Geometric Analysis of the Scaling of the Manganese Recovery Process Using Current Distribution and Potential Simulation Techniques
Abstract
1. Introduction
2. Materials and Methods
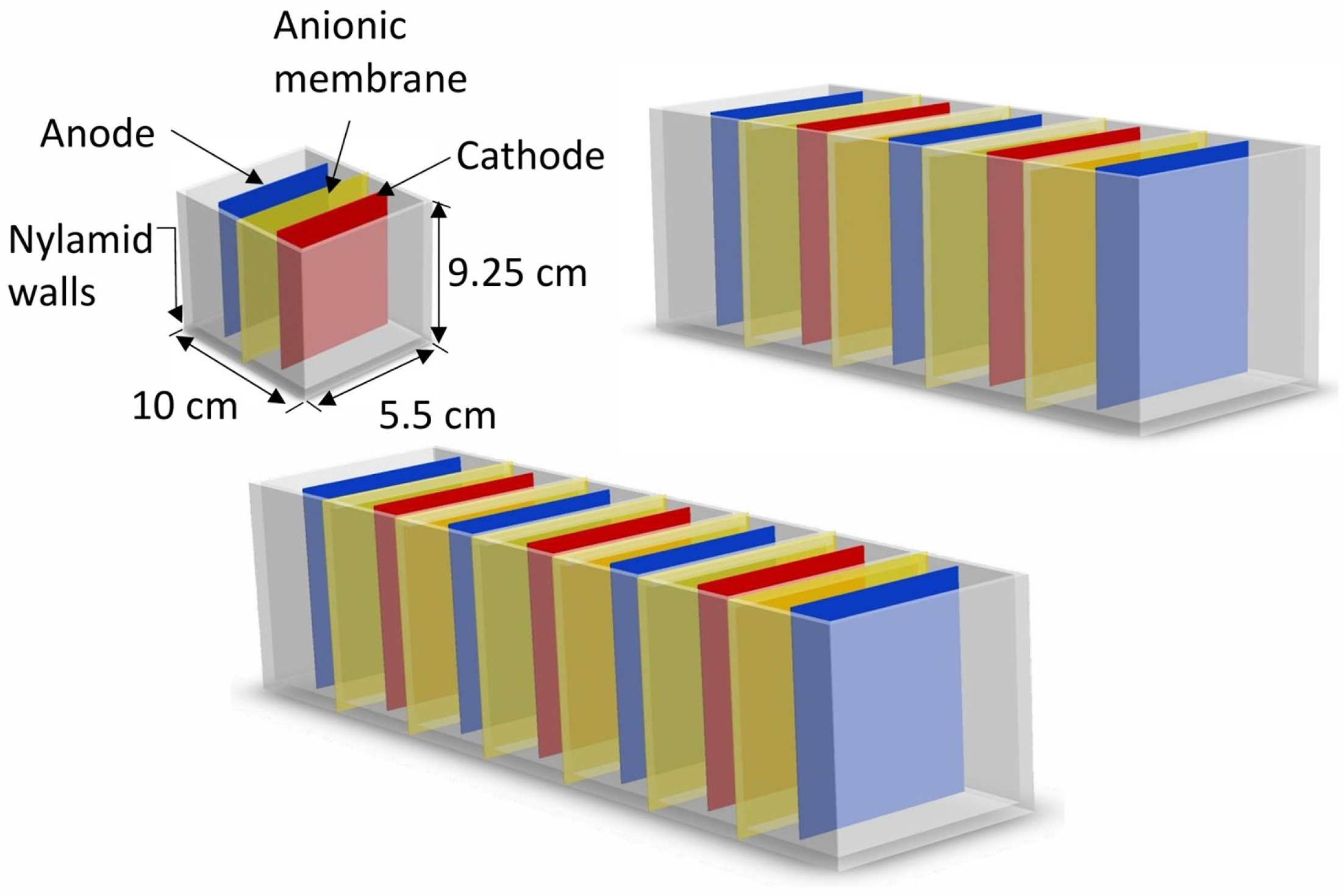
2.1. Studied Geometry
2.2. Mathematical Model
2.3. Simulation
2.4. Validation
3. Results
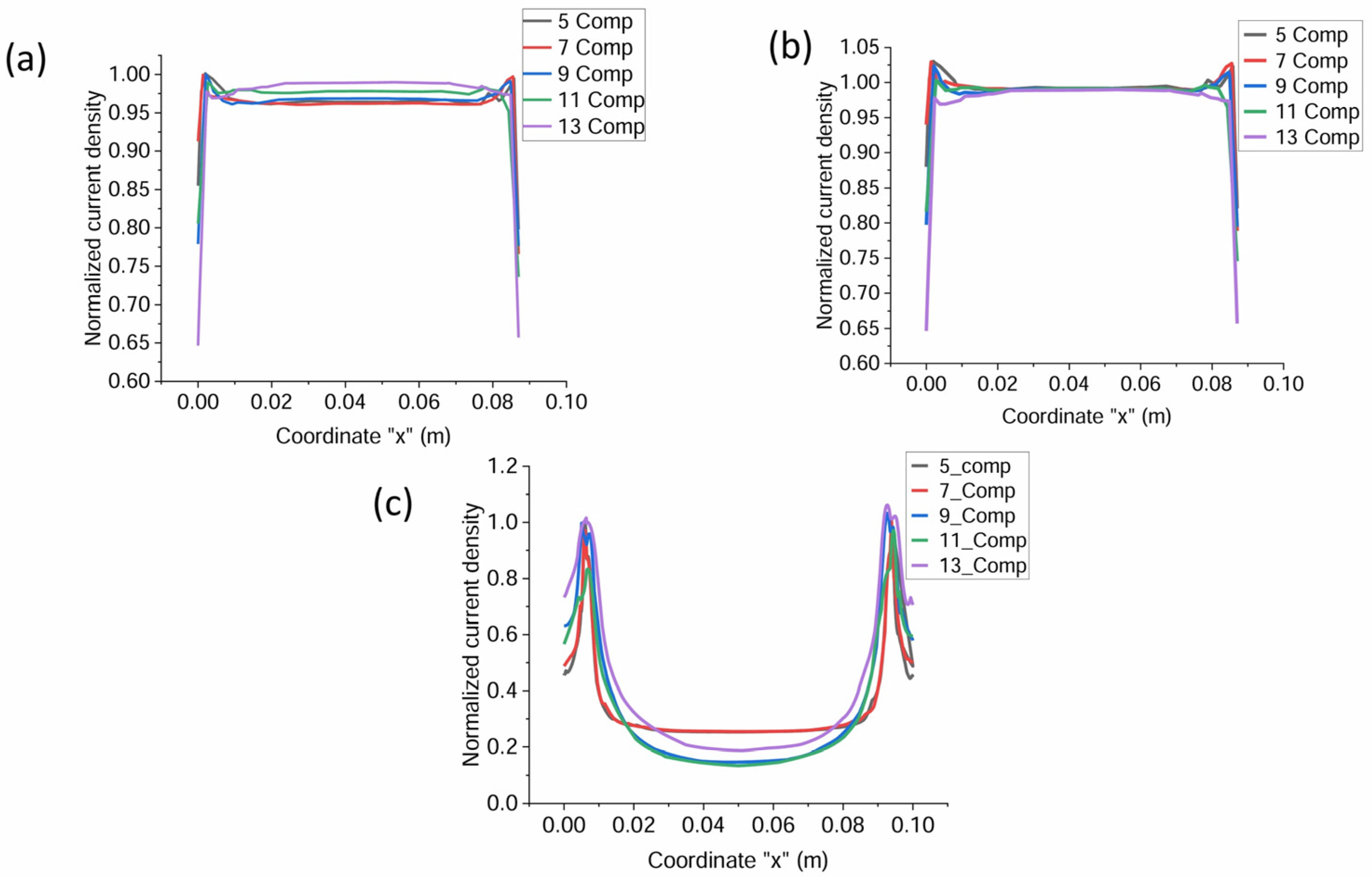
3.1. Current Density Distribution of the Electrolyte in Cell with Separate Compartments

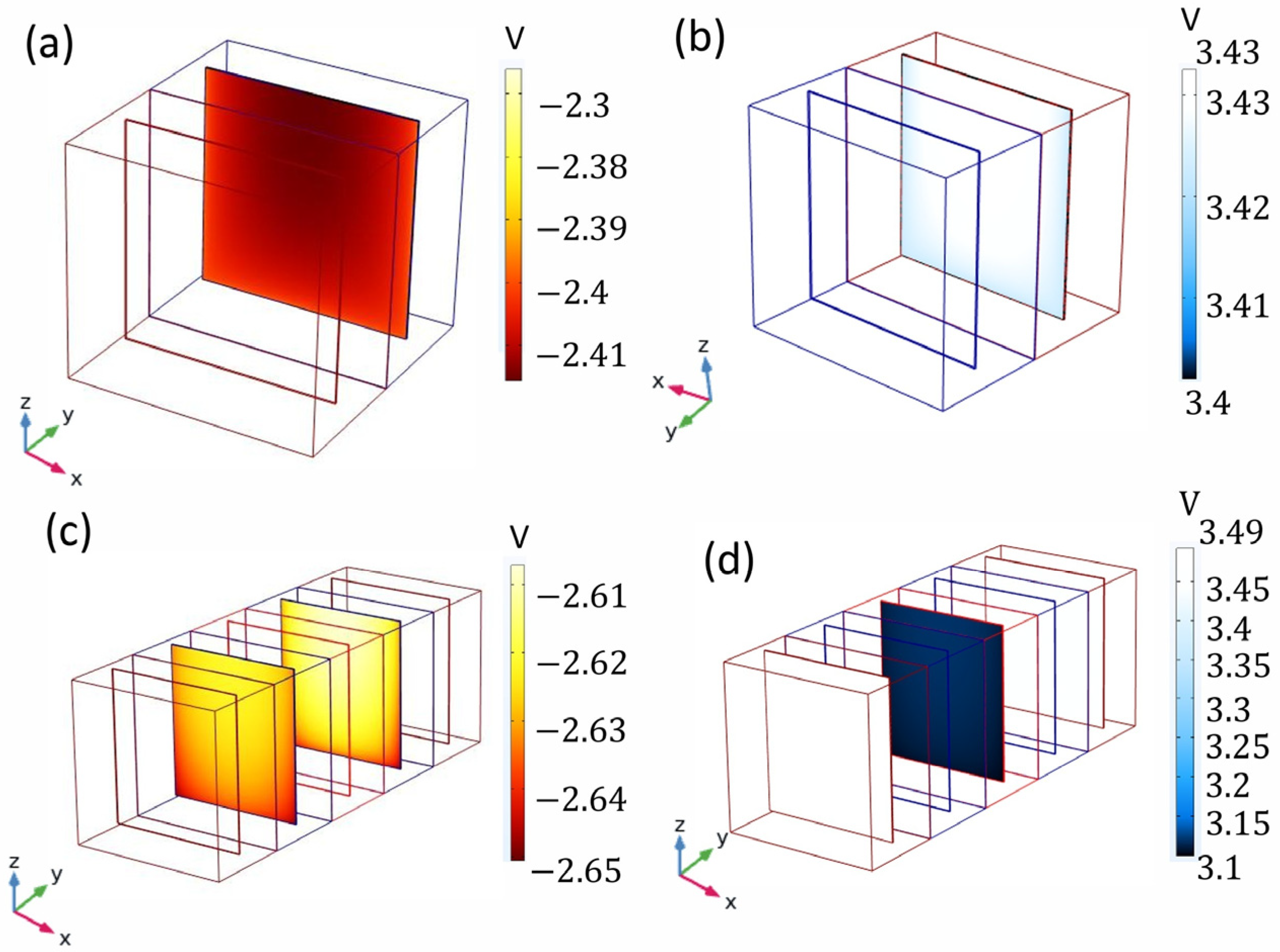
3.2. Distribution of the Electrochemical Potential in the Cells with Two Compartments and Five Compartments
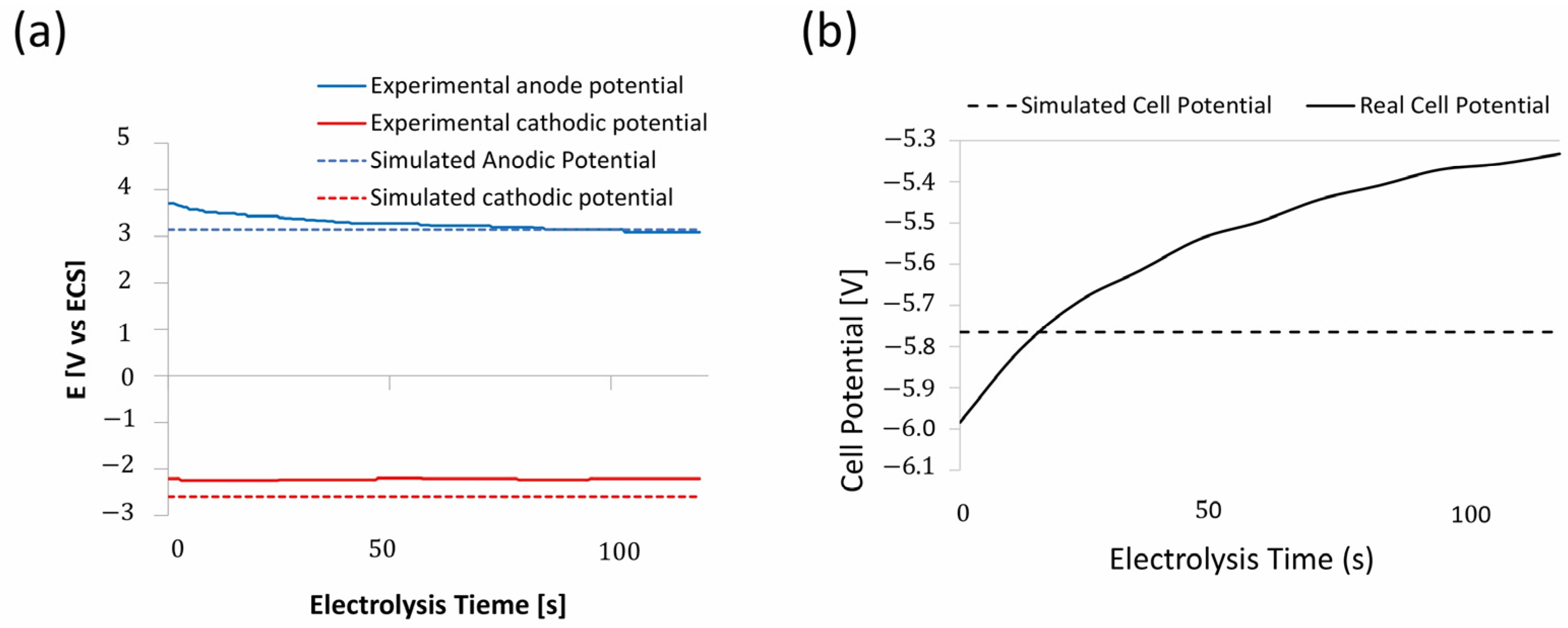
3.3. Experimental Validation
3.4. Projection of the Cell Operation with a Greater Number of Compartments
4. Conclusions
- ➢
- The similarity of the potential of cell, mass, and current density values of the two- and five-compartment cells of escalation simulation in COMSOL with the experimental results of the current-limiting controlled EMM validate the simulation programming parameters and thus the projection of the potential distribution of cathodes and anodes in cells with more than five compartments. As the number of compartments increases to five, energy consumption decreases from 9.2435 kWh/kg to 8.67 kWh/kg.
- ➢
- The 11-compartment cell is the most suitable for escalating the EMM deposit. This is because the normalized current density and potential distribution on the anodes are smaller compared to the 5-, 7-, 9-, and 13-compartment cells, resulting in a decrease in oxidation reactions and their impact on the cathode potential modifications.
- ➢
- Through simulation using the module current distribution secondary in COMSOL, it is possible to determine the potential distribution of anode and cathode electrodes in scaling electrochemical processes of electrowinning. This allows for scaling these processes to an industrial level, saving time, materials, and resources, as well as optimizing the electrochemical arrangement in diaphragm cells.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, V.; Chakraborty, T.; Tripathy, S.K. A Review of Low Grade Manganese Ore Upgradation Processes. Miner. Process. Extr. Met. Rev. 2019, 41, 417–438. [Google Scholar] [CrossRef]
- Aguirre, J.D.; Culotta, V.C. Battles with Iron: Manganese in Oxidative Stress Protection. J. Biol. Chem. 2012, 287, 13541–13548. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, X.; Chao, D.; Qiao, S.Z.; Alshareef, H.N.; Chen, W. Opportunities of Aqueous Manganese-Based Batteries with Deposition and Stripping Chemistry. Adv. Energy Mater. 2020, 11, 2002904. [Google Scholar] [CrossRef]
- Dashevskiy, V.Y.; Zhuchkov, V.I.; Zhdanov, A.V.; Leontyev, L.I. Production of Manganese Ferroalloys from Russian Manganese Ores. In Advanced Methods and Technologies in Metallurgy in Russia; Springer: Berlin/Heidelberg, Germany, 2018; pp. 103–111. [Google Scholar] [CrossRef]
- Karakurkchi, A.V.; Sakhnenko, N.D.; Ved, M.V.; Luhovskyi, I.S.; Drobakha, H.A.; Mayba, M.V. Features of Plasma Electrolytic Formation of Manganese- and Cobalt-Containing Composites on Aluminum Alloys. Adv. Mater. Sci. Eng. 2019, 2019, 1–13. [Google Scholar] [CrossRef]
- Sun, X.; Hao, H.; Liu, Z.; Zhao, F. Insights into the global flow pattern of manganese. Resour. Policy 2020, 65, 101578. [Google Scholar] [CrossRef]
- Fernández-González, D.; Sancho-Gorostiaga, J.; Piñuela-Noval, J.; González, L.F.V. Anodic Lodes and Scrapings as a Source of Electrolytic Manganese. Metals 2018, 8, 162. [Google Scholar] [CrossRef]
- Petersen, H.A.; Myren, T.H.; O’Sullivan, S.J.; Luca, O.R. Electrochemical methods for materials recycling. Mater. Adv. 2021, 2, 1113–1138. [Google Scholar] [CrossRef]
- Ayala, J.; Fernández, B. Recovery of manganese from silicomanganese slag by means of a hydrometallurgical process. Hydrometallurgy 2015, 158, 68–73. [Google Scholar] [CrossRef]
- Song, X.; Zhou, J.; Shui, B.; Wang, S.; Wu, N. Electrodeposition of Manganese Metal and Co-production of Electrolytic Manganese Dioxide Using Single-membrane Double-chamber Electrolysis. Electrochemistry 2021, 89, 447–454. [Google Scholar] [CrossRef]
- Wei, Q.; Ren, X.; Du, J.; Wei, S.; Hu, S. Study of the electrodeposition conditions of metallic manganese in an electrolytic membrane reactor. Miner. Eng. 2010, 23, 578–586. [Google Scholar] [CrossRef]
- Tsurtsumia, G.; Shengelia, D.; Koiava, N.; Lezhava, T.; Gogoli, D.; Beriashvili, L.; Hydrometallurgy, I.K. Novel hydro-electrometallurgical technology for simultaneous production of manganese metal, electrolytic manganese dioxide, and manganese sulfate monohydrate. Hydrometallurgy 2019, 186, 260–268. [Google Scholar] [CrossRef]
- Lu, J.; Dreisinger, D.; Glück, T. Electrolytic manganese metal production from manganese carbonate precipitate. Hydrometallurgy 2016, 161, 45–53. [Google Scholar] [CrossRef]
- Rojas-Montes, J.C.; Pérez-Garibay, R.; Uribe-Salas, A. The Effects of an Anionic Membrane on the Electrodeposition of Manganese Ions: A Kinetic and Statistical Study. J. Electrochem. Soc. 2013, 161, D67–D72. [Google Scholar] [CrossRef]
- Rojas-Montes, J.C.; Pérez-Garibay, R.; Uribe-Salas, A.; Teodoro, B.-S. Selenium reaction mechanism in manganese electrodeposition process. J. Electroanal. Chem. 2017, 803, 65–71. [Google Scholar] [CrossRef]
- Padhy, S.K.; Tripathy, B.C.; Alfantazi, A. Effect of sodium alkyl sulfates on electrodeposition of manganese metal from sulfate solutions in the presence of sodium metabisulphite. Hydrometallurgy 2018, 177, 227–236. [Google Scholar] [CrossRef]
- Montes, J.C.R.; Santillán, C.G.F.; Gómez, V.J.M.; Casillas, D.C.M.; Chigo, R.L.; Epistemus, F.A. Evaluación de Aditivos Alternativos Al SeO2 en la Electrodeposición de Manganeso Métalico. Epistemus 2021, 15, 49–55. [Google Scholar] [CrossRef]
- Reyes-Morales, Q.L.; Reyes-Cruz, V.E.; Trujillo-Estrada, A.; Cobos-Murcia, J.A.; Urbano-Reyes, G.; Pérez-Labra, M. Selenium and sulphur reactions involved in manganese reduction from sulphate solutions. RSC Adv. 2021, 11, 25542–25550. [Google Scholar] [CrossRef]
- Reyes-Morales, Q.L.; Reyes-Cruz, V.E.; Estrada, A.T.; Cobos-Murcia, J.A.; Reyes, G.U.; Labra, M.P.; Meneses, E.R. The effect of two anionic membranes (AMI 7001s and Neosepta AMX) on the electrodeposition of manganese from sulphate solutions. RSC Adv. 2022, 12, 17661–17674. [Google Scholar] [CrossRef]
- Zhong, H.; Zhang, Q.; Liu, Z.; Du, J.; Tao, C. Ti/Ti4O7 Anodes for Efficient Electrodeposition of Manganese Metal and Anode Slime Generation Reduction. ACS Omega 2023, 8, 38469–38480. [Google Scholar] [CrossRef]
- Lyu, K.; Zhang, W.; Chen, Y.; Wang, C. Energy-efficient and advanced electrowinning of metallic manganese within a novel H exchange membrane cell using a Ti/IrO−RuO−SiO anode. Sep. Purif. Technol. 2024, 347, 127489. [Google Scholar] [CrossRef]
- Cobos-Murcia, J.A.; Hernández-Aguilar, E.; Trujillo-Estrada, A.; Gallegos-Ortega, G.; Reyes-Cruz, V.E. Geometry optimization of an electrochemical reactor for bleaching kaolin. Clay Miner. 2022, 57, 183–191. [Google Scholar] [CrossRef]
- Tsai, T.H.; Babulal, S.M.; Wu, Y.F. Numerical simulation and experimental study on electrochemical recovery of copper using cylindrical and conical flow reactors. Int. J. Electrochem. Sci. 2024, 20, 100894. [Google Scholar] [CrossRef]
- Ma, C.W.; Yu, S.H.; Fang, J.K.; Yang, P.F.; Chang, H.C. The physical improvement of copper deposition uniformity with the simulation models. J. Electroanal. Chem. 2023, 948, 117790. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Tie, J. Numerical Simulation of Cathode Nodule Local Effects. Metals 2024, 14, 457. [Google Scholar] [CrossRef]
- Pérez, T.; León, C.P.; Walsh, F.C.; Nava, J.L. Simulation of current distribution along a planar electrode under turbulent flow conditions in a laboratory filter-press flow cell. Electrochim. Acta 2015, 154, 352–360. [Google Scholar] [CrossRef]
- Vázquez, A.; Rodríguez, I.; Lázaro, I. Primary potential and current density distribution analysis: A first approach for designing electrocoagulation reactors. Chem. Eng. J. 2011, 179, 253–261. [Google Scholar] [CrossRef]
- Giaccherini, A.; Caporali, S.; Berretti, E.; Marcantelli, P.; Sanzari; Perissi, I.; Innocenti, M. Finite Elements Analysis of an Electrochemical Coating Process of an Irregularly Shaped Cathode with COMSOL Multiphysics®. ECS Trans. 2015, 64, 1. [Google Scholar] [CrossRef]



| Parameter | Value |
|---|---|
| Manganese sulfate [M] | 0.27 |
| Ammonium sulfate [M] | 125.7 |
| Selenium dioxide [mM] | 5.4 |
| Acid sulfuric [M] | 0.5 |
| Chemical concentration equivalent [g/Ah] | 1.025 |
| Current density [A/m2] | 300 |
| Electrolysis time [min] | 120 |
| Temperature [T]/K | 293.15 |
| Parameter | Value |
|---|---|
| Anolyte conductivity [k]/Sm−1 | 203 |
| Catholyte conductivity [k]/Sm−1 | 125.7 |
| Membrane conductivity [k]/Sm−1 | 0.0363 |
| Exchange current density [i0]/Am−2 | 300 |
| Cathode external electrical potential [s, ext]/V | −2.5 |
| Anode external electrical potential [s, ext]/V | 3.5 |
| Cathodic transfer coefficient [αc] | 0.5 |
| Anodic transfer coefficient [αa] | 0.5 |
| Temperature [T]/K | 293.15 |
| Cell Configuration | Number of Elements (Domain) | Number of Elements (Contour) | Refinement and Solution Time |
|---|---|---|---|
| 2 compartments | 802,482 | 124,538 | 648 s |
| 5 compartments | 909,450 | 133,506 | 679 s |
| 7 compartments | 1,066,757 | 144,490 | 1213 s |
| 9 compartments | 1,146,220 | 126,504 | 1340 s |
| 11 compartments | 1,613,451 | 191,264 | 1808 s |
| 13 compartments | 2,401,282 | 284,600 | 1819 s |
| Cell | Real Mass [g] | , Real Current Efficiency [%] | Simulated Mass [g] | CE [kWh/kg] |
|---|---|---|---|---|
| 2 | 5.838 | 59 | 5.207 | 9.2435 |
| 5 | 11.88 | 60.7 | 11.616 | 8.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez Vigueras, E.M.; Reyes Cruz, V.E.; Galleguillos Madrid, F.M.; Cobos Murcia, J.A.; Reyes Morales, Q.L.; Urbano Reyes, G.; Vargas Ramírez, M.; Legorreta García, F.; Varas, M. Geometric Analysis of the Scaling of the Manganese Recovery Process Using Current Distribution and Potential Simulation Techniques. Metals 2025, 15, 562. https://doi.org/10.3390/met15050562
Rodríguez Vigueras EM, Reyes Cruz VE, Galleguillos Madrid FM, Cobos Murcia JA, Reyes Morales QL, Urbano Reyes G, Vargas Ramírez M, Legorreta García F, Varas M. Geometric Analysis of the Scaling of the Manganese Recovery Process Using Current Distribution and Potential Simulation Techniques. Metals. 2025; 15(5):562. https://doi.org/10.3390/met15050562
Chicago/Turabian StyleRodríguez Vigueras, Esaú M., Victor E. Reyes Cruz, Felipe M. Galleguillos Madrid, José A. Cobos Murcia, Quinik L. Reyes Morales, Gustavo Urbano Reyes, Marissa Vargas Ramírez, Felipe Legorreta García, and Marinka Varas. 2025. "Geometric Analysis of the Scaling of the Manganese Recovery Process Using Current Distribution and Potential Simulation Techniques" Metals 15, no. 5: 562. https://doi.org/10.3390/met15050562
APA StyleRodríguez Vigueras, E. M., Reyes Cruz, V. E., Galleguillos Madrid, F. M., Cobos Murcia, J. A., Reyes Morales, Q. L., Urbano Reyes, G., Vargas Ramírez, M., Legorreta García, F., & Varas, M. (2025). Geometric Analysis of the Scaling of the Manganese Recovery Process Using Current Distribution and Potential Simulation Techniques. Metals, 15(5), 562. https://doi.org/10.3390/met15050562








