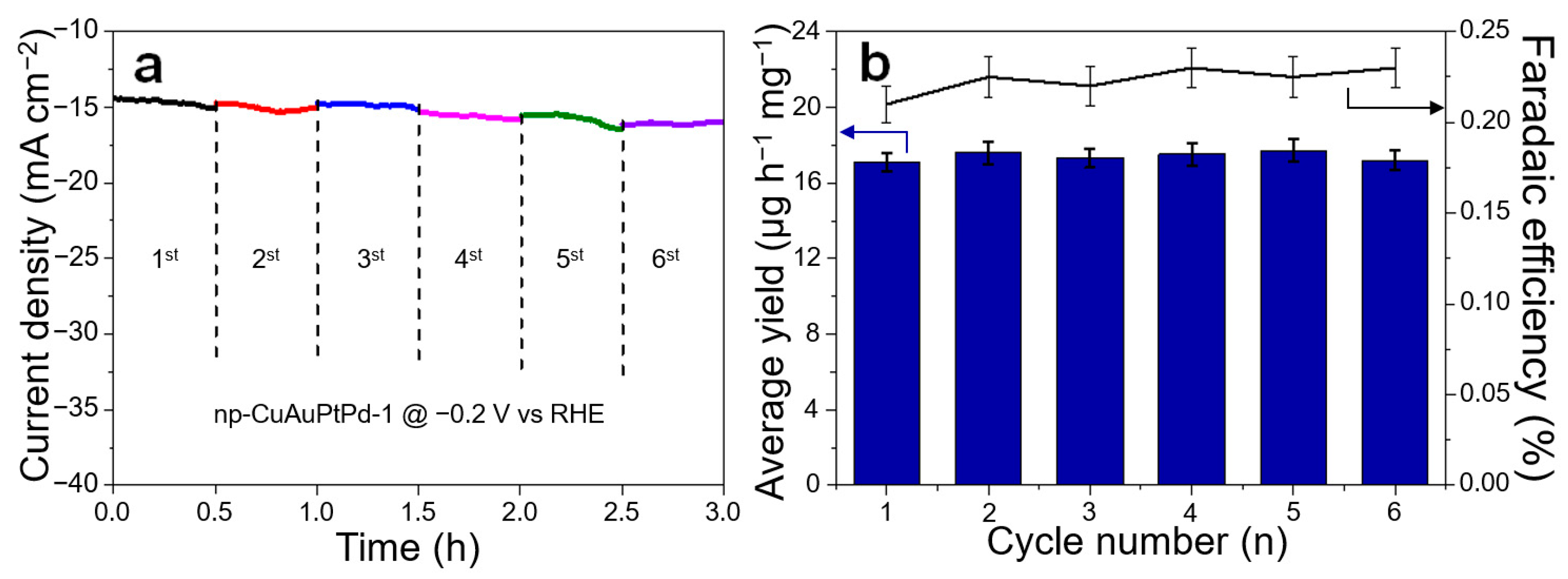
3.2. Electrocatalytic NRR Activity and Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kang, L.W.; Pan, W.G.; Zhang, J.K.; Wang, W.H.; Tang, C.W. A review on ammonia blends combustion for industrial applications. Fuel 2023, 332, 126150. [Google Scholar] [CrossRef]
- Xu, P.; Li, G.; Zheng, Y.; Fung, J.C.H.; Chen, A.P.; Zeng, Z.Z.; Shen, H.Z.; Hu, M.; Mao, J.F.; Zheng, Y.; et al. Fertilizer management for global ammonia emission reduction. Nature 2024, 626, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Ouyang, W.; Gao, A.; Yu, C.; Zhang, L. Promoting electrocatalytic nitrogen reduction to ammonia via dopant boron on two-dimensional materials. Rare Met. 2024, 43, 5117–5125. [Google Scholar] [CrossRef]
- Wu, X.; Tang, L.; Si, Y.; Ma, C.; Zhang, P.; Yu, J.; Liu, Y.; Ding, B. Smart interfacing between Co-Fe layered double hydroxide and graphitic carbon nitride for high-efficiency electrocatalytic nitrogen reduction. Energy Environ. Mater. 2023, 6, e12316. [Google Scholar] [CrossRef]
- He, Y.; Liu, S.; Wang, M.; Cheng, Q.; Ji, H.; Qian, T.; Yan, C. Advanced in situ characterization techniques for direct observation of gas-involved electrochemical reactions. Energy Environ. Mater. 2023, 6, e12552. [Google Scholar] [CrossRef]
- Wang, Z.F.; Wang, H.Y.; Liu, X.L.; Chen, Y.X.; Zhao, Y.; Zhang, Y.G.; Han, Q.Q.; Qin, C.L.; Bakenov, Z.; Wang, Y.C.; et al. Single Zn atoms anchored on hollow carbon nanofiber network for dendrite-free lithium metal anode of flexible Li-S full cell. Rare Met. 2023, 42, 3705–3717. [Google Scholar] [CrossRef]
- Awad, O.I.; Zhou, B.; Harrath, K.; Kadirgama, K. Characteristics of NH3/H2 blend as carbon-free fuels: A review. Int. J. Hydrogen Energy 2023, 48, 38077–38100. [Google Scholar] [CrossRef]
- Yin, Z.; Li, Y.; Ye, Y.; Liu, Y.; Li, M.; Yang, Z.; Zheng, X.; Wang, H.; Wang, Y.; Deng, Y. Sp/sp2 carbon ratio-driven high-throughput screening of electrocatalytic nitrogen reduction performance on transition metal single-atom catalysts. Rare Met. 2024, 43, 5781–5791. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, J.; Yang, Y.; Wang, X. Efficient electrocatalytic ammonia synthesis via theoretical screening of titanate nanosheet-supported single-atom catalysts. Materials 2024, 17, 2239. [Google Scholar] [CrossRef]
- Kojima, Y. Hydrogen storage materials for hydrogen and energy carriers. Int. J. Hydrogen Energy 2019, 44, 18179–18192. [Google Scholar] [CrossRef]
- Asif, M.; Bibi, S.S.; Ahmed, S.; Irshad, M.; Hussain, M.S.; Zeb, H.; Khan, M.K.; Kim, J. Recent advances in green hydrogen production, storage and commercial-scale use via catalytic ammonia cracking. Chem. Eng. J. 2023, 473, 145381. [Google Scholar] [CrossRef]
- Dong, G.; Huang, C.; Chen, F.; Liu, X.; Li, Z.; Su, X.; Zeng, T.; Chen, Y.; Chen, Y.; Wang, Y. Construction of electron rich Fe active sites by FeCu alloy anchoring on carbon nitride for photocatalytic nitrogen reduction. Rare Met. 2024, 43, 1570–1579. [Google Scholar] [CrossRef]
- Erfani, N.; Baharudin, L.; Watson, M. Recent advances and intensifications in Haber-Bosch ammonia synthesis process. Chem. Eng. Process 2024, 204, 109962. [Google Scholar] [CrossRef]
- Padinjarekutt, S.; Li, H.Z.; Ren, S.J.; Ramesh, P.; Zhou, F.L.; Li, S.G.; Belfort, G.; Yu, M. Na plus -gated nanochannel membrane for highly selective ammonia (NH3) separation in the Haber-Bosch process. Chem. Eng. J. 2023, 454, 139998. [Google Scholar] [CrossRef]
- Bianchi, F.R.; Bosio, B. Modelling of green ammonia production based on solid oxide cells as electrolyser and oxygen separator for Haber-Bosch loop decarbonization. Int. J. Hydrogen Energy 2024, 95, 1183–1193. [Google Scholar] [CrossRef]
- Zhang, C.L.; Li, J.Y.; Chen, R.M.; Shen, S.J.; Wang, J.L.; Sun, Y.J.; Dong, F. NH3 synthesis from N1 compounds by photocatalytic technology: Promotion mechanism, reaction pathways, and efficiency evaluation criteria. ACS Catal. 2024, 14, 15721–15742. [Google Scholar] [CrossRef]
- Shang, S.Y.; Wang, F.Z.; Sun, Z.Y.; Qiang, C.F.; Chu, K. Electrocatalytic nitrite reduction to ammonia on isolated bismuth alloyed ruthenium. J. Energy Chem. 2025, 100, 369–376. [Google Scholar] [CrossRef]
- Zhou, Y.B.; Wang, M.F.; Zhang, L.F.; Li, N.J.; Qian, T.; Yan, C.L.; Lu, J.M. Quaternary medium-entropy alloy metallene with strong charge polarization for highly selective urea electrosynthesis from carbon dioxide and nitrate. ACS Nano 2025, 19, 7273–7282. [Google Scholar] [CrossRef]
- Yin, H.B.; Peng, Y.; Li, J.H. Electrocatalytic reduction of nitrate to ammonia via a Au/Cu single atom alloy catalyst. Environ. Sci. Technol. 2023, 57, 3134–3144. [Google Scholar] [CrossRef]
- Cui, Z.J.; Zhao, P.W.; Wang, H.H.; Li, C.L.; Peng, W.C.; Fan, X.B.; Liu, J.P. Molten salts etching strategy construct alloy/MXene heterostructures for efficient ammonia synthesis and energy supply via Zn-nitrite battery. Appl. Catal. B 2024, 348, 123862. [Google Scholar] [CrossRef]
- Huang, Z.L.; Rafiq, M.; Woldu, A.R.; Tong, Q.X.; Astruc, D.; Hu, L.S. Recent progress in electrocatalytic nitrogen reduction to ammonia (NRR). Coord. Chem. Rev. 2023, 478, 214981. [Google Scholar] [CrossRef]
- Shen, H.D.; Choi, C.; Masa, J.; Li, X.; Qiu, J.S.; Jung, Y.; Sun, Z.Y. Electrochemical ammonia synthesis: Mechanistic understanding and catalyst design. Chemistry 2021, 7, 1708–1754. [Google Scholar] [CrossRef]
- Shi, L.; Yin, Y.; Wang, S.B.; Sun, H.Q. Rational catalyst design for N2 reduction under ambient conditions: Strategies toward enhanced conversion efficiency. ACS Catal. 2020, 10, 6870–6899. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, T.; Luo, Y.L.; Kong, Q.Q.; Li, T.S.; Lu, S.Y.; Alshehri, A.A.; Alzahrani, K.A.; Sun, X.P. Recent advances in strategies for highly selective electrocatalytic N2 reduction toward ambient NH3 synthesis. Curr. Opin. Electrochem. 2021, 29, 100766. [Google Scholar] [CrossRef]
- Ren, Y.W.; Yu, C.; Tan, X.Y.; Huang, H.L.; Wei, Q.B.; Qiu, J.S. Strategies to suppress hydrogen evolution for highly selective electrocatalytic nitrogen reduction: Challenges and perspectives. Energy Environ. Sci. 2021, 14, 1176–1193. [Google Scholar] [CrossRef]
- Wu, T.W.; Melander, M.M.; Honkala, K. Coadsorption of NRR and HER intermediates determines the performance of Ru-N4 toward electrocatalytic N2 reduction. ACS Catal. 2022, 12, 2505–2512. [Google Scholar] [CrossRef]
- Mushtaq, M.A.; Arif, M.; Yasin, G.; Tabish, M.; Kumar, A.; Ibraheem, S.; Ye, W.; Ajmal, S.; Zhao, J.; Li, P.Y.; et al. Recent developments in heterogeneous electrocatalysts for ambient nitrogen reduction to ammonia: Activity, challenges, and future perspectives. Renew. Sustain. Energy Rev. 2023, 176, 113197. [Google Scholar] [CrossRef]
- Chen, G.F.; Ren, S.Y.; Zhang, L.L.; Cheng, H.; Luo, Y.R.; Zhu, K.H.; Ding, L.X.; Wang, H.H. Advances in electrocatalytic N2 reduction-strategies to tackle the selectivity challenge. Small Methods 2019, 3, 1800337. [Google Scholar] [CrossRef]
- Yang, Y.J.; Wang, S.Q.; Wen, H.M.; Ye, T.; Chen, J.; Li, C.P.; Du, M. Nanoporous gold embedded ZIF composite for enhanced electrochemical nitrogen fixation. Angew. Chem. Int. Ed. 2019, 58, 15362–15366. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, S.Q.; Wang, H.J.; Li, H.; Shao, M.H. A spectroscopic study on the nitrogen electrochemical reduction reaction on gold and platinum surfaces. J. Am. Chem. Soc. 2018, 140, 1496–1501. [Google Scholar] [CrossRef]
- Pang, F.; Wang, F.; Yang, L.; Wang, Z.; Zhang, W. Hierarchical nanoporous Pd1Ag1 alloy enables efficient electrocatalytic nitrogen reduction under ambient conditions. Chem. Commun. 2019, 55, 10108–10111. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Li, C.; Tian, W.; Zhou, T.; Xu, Y.; Wang, Z.; Li, X.; Wang, L.; Wang, H. Modulating surface electronic structure of mesoporous Rh nanoparticles by Se-doping for enhanced electro chemical ammonia synthesis. J. Electroanal. Chem. 2022, 904, 115874. [Google Scholar] [CrossRef]
- Hsu, U.S.; Hung, U.D.; Yeh, J.W.; Chen, S.K.; Huang, Y.S.; Yang, C.C. Alloying behavior of iron, gold and silver in AlCoCrCuNi-based equimolar high-entropy alloys. J. Mater. Sci. Eng. A 2007, 460, 403–408. [Google Scholar] [CrossRef]
- Lu, Y.P.; Gao, X.Z.; Jiang, L.; Chen, Z.; Wang, T.; Jie, J.; Kang, H.; Zhang, Y.; Guo, S.; Ruan, H.; et al. Directly cast bulk eutectic and near-eutectic high entropy alloys with balanced strength and ductility in a wide temperature range. Acta Mater. 2017, 124, 143–150. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Fan, X.Y.; Xie, L.S.; Liang, J.; Ren, Y.C.; Zhang, L.C.; Yue, L.C.; Li, T.S.; Luo, Y.L.; Li, N.; Tang, B.; et al. In situ grown Fe3O4 particle on stainless steel: A highly efficient electrocatalyst for nitrate reduction to ammonia. Nano Res. 2022, 15, 3050–3055. [Google Scholar] [CrossRef]
- Ma, B.Y.; Zhao, H.T.; Li, T.S.; Liu, Q.; Luo, Y.S.; Li, C.B.; Lu, S.Y.; Asiri, A.M.; Ma, D.W.; Sun, X.P. Iron-group electrocatalysts for ambient nitrogen reduction reaction in aqueous media. Nano Res. 2021, 14, 555–569. [Google Scholar] [CrossRef]
- Xu, Y.; Ren, K.L.; Ren, T.L.; Wang, M.Z.; Wang, Z.Q.; Li, X.N.; Wang, L.; Wang, H.J. Ultralow-content Pd in-situ incorporation mediated hierarchical defects in corner-etched Cu2O octahedra for enhanced electrocatalytic nitrate reduction to ammonia. Appl. Catal. B 2022, 306, 121094. [Google Scholar] [CrossRef]
- Qiu, W.B.; Xie, X.Y.; Qiu, J.D.; Fang, W.H.; Liang, R.P.; Ren, X.; Ji, X.Q.; Cui, G.W.; Asiri, A.M.; Cui, G.L.; et al. High-performance artificial nitrogen fixation at ambient conditions using a metal-free electrocatalyst. Nat. Commun. 2018, 9, 3485. [Google Scholar] [CrossRef]
- Zhao, S.L.; Lu, X.Y.; Wang, L.Z.; Gale, J.; Amal, R. Carbon-based metal-free catalysts for electrocatalytic reduction of nitrogen for synthesis of ammonia at ambient conditions. Adv. Mater. 2019, 31, 1805367. [Google Scholar] [CrossRef]
- Balaji, V.; Xavior, M.A. Development of high entropy alloys (HEAs): Current trends. Heliyon 2024, 10, e26464. [Google Scholar]
- Wu, C.H.; Jin, M.H.; Guo, D.Y.; Wu, L.H.; Wang, C.; Chen, X.; Wang, S. Recent development and future perspectives of high entropy alloys for catalysts. Adv. Mater. 2023, 7, 2300192. [Google Scholar] [CrossRef]
- Ren, J.T.; Chen, L.; Wang, H.Y.; Yuan, Z.Y. High-entropy alloys in electrocatalysis: From fundamentals to applications. Chem. Soc. Rev. 2023, 52, 8319–8373. [Google Scholar] [CrossRef]
- Wang, Y.X.; Zhang, Y.; Xing, P.Y.; Li, X.Q.; Du, Q.Y.; Fan, X.Q.; Cai, Z.B.; Yin, R.; Yao, Y.G.; Gan, W.T. Self-encapsulation of high-entropy alloy nanoparticles inside carbonized wood for highly durable electrocatalysis. Adv. Mater. 2024, 36, 2402391. [Google Scholar] [CrossRef]
- Han, X.; Wu, G.; Zhao, S.Y.; Guo, J.J.; Yan, M.Y.; Hong, X.; Wang, D.S. Nanoscale high-entropy alloy for electrocatalysis. Matter 2023, 6, 1717–1751. [Google Scholar] [CrossRef]
- Mu, J.; Zhao, Z.; Gao, X.W.; Liu, Z.M.; Luo, W.B.; Sun, Z.; Gu, Q.F.; Li, F. Bimetallic PdFe3 nano-alloy with tunable electron configuration for boosting electrochemical nitrogen fixation. Adv. Energy Mater. 2024, 14, 2303558. [Google Scholar] [CrossRef]
- Lu, X.; Li, J.; Liu, F.; Wang, Y.; Tang, X.; Li, H.; Peng, Y.; Xu, C. Powerful orbital hybridization of copper-silver bimetallic nanosheets for electrocatalytic nitrogen reduction to ammonia. Inorg. Chem. 2023, 62, 12148–12156. [Google Scholar] [CrossRef]
- Pang, F.J.; Wang, Z.F.; Zhang, K.; He, J.; Zhang, W.Q.; Guo, C.X.; Ding, Y. Bimodal nanoporous Pd3Cu1 alloy with restrained hydrogen evolution for stable and high yield electrochemical nitrogen reduction. Nano Energy 2019, 58, 834–841. [Google Scholar] [CrossRef]
- Yang, L.Z.; Li, Y.Y.; Wang, Z.F.; Zhao, W.M.; Qin, C.L. Nanoporous quasi-high-entropy alloy microspheres. Metals 2019, 9, 345. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, L.; Wei, Z.; Wang, Z.; Liu, Q.; Hu, G.; Luo, J.; Liu, X. Coupling nitrate-to-ammonia conversion and sulfion oxidation reaction over hierarchical porous spinel MFe2O4 (M=Ni, Co, Fe, Mn) in wastewater. Small 2025, 21, 2411317. [Google Scholar] [CrossRef]
- Dong, L.; Luo, C.; Wang, Y.; Meng, S.; Yu, H.; Zhao, W.; Qin, C.; Wang, Z. Prelithiation enhanced initial Coulombic efficiency and cycling stability of spinel (FeCoCrNiMn)3O4 high entropy oxide anode. J. Energy Storage 2024, 98, 113185. [Google Scholar] [CrossRef]
- Mi, C.; Wang, Z.; Yang, S.; Liu, X.; Wang, Y.; Wang, Z. Porous Al11Ce3 intermetallics as effective sulphur host networks for stable lithium–sulphur batteries. J. Mater. Chem. C 2025, 13, 9014–9026. [Google Scholar] [CrossRef]
- Jin, Z.; Lv, J.; Jia, H.; Liu, W.; Li, H.; Chen, Z.; Lin, X.; Xie, G.; Liu, X.; Sun, S.; et al. Nanoporous Al-Ni-Co-Ir-Mo high-entropy alloy for record-high water splitting activity in acidic environments. Small 2019, 15, 1904180. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.; Park, Y.; Bhadouria, A.; Tackett, B. Effects of electrochemical active surface area of Cu on electrochemical CO reduction in acidic electrolyte using Cu nanoparticles on surfactant-treated carbon. J. Catal. 2024, 437, 115662. [Google Scholar] [CrossRef]
- Hochfilzer, D.; Tiwari, A.; Clark, E.; Bjørnlund, A.; Maagaard, T.; Horch, S.; Seger, B.; Chorkendorff, I.; Kibsgaard, J. In situ analysis of the facets of Cu-based electrocatalysts in alkaline media using Pb underpotential deposition. Langmuir 2022, 38, 1514–1521. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, Z.; Peng, G.; Hoenig, E.; Yan, G.; Wang, M.; Liu, Y.; Du, X.; Liu, C. Surface valence state effect of MoO2+x on electrochemical nitrogen reduction. Adv. Sci. 2022, 9, 2104857. [Google Scholar] [CrossRef]
- Wang, Y.C.; Ma, Z.Z.; Liu, K.; Yang, X.W.; Wang, J.X.; Wang, X.G. Rigid anchoring of highly crystallized and uniformly dispersed Pd nanocrystals on carbon fibers for ambient electrocatalytic reduction of nitrogen to ammonia. Dalton Trans. 2021, 50, 6975–6981. [Google Scholar] [CrossRef]
- Wan, Y.C.; Zheng, M.Y.; Lv, R.T. Rational design of Mo2C nanosheets anchored on hierarchically porous carbon for boosting electrocatalytic N2 reduction to NH3. Mater. Today Energy 2023, 32, 101240. [Google Scholar] [CrossRef]
- Yan, Y.; Qu, H.J.; Zheng, X.N.; Zhao, K.X.; Li, X.X.; Yao, Y.; Liu, Y. Amorphous core/shell Ti-doped SnO2 with synergistically improved N2 adsorption/activation and electrical conductivity for electrochemical N2 reduction. Chin. Chem. Lett. 2022, 33, 4655–4658. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Dai, Z.C.; Wang, S.Q.; Zhang, H.A.; Tian, W.J.; Xu, Y.; Li, X.I.; Wang, L.; Wang, H.J. Enhancing electrochemical ammonia synthesis on palladium nanorods through surface hydrogenation. Chem. Eng. J. 2021, 416, 129105. [Google Scholar] [CrossRef]
- Lu, X.Y.; Li, H.; Wang, Y.T.; Huang, J.F.; Xu, C.L. Amorphous ReS2 decorated TiO2 nanowire arrays for highly-efficient nitrogen reduction. Chem. Commun. 2021, 57, 6023–6026. [Google Scholar] [CrossRef] [PubMed]

| Material | Cu | Au | Pt | Pd |
|---|---|---|---|---|
| np-CuAuPtPd-1 | 34.5 | 20.9 | 19.3 | 25.3 |
| np-CuAuPtPd-2 | 30.2 | 22.9 | 22.0 | 24.9 |
| Electrocatalyst Materials | Potential (V vs. RHE) | NH3 Yield (μg h−1 mg−1) | Reference |
|---|---|---|---|
| MoO2+x | −0.2 | 3.95 | [56] |
| PdNCs@CNFs | −0.2 | 4.4 | [57] |
| Mo2C@C | −0.2 | 12.55 | [58] |
| Ti-doped SnO2 | −0.2 | 13.09 | [59] |
| PdH0.43NRs | −0.2 | 17.53 | [60] |
| ReS2/TiO2 | −0.2 | 49.8 | [61] |
| np-CuAuPtPd-1 | −0.2 | 17.5 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, S.; Meng, Z.; Zhao, W.; Wang, Z. Nanoporous CuAuPtPd Quasi-High-Entropy Alloy Prism Arrays for Sustainable Electrochemical Nitrogen Reduction. Metals 2025, 15, 568. https://doi.org/10.3390/met15050568
Hou S, Meng Z, Zhao W, Wang Z. Nanoporous CuAuPtPd Quasi-High-Entropy Alloy Prism Arrays for Sustainable Electrochemical Nitrogen Reduction. Metals. 2025; 15(5):568. https://doi.org/10.3390/met15050568
Chicago/Turabian StyleHou, Shuping, Ziying Meng, Weimin Zhao, and Zhifeng Wang. 2025. "Nanoporous CuAuPtPd Quasi-High-Entropy Alloy Prism Arrays for Sustainable Electrochemical Nitrogen Reduction" Metals 15, no. 5: 568. https://doi.org/10.3390/met15050568
APA StyleHou, S., Meng, Z., Zhao, W., & Wang, Z. (2025). Nanoporous CuAuPtPd Quasi-High-Entropy Alloy Prism Arrays for Sustainable Electrochemical Nitrogen Reduction. Metals, 15(5), 568. https://doi.org/10.3390/met15050568







